Abstract
Leukemic cancer stem cells (LSCs), as a unique cell population in acute myeloid leukemia (AML) marked by CD123 overexpression, are thought to play a key role in relapsed AML after chemotherapy. Thus, CD123 is considered as a particularly important target candidate for antibody-derived diagnosis and therapy. In the present work, we constructed an immunized murine antibody phage display library and isolated the functional anti-CD123 Single-chain fragment variable (scFv) clones. We also introduced fusing variable light (VL) and heavy (VH) chains with a new 18-amino acid residue linker as an alternative to conventional linkers. CD123-specific phage clones were progressively enriched through 4 rounds of biopanning, validated by phage ELISA, and anti-CD123 scFv clones with highest affinity were produced in Escherichia coli. The expression and purification of soluble scFv were verified by Western blot, and the results were indicative of the functionality of our proposed linker. The purified scFv specifically recognized CD123 by ELISA and flow cytometry, without any cross-reactivity with other related cell markers. Affinity of anti-CD123 scFv was measured to be 6.9 × 10−7 M, using the competitive ELISA. Our work, therefore, provides a framework for future studies involving biological functions and applications of our anti-CD123 scFv. It also reveals the feasibility of high throughput methods to isolate biomarker-specific scFvs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is a proliferative disease of immature myeloid lineage in the bone marrow [1]. Leukemic cancer stem cells (LSCs), as a unique cell population in AML, are thought to play a key role in the relapsed AML in patients after conventional chemotherapy. Intensive surface biomarker investigation of LSC population has previously demonstrated the elevated expression of cluster of differentiation 123 (CD123) on LSCs [2]. Clinical association with higher blast counts at diagnosis, a lower complete remission rate, poorer prognosis, and IL-3 dependence of LSC functions makes CD123 a particularly important target candidate for antibody-derived therapeutics [3, 4]. Consequently, antigen-targeted elimination of LSCs using antibodies has been previously explored [5, 6].
The alpha subunit of the interleukin-3 receptor (IL-3Rα)/CD123 consists of three extracellular domains, a transmembrane domain, and a short intracellular domain. The N-terminal domain of this molecule plays a significant role in binding to IL-3 and is a potential epitope for antibody production [5].
Monoclonal antibodies and their derivatives represent the fastest growing class of biopharmaceuticals [7], among which phage display technology has provided a high throughput screening for antibody development [8]. Single-chain fragment variable (scFv) is an engineered antibody in which the variable light (VL) and heavy (VH) chains of an antibody are fused by a short polypeptide linker, forming the antigen-binding domain [9]. scFv holds important potential for receptor blockade, pathogen neutralization, and therapeutic antigen targeting in vivo [10]. Reduced molecular weight, low immunogenicity, large-scale expression in Escherichia coli system, and yet superior tissue-penetrating capability make scFv an ideal antibody format for many diagnostic and therapeutic purposes [11].
So far, different formats of recombinant protein targeting CD123 have been developed [5]. To the best our knowledge, only one report on engineered anti-CD123 scFv has been published using anti-CD123 scFv isolated from phage display library, as part of a bi-specific anti-CD123-CD33 scFv protein [12]. It has been demonstrated that the proper folding of scFv and the association of VH/VL, expression level, and the solubility largely depend on the linker length and sequence and also significantly vary from one linker to another [13–15]. Several strategies have been developed to design the linker of scFv. One approach is to use flexible glycine-rich sequences, which, as mentioned above, has been used by Stein et al. for generating anti-CD123 scFv [12]. Alternatively, we have used linkers derived from multi-domain natural proteins [13]. In the current strategy, the VH and VL gene fragments were joined by a flexible 18-amino acid residue linker, containing the first six amino acids of the CH1 constant region domain and the hydrophilic pig brain alpha-tubulin peptide sequence EEGEFSEAR as a new linker [16]. Therefore, the goal of this study was to generate a new CD123-specific antibody scFv using the phage display library strategy. In order to achieve this goal, we aimed at (i) constructing an immune murine scFv library, (ii) exploring the feasibility of a new linker to fuse VH and VL fragments, (iii) identifying an scFv clone with a typically expected range of affinity in vitro.
Materials and Methods
Mice Immunization and Humoral Response Evaluation
Two 6–8-week-old female BALB/c mice (Pasteur Institute of Iran, Iran) were immunized with 10 μg of recombinant human IL-3Rα/CD123 (R&D Systems, USA) emulsified in an equal amount of complete Freund’s adjuvant (Sigma, Germany) at day zero. Boost immunizations were weekly administered with the decreasing doses of 10, 7.5, and 5 μg emulsified in an equal amount of incomplete Freund’s adjuvant (Sigma, Germany), for three consecutive weeks. Spleens of the immunized mice were dissected and used for further analysis 3 days after the last booster inoculation. The routes of administration were intraperitoneal for the first injection and subcutaneous for boosters and intravenous without adjuvant for the last booster.
Anti-CD123 in the serum of immunized mice was measured by ELISA. Briefly, a 96-well ELISA plate was coated with 100 ng/well of recombinant CD123 antigen (R&D Systems, USA) overnight at 4 °C, followed by blocking with 3 % bovine serum albumin (BSA) in Phosphate-Buffered Saline (PBS) buffer (pH 7.0) at 37 °C for 1 h. Wells were then washed 3 times with PBS-Tween (0.05 % v/v), and 100 μl of the diluted serum (1/1000 v/v in PBS) was added to the wells. One-hundred nanograms per well of 7G3 monoclonal mouse anti-human CD123 (BD Pharmingen, USA) was used as the positive control, and the serum obtained from mouse prior to immunization was used as the negative control. Wells were rinsed three times after 1 h of incubation at 37 °C. One-hundred microliters of diluted secondary goat HRP-conjugated anti-mouse antibody (Sigma, Germany) was added to each well and incubated at 37 °C for 1 h. Reactions were then developed by 100 μl of TMB (3, 30, 5, 50-tetramethylbenzidine) (Sigma, Germany) and stopped by the addition of 50 μl/well of 1 M sulfuric acid. The absorbance at 450 nm was determined using an ELISA microtiter plate reader (Stat Fax 2100, Awareness Technologies, USA).
