Abstract
Several metabolic engineered Escherichia coli strains were constructed and evaluated for four-carbon dicarboxylic acid production. Fumarase A, fumarase B and fumarase C single, double and triple mutants were constructed in a ldhA adhE mutant background overexpressing the pyruvate carboxylase from Lactococcus lactis. All the mutants produced succinate as the main four-carbon (C4) dicarboxylic acid product when glucose was used as carbon source with the exception of the fumAC and the triple fumB fumAC deletion strains, where malate was the main C4-product with a yield of 0.61–0.67 mol (mole glucose)−1. Additionally, a mdh mutant strain and a previously engineered high-succinate-producing strain (SBS550MG-Cms pHL413-Km) were investigated for aerobic malate production from succinate. These strains produced 40.38 mM (5.41 g/L) and 50.34 mM (6.75 g/L) malate with a molar yield of 0.53 and 0.55 mol (mole succinate)−1, respectively. Finally, by exploiting the high-succinate production capability, the strain SBS550MG-Cms243 pHL413-Km showed significant malate production in a two-stage process from glucose. This strain produced 133 mM (17.83 g/L) malate in 47 h, with a high yield of 1.3 mol (mole glucose)−1 and productivity of 0.38 g L−1 h−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Four-carbon (C4) dicarboxylic acid compounds such as succinic, malic and fumaric acids are traditionally produced from petrochemicals. These compounds can be used as building blocks for the synthesis of many different products such as resins, biodegradable polymers, surfactants as well as food additives, pharmaceutical formulations [1, 24] and cosmetics [5]. The petroleum marketplace, where oil prices are volatile and availability may turn unpredictable in the future, together with environmental concerns, has led to an increase in the interest for alternative sources for these compounds. Therefore, the development of biological processes to produce these compounds from renewable resources has been the goal of several research groups [3]. Different C4-dicarboxylic acids production strategies through metabolic engineering have been discussed in detail in a previous work [19].
The production of succinate by a high producer as well as the evaluation of operational conditions that influence succinate production has been discussed extensively in previous reports [8, 10, 11, 15, 20]. The present study is focused on the production of other C4-dicarboxylic acids, such as malic and fumaric acids, using metabolic engineered Escherichia coli strains.
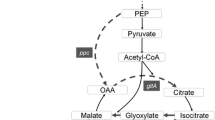
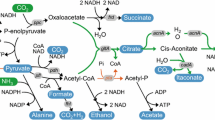
Malate is naturally produced by microorganisms such as Aspergillus, Schizophyllum commune, Monascus araneosus, and Saccharomyces cerevisiae, among others. Efforts have been made to increase the production of malate by metabolic engineering in some of these organisms. For instance, Aspergillus oryzae NRRL 3488 has been engineered to overexpress the native C4-dicarboxylic acids transporter, cytosolic pyruvate carboxylase and malate dehydrogenase, increasing the malate titer to 154 g/L in 164 h, with a yield of 1.38 mol (mole glucose)−1, corresponding to 69% of the theoretical yield [2]. On the other hand, metabolic engineered S. cerevisiae has been reported to produce malate at a molar yield of 0.48 mol (mole glucose) −1 under optimized conditions in laboratory-scale bioreactors [25]. However, E. coli also has the potential of producing malate or fumarate. These compounds are intermediates in the tricarboxylic acid (TCA) cycle as well as intermediates of the mixed anaerobic fermentation in E. coli (Fig. 1). The production of malate or fumarate, through the fermentative pathway of E. coli, is redox balanced, i.e., the maximum malate theoretical yield is two mole of malate per mole of glucose consumed. One mole of glucose can generate two mole of NADH and two mole of pyruvate. One mole of pyruvate is required to combine with one mole CO2 to generate one mole of malate, which could later be converted into fumarate (Fig. 1). Therefore, neither carbon nor reducing equivalents is limiting or in excess in this pathway. However, E. coli does not accumulate malate or fumarate under normal circumstances. Thus, the present study aimed to develop a process for malate and fumarate production using strategically engineered E. coli strains.
