Abstract
In the present study, silver nanoparticles were biosynthesized using banana (Musa spp.) peel extract as the reducing agent. The biosynthesized particles were then characterized using scanning electron microscopy, X-ray diffraction, transmission electron microscopy and Fourier transform infrared spectroscopy. Biosynthesized silver nanoparticles were then evaluated for their antibacterial activity against Gram-positive and Gram-negative cocci bacteria through agar diffusion study and minimum inhibitory concentration, and minimum bactericidal concentration was determined. Low density polyethylene film was coated with the biosynthesized silver nanoparticles following the dip coating method. The nanoparticle incorporated polymer films were then evaluated for antibacterial and antioxidant activity by agar diffusion studies, dynamic shake flask studies and 2, 2- diphenyl-1-picryl-hydrazyl-hydrate free radical scavenging assay, respectively. The surface characterization of the prepared films was also performed to ensure the presence of silver nanoparticle coating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Packaging is the final process in the food industry, which primarily aids in food preservation. The packaging business is constantly seeking solutions to boost quality and increase the shelf life of packaged food. Among the different packaging materials generally used in the food industry, petrochemical-based plastics have an increased acceptance due to their low cost and ease of processing. Because of its flexibility, thermal stability, low permeability to water and transparency, low density polyethylene (LDPE), is usually preferred in food industries.1 Although aseptic food packaging lengthens the completed product's shelf life, the possibility of contamination still exists.2,3 In recent times, the active packaging materials prepared using the addition of antibacterial agents into the packaging matrix has attracted the attention of food researchers. Apart from the protective function of the polymer packaging matrix, these antibacterial components also provide a preservative function.4 Antibacterial agents can be added into the polymer matrix through direct method or thermal processing. Direct method involves surface modification, surface coating and physical vapor deposition, while in thermal processing, antibacterial agents are incorporated into the polymer through injection molding, melt mixing and extrusion.5 Among the various antibacterial films studied by the researchers, polymer nanocomposites prepared by adding inorganic metal nanoparticles are considered to be more advantageous due to their enhancement of thermal, mechanical and barrier properties when applied in low concentrations.6 Silver nanoparticles are known for their antibacterial properties along with unique optical and catalytic properties in comparison with bulk precursor materials.7 Additionally, research has proved that silver nanoparticles are less cytotoxic to human cells than other inorganic nanoparticles.5 Investigations on the synthesis of silver nanoparticles through physical, chemical and biological approaches were reported by many of the researchers.8 Among these, the biosynthesis route of silver nanoparticles has proven to be more sustainable due to its single step conversion of precursor salt along with the remarkable benefits involving cost-effectiveness, low energy requirements, environmental friendliness and safety in human therapeutic usage.9,10
Apart from the microbial interventions, oxidation of diverse food components is the major factor controlling the quality of food products. Exposure of packed food items to excess light, temperature and moisture will generate singlet oxygen, which in turn produces alkyl-free radicals by combining with the hydrogen atom of unsaturated fatty acids. This lipid oxidation has a negative impact on the sensory characteristics of the food items by causing rancidity, the production of harmful aldehydes, and the loss of nutritional content.11 Hence, addition of antioxidant agents to the food packaging material can prevent rancidity of the processed food items to a certain extent. Biosynthesized silver nanoparticles are also known for their unique antioxidant property, which will provide an additional benefit to the existing antibacterial packaging material. In the studies reported by Akintola et al.,12 Keshari et al.,13 and Mohanta et al.,14 biosynthesized silver nanoparticles reported enhanced free radical scavenging capacity in comparison to the vitamin C used as a standard reference.
In the present paper, silver nanoparticles were biosynthesized using banana [Musa spp.] peel extract as the reducing agent. After characterization and evaluation for its antibacterial and antioxidant potential, the silver nanoparticle colloidal solution was coated onto low density polyethylene film following dip coating method. This prepared polymer film with proven antibacterial and antioxidant properties can be suggested as a novel food packaging material after verifying the same with further migration studies.
Materials and methods
Processing of plant resource material
Banana peels [Musa spp.] were collected from Karthika Chips, Panamukku, Kanimangalam, Thrissur, Kerala, India after being disposed of as leftover waste after being used to make chips. Collected peels were washed under tap water followed by distilled water. Cleaned plant peel materials were then sliced into 10 mm × 2 mm measuring pieces and shade dried in dust-free conditions. The shade-dried peels were ground into fine powder using a Preethi Platinum domestic mixer and stored in dry airtight containers for future use.
Preparation of aqueous banana peel extract
The extract was prepared by mixing 3 g of the processed powder in 100 mL of distilled water, and the mixture was allowed to boil under continuous mixing conditions. The suspension was allowed to cool to room temperature and then filtered through Whatman No. 1 filter paper to eliminate the residual plant material. The obtained extract was stored in amber colored bottles at 4°C for further usage.