RNA Extraction, cDNA Synthesis, and Variable Region Amplification
Total RNA was extracted from homogenized spleen tissue using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The first-strand Complementary DNA (cDNA) was generated from the extracted RNA using QuantiTect Reverse Transcription Kit (Qiagen, Germany) according to the manufacturer’s guidelines.
Two sets of primers in a two-step procedure were used to amplify the immunized mouse rearranged IgG variable domain of light and heavy chains (PROGEN, Biotechnik GmbH, Heidelberg, Germany). For the primary PCR reaction, total cDNA derived from the purified lymphocytes of immunized mouse was used as a template and the VH and VL repertoire was separately amplified using specific primers in 22 reactions (11 VH, 11 VL). A secondary PCR reaction of each product was performed using primers with embedded restriction sites for subcloning. Consequently, NcoI (5′), HindIII (3′) and MluI (5′), and NotI (3′) were added to the amplified VH and VL, respectively. The second PCR reactions were performed in 22 separate reactions. The amplified VH/VL was linked by flexible linker from pSEX81 phagemid sequence (PROGEN Biotechnik GmbH, Heidelberg, Germany). The pSEX81 plasmid has been described elsewhere [16].
Library Construction
Equal DNA amounts (~200 ng) from each individual secondary VL and VH PCR product repertoires were combined into two pools (one VL, one VH), respectively. Both pools were initially purified and concentrated using a gel extraction kit (Qiagen, Germany). The digested DNA (NcoI/HindIII for VH and MluI/NotI for VL) was further purified by PCR Cleanup kit (Qiagen, Germany). The purified MluI/NotI digested light chains were cloned into the pSEX81 plasmid. Library ligation reactions were prepared using 1.4 μg of pSEX81 vector and 700 ng of scFv insert. Twelve reactions of 10 μg of plasmid were performed, and the products of one ligation reaction were used for the electroporation of 50 μl of electrocompetent E. coli TG1 according to the manufacturer’s protocol, followed by an overnight incubation at 37 °C on SOB-GA agar plates (LB agar containing 100 mg/ml ampicillin and 100 mM glucose) to generate light chain sublibrary. VL gene insertions were confirmed by PCR using templates of randomly selected colonies. The number of transformants was calculated from bacterial colony counts to determine sublibrary size as previously described [17]. The total bacterial library was harvested by scraping the colonies, and the vector DNA containing the light chain sublibrary was then prepared. Subsequently, the NcoI/HindIII digested H chain pools were cloned into the light chain containing vectors, creating a scFv library. The ligation of the heavy chain PCR products into pSEX81 containing the light chain library was performed under the same conditions as used for cloning the light chains into pSEX81. The size of library along with the transformation efficiency was determined as previously described [17].
Phage Amplification and Biopanning
To rescue scFv-displaying phages, 100 ml of 2xYT supplemented with ampicillin (100 mg/ml) and 1 % glucose (NE medium) was inoculated with approximately 4 × 106 cells (100 μl) from the glycerol library stock and incubated at 37 °C until an OD600 of 0.4 to 0.6 was obtained. The culture was superinfected with VCSM13 helper phage (Stratagene, USA), to rescue the phagemid with its scFv insert at a multiplicity of infection (MOI) of 20 and incubated for 1 h at 37 °C, followed by centrifuging and resuspending the cells in fresh 2xYT-AK medium (2xYT medium containing 100 mg/ml ampicillin and 50 mg/ml kanamycin). After overnight cultivation, the rescued phage was precipitated by the addition of PEG/NaCl (PEG 6000 in 2.5 M, NaCl (20 % w/v)) for 1 h on ice and pelleted by centrifugation at 4000 rpm, for 30 min at 4 °C and resuspended in 1 × PBS, which was further spun at 14,000 rpm for 5 min at 4 °C. The supernatant was used for panning, as described by the standard protocol [17, 18]. Four rounds of panning were performed on recombinant human IL-3Rα/CD123 (R&D Systems, USA). Briefly, panning of our phage scFv library was performed against CD123 which had been coated on microtiter plates (Maxisorp, Nunc, Denmark) at a concentration of 5 μg/well overnight at 4 °C. After rinsing three times with PBS, the immune tube was blocked with 3 % BSA in PBS for 1 h at room temperature. Then, the prepared phage was added and incubated at room temperature for 2 h. Unbound phage was removed by rinsing five times with PBST (PBS containing 0.05 % v/v, Tween 20). The CD123-binding phage was eluted from the immune tube with 100 μl of 100 mM triethylamine followed by neutralization with 100 μl of 1 M Tris–HCl (pH 7.5). The eluted phage was then used to infect E. coli TG1 (in logarithmic phase) for 1 h at 37 °C, and then the enriched phagemids were rescued using VCSM13 helper phage (Stratagene, USA), as described above. Amplified phage was titrated and used as the input phage for the next consecutive round of panning. Input and eluted phage titers were calculated for each panning round according to the number of colonies. While phage input for all rounds during panning was maintained at approximately 1012 pfu/100 μl, the antigen concentration was reduced to 5, 2.5, 1, and 1 μg per round of panning and washing steps were increased to 5, 10, 15, and 20 times for stringent selection. In addition to this, in all rounds, a well without coating antigen was considered as the negative control.
Polyclonal Phage ELISA
CD123 specificity of the isolated phage was assessed by ELISA, using the pooled phage from each subsequent rounds of panning. Wells of a microtiter plate (Maxisorp, Nunc, Denmark) were coated with 100 μl of 1 μg/ml CD123 in PBS or PBS alone as negative controls. After incubating the plates for 16–20 h at 4 °C, wells were blocked with 200 μl of 3 % BSA and incubated for 1 h at room temperature. Such wells were washed three times with PBST (0.05 % Tween 20 v/v) and then added with a 109 phage solution and incubated for 2 h at room temperature. After washing the plate five times with PBST, HRP-conjugated anti-M13 antibody (1:3000 v/v) (GE Healthcare, USA) was added and incubated for 1 h at room temperature. Following washing, TMB (3, 30, 5, 50-tetramethylbenzidine) (Sigma, Germany) was added, the developing reaction was stopped by 1 M sulfuric acid, and the absorbance was measured at OD450.