Aerobic (a) and anaerobic (b) Escherichia coli metabolic routes. G-6P, glucose 6 phosphate; GAP, glyceraldehyde phosphate; PEP, phosphoenolpyruvate; fumA, fumB, fumC, fumarases A, B and C; mdh, malate dehydrogenase, ldhA lactate dehydrogenase; adhE, alcohol dehydrogenase; ackA, acetate kinase; pta, phosphate acetyltransferase
An E. coli C4-dicarboxylic acid-producing strain has been developed in the past by Jantama and collaborators [8] by a combination of metabolic engineering and metabolic evolution. The metabolic evolution involved the multiple subculturing of the cells and the selection of the ones that grew faster and presented promising product profiles together with the addition of gene inactivations at different stages of the evolution. The final evolved E. coli mutant strain KJ071 with deletions in ldhA, adhE, ackA, focA, pflB and mgsA showed the production of a mixture of C4-dicarboxylic acids, malate and succinate [8]. In addition, this approach has the limitation of using non-genetically defined strains due to unknown mutations generated through metabolic evolution that resulted in the final strain phenotype.
Song and collaborators [18] investigated the production of fumaric acid from glucose in anaerobic conditions using a ldhA adhE frdABCD, but it was unsuccessful. Then, they performed metabolic engineering of E. coli to produce fumaric acid through the glyoxylate shunt in aerobic conditions and deleted fumA, fumB, fumC, and aspA (to avoid aspartate formation from fumaric acid) and iclR (glyoxylate shunt repressor), among other genes and overexpressing the native phosphoenolpyruvate carboxylase (ppc). The authors reported a yield of about 0.5-0.6 mol (mol glucose)−1.
Three fumarases denominated fumarase A, fumarase B and fumarase C have been described in E. coli [7, 12, 22, 23]. These enzymes catalyze the reversible conversion of fumarate into (l)-malate with similar catalytic efficiencies and higher affinity for fumarate than malate in all cases [9]. Fumarases participate in the TCA cycle and in anaerobic fermentation. Fumarase A and fumarase B show high sequence similarity (90% identity) [6], form dimers, are part of the Class I (iron-dependent) fumarases and are oxygen-sensitive, whereas fumarase C does not show sequence similarity to the other two, is iron-independent, forms tetramers and is part of the Class II fumarases [23]. Woods and collaborators [23] studied the fumarase activity of a series of plasmids encoding the different fumarases (A, B and C) separately under different culture conditions. Fumarases A and B have been found to be thermolabile, whereas fumarase C was stable with 70% of the activity retained after 80 min at 50 °C. FumA and FumC have higher affinity for fumarate than for malate in in vitro experiments; however, the opposite was found for FumB, suggesting than FumA and FumC participate mainly in the TCA cycle, and FumB may act primarily in anaerobic fermentation, converting malate into fumarate [23]. This result disagrees with Kronen and Berg [9] findings where all three fumarases were shown to prefer fumarate as substrate over malate.
In the present work, a series of strategically selected genes were inactivated to assess the relative importance of the corresponding enzymes in C4-dicarboxylic acids biosynthesis. The effect of single, double and triple fumarase deletions in the anaerobic product profile was investigated in a ldhA adhE mutant E. coli strain overexpressing the pyruvate carboxylase from Lactococcus lactis (pyc). On the other hand, as the malate dehydrogenase (MDH) catalyzes the reversible conversion of malate into oxaloacetate we might expect that in a culture of a mdh mutant strain in aerobic conditions, succinate should be converted to fumarate and finally to malate, avoiding the conversion of malate into oxaloacetate (Fig. 1). Therefore, the effect of a mdh knockout was evaluated in the present work, in aerobic conditions, for malate production in E. coli using succinate as carbon source. Finally, a high-succinate-producing strain (SBS550MG-Cms243 pHL413-Km) [21] was also assessed for C4 dicarboxylic acids production under various conditions.