Biosynthesis of silver nanoparticles
Biosynthesis of silver nanoparticles was carried out using prepared aqueous banana [Musa spp.] peel extract as the reducing agent. One milliliter of the prepared extract was allowed to react with 10 mL of 10 mM silver nitrate solution in the volumetric mix ratio 1:10 with the initial pH of the extract adjusted to 11. The reaction mixture was incubated for a period of 48 h at 70°C in a hot air oven (Betson, Model: BHO-02D). The solution was observed for the color change from light yellow to dark brown at regular intervals with reference to the banana peel extract as control.
UV-Visible spectrophotometer analysis
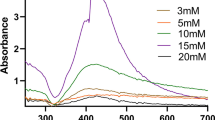
Aliquots of the samples measuring 1 mL were withdrawn to quartz cuvettes from the reaction mixture after the required incubation time and diluted in order to primarily characterize the presence of silver nanoparticles. The spectral analyses were carried out at a resolution of 1 nm from 350 to 700 nm using dual beam UV–Visible spectrophotometer (Shimadzu UV-1800). The experiments were performed in triplicate to determine the surface plasmon resonance peak, and the mean values were plotted.
Characterization of biosynthesized silver nanoparticles
Biosynthesized silver nanoparticles were recovered by centrifuging the colloidal suspension of nanoparticles at 12000 rpm using a REMI-C24BL centrifuge for 20 min. The pellet obtained after centrifugation was washed with 70% ethanol followed by distilled water washing and dried in a hot air oven (Betson, Model: BHO-02D) at 70°C for 48 h. These dried particles were used for characterization studies. The recovered particles were characterized using scanning electron microscopy (SEM) analysis, energy-dispersive X-ray (EDX) analysis, X-ray diffractometer (XRD) analysis, transmission electron microscopy (TEM) and Fourier transform infrared (FTIR) spectroscopy analysis to determine the shape, chemical constituents, size and surface moieties.
SEM and EDX analysis
Surface morphology of the particles were characterized using scanning electron microscopy performed by operating a JEOL 6390LA scanning electron microscope at 30.0 kV under 25 kx magnification after sputter coating the sample with gold. EDX analysis was carried out along with SEM to confirm the presence of silver.
XRD analysis
X-ray diffractogram of biosynthesized silver nanoparticles prepared under optimum conditions was obtained using Aeris research benchtop X-ray diffractometer with 2θ in the range of 20°–90° with Cu Kα radiation of wavelength 1.540598 Å. Crystalline size of the nanoparticles was calculated using the Scherrer’s formula15 as described in equation (1) given below.
where Dp = average crystalline size in nm, K = 0.94 (constant), \(\lambda\) = wavelength of X-ray, \(\frac{\beta 1}{2}\) = full width half maximum (FWHM) of the peak and cos \(\theta\) = peak angle.
TEM analysis
For transmission electron microscopy (TEM), a drop of the aqueous silver nanoparticle sample was applied to a carbon-coated copper grid and left to air dry at room temperature. Micrographs were acquired using a TEM instrument (JEOL JEM-F200) operating at accelerating voltage of 200 kV.
FTIR analysis
Biosynthesized silver nanoparticles prepared under optimum conditions were analyzed on an ATR-FTIR Shimadzu IR Prestige 21 spectroscope operating in the mid infrared region of 600–4000 cm−1 with 2 cm−1 resolution. FTIR analysis was performed to identify the functional groups on the surface of the nanoparticles. The data gives information about the interaction between biomolecules of the plant extract with the silver nanoparticles as capping agents.
Antibacterial activity of biosynthesized silver nanoparticles solution
Antibacterial activity of biosynthesized silver nanoparticle colloidal suspension was evaluated by agar diffusion assay and minimum inhibitory concentration and minimum bactericidal concentration were computed against Gram-positive and Gram-negative cocci bacteria isolated from shelf life expired chips.
Antibacterial activity studies by agar diffusion assay
Biosynthesized silver nanoparticle solution was evaluated for its antibacterial activity against Gram-positive and Gram-negative cocci bacteria following agar diffusion method on Mueller–Hinton agar medium. A 24-h-grown bacterial culture inoculum was spread on the solid agar plates using the Kirby–Bauer method. Chloramphenicol (10 mcg) was maintained as the positive control along with the banana peel extract and 10 mM silver nitrate solutions as control samples. One hundred microliters of each sample were added to wells with a 6-mm diameter created in inoculated plates using a sterile well borer. All the plates were incubated for 24 h at 37°C, and zone of inhibition around the wells was measured in mm.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determination
Minimal inhibitory concentration (MIC) was determined by using a twofold serial dilution method with the growth of stock inoculum adjusted to 1% McFarland Standard. The assay was performed in 96 well microtiter plate. Each well was added with increasing concentrations of the silver nanoparticle solution ranging from 62.5 to 1000 µg/mL, respectively. The plates were then incubated overnight for 24 h at 37°C with positive control as Muller–Hinton broth medium inoculated with microorganisms. Optical density of the plates was read at 630 nm using ERBA, LisaScan ELISA plate reader. The growth inhibition for the test wells at each sample dilution was determined by the formula (2)16:
Twenty microliters from each well were swabbed onto Muller–Hinton agar plates after the period of incubation. The plates were then incubated at 37°C for 48 h and observed for the bacterial growth. The MBC endpoint is characterized by the minimum concentration of the antimicrobial agent required to eliminate 99.9% of the initial bacterial population.