Screening of CD123-Specific Clones by Monoclonal Phage ELISA
After four rounds of panning, 200 individual clones from rounds 2 to 4 of panning were randomly selected to assess the specificity by ELISA. Briefly, single colonies were picked up and cultured in 200 μl of 2xYT-AG medium (2xYT medium containing 1 % glucose and 100 mg/ml ampicillin) overnight at 37 °C and 200 rpm, using 96-microwell plates. Then, individual phage clones were produced by the addition of VCSM13 helper phages, as described in the phage amplification section. Screening of antigen-specific clones was carried out as described above for polyclonal phage ELISA.
Expression of Soluble Anti-CD123 scFv
The highest affinity phage clones were selected for further analysis. ScFv sequence fragment was digested from the pSEX81 vector and subcloned into the NcoI/NotI linearized expression vector pOPE101-215 (Yol) (PROGEN Biotechnik GmbH, Heidelberg, Germany), fused to the C-terminal His-tag and C-myc-tag sequence in frame, and E. coli HB2151 was transformed for soluble protein expression. Each clone was grown in 1 ml of TB medium containing ampicillin at 37 °C until the OD600 reached 0.7–0.8, followed by the induction with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and incubation for 16 h at 28 °C. Since the vector has pelB leader signal sequence, the soluble scFv was expressed as periplasmic protein. Overnight cultures were centrifuged at 3000 rpm for 30 min, and soluble protein from the periplasm was extracted by resuspending the pellet in ice-cold 1X TES buffer (0.2 M Tris–HCl pH 8.0, 0.5 mM EDTA, 0.5 M sucrose). After adding TES, cells were vortexed for 30 s and incubated on ice for 1 h, followed by resuspension in 2 volumes of TES/4 (TES diluted 1:4 in water), incubated on ice for 12 h. ScFv protein was collected by centrifugation at 3000 rpm for 30 min. Functional scFv production in the supernatant of each culture was evaluated by ELISA using HRP-conjugated protein L (Thermo Fisher Scientific, USA). Subsequently, the highest affinity anti-CD123 clone according to the previous ELISA was selected and grown in 300-ml cultures and the periplasmic fraction was extracted as previously described. Soluble scFv antibodies were purified using Ni-Nta metal affinity chromatography (Qiagen, Germany) according to the manufacturer’s instructions. The eluted scFv with 500 mM imidazole was dialyzed against 1X PBS for 24 h at 4 °C.
Characterization of Purified Anti-CD123 scFv Antibody
SDS-PAGE, Western Blot
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed based on the standard protocols using the Mini-PROTEANTM system (Bio-Rad, USA). The purified scFv samples were subjected to SDS-PAGE (12 %). Coomassie Brilliant Blue staining and then Western blot assay were carried out using pre-stained standards (Thermo Fisher Scientific, USA). After electrophoresis, the proteins were transferred to a nitrocellulose membrane and blocked for 16 h at 4 °C using 3 % BSA in TBS buffer, followed by washing four times for 5 min in 20 ml of TBS, 0.1 % (v/v) Tween 20. HRP-conjugated protein L diluted (1:3000) in TBS buffer (Thermo Fisher Scientific, USA) was added to detect the scFv antibodies. After 1 h of incubation, the membrane was washed four times with TBS 0.1 % (v/v) Tween 20 and developed by 3, 3-diaminobenzidine (DAB) (Sigma, Germany).
Antigen-Binding Activity and Specificity
Indirect ELISA was performed using a Protein L-HRP conjugate (Thermo Fisher Scientific, USA) to evaluate the antigen-binding activity of purified CD123-specific scFv. The experiment was performed twice and each time ELISA measurements were carried out in duplicate as previously described.
Moreover, specificity of anti-CD123 scFv was determined against markers such as CD20 and CD47, frequently expressed by cancer stem cells. Ninety-six-well microplates were coated with 100 μl of 1 μg/ml CD20, CD47, CD123, BSA, and casein, to evaluate cross-reactions of purified soluble scFv antibody by ELISA.
Affinity Determination
Affinity constant (Kaff) of scFv against CD123 was determined using the protocol described by Pierre Martineau [19]. Binding experiment by ELISA was performed as described above with some modifications. CD123-coated wells were probed with scFv (2 μg/ml) followed by adding 0.39, 6.25, 25, and 100 nM of free CD123 antigen for 1 h at room temperature to complete the binding.
Cell Culture and Cell-Binding Assay by Flow Cytometry
Human hematopoietic cancer cell lines, CD123+ KG1 and CD123− MOLT-4 (Pasteur Institute of Iran, Tehran, Iran), were cultured in RPMI medium supplemented with 10 % fetal bovine serum, at 37 °C. Cell surface antigen binding of selected scFv was determined by flow cytometry analysis using PBS as the negative or isotype control. Briefly, 5 × 105 cells were suspended in staining buffer (PBS, 4 % FBS v/v). The prepared scFv at a concentration of 10 μg/ml was pre-incubated with anti His-tag monoclonal antibody (Qiagen, Germany) followed by 1 h of incubation at 4 °C with cells. The cells were then washed three times with ice-cold wash buffer (PBS, 4 % FBS v/v) and centrifuged at 300 ×g. Thereafter, they were resuspended in 500 μl of ice-cold wash buffer and stained at 1:500 (v/v) goat anti-mouse FITC antibody (Abcam, UK) for 1 h at 4 °C. Cells were washed twice, acquired on FACS Calibur (Partec, Germany), and the obtained data was analyzed using FlowJo 9.7 (Treestar, Ashland, OR, USA). Statistical analysis was performed by GraphPad Prism 5 software (La Jolla, San Diego, USA).
