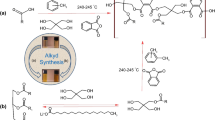
Abstract
Due to the significant importance of waterborne polymers in terms of sustainability, researchers have undertaken the process of transferring alkyd resins into waterborne latex. In this study, two distinct types of alkyd resins were meticulously prepared through the esterification of sustainable sources, namely hemp seed oil (HSO) and pine fatty acid (TOFA), with polyols and dibasic acids. The goal of this research was to synthesize hydrophobic waterborne hybrid polymer emulsions, combining alkyd and styrene acrylic components. This was achieved through a semibatch mini-emulsion polymerization process involving styrene, butyl acrylate, and acrylic acid. The alkyd resins, derived from sustainable sources, were integrated into the polymerization process along with hydrophobic monomers that contained either fluorine or silane groups. Based on the alkyd content, polymer emulsions were examined in three classes: pure polymer emulsions without alkyd (0% alkyd), 10% HSO-based alkyd-containing polymer emulsions (10% HSO-based), and 10% TOFA-based alkyd-polymer emulsions (10% TOFA). Polymer emulsions in each class were first modified with 3% and 6% triethoxyvinyl silane (VTES) and 2,2,2-trifluoroethyl methacrylate (TFEMA) to obtain a total of 12 different modified latexes. The structure of the synthesized latex was characterized by FTIR and 1H-NMR, while the morphology of the latex was examined by AFM, STEM, EDAX, particle size, and zeta potential measurements. The thermal and mechanical properties of the materials were assessed using DSC, TGA, and tensile testings. Lastly, the water contact angle was used to gauge the hydrophobicity of the latexes. The findings revealed that TFEMA and VTES hydrophobic monomers significantly influenced the mechanical, thermal, and hydrophobic properties of hybrid (alkyd/styrene acrylic) polymer emulsions and films.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coating formulations applied in the coating and paint industries play a crucial role in terms of compatibility with the applied surfaces and their overall carbon footprint.1,2,3 In recent years, there has been a growing emphasis on eco-friendly options, and one such trend is the use of waterborne alkyd resin. Although waterborne alkyd resin is oil-based, it has gained significant attention from researchers, especially in the development of weather-resistant coating products where water compatibility is essential.4,5,6,7
Waterborne alkyd resin offers many good properties that make it very valuable due to its chemical composition. However, it is important to acknowledge that certain challenges exist in coating applications. Emulsion polymers containing hydrophilic components can present issues such as poor adhesion and limited thermal resistance. Over time, these factors can render coatings susceptible to various environmental elements, including heat, water, oxygen, ions, and weather conditions, leading to problems experienced by the materials beneath the coating. As a result, the materials on which the coating is applied experience some problems over time.8,9,10,11,12 Therefore, harmony between the coating and the surface is important. According to research, the compatibility of a coating with the surface depends on how chemicals affect the energy level of the surface and the micro- and nanostructures on the surface. Consequently, when developing protective coatings, the hydrolytic stability of the coating becomes a critical consideration. In this context, we come across two criteria: hydrophilic and hydrophobic. These two concepts enable us to describe how water droplets interact with solid surfaces, including the angle at which they make contact, the degree of wetting, and their overall behavior on the surface. On hydrophilic surfaces, a water droplet will penetrate the material's pores and spread across the surface, making it highly wettable. Conversely, water droplets on hydrophobic surfaces will be repelled, forming spherical shapes. These two concepts are of paramount importance, as coatings, including paints and surface treatments, act as protective barriers shielding the underlying surfaces from external influences.12,13,14 In addition, hydrophobic films reinforced with various materials have been an alternative solution for eliminating the material and environmental problems caused by the low durability of waterborne hybrid alkyd composites on external surfaces. Numerous studies have focused on the modification of hydrophilic polymers using substances such as long alkyl chain thiols, alkyl or fluorinated organic silanes, perfluorinated alkyl agents, long alkyl chain fatty acids, silicones, fluorocarbons, and long-chain fatty acids.12,15,16,17
Kanai et al. utilized the synthesized silicon acrylate-soy alkyd resin as a binder for durable exterior coatings and found that it led to an enhancement in the mechanical properties of the coating.18