Preparation of silver nanoparticle-coated low density polyethylene (LDPE) film
A colloidal suspension of biosynthesized silver nanoparticles was coated on to commercially purchased LDPE film (Gregory Polymers, Kaninad, Puthencruz, Ernakulam, India) with 70 micron thickness following the dip coating method.17 The coating is obtained by dipping the polymer film of 10 cm × 10 cm (L × W) in 50 mL of biosynthesized solution of silver nanoparticles for a period of 5 h. The film material after the process of coating was air dried in room temperature for 2–3 days to remove the moisture content. The dried product was stored in moisture-free conditions for further analysis and characterization studies.
Characterization of silver nanoparticle-coated LDPE film
Characterization of silver nanoparticle-coated LDPE film was performed using SEM and EDX analysis. Morphological characteristics of the prepared film along with the control were carried out using TESCAN VEGA-3LMV scanning electron microscope operating at 20.0 kv with 23 kx magnification. EDX spectrum of control and sample films were also plotted to confirm the presence of coating of silver nanoparticles with reference to that of control.
Antibacterial activity of silver nanoparticle-coated LDPE film
Antibacterial activity of silver nanoparticle-coated LDPE film was determined by agar diffusion method in solid agar medium and dynamic shake flask studies in liquid broth.
Antibacterial activity studies by agar diffusion method
A 24-h-grown Gram-positive and Gram-negative cocci bacteria was spread onto Muller–Hinton agar plates following Kirby–Bauer method. 1.5 × 1.5 cm2 measuring silver nanoparticle-coated LDPE film (AgNp-LDPE) was placed on the agar plates,5 along with the control film (Control-LDPE) samples. Plates were incubated at 37°C for 24 h. Zone of clearance around the LDPE film was recorded as an indication of antibacterial activity of the films.
Antibacterial activity studies by dynamic shake flask method
Antibacterial activity of biosynthesized silver nanoparticles coated LDPE film along with the control film was carried out using modified dynamic shake flask method18 following time-dependent methodology against Gram-positive and Gram-negative cocci bacteria isolated from shelf life expired chips. 20 mL of Muller–Hinton Broth (MHB) was inoculated with 0.4 mL of overnight grown inoculum and incubated for 3 h at 37°C at 110 rpm. Then, 0.4 g of silver nanoparticle-coated LDPE (AgNp-LDPE) along with the control film (Control-LDPE) were placed into sterile Erlenmeyer flasks and inoculated with 0.2 mL of the twofold serially diluted 3-h-grown culture. Samples were soaked in 40 mL of MHB, and the flasks were shaken at 37°C in a rotary shaking incubator (REMI, CIS-24 PLUS) and absorbance was taken at 660 nm for varied time intervals for a period of 24 h.
Antioxidant activity of silver nanoparticle-coated LDPE film
Antioxidant activity of AgNp-LDPE film along with the control was determined by dose-dependent 2, 2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay as per reference 19. Then, 10 mg of the film samples were vortexed for 3 min, immersed in 1 mL of methanol solution and allowed to stand at room temperature for 3 h. The mixture was again vortexed for 3 min followed by centrifugation at 2300 rpm for 10 min. Five hundred microliters of thus obtained supernatant was used for analyzing the antioxidant activity by mixing with an equal volume of freshly prepared DPPH reagent. Absorbance of the reaction mixture at 517 nm was recorded after incubating in the dark for 30 min. DPPH radical scavenging activity was calculated using the following equation (3).
Ao = Absorbance of blank
A1 = Absorbance of reaction solution
Statistical analysis
All independent analyses were performed in triplicate, and the data were expressed as mean + /− standard deviation. Final experiments on antioxidant activity of silver nanoparticle-coated film were done in triplicate and results were analyzed by one-way ANOVA and Duncan test were performed to analyze data, with p < 0.001 compared to the control group.
Results and discussion
Phytosynthesis of silver nanoparticles was reported by a vast number of researchers20,21 and among them sustainable methods for the synthesis of silver nanoparticles have been gaining greater importance in the recent years. In the current study, silver nanoparticles were biosynthesized using plant extract prepared from the waste resource material collected from the savory chips factory as the reducing agent. Visual confirmation of the formation of silver nanoparticles was observed by the change in the color of reaction mixture from pale yellow to dark brown after 48 h, as presented in Fig. 1. Similar studies on bio reduction of silver nitrate using banana peel extract were reported by Ibrahim9 and Kokila et al.10 It also suggested the role of active components in banana peel extracts including pectin, cellulose and hemicellulose in reducing silver ions to their zerovalent state.