DNA Sequence Analysis of Heavy and Light Chain Variable Regions of the Selected Clones
Nucleotide sequence of extracted plasmid DNA of the selected clones was determined by Bioneer biotech company (Korea). Immunoglobulin BLAST (IgBlast) was used to search for mouse Ig homologues in the database. The sequences of VH and VH genes were compared with the germline sequences in the V BASE database (MRC Centre for Protein Engineering, Cambridge, UK).
Results
Construction of scFv Phage Display Library
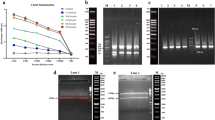
ELISA analysis showed that titer of 1:1000 (v/v in PBS) of anti-CD123 polyclonal antibody raised in Balb/C mice exhibited a significant binding activity to the given antigen, showing successful immunization (Fig. 1). Subsequently, in all 11 VH reactions, PCR products of ~400 bp were amplified (Fig. 2a). Similarly, a ∼380 bp of 11 VLs was amplified (Fig. 2b).
Immunoassay results of antibody immune response to CD123. Sera from two mice immunized with CD123, on days 0 (non-IS) and 49 (IS). Non-immune serum (day 0) was collected before the immunization. Immune sera, IS (day 49), were collected after the third boost. Results are two independent tests on two mice
Analysis of amplified mouse variable fragment of light and heavy genes. PCR products of a VH (400 bp) and b VL (~380 bp) were run on 1.5 % agarose gel using GeneRuler 50 bp DNA marker (Thermo Fisher Scientific, USA). c Colony PCR of sublibrary; PCR on 30 clones revealed 89 % of the clones contained an insert representing by bands at ~1000 bp. d Twenty-nine randomly selected colonies showed amplification of 28 scFv inserts of ~1000 bp, indicating efficiency of 96 %. Results are compared with GeneRuler 1 kb DNA Ladder (Thermo Fisher Scientific, USA)
Following VL and pSEX81 digestion, ligation, and transformation and making use of the pair of primers for MCS of vector, the insertion of VL in the plasmid construct was confirmed by PCR amplification from 30 randomly selected colonies. Twenty six out of 29 clones contained a ~1 kb DNA fragment which confirmed the presence of ~400 bp of VL (Fig. 2c). This implied that the recombination frequency of sublibrary (vector + VL) was about 89 %. Similarly, colony PCR screening of library confirmed that 28 out of 29 individual clones randomly picked from the library contained an insert of the correct size (Fig. 2d). This implied that the recombination frequency of scFv library was about 96 %. Taking such percentage of successful cloning into account, the actual size of the library was calculated following the counting of the colonies on plates, to be approximately 4 × 107 cfu/ml clones with 96 % efficiency and large enough to be considered representative of the murine antibody repertoire.
scFv Phage Library Screening and Identification of CD123-Specific scFv
To determine whether Ag-specific scFv clones could be isolated from the constructed library, we screened the scFv phage display library for the presence of scFv clones that bound to CD123, through a biopanning process.
Input and output numbers of phage were determined before and after each round of panning procedure to determine the enrichment of the binders specific to CD123 (Table 1). Following each panning, the number of eluted phage steadily increased suggesting that specific binding clones were selected and enriched during panning. As shown in the Table 1, phage enrichment was successfully achieved in every panning round through a stringent wash step and decreasing the amount of antigen for consecutive rounds of panning. Output rates of the first and second round had the same order of magnitude, indicating that sufficient high affinity antibodies were favored after three rounds of panning. The results showed that the titer of phage bound to CD123 steadily increased from 106 pfu in the first round to 1.3 × 108 pfu in the fourth one.
In order to confirm the progressive enrichment of CD123-specific scFv, pooled amplified phage from each consecutive round of panning was assessed by ELISA (Fig. 3a). Following each round of panning, phage isolates showed a steady increase in Ag-specific phage binding. In contrast, binding of panned phage libraries to negative wells remained at background levels. As expected, the highest signal was observed in the fourth round of panning on the immobilized antigen.
Screening of specific CD123 binding. a Polyclonal phage-scFv ELISA using the amplified pool phage from each round of panning. Results demonstrated that increasing signal in each round, with a strong specific response observed after the fourth round comparing to the first round. The background (wells without antigen) was subtracted from the absorbance to the respective CD123-coated wells. b Monoclonal phage ELISA. Absorbance at 450 nm for randomly selected phage clones showed ratio binding over negative control. c Selected clones for further analysis based on higher absorbance over the background (wells without coated antigen). d The periplasmic extract of selected clones containing soluble scFv was directly used for an ELISA to identify monovalent binding. Clone 110 with the highest affinity was selected for further study. Results are one-way ANOVA test. All analysis representative of 2 independent experiments ± SD. e A phylogenetic tree constructed by MEGA software version 6 (Tamura, USA), using neighbor-joining, Dayhoff model. Five-hundred bootstrap replicate performed based on amino acid sequence of 6 clones indicating diverse amino acid composition of them
Furthermore, to identify individual CD123 specificity, scFv clones derived from eluted phage were screened for CD123 specificity by monoclonal phage ELISA. More than 200 clones were randomly picked and assessed for binding to CD123 by ELISA among which 30 clones displayed CD123-binding activity (Fig. 3b). Subsequently, 6 clones with relatively higher binding affinity to CD123 compared to the negative control were chosen for further analysis (Fig. 3c).
Subcloning, Expression, and Purification
In order to induce the expression of functionally soluble scFv protein and add detection tag peptide, DNA phagemid of six strongly positive clones with the highest signals was subcloned into the pOPE101 expression vector. The presence of functional expression of scFv was detected by SDS-PAGE of recombinant E. coli periplasmic extract (Fig. 4a). Also, binding of the six periplasmic extract soluble scFv antibodies to the antigen was determined by ELISA. The results were indicative of CD123 binding of all scFv antibodies to a variable degree, whereas none of them exhibited significant binding to negative control wells (Fig. 3d).