Very recently, during the writing of the present article, Sévin and collaborators (2017) have reported fumarase activity (fumarate + H2O → malate) in two other gene products, previously annotated as YdhZ and YggD suggesting the new annotation as fumarase D and fumarase E, respectively [17].
Materials and methods
Strains and plasmids
The strains and plasmids used in this study are described in Table 1.
Fumarase single-mutant strains were constructed by P1 phage transduction using the Keio Collection donor strains JW1604, JW4083 and JW1603 for fumA, fumB and fumC gene inactivation, respectively [1]. The fumAC double-mutant strain was constructed using the single-step inactivation method by Datsenko and Wanner [4], and the following primers: forward: 5′GTGGTGGTTCGGCAAACAAGACGTATCTCTATCAGGAAACGTGTAGGCTGGAGCTGCTTC-3′; reverse: 5′-AAACGGTGCACAGGTAATGACTGCCAGTTCATCTGCTACGCATATGAATATCCTCCTTAG-3′. The underlined sequences are homologous to the fumAC sequence.
The mdh mutant, MBS440, was constructed by P1 phage transduction using the Keio Collection donor strain JW3205 [1].
Experimental procedures
Different strains and processes were evaluated for their potential for C4-dicarboxylic acid production. The first approach was to perform anaerobic cultures with fumarase mutant strains (simple, double and triple fumarase mutants) using glucose as carbon source. The second approach was to evaluate the capability of several engineered E. coli strains, including the fumarase mutants, a malate dehydrogenase mutant and a high-succinate-producing strain to produce C4 dicarboxylic acids using succinate as carbon source under aerobic conditions. And finally, the high-succinate-producing strain was evaluated in a two-stage production process using glucose as carbon source.
Anaerobic production of C4-dicarboxylic acids
The effect of fumarase mutations on anaerobic product profiles was investigated in shake flask experiments. First, the cells were grown aerobically for biomass accumulation and then product biosynthesis was performed under anaerobic conditions as described previously [15, 16]. The strains were initially streaked on Luria–Bertani (LB) agar plates containing 66.7 mg/L of each ampicillin, carbenicillin and oxacillin. A 5 ml LB medium culture containing the mixture of antibiotics was inoculated using a single colony from the plate mentioned above. The cultures were grown at 37 °C and 250 rpm overnight. Cultures were then centrifuged and washed with fresh LB medium three times and finally resuspended in the same medium to the same initial volume. Two-liter flasks containing a volume of 400 ml of LB medium with 200 mg/L ampicillin (Ap) were inoculated to 1% in volume using the cells prepared above. These cultures were grown overnight at 37 °C and 250 rpm. A calculated volume of this grown culture was centrifuged and resuspended in 10 ml of glucose medium (LB + 1 g/L NaHCO3 + 19.8 g/L glucose + 100 mg/L Ap) to a cell concentration of 20 OD600. This suspension was poured into 250-ml flasks containing 0.5 g MgCO3, purged for 1 min with 1 L/min CO2 and sealed with rubber stoppers. These cultures were incubated at 37 °C and 250 rpm for 24 h.
Aerobic production of C4-dicarboxylic acids
The ability of several engineered E. coli strains, including the fumarase mutants, a malate dehydrogenase mutant and a high-succinate-producing strain to produce C4 dicarboxylic acids was investigated using succinate as carbon source under aerobic conditions.
Shake flask experiments were performed as described above, with the exception of the production medium, where a concentration of 100 mM (11.81 g/L) succinate was used instead of glucose and the conditions were aerobic instead of anaerobic; for example, foam stoppers were used instead of rubber stoppers and no CO2 was added.