Characterization of biosynthesized silver nanoparticles
Biosynthesized silver nanoparticles using banana peel extract were initially characterized using a UV-Visible spectrophotometer to confirm the formation of the silver nanoparticles. The spectra depicted in Fig. 2 presented a surface plasmon resonance peak at 430 nm for biosynthesized silver nanoparticles in comparison to the banana peel extract, and the bio reduction of silver nitrate was aided through the active components of banana peel extract. Similar reports on the surface plasmon resonance band at 430 nm were reported by Dang et al.22 and Kokila et al.10 As discussed by Ibrahim,9 a single peak in the surface plasmon resonance spectrum indicates the formation of spherical nanoparticles, whereas the multiple peaks correspond to anisotropic particle formation. In the present study, a single peak was formed which suggested the formation of spherical nanoparticles.
Morphological characteristics of the biosynthesized particles using banana peel extract were analyzed using scanning electron microscopy and, as featured in Fig. 3a, monodispersed spherical particles were observed. Similarly, reports on the biosynthesis of spherical nanoparticles within the size range of 23–30 nm were confirmed by Kokila et al.10 using Cavendish banana peel extract, and Bhuyar et al.23 revealed the formation of spherical silver nanoparticles with an average mean diameter of 33.75 nm using marine microalgae extract as the reducing agent. The EDX spectrum depicted in Fig. 3b denotes higher absorption signals at 3 kV which indicates the elemental profile of silver and thereby confirms the formation of silver nanoparticles. The spectrum also revealed weaker signals for carbon and oxygen in response to the presence of biomolecules from banana peel extract on the surface of nanoparticles. Another major peak of chlorine in the spectrum is due to the presence of non-metallic compounds in banana peel extract.24,25
The transmission electron micrographic image of biologically synthesized silver nanoparticles in Fig. 4 unveiled a spherical morphology, with the average particle size determined as 11.82 nm through Gatan Digital Micrograph. A similar investigation conducted by Ibrahim9 reported the formation of spherical silver nanoparticles measuring 23.7 nm, utilizing banana peel extract as a reducing agent. In the study by Otunola and Afolayan,19 TEM analysis revealed polydispersed silver nanoparticle formation in the range of 6–28 nm using an aqueous spice blend formulation as the reducing agent.
XRD spectrum of recovered nanoparticles plotted in Fig. 5 representing Bragg’s reflection peaks at 27.63°, 31.979°, 38.193°, 46.097°, 54.599°, 64.375° and 77.063° corresponds to (210), (122), (111), (200), (142), (220) and (311) planes of silver nanoparticles which suggested a face centered cubic crystal system of silver nanoparticle formation as per International Centre for Diffraction Data (ICDD:00-004-0783)26,27,28 along with 2θ excitation at 57.38° which indicates the existence of hexagonal silver nanoparticles with reference to International Centre for Diffraction Data (ICDD:00-041-1402).26
These results suggested the biphasic nature of the biosynthesized silver nanoparticles. The existence of strain in the crystal structure was indicated by the slight shift in the peak locations. Also, the average particle size obtained was calculated to be in the range of 10.08 nm. The result was satisfactory in comparison to the particle size determined by TEM analysis. Biosynthesis of silver nanoparticles within the average particle size of 11.74 nm was reported by Kalpana et al.29 using Torreya nucifera as the reducing agent.
FTIR spectrometry was performed in order to understand the role of biomolecules present in the banana peel extract acting as capping and reducing agents aiding in the biosynthesis of silver nanoparticles.19,29 FTIR analysis of biosynthesized silver nanoparticles was carried out in order to identify the functional groups on the surface of nanoparticles. As visible in the FTIR spectrum depicted in Fig. 6, the broad absorption band at 3625.47 cm−1 indicates the presence of hydroxyl (–OH) and amino groups and the peak at 3205.19 cm−1 corresponds to –OH stretching due to hydrogen bonds. The bands at 2895.79 cm−1 and 1459.71 cm−1 are due to the existence of aliphatic –CH stretching and asymmetric bending vibrations, respectively. Also, the peak at 2704.22 cm−1 could be assigned to the presence of aldehydes.30,31 The presence of pectin, cellulose, and hemicellulose in the banana peel extracts serving as stabilizing factors aiding in nanoparticle formation is responsible for the occurrence of these linkages on the surface of biosynthesized silver nanoparticles.32 The peaks located at 1687.69 cm−1 and 1628.76 cm−1 are attributed to C=C bond stretching. The absorption peaks at 2173.88 cm−1, 1548.11 cm−1 and 1032.46 cm−1 correspond to aromatic ring stretching and cyclohexane ring vibrations. Also, the multiple peaks between 900 and 670 cm−1 with a prominent peak at 678.94 cm−1 are due to the existence of aromatic C–H out of plane bending.30,31 Alkaloids and terpenoids from the extract serve as reducing agents on the silver nanoparticles, which results in unsaturated bonding and aromatic ring stretching. The presence of such alkaloids and terpenoids in banana peel extract was reported by Uzairu and Kano.33
Antibacterial activity of biosynthesized silver nanoparticles
Antibacterial activity of biosynthesized silver nanoparticles against Gram-positive and Gram-negative bacteria was initially studied by the agar diffusion method followed by evaluation of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC).
The antibacterial activity of biosynthesized silver nanoparticles against Gram-positive and Gram-negative was demonstrated by the notable diameter of the inhibition zone as given in Table 1.