Zhao et al. synthesized styrene a vinyltrimethoxysilane copolymer by emulsifier-free emulsion polymerization to produce hybrid organic and inorganic materials. It has been observed that the siloxane segments within the copolymer chains alter the structure of the copolymer transitioning it from a linear configuration to a crosslinked network.19
Huang et al. designed a new method for preparing polyacrylate modified with polysiloxane latex particles using methyl methacrylate, butyl acrylate, vinyltriethoxysilane, and octamethylcyclotetrasiloxane. Static contact angle, water absorption, and TGA test results of the films showed that the latexes with a uniform spherical structure exhibited a narrow particle size distribution. Furthermore, it was observed that the introduction of polysiloxane content significantly enhanced hydrophobicity, low surface free energy, and improved thermal stability.20
Bao et al. demonstrated that the thermal stability of organic silicon-modified phosphor acrylic latex, prepared by semibatch emulsion polymerization, exhibited improvement when compared to an unmodified counterpart.21
Lü et al. prepared and characterized a series of self-crosslinking fluorinated polyacrylate latex particles with a core-shell structure by mini-emulsion polymerization. They determined that latex films exhibit good hydrophobicity and improved thermal stability, and making them suitable for the development of advanced multifunctional protective coatings, including those with antiwetting, antiicing, antifog, and anticorrosion properties.22
Zhao et al. synthesized waterborne alkyd hybrid resins modified with fluorinated acrylate-siloxane by surfactant-free mini-emulsion polymerization process. They produced films with low water absorption, high water contact angles, improved mechanical and thermal properties, and, notably, superior corrosion resistance performance.23
Zhong et al. synthesized waterborne alkyd hybrid resins modified with fluorinated acrylate-siloxane by surfactant-free mini-emulsion polymerization process, and according to the results of electrochemical corrosion studies, the synthesized emulsions exhibited high waterproofing properties and excellent anticorrosion performance.24
Gao et al. improved the hydrophobicity and water resistance of the melamine-formaldehyde (MF) resin by modifying it with specific chemical additives such as siloxane, silane, fluorine compounds, and nanoparticles.25
Xu et al. reported that various hydrophobic fluorinated polysiloxane coatings were showed good microscopic homogeneity, adhesion, and thermal stability.26
Zhu et al. prepared polyacrylate amphiphilic polymers modified with fluorine and silane-containing monomers and investigated their corrosion inhibitor and antifouling performances.27
Wu et al. synthesized silicon-modified styrene-acrylate latex, and all the results obtained indicate that the synthesized latex holds significant promise for use in weather-proof and anticorrosive coatings.28
Huang et al. synthesized fluorine-containing polyacrylate hybrid coating materials by free radical polymerization. Their work demonstrated a significant enhancement in the hydrophobicity of the resulting fluorine-containing nanohybrid silica polyacrylate coating films.16
Wang et al. synthesized crosslinked fluorinated acrylate-modified waterborne polyurethane and investigated its effect on metal protection coatings. The results show that the modified waterborne polyurethane coating is a potential alternative for metal corrosion inhibition.29
Likewise, Wu et al. synthesized fluorinated waterborne polyurethane and investigated its hydrophobicity and antifouling properties.30
Dong et al. synthesized the vinyltrimethoxysilane-modified styrene-acrylic polymer emulsion by semicontinuous emulsion polymerization to improve the weatherability and thermal stability of styrene-acrylic polymer emulsion coatings. According to the results, they observed that the thermal stability and water resistance of the coatings were improved with incorporation of fluorine and silicon monomers.31
Considering all the data, it is predicted that with the addition of hydrophobic monomers such as silicon, fluorine, and acrylic, will lead to the development of waterborne alkyd hybrid coatings that are more resistant to environmental conditions and chemicals in the future. In addition, it is clearly seen in the studies that the modification of styrene acrylic polymers with hydrophobic monomers containing silicone, epoxy, phosphorus, or fluorine helps address numerous challenges in the field of coatings. Studies have shown that these modifications improve the bonding strength, water resistance, and film-forming abilities of styrene acrylic polymer latexes. Moreover, based on the literature, it might be inferred that high surface roughness and low surface energy are directly related to hydrophobicity. Also, silane and fluorine-containing groups are frequently employed as coating modifiers and crosslinking agents.15 In addition, studies have consistently demonstrated that fluorine and silane-containing substances reduce the energy of the air–film interface during film formation, ultimately improving the hydrophobic properties of polymers treated with these groups.