Two-stage production process (anaerobic/aerobic)
Malate production was assessed using the high-succinate-producing strain SBS550-Cms243 pHL413-Km [21] in a two-stage production process. The cells were grown in shake flasks under aerobic conditions as described previously [15]. Then the first production stage was anaerobic, where glucose was converted into succinate, and the second stage was aerobic for succinate conversion into malate. In this approach, we exploit the high-succinate production feature of the strain SBS550-Cms243 pHL413-Km which can produce succinate close to the maximum theoretical yield of 1.72 mol (mole glucose)−1. It is of interest to point out that the anaerobic succinate production process is a carbon fixation process. For example, at a molar yield of 1.6, the strain will incorporate 80 mol of carbon dioxide per 100 mol of glucose consumed. As such, further conversion of succinate to other products, such as malate, will become very appealing.
Analytical methods
Cell growth
Cell growth was monitored by measuring the optical density at 600 nm and expressed as dry cell weight (DCW) using a corresponding calibration curve.
Extracellular metabolite analysis
A volume of 2 ml of sample from each shake flask was centrifuged in a microcentrifuge at 13,000×g for 1 min, filtered through a 0.2-μm filter and stored at − 20 °C until analysis.
Glucose, succinate and extracellular metabolites such as malate, fumarate and acetate, among others, were analyzed by HPLC as previously described [1, 15]. In brief, the HPLC system (Shimadzu-10A System, Shimadzu, Columbia, MD) was equipped with a cation-exchange column (HPX-87H, Bio-Rad Laboratories, Hercules, CA), a differential refractive index (RI) detector (Waters 2410, Waters, Milford, MA) and an ultraviolet (UV) detector (Shimadzu SPD-10A). The mobile phase was 2.5 mM (0.24 g/L) H2SO4 solution at a 0.5 mL/min flow rate. The column was operated at 55 °C.
Results and discussion
Anaerobic production of C4-dicarboxylic acids
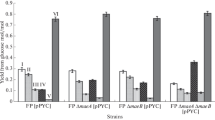
E. coli fumarases A, B and C catalyze the reversible conversion of fumarate into malate. The effect of inactivating one or more fumarases in a ldhA adhE mutant, on anaerobic product profiles, was investigated. These strains overexpressed the pyruvate carboxylase gene from Lactococcus lactis, which converts pyruvate into oxaloacetate to favor C4-dicarboxylic acid synthesis. The experiments were performed in shake flasks as previously described using glucose as carbon source. The results are shown in Fig. 2.
Single fumarase mutants showed no significant difference in product profiles compared to the control strain (SBS110MG pHL413). All the fumarase mutant strains consumed nearly 100% of the glucose in the medium with the exception of the fumAC mutant and the fumB fumAC triple-mutant strain, which only consumed about 20–25% of the glucose. These two strains also showed significantly lower cell growth after aerobic overnight culture in LB medium, with values of 1.6 and 1.2 OD600, respectively, compared to 3.2–4.7 OD600 for the other fumarase mutants and the control strain, SBS110MG (pHL413). The growth impairment in aerobic conditions in the fumAC mutants may indicate that fumarases A and C are the important fumarases functioning in the TCA cycle during aerobic growth; however, it is not clear why these strains did not consume all the glucose in the anaerobic stage converting glucose into malate, which should be redox and carbon balanced. The recently identified fumarase D and fumarase E [21] may not play an important role in aerobic growth as they were not able to support the level of growth achieved when FumA and FumC were present.
All fumarase mutant strains showed succinate to be the major anaerobic product together with acetate and formate, with the exception of the fumAC mutant strains. Concentrations of succinate ranging from 101 to 113 mM (11.93–13.34 g/L) and a yield of 0.9–1.0 mol of succinate per mol of glucose were achieved. Succinate production yield is limited by redox balance to be 1.0 mol per mol of glucose consumed; therefore, the succinate yield achieved was close to the theoretical. Acetate concentrations were high, of about 140–150 mM (8.41–9.01 g/L) for all the strains except the fumAC mutants; probably due to the low growth. Production of malate or fumarate from glucose is carbon and redox balanced; on the contrary, the production of succinate is limited by NADH (see Fig. 1b). Therefore, the accumulation of malate or fumarate should have been favored over the accumulation of succinate under anaerobic conditions using glucose as carbon source in a wild-type strain. Nevertheless, succinate was the main product with a yield limited by the redox balance.