The antibiotic Chloramphenicol 10 mcg was maintained as positive control and banana peel extract as negative control throughout the study. The findings demonstrated a notable expansion in the diameter of the inhibition zone for Gram-positive bacteria when compared to Gram-negative organisms. The Gram-negative bacteria exhibited greater resistance to the silver nanoparticle solution, attributed to the protective outer coating on their surfaces, as elucidated in previous research.35,36 This resistance was subsequently verified in antibacterial investigations conducted through dynamic shake flask studies.
Minimum inhibitory concentration of biosynthesized silver nanoparticle solution required to inhibit the growth of both Gram-positive and Gram-negative cocci bacteria was calculated using ED50 PLUS V1.0 software from the percentage growth inhibition of bacteria when in contact with concentration of silver nanoparticles varied from 62.5 µg/mL to 1000 µg/mL. The inhibition of Gram-positive bacterial growth ranged from 46.33 to 81.78% with MIC 50 as 91.844 µg/mL, while for Gram-negative bacteria, the inhibition ranged from 4.70 to 76.49% with MIC 50 as 364.67 µg/mL, corresponding to ascending concentrations. The MBC 90 values represent the minimum bactericidal concentration of biosynthesized silver nanoparticles against Gram-positive and Gram-negative cocci bacteria. Twenty microliters of sample from each well with different concentrations were swabbed onto sterile Muller–Hinton agar plates and the minimum concentration of silver nanoparticle solution inhibited the bacterial growth was computed as 174.04 µg/mL for Gram-positive bacteria and 650.399 µg/mL for Gram-negative bacteria using ED50 PLUS V1.0 software, respectively. In the study by Parvekar et al.37 MIC and MBC of commercially purchased silver nanoparticles with 5 nm size was in the range of 625 µg/mL against S. aureus. Also, microbial mediated biosynthesized silver nanoparticles exhibited MIC and MBC in the range of 31.25–125 µg/mL and 62.5–250 µg/mL against human pathogenic microorganisms.38
Preparation and characterization of silver nanoparticle-coated LDPE film
Low density polyethylene film coated with silver nanoparticles was prepared by dip coating method. Prepared films were characterized by means of scanning electron microscopy and energy dispersive X-ray analysis. Electron microscopic imaging of the sample (AgNp-LDPE) revealed the morphological existence of silver nanoparticles in comparison with the control film (Control-LDPE), as indicated in Fig. 7. The presence of silver nanoparticles was later verified by EDX analysis.
As observed in Fig. 8b, energy-dispersive X-ray analysis of the AgNp-LDPE film revealed the presence of silver in addition to the peak of carbon in Fig. 8a. The peak of carbon in the EDX spectrum is due to the elemental composition of polyethylene and additional peaks of sodium, chlorine and aluminum indicate the compounds present in the banana peel extract. Similar reports on the existence of the silver peak in the EDX spectrum was observed by Becaro et al.34
Antibacterial activity of silver nanoparticle-coated LDPE film
Antibacterial activity of biosynthesized silver nanoparticle-coated polymer films was performed using dynamic shake flask studies with dose dependency of film as 10 mg/mL and by agar diffusion study.
As shown in Fig. 9, a clear zone was observed around the AgNp-LDPE film against both Gram-positive and Gram-negative cocci bacteria. In comparison to Gram-negative bacteria, Gram-positive bacteria exhibited a larger area of inhibition in bacterial growth. The presence of a distinct clear zone surrounding the silver-coated films serves as evidence of the antimicrobial effectiveness of these films in contrast to the control sample. The microbial inhibition observed suggests that silver ions are released from the coated film, into the agar layer and effectively hinder the growth of microbial colonies within the agar medium.5
In dynamic shake flask studies, bacteria grown in Muller–Hinton Broth media was monitored for its growth for a period of 24 h at varied time intervals. The effect of biosynthesized silver nanoparticle-coated LDPE films on the growth profile of Gram-negative and Gram-positive cocci bacteria in comparison to the Control-LDPE is shown in Fig. 10. As visible in Fig. 10, the silver-coated LDPE film had an impact on the kinetics of bacterial growth. According to the study, bacterial growth was inhibited for the sample in conjunction with AgNp-LDPE film after a lag period for Gram-positive bacteria, and in the time that followed, there was a progressive transition towards the stationary phase. In comparison with the growth pattern of Gram-positive bacteria, the Gram-negative organism was resistant towards silver nanoparticles throughout the growth period of 24 h. The resistance is mainly due to the presence of an outer membrane with phospholipids and lipopolysaccharides on the bacterial surface.35
In a similar study by Dehnavi et al.,5 the growth of Staphylococcus aureus bacteria was extended in the lag phase with the contact of silver nanoparticle-coated polyethylene film. Reduction in growth of P. oleovorans was observed for samples in contact with silver nanoparticle-coated LDPE film by spraying method. The study reported 30% inhibition on the growth of bacteria.6
Antioxidant activity of silver nanoparticle-coated LDPE film
Reports on the free radical scavenging potential of silver nanoparticles were discussed by Keshari et al.13 and Mohanta et al.14.They also discussed the 5.27% increase in free radical scavenging potential of silver nanoparticles with respect to the vitamin C standard. These reports recommended the usage of phytosynthesized silver nanoparticles as free radical scavenger which will be beneficial in food packaging applications. In the DPPH-free radical scavenging assay carried out in the present experiment, using the prepared biosynthesized silver nanoparticle-coated polymer films, the sample of interest [AgNp-LDPE] revealed an increase in scavenging of 13.97% as compared to Control-LDPE (Fig. 11). This increase in the antioxidant activity of the AgNp-LDPE film is due to the bioreductive agents on the surface of biosynthesized nanoparticles. The presence of bioactive anthocyanins and silver nanoparticles on the surface of chitosan films39 cited an enhancement of 30% scavenging potential in comparison to the control. Also, in a study by Hajji et al.,40 the researcher reported on the role of residual-free amino groups on the surface of chitosan film acting as free radical scavengers. In agreement to the study, the FTIR analysis spectrum illustrated in Fig. 6 confirms the existence of amino groups on the surface of biosynthesized silver nanoparticles, thereby contributing to the observed enhancement in antioxidant activity as demonstrated in this study.
