Abstract
The bacterial strains of the genus Nocardia were used for the bioconversion of fumaric acid to l-malic acid. The ability of the bacterial strain Nocardia sp. CCM 4837/A to produce l-malic acid from fumaric acid was investigated under various conditions. The optimal temperature for the bioconversion was approximately 37 °C, and the optimal pH was around 8.0. The addition of an inductor (fumarate salt) to the fermentation medium was necessary to enhance enzyme activity. The presence of detergent Triton X-100 (0.02–0.1 %) in the reaction mixture rapidly increased the enzyme activity of fumarase. The specific fumarase activity of intact cells Nocardia sp. CCM 4837/A increased from 2.8 to 75 U/mg after optimising the experimental conditions described here. Pretreatment of the Nocardia cells with malonate was not necessary because succinate was not detected as a by-product under our experimental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-malic acid is an essential intermediate of the cell metabolism and is widely used in food and beverage industries (food additives), metallurgy (chelatation agent), pharmacy (treatment of the liver dysfunction and hyperammonemia, a component for amino acid infusions) and cosmetics (sweet perfumes, anti-wrinkle creams). Recently, it has also been used as a raw material for the biodegradable polymers [1]. Nowadays, l-malic acid can be produced by two technological processes: chemical synthesis and fermentation. Malic acid is prepared by chemical synthesis via hydration of fumaric or maleic acid at high pressures and high temperatures. Malic acid obtained by such a method is of racemic form (d,l-form), which is much less soluble than its d- or l-form, therefore limiting its commercial application.
The bioconversion of fumaric acid to l-malic acid can also be performed by various species of yeasts such as Saccharomyces, Schizosaccharomyces Candida, Pichia and Dipodascus. The most common strain for this bioconversion is the yeast Saccharomyces cerevisiae. Peleg et al. [2] described the strain Saccharomyces cerevisiae engineered to overproduce fumarase and without formation of succinic acid. Good results for production of l-malic acid were obtained with immobilised cells of S. cerevisiae in polyacrylamide gel without producing succinic acid after malonate pretreatment [3], and almost 100 % of conversion rate was achieved using S. cerevisiae in a supported liquid membrane bioreactor [4]. In 1999, the yeast of the genus Dipodascus was found to produce l-malic acid from fumaric acid without formation of any by-products [5].
The microorganisms of the genus Nocardia have the ability produce cis-epoxysuccinate hydrolase that can convert cis-epoxysuccinic acid to l-isomer of tartaric acid with superior activity [6]. It is the most effective way for production of l (+)-tartaric acid. The substrate can be easily obtained by epoxidation of maleic acid with hydrogen peroxide using tungsten acid as a catalyst. l-tartaric acid is subsequently produced from cis-epoxysuccinic acid by free, permeabilised or immobilised bacterial cells [7].
In this paper, we describe for the first time the possibility of using microorganisms of the genus Nocardia for the production of l-malic acid. We studied optimal conditions for fumarase in Nocardia sp. CCM 4837/A during bioconversion of fumarate to l-malate. The advantage of using this bacterial strain in described bioconversion is no by-product formation (mainly succinic acid is the undesirable by-product formed by l-malic acid-producing bacteria and yeasts).
Materials and Methods
Microorganisms
Nocardia sp. CCM 4837/A, Nocardia otitidiscaviarum CCM 2779, Nocardia carnea CCM 2756 and Nocardia carnea CCM 3470 were obtained from the Czechoslovak Collection of Microorganisms, Brno (Czech Republic). All strains were maintained on agar slants at 4 °C. The slant agar consisted of yeast extract (Oxoid, England), 5 g/L; peptone (Imuna, Slovak Republic), 5 g/L; glucose, 10 g/L; agar, 20 g/L; and distilled water, pH 7.2.
Cultivation of Microorganisms
The strains were grown aerobically at 30 °C in the 90 mL medium (pH 7.0–7.2) containing 0.3 % yeast extract, 1 % peptone, 0.3 % NaCl, 0.3 % KH2PO4, 0.2 % MgSO4.7H2O, 0.001 % FeSO4.7H2O, 0.003 % CaCl2.2H2O, 0.003 % MnSO4.4H2O and distilled water. The 500 mL cultivation flasks filled with 90 mL of culture medium were used for all experiments. The sterile medium was inoculated with one loopful of microorganism and cultivated at 30 °C on a rotary shaker (180 rpm) for 24 h. Then, the sterile 90 mL of culture medium in 500 mL cultivation flasks were inoculated with 3 % of the obtained inocula, and the flasks were shaken on a rotary shaker by 180 rpm at 30 °C for 32 h (during cultivation, ammonium fumarate was added to the medium). After cultivation, the cells were centrifugated (7,000 g, 7 min), washed (once) and resuspended in 1 mol/L ammonium fumarate (pH 8.0) and used for bioconversion.