VTES possesses a unique structure characterized by organic–inorganic functional groups, offering advantages such as high gas permeability, a low-temperature coefficient, and a low dielectric constant, attributed to the presence of silicon (Si), oxygen (O), and nonpolar methyl side groups. Additionally, VTES contributes to the reduction of surface free energies.2,31 Due to the high bond energy and low surface energy of Si-O, it is preferred to improve the physical, chemical, and thermal properties of poly(styrene acrylate) emulsion. Silane agents, known for their high crosslinking effect due to their structural composition, further improve crosslinking density and resin adhesion properties.32,33
On the other hand, studies have demonstrated that fluorine-containing polymers, owing to their unique structure, significantly enhance resistance to external factors such as water, air, and corrosion, thanks to their superior hydrophobicity. It is worth noting that there have been relatively few reports examining the use of fluorine-containing polymers to improve hydrophobic and anticorrosion properties.14,16,19 Similar to VTES, TFEMA, with its high surface energy and large specific surface area, also influences surface wettability by reducing surface free energies through enhanced crosslinking density, thanks to the presence of fluorine groups.
Herein, polymer emulsions modified with triethoxyvinyl silane (VTES) and 2,2,2-trifluoroethyl methacrylate (TFEMA) monomers were synthesized by using mini-emulsion polymerization. We aimed to investigate the impact of VTES and TFEMA on waterborne hybrid (alkyd/styrene acrylic) latex.
When we examine the contributions of agents like silane and fluorine to polymer structures, it becomes evident that these monomers impart several distinctive properties. These properties include excellent water repellency, enhanced durability on external surfaces, reduced surface free energy, heightened flexibility, and superior thermal stability. Therefore, synthesizing hybrid polymers by combining the extraordinary properties of VTES and TFEMA agents with styrene and butyl acrylate monomers becomes crucial in addressing prevailing challenges.20,34,35 As a result, in addition to the copolymerization of three materials (alkyd, styrene, and acrylate) with high complementarity, the modification with silane and fluorine agents is expected to yield advantageous composite materials and expand the range of potential applications.
Experimental
Materials
Tall oil fatty acid (TOFA) was kindly supplied from EBS Paint and Chemical Industry Inc (Turkey). Cannabis seeds were supplied within the borders of Yozgat Province. Hemp seed oil (HSO) was extracted from hemp seeds using cold pressing. The oil was bleached by treating it with clay.
In alkyd synthesis, phthalic anhydride (PA), pentaerythritol (PENTA), lithium stearate (LS), xylene, and ortho-phosphoric acid (o-PA) were used. In hydrophobic waterborne hybrid (alkyd/styrene acrylic) latexes synthesis, butyl acrylate (BA), styrene (Sty), acrylic acid (AA), 2,2,2-trifluoroethyl methacrylate (TFEMA), triethoxyvinyl silane (VTES), Exosel 073, Span 80, potassium persulfate (KPS), and hexadecane (HD) were used directly without further purification. All chemicals used are given in Table 1. Deionized water (DIW) was used in all experiments (Mes, MP MINIpure, Turkey).
Alkyd resins synthesis
TOFA-based and HSO-based long oil alkyd resins were synthesized based on our previous work.36 The composition of the synthesized oil-based alkyd resins was formulated to be K > 1 according to the K alkyd constant system (Table 2).36,37 Synthesis of both alkyd resins was carried out in a total amount of 100 g of substance, as given in Table 2. Polycondensation was carried out in an inert and azeotropic environment at 240–245°C using a Dean-Stark apparatus, mantle heater, thermometer, and mechanical stirrer in a three-necked flask (xylene) medium. The reaction was terminated when the acid number fell below 20 mg/KOH. Based on the TS 4862 standard, the acid number was determined.38 Xylene was added under reflux to remove all the water released at the end of the reaction from the reaction medium and to stop the reversible reaction caused by the water. The reaction temperature was rapidly reduced to 150–180°C to prevent gelation.
TOFA-based long oil alkyd resin was synthesized by the fatty acid method. Polycondensation was carried out in one step in an inert and azeotropic environment at 240–245°C by adding all chemicals to the reaction medium.
HSO-based long oil alkyd resin was synthesized in two stages by the alcoholysis (monoglyceride) method. The first step is the alcoholysis step, performed at 240–245°C in the presence of hemp seed oil, pentaerythritol, and lithium stearate. The alcoholysis process was checked by the methanol test39. According to the methanol test, the reaction was terminated when a clear solution was obtained when the sample and methanol were mixed at a 1:3 volume ratio. In the second step, the reaction medium was cooled to 150°C. After adding phthalic anhydride and xylene, the temperature was increased to 240–245°C and the reaction continued in an azeotropic environment. Finally, xylene, which formed the azeotropic environment at the end of both synthesis processes, was removed from the reaction medium by a rotary evaporator under vacuum distillation.