Conclusions
Biosynthesized silver nanoparticle incorporated LDPE films were prepared by dip coating method. A stable colloidal solution of green synthesized silver nanoparticles was prepared using banana (Musa spp.) peel extract as the reducing agent. Analysis of the colloidal solution reported surface plasmon resonance peak for absorption maxima at 430 nm. Characterization using transmission electron microscopy revealed spherical surface morphology of the particles with the size in the range of 11.82 nm. XRD spectrum reported the existence of hexagonal and face-centered cubic crystal systems with the slight shift in the peak locations indicating strain in the crystal structure and particle size was calculated as 10.08 nm using Scherrer’s formula. The absorption bands in the FTIR data with hydroxyl, amino and aliphatic –CH stretching were due to the presence of pectin, cellulose and hemicellulose in the plantain peel extract. Similarly, the absorption peaks due to C=C stretching, aromatic ring stretching and C–H out of plane bending justified the existence of alkaloids and terpenoids in the extract acting as capping agents on the surface of nanoparticles. Antibacterial activity of biosynthesized silver nanoparticles was studied by agar diffusion assay and minimum bactericidal concentration was determined as 174.04 and 650.399 µg/mL against Gram-positive and Gram-negative bacteria, respectively. Surface modification of the LDPE films was observed through SEM analysis. In addition to the observed inhibition of bacterial growth in the agar film diffusion study, antibacterial investigations conducted through dynamic shake flask studies exhibited an initial suppression of microbial growth, commencing from the lag phase. This inhibition transitioned towards the stationary phase at a later stage. In addition to the antibacterial studies, prepared films also reported a 13.97% increase in the scavenging capacity of the free radicals with respect to the control samples. In the present study, another interesting point to be highlighted is the sustainability of the packaging method as the nanoparticles are biosynthesized using the waste peel of the banana utilized for manufacturing the banana chips being packaged. Thus, the biogenic prepared films with antibacterial and antioxidant potential can be suggested as an alternative eco-friendly and sustainable food packaging material after further migration studies are completed.
References
Essa, RY, Elsebaie, EM, “Immobilization of Synthesized Silver Nanoparticles Using Mango Peel Extract on Low Density Polyethylene Surface and Its Application as Biologically Active Packages.” Alex. J. Fd. Sci. Technol, 13 (1) 31–38 (2016)
Lomate, GB, Dandi, B, Mishra, S, “Development of Antimicrobial LDPE/Cu Nanocomposite Food Packaging Film for Extended Shelf Life of Peda.” Food Packag. Shelf Life, 16 211–219 (2018)
Olmos, D, Pontes-Quero, GM, Corral, A, González-Gaitano, G, González-Benito, J, “Preparation and Characterization of Antimicrobial Films Based on LDPE/Ag Nanoparticles with Potential Uses in Food and Health Industries.” Nanomaterials, 8 (2) 60 (2018)
Persico, P, Ambrogi, V, Carfagna, C, Cerruti, P, Ferrocino, I, Mauriello, G, “Nanocomposite Polymer Films Containing Carvacrol for Antimicrobial Active Packaging.” Polym. Eng. Sci., 49 (7) 1447–1455 (2009)
Dehnavi, AS, Aroujalian, A, Raisi, A, Fazel, S, “Preparation and Characterization of Polyethylene/Silver Nanocomposite Films with Antibacterial Activity.” J. Appl. Polym. Sci., 127 (2) 1180–1190 (2013)
Valdes, SS, Ortega-Ortiz, H, Ramos-de Valle, LF, Medellín-Rodríguez, FJ, Guedea-Miranda, R, “Mechanical and Antimicrobial Properties of Multilayer Films with a Polyethylene/Silver Nanocomposite Layer.” J. Appl. Polym. Sci., 111 (2) 953–962 (2009)
Haider, A, Kang, IK, “Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review.” Adv. Mater. Sci. Eng., 2015 1–16 (2015)
Iravani, S, Korbekandi, H, Mirmohammadi, SV, Zolfaghari, B, “Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods.” Res. Pharm. Sci., 9 (6) 385–406 (2014)