Expression of soluble scFv antibody. a Silver staining of periplasmic extract of 6 selected clones; the last lane is negative control (E. coli without pOPE101); the ~28 kDa band was observed in all 6 selected clones, except for negative control (pOPE-). Results are compared with PageRuler pre-stained protein ladder (Thermo Fisher Scientific, USA). b SDS-PAGE of purified anti-CD123 scFv clone 110, Coomassie Brilliant Blue staining. From left to right: eluted scFv from Ni-NTA column (1), periplasmic extract of E. coli HB2151 without pOPE-scFv plasmids (negative control), periplasmic extract before purification (3), and PageRuler pre-stained protein ladder (Thermo Fisher Scientific, USA) (M). c Western blot of purified anti-CD123 scFv, clone 110, by protein L-HRP conjugate. PageRuler pre-stained protein ladder (Thermo Fisher Scientific, USA) (M), purified scFv (1), periplasmic extract before purification (2), and periplasmic extract of E. coli HB2151 without pOPE-scFv plasmid as negative control (3)
In this regard, selection of the best clone was performed based on the binding affinity and therefore, clone 110 was chosen for further characterization. The protein expression of clone 110 was analyzed by SDS-PAGE with Coomassie Brilliant Blue, as well as the Western blot using protein L-HRP conjugate. A distinct band of ~28 kDa within the expected molecular weight range of soluble scFv was visualized. The SDS-PAGE analysis indicated that the vast majority of anti-CD123 scFv clone 110 protein formed soluble monomer proteins. No fractions corresponding to the oligomeric forms of scFv were eluted from the column. This indicated the low tendency of generated anti-CD123 scFv to form multimeric structures (Fig. 4b) and confirmed the successful expression of soluble scFv with the yield of 1 mg/l of bacterial culture.
Characterization of Purified Soluble Anti-CD123, a Selected Clone
Sequence analysis of the selected clones confirmed that scFv VH gene fragment had linked to a VL without mutations or deletions. In addition, all amino acid sequences in the CDR3 regions of both heavy and light variable chains were diverse (Table 2).
Antigen-specific binding of the purified scFv protein was further measured by ELISA as shown in Fig. 5a. Non-specific binding was not observed in the case of negative controls (wells without CD123 coating) indicating the binding specificity of scFv with a fivefold higher signal compared to negative control. In addition, the scFv clearly showed the binding only to CD123 but not to other available cancer stem cell markers, such as CD47, CD20, or complex proteins such as BSA and casein. Thus, scFv could be used to detect CD123 without cross-reactivity to other tested proteins (Fig. 5b).
Characterization of purified scFv-110. a Purified soluble scFv binding to CD123 in indirect ELISA. Soluble scFv clone showed significant binding to CD123 as little as 100 ng/well compared to the background (wells without coated antigen). Results are unpaired t test analysis representative of 2 independent experiments ± SD. b Specificity analysis of scFv by ELISA. Different tumor-associated antigens such as CD47, CD20, and complex proteins such as BSA and casein were coated at 100 ng/well. Clone 110, specifically bound to CD123 antigen. Results are one-way ANOVA test analysis representative of 2 independent experiments ± SD. c Flow cytometry analysis of purified scFv on CD123+ KG1 and CD123− MOLT-4 cell lines. Histograms are gated on forward scatter and side scatter and are representative of 10,000 events. d Mean fluorescence intensity (MFI) of stained cells
The relative affinity constant for the scFv against CD123 was calculated using a competitive ELISA method [19]. The affinity constants were calculated for scFv to be 693 nM (6.9 × 10−7 M).
To further evaluate the CD123-binding specificity of scFv-110, flow cytometry analysis on cell lines was performed. As indicated by the shift in fluorescent intensity in Fig. 5c, flow cytometry analysis revealed that scFv-110 specifically binds to CD123-expressing KG1 cells compared to the control cell line, MOLT-4. These results further demonstrate that our scFv-110 derived from our library is biologically functional and specific to CD123.
Taken together, these results indicate that antigen-specific scFv clone can be generated and isolated by phage display strategy, and that our new linker can function as an alternative to the conventional Gly-Ser poly linker in the production of scFv antibodies.
Discussion
Twenty-five years after the introduction of phage display technology, this approach is still a powerful tool with potential applicability in a wide range of research fields such as preparation of monoclonal antibodies without the restraints of conventional hybridoma technology [20]. Consistently, this technology confers rapid culture of phage clones, easy handling, genetic stability, lower production costs, and ease of modification by protein engineering to produce fusion proteins with desirable properties.
The phage library in which the scFv gene repertoires are expressed on the surface of phages has been considered as a powerful tool for isolation and identification of antigen-specific scFv. Therefore, in the present work, we have used this method to isolate CD123-specific VL and VH. This study has described the production and characterization of specific murine scFv directly obtained by phage display technology against CD123, the most important target for LCSs. In addition, we have used PROGEN phage display system with a new linker as previously described.
To construct the immune scFv library for selection of specific binders to CD123, two mice were immunized with recombinant CD123. Mice elicited a response against the inoculated antigen after the third boost as shown in Fig. 1. These results indicated that after the third boost, a robust antibody response and specific B cell enrichment were achieved towards our desired antigen. Thus, it is reasonable to assume that after the fourth boost, there was enough affinity maturation and specificity of antibody against the immunogene [21]. Subsequently, splenocytes of those mice were used to amplify VH and VL genes and phage display scFv library construction. The success of this approach to identify and isolate antigen-specific scFv clones depends on the ability of the designed primers to amplify a diverse immunoglobulin repertoire. Therefore, two sets of primers were used to construct the library. VL and VH amplicons were connected by a flexible linker within the pSEX81 vector sequence, between the cloning site of VH and VL in a two-step cloning process to construct the scFv phage library against CD123.
It has been long appreciated that the actual library size must correspond to the functional antibody. Using standard techniques to estimate library size, we demonstrated that our method led to murine scFv library of 4 × 107 cfu/ml, consistent with library size in previous reports of naive and immunized phage display libraries ranging from 106 to 109 [22–27]. Furthermore, as we have shown in Fig. 2d, our generated scFv library contains VH and VL genes with a 96 % recombinant rate confirming the sufficient repertoire of recombinant clones for further analysis.