Malate was produced in all the mutant strains, with the highest values, 13.5–16.6 mM (1.81–2.23 g/L) found in the fumB mutant (MBS420), the fumAC mutant (MBS406) and the triple fumarase mutant (MBS426). An important difference among these strains was the malate yield achieved, MBS420 showed a yield of only 0.13 mol of malate per mol of glucose consumed, whereas the fumAC mutants (MBS406 and MBS426) showed a significant increase in malate yield up to 0.61–0.67 mol malate per mol of glucose; however, the fumAC mutants, as mentioned earlier, grew slower and did not consume all the glucose in 24 h.
In this work, the presence of either one of the three fumarases (A, B or C) was sufficient to allow the conversion of malate into fumarate for succinate production and only when both fumA and fumC, or all three fumarases genes were inactivated, did cultures show a lowered amount of succinate production. The prevention of succinate synthesis under these circumstances led to a significant increase in malate yield, and some increase in final malate concentration, but glucose consumption decreased to only 20–25% as mentioned earlier. Therefore, the elimination of fumA and fumC or all three fumarase genes resulted in limited aerobic cell growth and anaerobic fermentation.
Zhang and collaborators [26] showed succinate as the major C4-dicarboxylic compound produced in anaerobic fermentation with a fumB mutant in a different background strain (∆ldhA ∆ackA ∆adhE ∆pflB) which contained spontaneous mutations in pck, ptsI and galP. On the other hand, the fumB fumAC mutant showed mainly malate accumulation in a 6-day experiment and succinate accumulation after 9 days, despite the inactivation of all three fumarases. The authors did not study the fumAC deletion without the fumB deletion. Our results showed that malate accumulation is achieved due to the fumAC mutation; and no major differences in glucose consumption, growth and anaerobic product profile between the fumB fumAC and the fumAC mutants were observed (Fig. 2). Even though anaerobic malate production is carbon and redox balanced, ATP availability may have been limiting in the system due to the synthesis of oxaloacetate from PEP, decreasing the formation of ATP by the pyruvate kinase and/or the acetate kinase. In this context, Moon et al. [13] have performed metabolic flux analysis to find a strategy for malate production in E. coli. The authors found that the overexpression of a PEP carboxykinase (pckA) for the conversion of PEP into oxaloacetate, with the concomitant formation of ATP, favored malate synthesis in aerobic cultures both in silico and experimentally. The authors overexpressed the pckA gene from Mannheimia succiniciproducens in a pta mutant strain resulting in the production of malate as the main product, from glucose, in aerobic conditions (0.75 mol mol−1).
On the other hand, succinate can be used as substrate for fumarate or malate production under aerobic conditions. Hence, the production of these C4-dicarboxylic acids was first evaluated using succinate as substrate by adding it to the culture medium and followed by using an efficient succinate-producing strain as described below.
Aerobic production of C4-dicarboxylic acids
The ability of several engineered E. coli strains, including the fumarase mutants, malate dehydrogenase mutant and a high-succinate-producing strain to produce the C4-dicarboxylic acids malate and fumarate was investigated, using succinate as carbon source under aerobic conditions. Results are shown in Fig. 3.
The fumAC mutant strains showed the highest fumarate production, 69.37 and 29.88 mM (8.05 and 3.47 g/L) for MBS406 and MBS426, respectively. Fumarate yields were close to stoichiometric (0.86 and 0.93 mol/mol succinate, respectively) when succinate was used as a precursor in aerobic conditions (Fig. 3). The inactivation of fumA and fumC led to the accumulation of fumarate instead of malate, reassuring that fumA and fumC are the main fumarases in aerobic conditions [23]. The presence of fumA (MBS432) allowed for the synthesis of malate from succinate, but succinate consumption was limited. Not all the succinate could be converted to fumarate or malate, perhaps this was due to limited NAD+ availability (or NADH accumulation). Fumarase single mutants and the fumAB mutant showed less than 20% of succinate conversion (data not shown).