Ibrahim, HM, “Green Synthesis and Characterization of Silver Nanoparticles Using Banana Peel Extract and Their Antimicrobial Activity Against Representative Microorganisms.” J. Radiat. Res. Appl. Sci., 8 (3) 265–275 (2015)
Kokila, T, Ramesh, PS, Geetha, D, “Biosynthesis of Silver Nanoparticles from Cavendish Banana Peel Extract and Its Antibacterial and Free Radical Scavenging Assay: A Novel Biological Approach.” Appl. Nanosci., 5 911–920 (2015)
Lai, WF, “Design of Polymeric Films for Antioxidant Active Food Packaging.” Int. J. Mol. Sci, 23 (1) 12 (2021)
Akintola, AO, Kehinde, BD, Ayoola, PB, Adewoyin, AG, Adedosu, OT, Ajayi, JF, Ogunsona, SB, “Antioxidant Properties of Silver Nanoparticles Biosynthesized from Methanolic Leaf Extract of Blighia sapida.” Mater. Sci. Eng., 805 (1) 012004 (2020)
Keshari, AK, Srivastava, R, Singh, P, Yadav, VB, Nath, G, “Antioxidant and Antibacterial Activity of Silver Nanoparticles Synthesized by Cestrum nocturnum.” J. Ayurveda Integr. Med., 11 (1) 37–44 (2020)
Mohanta, YK, Panda, SK, Jayabalan, R, Sharma, N, Bastia, AK, Mohanta, TK, “Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina suberosa (Roxb.).” Front. Mol. Biosci, 4 14 (2017)
Alexander, L, Klug, HP, “Determination of Crystallite Size with the XRay Spectrometer.” J. Appl. Phys., 21 (2) 137–142 (1950)
Ediyilyam, S, Lalitha, MM, George, B, Shankar, SS, Wacławek, S, Černík, M, Padil, VVT, “Synthesis, Characterization and Physicochemical Properties of Biogenic Silver Nanoparticle-Encapsulated Chitosan Bionanocomposites.” Polymers, 14 (3) 463 (2022)
Chougule, SS, Gurme, ST, Jadhav, JP, Dongale, TD, Tiwari, AP, “Low Density Polyethylene Films Incorporated with Biosynthesized Silver Nanoparticles Using Moringa oleifera Plant Extract for Antimicrobial, Food Packaging, and Photocatalytic Degradation Applications.” J. Plant Biochem. Biotechnol., 30 208–214 (2021)
Singh, G, Joyce, EM, Beddow, J, Mason, TJ, “Evaluation of Antibacterial Activity of ZnO Nanoparticles Coated Sonochemically onto Textile Fabrics.” J. Microbiol. Biotechnol. Food Sci., 2 (1) 106–120 (2012)
Otunola, GA, Afolayan, AJ, “In Vitro Antibacterial, Antioxidant and Toxicity Profile of Silver Nanoparticles Green-Synthesized and Characterized from Aqueous Extract of a Spice Blend Formulation.” Biotechnol. Biotechnol. Equ., 32 (3) 724–733 (2018)
Ahmed, S, Ahmad, M, Swami, BL, Ikram, S, “A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise.” J. Adv. Res., 7 (1) 17–28 (2016)
Bala, A, Rani, G, “A Review on Phytosynthesis, Affecting Factors and Characterization Techniques of Silver Nanoparticles Designed by Green Approach.” Int. Nano Lett, 10 (3) 159–176 (2020)
Dang, H, Fawcett, D, Poinern, GEJ, “Biogenic Synthesis of Silver Nanoparticles from Waste Banana Plant Stems and Their Antibacterial Activity against Escherichia coli and Staphylococcus epidermis.” Int. J. Res. Med. Sci., 5 (9) 3769–3775 (2017)
Bhuyar, P, Rahim, MHA, Sundararaju, S, Ramaraj, R, Maniam, GP, Govindan, N, “Synthesis of Silver Nanoparticles Using Marine Macroalgae Padina sp. and Its Antibacterial Activity towards Pathogenic Bacteria.” Beni-Suef Univ. J. Basic Appl. Sci., 9 1–15 (2020)
Moshahary, S, Mishra, P, “Synthesis of Silver Nanoparticles (AgNPs) Using Culinary Banana Peel Extract for the Detection of Melamine in Milk.” J. Food Sci. Technol., 58 (2) 797–804 (2021)
Valsalam, S, Agastian, P, Esmail, GA, Ghilan, AKM, Al-Dhabi, NA, Arasu, MV, “Biosynthesis of Silver and Gold Nanoparticles Using Musa acuminata Colla Flower and Its Pharmaceutical Activity against Bacteria and Anticancer Efficacy.” J. Photochem. Photobiol. B: Biol., 201 111670 (2019)
Jayaseelan, C, Rahuman, AA, “Acaricidal Efficacy of Synthesized Silver Nanoparticles Using Aqueous Leaf Extract of Ocimum Canum against Hyalomma anatolicum anatolicum and Hyalomma marginatum isaaci (Acari: Ixodidae).” Parasitol. Res., 111 1369–1378 (2012)
Lee, KJ, Park, SH, Govarthanan, M, Hwang, PH, Seo, YS, Cho, M, Oh, BT, “Synthesis of Silver Nanoparticles Using Cow Milk and Their Antifungal Activity against Phytopathogens.” Mater. Lett., 105 128–131 (2013)