In order to select CD123-specific scFv, a biopanning process was performed for several rounds. To enrich high affinity binders, we applied two strategies: washing steps were increased while concentrations of the coated CD123 were progressively decreased from 5 μg/well to 2.5, 1, and 1 μg/well as the best setup condition for our work. Enrichment of the library with specific binding to CD123 was observed after three rounds of panning as shown in Fig. 3a. Although our library is considered to represent a sufficiently large diversity, among more than 200 random phage clones from the scFv library against CD123 screened, the inserted scFv genes of six phages were good enough for further analysis. This demonstrates that there may have been some non-recombinant phages in the constructed scFv phage library.
Recombinant proteins expressed in bacteria often form inclusion bodies, especially when they are expressed at high levels [28]. Moreover, since scFv consists of the variable heavy chain (VH) and the variable light chain (VL) domains with intramolecular and intermolecular disulfide bridges for correct folding and structural stability, they must be secreted in the bacterial periplasm because its oxidizing environment allows the formation of their disulfide bridges [29, 30]. Also, to exclude the effect of avidity-based binding due to phage display, all six positive scFv clone gene fragments were subcloned into pOPE101 expression vector in frame with pelB leader sequence which exports the expressed scFv into the bacterial periplasmic space. Our results show (Fig. 4a) the soluble and correct folding of the scFv. All six selected clones expressed ~28 kDa soluble monomeric scFv in E. coli. It has been demonstrated that expression conditions are important for heterologous proteins to be highly expressed in E. coli. Some factors including cell density, temperature, IPTG concentration, and induction time should be experimentally optimized to improve the protein expression [31]. In this regard, in the present study, overnight cultivation at 28 °C following the induction of 1 mM IPTG was considered as the optimized condition for expression.
It has been previously reported that successful construction of scFv relies on a linker that neither interferes with the folding and association of VH and VL domains nor reduces the stability and specificity of the scFv molecule [9, 13, 32]. To satisfy these requirements, one approach is to use the flexible glycine-rich sequences and another is to utilize a set of useful linkers derived from the existing peptide linkers in natural multi-domain proteins [13, 14, 33, 34], similar to our proposed linker driven from hydrophilic pig brain alpha-tubulin peptide sequence EEGEFSEAR.
In general, fusion proteins lacking suitable linkers may misfold and express at low levels [35]. Moreover, the linker mediates the tendency of scFv to aggregate at high concentrations [36]. Indeed, the expression level, solubility, folding, and stability of scFv largely depend on amino acid composition and length of the linker and significantly vary from one linker to another [13, 15, 32, 34, 37–41]. Although E. coli is frequently used as a host strain for scFv production, most scFvs that are overexpressed in E. coli form inclusion bodies, inactive and insoluble aggregates [42]. Several expression vectors proposed for the effective production of soluble and active scFvs have not been successful. Alternatively, various screening strategies for identifying effective polypeptide linkers have been proposed to overcome these problems [42].
In studies by Gustavsson, linkers with lengths of 4–44 amino acid residues have been shown to be ideal [41], whereas Le Gall has reported 6–27 residue linkers [39]. On the other hand, Klement, reported poor expression level and stability of anti-CD16 scFv with common linker peptide (Gly4Ser) 3 [34].
Moreover, the linker is helpful in the refolding of scFv within the bacterial periplasm. Such an improvement in refolding will be reflected by an increased yield of active material. Also, the linker may be altering the properties of the fully folded molecule, again by interacting or masking crucial surface residues [32]. Changing the linker composition might also limit proteolysis that occurs in the G4S linker sequences [40, 43].
Hence, it is important to investigate the effects of relevant linker design on the functionality of antibody fragments. These findings prompted us to pursue a new linker and investigate its efficacy. Our observations show that all clones, with respect to their different amino acid composition (Fig. 3e), were secreted to the periplasm at approximately the same level (Fig. 4a). Also, it ruled out the multimeric forms of scFv, supporting the correct monomeric folding of the molecule. These observations allowed us to infer the validation of linker’s functionality and its ability to improve the expression of soluble scFv antibodies in the periplasm which is clearly of great technological importance for scFv production.
Furthermore, antigen binding of monomeric scFv (Fig. 5) demonstrated the in-frame translation and adequate flexibility of linker to join VH and VL, leading to proper scFv folding and retained specificity. Although many inherent properties of scFv by itself, as well as its amino acid composition, length, flexibility, conformation, and charge, can affect the efficiency of the linker [44], this linker can be used as an alternative to common linker peptide (Gly4Ser). Furthermore, our cloning strategy of joining VH and VL by a two-step cloning procedure avoided potential errors of PCR-based fragment assembly.
Another challenge of antibody display technology, which often goes unreported, is the affinity discrepancy between the phage surface scFv and the monomeric scFv. In fact, a single phage particle displays one to five copies of a scFv molecule fused to the M13 minor coat protein (PIII) [45]. Increasing valency and possible structural stability would contribute to the improvement of avidity. The periplasmic-extracted scFv binding to CD123 as soluble scFv was further investigated by ELISA. Consistently, the relatively low apparent OD of all clones obtained here in comparison with their phage ELISA counterparts may be due to their monovalent nature [46–48] (Fig. 3c, d). Moreover, it explains the decreased affinity of clone 45 in soluble form compared to the phage clone, while it had less effect on other clones such as clone 110. Since clone 110 was least impacted upon conversion to soluble form, it was chosen for further analysis.