Synthesis of hydrophobic waterborne hybrid (alkyd/styrene acrylic) emulsion polymers modified with fluorine and silane-containing monomers
The synthesis of hydrophobic waterborne hybrid latex (alkyd/styrene acrylic) modified with fluorine and silane-containing monomers was carried out in a 3-neck reactor using feed pumps and mixing equipment by semibatch mini-emulsion polymerization in a temperature-controlled and inert atmosphere. The amounts of chemicals have been calculated as 50% by weight of solids (Table 3). As seen in Table 3, as the amount of alkyd, VTES, and TFEMA in the formulation increased, the amount of Sty and BA monomers decreased. However, the amounts of DIW, AA, HD, KPS, and surfactants remained constant throughout the formulation. In the first vessel, the aqueous phase was obtained by mixing the mixture of Exosel 073 and DIW at room temperature. In the initial vessel, the aqueous phase was prepared by mixing Exosel 073 and DIW at room temperature. In the second vessel, the oil phase was obtained by blending alkyd resin (either TOFA-based or HSO-based), monomers, comonomers, cosurfactants, and hydrophobic monomers (either VTES or TFEMA), along with a mixture of Span 80. Subsequently, the oil phase was slowly introduced into the aqueous phase. The preemulsion was prepared by homogenizing the oil and water phases at 15,000 rpm. After keeping the temperature of the reaction medium constant at 78°C, some DIW, initiator-I, and 14% of the prepared preemulsion were added to the reactor. After half an hour, the remaining preemulsion was gradually introduced into the polymerization medium over a three-hour period, utilizing a dosage pump. After the monomer addition is complete, the emulsion is stirred for more 30 min. Initiator solution II was then added to convert remaining monomer, and the reaction temperature was raised to 85°C and stirred for an additional hour. The polymerization was terminated by cooling the latex to room temperature. Finally, the pH was adjusted to range of 8–9 using an ammonia solution.
All of the latex was passed through a 100-m polyester fabric sieve, and the coagulum formed in the reaction medium was removed. The filtered latex was stored in a closed container in the dark.
The synthesized polymer emulsions were abbreviated as HHL-1 to HHL-12. Specifically, latexes synthesized using alkyd-free styrene (Sty), butyl acrylate (BA), and acrylic acid (AA) were designated as HHL-1 to HHL-4.
Tests and measurements
Physicochemical properties were determined using standard methods. The pH value of the hydrophobic hybrid polymer emulsions was adjusted to range of 8–9 using a pH meter from HANNA brand. Subsequently, the solid content (SC) of the polymer emulsions was measured at 150°C using a Shimadzu MOC63u brand moisture analyzer. Also, kinematic viscosity of the emulsions was measured using an R2 spindle and a Fungilab viscometer at 20°C and 100 rpm. Particle size and zeta potential of waterborne hydrophobic hybrid latexes were analyzed with a Malvern Nano ZS90. The particle size of the emulsion, samples were diluted to 1:10 with distilled water, was determined by the dynamic light scattering approach. PerkinElmer brand gel permeation chromatography (GPC) was used to determine the molecular weight of the synthetic polymer emulsions using the polystyrene standards. Measurements were taken in the organic phase (THF) with a combined RI and UV detector at 25°C at a flow rate of 0.60 mL/min. In order to clarify the structure of the synthetic hydrophobic hybrid polymer emulsions and evaluate their film properties, latex films were produced. The synthesized polymer emulsions were left to dry for a period of 7 days at 25°C in silicone molds to obtain latex film approximately 1 mm thickness under controlled conditions. FTIR measurements and 1H-NMR analysis were used to identify the chemical composition of the latex films and alkyd resins. The functional groups in the structures were determined with the PerkinElmer Spectrum Two-model FTIR spectrometer. Measurements were taken within the frequency range of 400–4000 cm−1. In addition, a Bruker 400 model NMR device was used to elucidate the structures. NMR measurements were taken in deuterated chloroform. The surface morphology of the latex films, which were applied onto glass plates using a 200-µm applicator, was examined in 5 × 5 µm2 areas utilizing a Veeco Multimode 8 model atomic force microscope (AFM). Using a PerkinElmer 6000 DSC with an internal cooling system, the glass transition temperatures (Tg) of the latexes were measured from − 40 to 110°C in a nitrogen atmosphere. Heating and cooling rates were set at 10°C/min. Thermal analyses were performed using PerkinElmer Diamond model equipment in a nitrogen atmosphere with a heating rate of 10°C /min and a temperature range of 30–900°C. The hydrophilic or hydrophobic characteristics of the latex films were determined by measuring the contact angle (CA). In this procedure, deionized water was dropped on the surface of the polymer film, and the contact angle of the formed between the drop and the surface was measured at three different places within an image captured from the polymer's surface. An average of these measurements was then calculated. The microstructure of the materials was investigated with the images obtained with the ZEISS GEMINI 500 field emission scanning electron microscope (FESEM) and STEM and EDAX detectors.