Suman, TY, Rajasree, SR, Kanchana, A, Elizabeth, SB, “Biosynthesis, Characterization and Cytotoxic Effect of Plant Mediated Silver Nanoparticles Using Morinda citrifolia Root Extract.” Coll. Surf. B: Biointerfaces, 106 74–78 (2013)
Kalpana, D, Han, JH, Park, WS, Lee, SM, Wahab, R, Lee, YS, “Green Biosynthesis of Silver Nanoparticles Using Torreya nucifera and Their Antibacterial Activity.” Arabian J. Chem., 12 (7) 1722–1732 (2019)
Coates, J, “Interpretation of Infrared Spectra, a Practical Approach.” In: Meyers, R A (ed.) Encyclopedia of Analytical Chemistry: Applications, Theory, and Instrumentation. Wiley. https://doi.org/10.1002/9780470027318.a5606 (2007)
Nandiyanto, ABD, Oktiani, R, Ragadhita, R, “How to Read and Interpret FTIR Spectroscope of Organic Material.” Indones. J. Sci. Technol., 4 (1) 97–118 (2019)
Bankar, A, Joshi, B, Kumar, AR, Zinjarde, S, “Banana Peel Extract Mediated Novel Route for the Synthesis of Silver Nanoparticles.” Coll. Surf. A: Physicochem. Eng. Aspects, 368 (1–3) 58–63 (2010)
Uzairu, SM, Kano, MA, “Assessment of Phytochemical and Mineral Composition of Unripe and Ripe Plantain (Musa paradisiaca) Peels.” Afr. J. Food Sci, 15 (3) 107–112 (2021)
Becaro, AA, Puti, FC, Correa, DS, Paris, EC, Marconcini, JM, Ferreira, MD, “Polyethylene Films Containing Silver Nanoparticles for Applications in Food Packaging: Characterization of Physico-Chemical and Anti-Microbial Properties.” J. Nanosci. Nanotechnol, 15 (3) 2148–2156 (2015)
Breijyeh, Z, Jubeh, B, Karaman, R, “Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It.” Molecules, 25 (6) 1340 (2020)
Rahisuddin, AL-Thabaiti, SA, Khan, Z, Manzoor, N, “Biosynthesis of Silver Nanoparticles and Its Antibacterial and Antifungal Activities towards Gram-Positive, Gram-Negative Bacterial Strains and Different Species of Candida Fungus.” Bioprocess Biosyst. Eng., 38 1773–1781 (2015)
Parvekar, P, Palaskar, J, Metgud, S, Maria, R, Dutta, S, “The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Silver Nanoparticles against Staphylococcus aureus.” Biomater. Investigat. Dent., 7 (1) 105–109. https://doi.org/10.1080/26415275.2020.1796674 (2020)
Jalal, M, Ansari, MA, Alzohairy, MA, Ali, SG, Khan, HM, Almatroudi, A, Raees, K, “Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida Glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi.” Nanomaterials, 8 (8) 586 (2018)
Qin, Y, Liu, Y, Yuan, L, Yong, H, Liu, J, “Preparation and Characterization of Antioxidant, Antimicrobial and pH-Sensitive Films Based on Chitosan, Silver Nanoparticles and Purple Corn Extract.” Food Hydrocolloids, 96 102–111 (2019)
Hajji, S, Salem, RBSB, Hamdi, M, Jellouli, K, Ayadi, W, Nasri, M, Boufi, S, “Nanocomposite Films Based on Chitosan–Poly(vinyl Alcohol) and Silver Nanoparticles with High Antibacterial and Antioxidant Activities.” Process Saf. Environ. Prot, 111 112–121 (2017)
Acknowledgments
We would like to express our sincere gratitude towards Kerala Development and Innovation Strategic Council (K-DISC) for giving financial support to our work under the Young Innovators Programme -YIP 2020 Scheme (Idea ID: 20YIP2494). We are also thankful towards St. Thomas College (Autonomous) Thrissur, Central Instruments Laboratory, Kerala Veterinary and Animal Science University, Mannuthy, Central Research Facility, NITK surathkal and Sophisticated Test and Instrumentation Centre, DST-SAIF, Cochin for their extended support towards characterization studies.
Funding
Sumi Maria Babu declares the report on financial support received in the form of Award provided by Kerala State Government (Kerala Development and Innovation Strategy Council) under the scheme of Young Innovators Programme (YIP 2020-Idea ID-20YIP2494).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Babu, S.M., Ittiachen, L. Biogenic silver nanoparticle-coated low density polyethylene film with antibacterial and antioxidant properties. J Coat Technol Res 21, 1409–1420 (2024). https://doi.org/10.1007/s11998-023-00903-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-023-00903-2