The results shown in Fig. 3 demonstrate the ability of MBS440 (mdh mutant) and SBS550MG-Cm243 pHL413-Km to produce malate from succinate as the main C4-product, 40.38 mM (5.41 g/L) and 50.24 mM (6.73 g/L), 0.53 and 0.55 mol (mole succinate)−1, respectively. As SBS550MG-Cm243 pHL413-Km is a high-succinate-producing strain [21], the next step was to develop a two-stage production process where the first stage is anaerobic for succinate production from glucose and the second stage was aerobic for the final conversion of succinate into malate. The results are shown in Fig. 4. The mdh mutant strain was not used in a two-stage production process because it is unable to produce succinate under anaerobic conditions (Fig. 1). In addition, the use of the high-succinate producer, SBS550MG-Cm243 pHL413-Km, has the advantage over the mdh mutant strain of fixating CO2 incorporating it into the C4-dicarboxylic acids formed.
In the two-stage production process, after succinate accumulation, the switch from anaerobic to aerobic conditions lets the reverse reaction take place and succinate was transformed into malate. The reaction from succinate to fumarate has a ∆G0 of 0 kJ mol−1 and the reaction from fumarate to malate has ∆G0 of − 3.8 kJ mol−1, whereas the ∆G0 of malate to oxaloacetate is positive 29.7 kJ mol−1 [14] leading malate as the most favorable product from the thermodynamic perspective. The overall malate yield was 1.30 mol (mole glucose)−1. Malic acid was the only C4-dicarboxylic acid product; no other C4-dicarboxylic acids were detected. The malate yield from succinate was 0.74 mol mol−1 (74% of the theoretical yield), and the final malate productivity was 0.38 g L−1 h−1. Some of the succinate may have been metabolized through the TCA cycle where the malate formed could be converted into pyruvate by the malic enzymes, or to oxaloacetate which could later be converted into PEP by the PEP carboxykinase, and then into pyruvate through glycolysis. The pyruvate formed under aerobic conditions can finally be converted into acetate. Microaerobic conditions might improve malate yield by controlling the loss of succinate.
The advantage of the two-stage process is the use of the same tank for all stages of the process. The shift in conditions for the final stage allows a different product to be formed, and the relative economics of succinate or malate production could allow adjustment of product based on factors of feedstock availability, configuration and operation costs of the bioproduction facility. The overall process is longer for malate than succinate; however, the potential high molar yield of malate (molecular weight 134 and current bulk prices ($2200-4000/MT) may compare well with the value of succinic acid (molecular weight 118 and bulk price 1550–2600/MT, somewhat lower than l-malate) making production of l-malate more profitable under certain circumstances.