Antibody affinities are typically expected to be in the range of 10−7–10−10 M [49]. Recombinant scFvs frequently exhibit markedly decreased binding affinity compared to the full-length monoclonal antibodies perhaps due to monovalent nature of binding activity while mAb is divalent and in part due to deficiency in constant regions of the antibody structural domains [50, 51]. Consistent with the literature, our calculated affinity was 693 nM = 6.9 × 10−7 M [26, 48, 52, 53]. Despite this affinity, we ruled out cross-reactivity with structurally related proteins such as available cancer stem cell markers, CD47, CD20, or mixture of complex proteins such as BSA and casein. The lack of a significant interaction between scFv-110 and other antigens proved its high specificity towards CD123. Moreover, the flow cytometry analysis revealed that scFv-110 can specifically bind to CD123-positive cells (KG1) compared to CD123-negative cells (MOLT-4) and thus confirmed its targeting ability. This in turn means that scFv-110 affinity could significantly be improved through an affinity maturation process [24, 26, 54, 55]. In fact, in vitro affinity maturation of phage antibodies has been reported with approximately tenfold higher affinity (1011 M−1) compared to the highest affinity obtained from in vivo selection of B cells [11]. Furthermore, it is possible to engineer dimeric or multimeric scFv with the same enhanced avidity properties as bivalent antibodies to improve its function [37, 56, 57]. Thus, we propose those strategies to increase the binding affinity of our scFv-110, which promises a diagnostic reagent.
On the whole, we have described a simple and rapid method to produce and evaluate a murine scFv library specific for CD123, using the phage display system. We have also evaluated a new flexible peptide linker as an alternative for challenging fusion protein engineering. Our scFv-110 specifically binds to its target CD123 in vitro, suggesting a diagnostic reagent for residual AML cells. We also propose that with some modifications, such as affinity enhancement and humanization, anti-human IL-3Rα (CD123) scFv-110 may represent promising leads for the development of new tools for use in targeting LCSs. In this scope, detailed assessment of biological activity of the scFvs reported here together with improvement of its binding properties remains the main goal of our future work.
Abbreviations
- AML:
-
acute myeloid leukemia
- CD123:
-
cluster of differentiation 123
- LSCs:
-
leukemic cancer stem cells
- scFv:
-
single-chain fragment variable
- VH:
-
variable heavy chains of an antibody
- VL:
-
variable light chains of an antibody
References
Rubnitz, J. E., Gibson, B., & Smith, F. O. (2010). Acute myeloid leukemia. Hematology/Oncology Clinics of North America, 24(1), 35–63.
Jordan, C., et al. (2000). The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia, 14(10), 1777–1784.
Testa, U., et al. (2004). Interleukin-3 receptor in acute leukemia. Leukemia, 18(2), 219–226.
Ravandi, F., & Estrov, Z. (2006). Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clinical Cancer Research, 12(2), 340–344.
Liu, K., et al. (2015). CD123 and its potential clinical application in leukemias. Life Sciences, 122, 59–64.
Taussig, D. C., et al. (2005). Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood, 106(13), 4086–4092.
Hammers, C. M., & Stanley, J. R. (2014). Antibody phage display: technique and applications. The Journal of Investigative Dermatology, 134(2), e17.
Sidhu, S. S. (2000). Phage display in pharmaceutical biotechnology. Current Opinion in Biotechnology, 11(6), 610–616.
Bird, R. E., et al. (1988). Single-chain antigen-binding proteins. Science, 242(4877), 423–426.
Carter, P. J. (2006). Potent antibody therapeutics by design. Nature Reviews Immunology, 6(5), 343–357.
Pansri, P., et al. (2009). A compact phage display human scFv library for selection of antibodies to a wide variety of antigens. BMC Biotechnology, 9(1), 6.
Stein, C., et al. (2010). Novel conjugates of single‐chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. British Journal of Haematology, 148(6), 879–889.
Gu, X., et al. (2010). Molecular modeling and affinity determination of scFv antibody: proper linker peptide enhances its activity. Annals of Biomedical Engineering, 38(2), 537–549.
Alfthan, K., et al. (1995). Properties of a single-chain antibody containing different linker peptides. Protein Engineering, 8(7), 725–731.
Shan, D., et al. (1999). Characterization of scFv-Ig constructs generated from the anti-CD20 mAb 1F5 using linker peptides of varying lengths. The Journal of Immunology, 162(11), 6589–6595.
Breitling, F., et al. (1991). A surface expression vector for antibody screening. Gene, 104(2), 147–153.
Barbas, C. F. (2001). Phage display: a laboratory manual. New York: Cold Spring Harbor Laboratory Press.
Lee, C. M., et al. (2007). Selection of human antibody fragments by phage display. Nature Protocols, 2(11), 3001–3008.
Martineau, P. (2010). Affinity measurements by competition ELISA. In Antibody engineering Vol 2 (pp. 657–665). Berlin Heidelberg: Springer.
Roncolato, E. C., et al. (2015). Phage display as a novel promising antivenom therapy: a review. Toxicon, 93, 79–84.
Kellner, C., Nodehi, S. M., Peipp, M. (2010) Mouse immune libraries for the generation of ScFv fragments directed against human cell surface antigens. In Antibody engineering Vol 1 (pp. 47–63). Berlin Heidelberg: Springer.
Hussein, A. H., et al. (2007). Construction and characterization of single-chain variable fragment antibodies directed against the Bordetella pertussis surface adhesins filamentous hemagglutinin and pertactin. Infection and Immunity, 75(11), 5476–5482.
Dong, L., et al. (2003). Generation of affinity matured scFv antibodies against mouse neural cell adhesion molecule L1 by phage display. Biochemical and Biophysical Research Communications, 301(1), 60–70.
Wang, N., et al. (2008). Construction and characterization of phage display library: recognition of mouse serologically detected male (SDM) antigen. Animal Reproduction Science, 104(1), 93–110.
Sepulveda, J., & Shoemaker, C. B. (2008). Design and testing of PCR primers for the construction of scFv libraries representing the immunoglobulin repertoire of rats. Journal of Immunological Methods, 332(1), 92–102.
Nishi, M., et al. (2014). Ligation-based assembly for constructing mouse synthetic scFv libraries by chain shuffling with in vivo-amplified V H and V L fragments. Journal of Immunological Methods, 412, 53–69.
Shahsavarian, M. A., et al. (2014). Exploitation of rolling circle amplification for the construction of large phage-display antibody libraries. Journal of Immunological Methods, 407, 26–34.