Conditions for Optimalisation
Influence of inductor on the fumarase activity in the intact cells was investigated. The cells were cultivated under the cultivation conditions described above. The cultivation was conducted without the inductor for the first 24 h. The inductor (ammonium fumarate at concentration 0–12 g/L) was added to the medium after 24 h, and cultivation continued eight more hours. Samples were taken regularly, and supernatants were used after centrifugation to determine fumarase activity.
Effect of pH on enzyme activity in the intact cells during bioconversion was studied in the range 7.5–9.5.
Effect of temperature on enzyme activity in the intact cells during bioconversion was observed in the temperature range 25–60 °C. Other experimental conditions were similar to those described in the assay of fumarase activity.
Effect of fumarate concentration on l-malic acid production was investigated by incubating the bacterial cells with various concentrations of fumaric acid neutralised with ammonium hydroxide to pH 8.0.
Effect of detergents on fumarase activity in the intact cells was studied. The cells were resuspended in 1 mol/L ammonium fumarate solutions (pH 8.0, temperature 37 °C) with the detergents. Three detergents—Triton X-100, sodium deoxycholate and Tween 80—were tested in the concentration range 0.02–0.1 % (w/v).
Bioconversion of Fumaric Acid to l-malic Acid
After the cultivation, the cells were washed and resuspended in 1 mol/L ammonium fumarate (25 mL, pH 8.0). The intact cells were used for the bioconversion at 37 °C. The effect of detergents on the enzyme activity was tested by the addition of these compounds to the reaction mixture. Samples were taken regularly, immediately cooled and centrifuged (3 min, 10,000 g) and used for the determination of organic acids in supernatant. All experiments were repeated three times, and given data are the averages of all measurements.
Fumarase Activity Assay
One unit of the enzyme activity was defined as the amount of enzyme capable of generating one micromole of l-malic acid per hour under the experimental conditions. Specific activity was calculated as U per mg of dry cell weight.
Analytical Methods
Biomass Concentration
For the determination of dry weight, the cells were washed twice with distilled water. The biomass was collected by centrifugation (7,000 g, 7 min) after each operation, and the dry weight was determined by heating the biomass at 105 °C for 3 h.
HPLC Analysis
Concentrations of organic acids were determined by HPLC with refractive index detector K-2301 (Knauer, Germany), Ionex column (Watrex 250 mm × 8 mm), and Polymer IEX 8 μm H form (Watrex, Czech Republic) with 1.3 mM H2SO4 as mobile phase at 30 °C and 0.7 cm3/min.
Results and Discussion
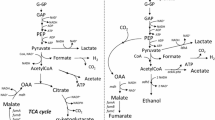
The bacterial strains of the genus Nocardia showed the ability to convert fumarate to l-malate salts (Fig. 1). Succinic acid is the undesirable by-product formed by some l-malic acid-producing bacteria and yeasts [8]. The advantage of using Nocardia genus in the described bioconversion is that there is no by-product formation. The strain Nocardia sp. CCM 4837/A with the maximum specific fumarase activity (2.9 U/mg) was selected and used to further optimise bioconversion conditions and the yield of l-malic acid from fumaric acid.
Effect of Inductor
We tested ammonium salt of fumaric acid as an inductor and found that the enzyme activity of fumarase in the intact cells of Nocardia sp. strongly depends on the presence of an inductor in the fermentation medium. The effect depends on the inductor concentration and time when it was added as well. We found that the optimal time to add the inductor (to achieve maximum biomass concentration as well as specific enzyme activity) is the interval 20 to 24 h during the 32 h fermentation process. Figure 2 shows the effect of various concentrations of ammonium fumarate (in the range of 0–12 g/L) on specific fumarase activity when the inductor was added to the fermentation medium 8 h before the end of cultivation. After 32 h of cultivation, the cells were used for bioconversions of 1 M fumarate to l-malate. The optimal concentration of the inductor was found to be 3 g/L, and maximum fumarase activity 73.2 U/mg was reached using this protocol.
Effect of Detergents
Detergents are known to enhance the rates of bioconversion of fumarate to l-malate [8]. They remove the permeability barriers for substrate and/or product across the cell’s membrane, and thus the yields of l-malic acid can be increased. The effect of detergents on the enzyme activity of fumarase in the intact cells Nocardia sp. CCM8437/A is shown in Table 1. We tested three detergents (Triton X-100, Sodium deoxycholate and Tween 80) at various concentrations in the range of 0.05–0.1 %. The specific activity of intact cells without treatment was found to be 2.84 U/mg. An increase in specific activity was observed when Triton X-100 or sodium deoxycholate were added. Triton X-100 exhibited a strong enhancement effect on the specific fumarase activity even at the low concentration of 0.02 % (w/v). The highest increase in specific enzyme activity was observed when 0.05 % (w/v) Triton X-100 was used (75 mmol/h/g). Bioconversion of fumaric acid to l-malic acid with and without the addition of this detergent is shown in Fig. 3. Fumarase activity in the presence of Tween (0.02 %, w/v) decreased around 40 % (compared to the control). Significant effect of detergents on specific enzyme activity in bacteria of the genus Nocardia was also shown in previous publications [9, 10].