References
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2(2006):0008
Brown SH, Bashkirova L, Berka R, Chandler T, Doty T, McCall K, McCulloch M, McFarland S, Thompson S, Yaver D, Berry A (2013) Metabolic engineering of Aspergillus oryzae NRRL3488 for increased production of l-malic acid. Appl Microbiol Biotechnol 97:8903–8912
Cao Y, Cao Y, Lin X (2011) Metabolically engineered Escherichia coli for biotechnological production of four-carbon 1,4-dicarboxylic acids. J Ind Microbiol Biotechnol 38:649–656
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
Fiume Z (2001) Final report on the safety assessment of malic acid and sodium malate. Int J Toxicol 20(Suppl 1):47–55
Flint DH, Emptage MH, Guest JR (1992) Fumarase a from Escherichia coli: purification and characterization as an iron–sulfur cluster containing enzyme. Biochemistry 31:10331–10337
Guest JR, Miles JS, Roberts RE, Woods SA (1985) The fumarase genes of Escherichia coli: location of the fumB gene and discovery of a new gene (fumC). J Gen Microbiol 131:2971–2984
Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO (2008) Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99:1140–1153
Kronen M, Berg IA (2015) Mesaconase/fumarase FumD in Escherichia coli O157:H7 and promiscuity of Escherichia coli class I fumarases FumA and FumB. PLoS ONE 10(12):e0145098
Lin H, Vadali RV, Bennett GN, San K-Y (2004) Increasing the acetyl-CoA pool in the presence of overexpressed phosphoenolpyruvate carboxylase or pyruvate carboxylase enhances succinate production in Escherichia coli. Biotechnol Prog 20:1599–1604
Martinez I, Lee A, Bennett GN, San K-Y (2011) Culture conditions’ impact on succinate production by a high succinate producing Escherichia coli strain. Biotechnol Prog 27:1225–1231
Miles JS, Guest JR (1984) Complete nucleotide sequence of the fumarase gene fumA, of Escherichia coli. Nucleic Acids Res 12:3631–3642
Moon SY, Hong SH, Kim TY, Lee SY (2008) Metabolic engineering of Escherichia coli for the production of malic acid. Biochem Eng J 40:312–320
Nelson D, Cox M (2005) Lehninger Principles of Biochemistry, 4th edn. W.H. Freeman and Company, New York, p 612
Sanchez AM, Bennett GN, San K-Y (2005) Efficient succinic acid production from glucose through overexpression of pyruvate carboxylase in an Escherichia coli alcohol dehydrogenase and lactate dehydrogenase mutant. Biotechnol Prog 21:358–365
Sanchez AM, Bennett GN, San K-Y (2005) Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity. Metab Eng 7:229–239
Sévin DC, Fuhrer T, Zamboni N, Sauer U (2017) Nontargeted in vitro metabolomics for high-throughput identification of novel enzymes in Escherichia coli. Nat Methods 14:187–194
Song CW, Kim DI, Choi S, Jang JW, Lee SY (2013) Metabolic engineering of Escherichia coli for the production of fumaric acid. Biotechnol Bioeng 110:2025–2034
Thakker C, Martínez I, Li W, San K-Y, Bennett GN (2015) Metabolic engineering of carbon and redox flow in the production of small organic acids. J Ind Microbiol Biotechnol 42:403–422
Thakker C, Martínez I, San K-Y, Bennett GN (2012) Succinate production in Escherichia coli. Biotechnol J 7:213–224
Thakker C, Zhu J, San K-Y, Bennett GN (2011) Heterologous pyc gene expression under various natural and engineered promoters in Escherichia coli for improved succinate production. J Biotechnol 155:236–243
Tseng CP, Yu C-C, Lin H-H, Chang C-Y, Kuo J-T (2001) Oxygen- and growth rate-dependent regulation of Escherichia coli fumarase (FumA, FumB, and FumC) activity. J Bacteriol 183:461–467
Woods SA, Schwartzbach SD, Guest JR (1988) Two biochemically distinct classes of fumarase in Escherichia coli. Biochim Biophys Acta 954:14–26
Zeikus JG, Jain MK, Elankovan P (1999) Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552
Zelle RM, de Hulster E, Kloenzen W, Pronk JT, van Maris AJA (2010) Key process conditions for production of C4 dicarboxylic acids in bioreactor batch cultures of an engineered Saccharomyces cerevisiae strain. Appl Environ Microbiol 76:744–750
Zhang X, Wang X, Shanmugam KT, Ingram O (2011) l-Malate production by metabolic engineering Escherichia coli. Appl Environ Microbiol 77:427–434
Acknowledgements
The authors acknowledge funding from grants from the National Institutes of Health (NIH GM090152) and NSF (CBET-0828516). The authors would like to thank Auritra Mallick for performing preliminary experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinez, I., Gao, H., Bennett, G.N. et al. High yield production of four-carbon dicarboxylic acids by metabolically engineered Escherichia coli . J Ind Microbiol Biotechnol 45, 53–60 (2018). https://doi.org/10.1007/s10295-017-1991-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1991-3