Shi, J., et al. (2015). Expression, purification, and characterization of scar tissue neovasculature endothelial cell-targeted rhIL10 in Escherichia coli. Applied Biochemistry and Biotechnology, 175(1), 625–634.
Sonoda, H., et al. (2011). Cytoplasmic production of soluble and functional single-chain Fv-Fc fusion protein in Escherichia coli. Biochemical Engineering Journal, 53(3), 253–259.
Miethe, S., et al. (2013). Production of single chain fragment variable (scFv) antibodies in Escherichia coli using the LEX™ bioreactor. Journal of Biotechnology, 163(2), 105–111.
Sun, Y., et al. (2015). Expression and characterization of the extracellular domain of human HER2 from escherichia coli, and production of polyclonal antibodies against the recombinant proteins. Applied Biochemistry and Biotechnology, 176(4), 1029–1043.
Turner, D. J., Ritter, M. A., & George, A. J. (1997). Importance of the linker in expression of single-chain Fv antibody fragments: optimisation of peptide sequence using phage display technology. Journal of Immunological Methods, 205(1), 43–54.
Wang, S.-H., et al. (2006). Construction of single chain variable fragment (scFv) and BiscFv-alkaline phosphatase fusion protein for detection of Bacillus anthracis. Analytical Chemistry, 78(4), 997–1004.
Klement, M., et al. (2015). Effect of linker flexibility and length on the functionality of a cytotoxic engineered antibody fragment. Journal of Biotechnology, 199, 90–97.
Chen, X., Zaro, J. L., & Shen, W.-C. (2013). Fusion protein linkers: property, design and functionality. Advanced Drug Delivery Reviews, 65(10), 1357–1369.
Schmiedl, A., Breitling, F., & Dübel, S. (2000). Expression of a bispecific dsFv–dsFv’ antibody fragment in Escherichia coli. Protein Engineering, 13(10), 725–734.
Atwell, J. L., et al. (1996). Design and expression of a stable bispecific scFv dimer with affinity for both glycophorin and N9 neuraminidase. Molecular Immunology, 33(17), 1301–1312.
Feng, J., et al. (2003). Design and assembly of anti-CD16 scFv antibody with two different linker peptides. Journal of Immunological Methods, 282(1), 33–43.
Le Gall, F., et al. (2004). Effect of linker sequences between the antibody variable domains on the formation, stability and biological activity of a bispecific tandem diabody. Protein Engineering Design and Selection, 17(4), 357–366.
Albrecht, H., DeNardo, G. L., & DeNardo, S. J. (2006). Monospecific bivalent scFv-SH: effects of linker length and location of an engineered cysteine on production, antigen binding activity and free SH accessibility. Journal of Immunological Methods, 310(1), 100–116.
Gustavsson, M., et al. (2001). Stable linker peptides for a cellulose-binding domain–lipase fusion protein expressed in Pichia pastoris. Protein Engineering, 14(9), 711–715.
Kumada, Y., et al. (2007). Polypeptide linkers suitable for the efficient production of dimeric scFv in Escherichia coli. Biochemical Engineering Journal, 35(2), 158–165.
Whitlow, M., et al. (1993). An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Engineering, 6(8), 989–995.
Zhang, J., et al. (2009). Design and optimization of a linker for fusion protein construction. Progress in Natural Science, 19(10), 1197–1200.
Kiss, M. M., et al. (2011). Phage ESCape: an emulsion-based approach for the selection of recombinant phage display antibodies. Journal of Immunological Methods, 367(1), 17–26.
Alirezapour, B., et al. (2013). Production and characterization of recombinant scFv against digoxin by phage display technology. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 32(3), 172–179.
Min, W.-K., et al. (2010). Production and characterization of monoclonal antibody and its recombinant single chain variable fragment specific for a food-born mycotoxin, fumonisin B1. Bioprocess and Biosystems Engineering, 33(1), 109–115.
Martsev, S. P., et al. (1998). Antiferritin single-chain antibody: a functional protein with incomplete folding? FEBS Letters, 441(3), 458–462.
Drees, J. J., et al. (2014). Soluble production of a biologically active single-chain antibody against murine PD-L1 in Escherichia coli. Protein Expression and Purification, 94, 60–66.
Schaefer, J. V., & Pluckthun, A. (2012). Transfer of engineered biophysical properties between different antibody formats and expression systems. Protein Engineering Design and Selection, 25(10), 485–506.
Renaut, L., et al. (2012) Affinity maturation of antibodies: optimized methods to generate high-quality ScFv libraries and isolate IgG candidates by high-throughput screening. In Antibody engineering Vol 1 (pp. 451–461). Berlin Heidelberg: Springer.
Xia, J., et al. (2013). Isolation, identification and expression of specific human CD133 antibodies. Scientific Reports, 3, 3320.
Blazek, D., et al. (2004). Generation and characterization of single-chain antibody fragments specific against transmembrane envelope glycoprotein gp46 of maedi-visna virus. Journal of Virological Methods, 115(1), 83–92.
Kim, H. S., et al. (2011). Improvement of anti-Burkholderia mouse monoclonal antibody from various phage-displayed single-chain antibody libraries. Journal of Immunological Methods, 372(1–2), 146–161.
Boder, E. T., Midelfort, K. S., & Wittrup, K. D. (2000). Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proceedings of the National Academy of Sciences, 97(20), 10701–10705.
Hudson, P. J., & Kortt, A. A. (1999). High avidity scFv multimers: diabodies and triabodies. Journal of Immunological Methods, 231(1), 177–189.
Power, B. E., & Hudson, P. J. (2000). Synthesis of high avidity antibody fragments (scFv multimers) for cancer imaging. Journal of Immunological Methods, 242(1), 193–204.
Acknowledgments
This work was supported by Postgraduate Office, Pasteur Institute of Iran, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Moradi-Kalbolandi, S., Davani, D., Golkar, M. et al. Soluble Expression and Characterization of a New scFv Directed to Human CD123. Appl Biochem Biotechnol 178, 1390–1406 (2016). https://doi.org/10.1007/s12010-015-1954-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1954-y