Activity of fumarase achieved in our work (75 mmol/h/g of cells treated with Triton X-100) is about three times higher than that reported by Presecki et al. [11]: 20.8 mmol/h/g of permeabilised yeast cells or Rosenberg et al. [5], 18.78 mmol/h/g of pretreated yeast cells with Triton X-305 and around 10 times higher than the one obtained by Yamamoto et al. [12] with immobilised B. ammoniagenes (7.48 mmol of l-malic acid/h/g). Activity of fumarase in the treated cells of Nocardia sp. presented here are in good accordance with the yeast S. cerevisiae (65 mmol/h/g) reported by Neufeld et al. [13] and also with the results described by Oliveira et al. [3]: 60.6 mmol/h/g of immobilised S. cerevisiae within polyacrylamide gel beads. Based on our results, Triton X-100 was added to the reaction mixture in concentration 0.05 % (w/v) in all subsequent experiments.
Effect of pH and Temperature
With the aim to find the optimal conditions of bioconversion of fumarate to l-malate, the effect of pH and temperature on fumarase activity was tested. The effect of pH was studied in the range 7.5–9.5. The maximum enzyme activity (56.68 U/mg) was reached at pH 8.0 (Fig. 4). At higher pH values, the rates of bioconversion declined rapidly. Incubation temperature does not have a very significant effect in the range 30–40 °C. The specific enzyme activity of fumarase reached 62.13 U/mg at the optimum temperature 37 °C (Fig. 5).
Effect of Substrate Concentration
The effect of initial substrate concentration on the production of l-malic acid and possible substrate inhibition was examined in the range 20–120 g/L. Bioconversions were realised with constant biomass concentration and with the addition of Triton X-100 (0.05 %, w/v) to the reaction mixture. The specific production and volumetric rates and other parameters are shown in Table 2. The greatest specific production rate (4.281 g/g.h) and volumetric production rate (15.412 g/L.h) were obtained when the highest initial substrate concentration (120 g/L) was used. The product yield (0.988) corresponding to 86 % of the theoretical yield (1.155) was obtained at 120 g/L.
Conclusion
Fumaric acid was converted to l-malic acid by the bacteria Nocardia sp. CCM 4837/A. During the biomass production phase (32-h cultivation), the addition of an inductor ammonium fumarate (3 g/L) to the fermentation medium was essential for the fumarase induction. The bioconversion of 1 mol/L ammonium fumarate at pH 8.0 and 37 °C with cells permeabilised with Triton X-100 (0.05 %) lead to maximal specific fumarase activity (75 U/mg). The presence of succinic acid as an undesired by-product was not detected.
References
Lee, B.-S., Vert, M., & Holler, E. (2002). in Doi V. In A. Steinbuechel (Ed.), Biopolymers (pp. 75–103). New York: Wiley-VCH.
Peleg, Y., Rokem, J. S., Goldberg, I., & Pines, O. (1990). Applied and Enviromental Microbiology, 56, 2777–2783.
Oliveira, E. A., Costa, A. A. R., Figueiredo, Z. M. B., & Carvalho, L. B. (1994). Applied Biochemistry and Biotechnology, 47, 65–72.
Bressler, E., Pines, O., Goldberg, I., & Braun, S. (2002). Biotechnology Progress, 18, 445–450.
Rosenberg, M., Miková, H., & Krištofíková, Ľ. (1999). Letters in Applied Microbiology, 29(4), 221–223.
Rosenberg, M., Hronská, H., & Krištofíková, Ľ. (2002). Strain of Nocardia sp. microorganism. Patent CZ, 289, 404.
Wang, Z., Wang, Y., & Su, Z. (2012). Applied Microbiology and Biotechnology, 97, 2433–2441.
Gong, C. S., Cao, N., Sun, Y., & Tsao, G. T. (1996). Applied Biochemistry and Biotechnology, 57/58, 481–487.
Kroutil, W., Genzel, Y., Pietzsch, M., Syldatk, C., & Faber, K. (1998). Journal of Biotechnology, 61, 143–150.
Rosenberg, M., Miková, H., & Krištofíková, Ľ. (1999). Biotechnology Letters, 21(6), 491–495.
Presečki, A. V., Zelić, B., & Vasić-Rački, Ð. (2007). Enzyme Microbial Technology, 41, 605–612.
Yamamoto, K., Tosa, T., Yamashita, K., & Chibata, I. (1976). European Journal of Applied Microbiology, 3, 169–183.
Neufeld, R. J., Peleg, Y., Okem, J. S., Pines, O., & Goldberg, I. (1991). Enzyme Microbiology and Technology, 13, 991–996.
Acknowledgments
The work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Academy of Sciences, registration number 1/0229/12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hronská, H., Tokošová, S., Pilniková, A. et al. Bioconversion of Fumaric Acid to l-malic Acid by the Bacteria of the Genus Nocardia . Appl Biochem Biotechnol 175, 266–273 (2015). https://doi.org/10.1007/s12010-014-1251-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1251-1