Results and discussion
Evaluation of TOFA and HSO
TOFA and HSO are very rich in unsaturated fats, which is important in alkyd resin synthesis. Notably, these oils contain a substantial amount of oleic acid (C18:1) and linoleic acid (C18:2) in their molecular structures. Moreover, the physicochemical properties of TOFA and HSO, including their acid number, iodine number, viscosity, and oil length, establish alkyds derived from these oils as ideal binders for numerous coating applications, particularly those intended for exterior use.36
Evaluation of waterborne hydrophobic hybrid (alkyd/styrene acrylic) polymer emulsions containing VTES and TFEMA
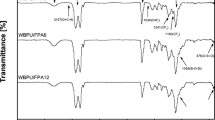
The structures of the synthesized composite polymers were elucidated by FTIR and 1H-NMR analyses (Figs. 1, 2, 3 and detailed representation in the range of 500–1500 cm-1 is given in Figs. S1 and S2.). In general, when both FTIR spectra were examined, the characteristic bands belonging to Sty-BA and alkyd resin units show that polymerization has occurred. The sharp peak around 1200 cm−1 belongs to the C–O–C asymmetric characteristic tensile vibration in BA and TFEMA.16 The sharp peak around 1730 cm−1 indicates the stretching vibration belonging to the C–O group. The peaks observed between 3000 and 2750 cm−1 represent the saturated C–H group, while the peaks at 3030 cm−1 indicate the unsaturated C–H group of phenyls. The vibration peak of the benzene ring is observed at 1455 cm−1, and the C–H vibration peak of the benzene ring is observed at 760 cm−1 and 700 cm−1. In addition, the bands around 3500 cm−1 indicate the hydroxyl group caused by AA and moisture.40,41,42,43 In Fig. 1, weak absorption peaks at 840 cm−1 attributed to Si–C bonds and weak absorption peaks at 1020 cm−1 attributed to Si-O-Si bonds show the VTES content. These results show that the VTES monomer is copolymerized with Sty and BA.28,36 In Fig. 2, characteristic peaks of C–F at 650 cm−1 and 1280 cm−1 are evidence that TFEMA participates in copolymerization.12,30,36,44
Chemical shift values of 1H-NMR spectra of waterborne hydrophobic hybrid (alkyd/styrene acrylic) polymer emulsions containing TFEMA and VTES are evaluated in Fig. 3. Deuterated chloroform (CDCl3) was the solvent for all experiments. The peak of the solvent is seen around 7.27 ppm. The peaks in the range of δ = 3.50–4.00 ppm represent the protons of the carbon atoms adjacent to Si and F. Peaks corresponding to aromatic ring protons are seen around 7.12 ppm.28,30,45 The peaks in the range of δ = 0.00–2.00 ppm belong to allylic and saturated carbon protons. δ Weak signals around 5.30 ppm could be attributed to alkyd groups in the structure of the copolymer46 and vinyl protons which were not converted to the polymer. It is attributed to the protons of the unsaturated carbons in the fatty acid chains it contains.
The obtained FTIR and 1H-NMR spectra show that the reactions took place as planned when compared with the literature. In addition, it was supported that VTES and TFEMA were included in the latex structures with the obtained EDAX images (Fig. 4).
The presence of Si and F elements incorporated into the latex structure was determined by the EDAX spectrum (Fig. 4). C and O signals were attributed to BA, Sty, and AA compounds. The characteristic peaks of Si and F indicated that VTES and TFEMA were included in the hybrid latex, respectively.
The physicochemical properties of polymer emulsions [solid content (SC), pH, viscosity, etc.] are presented in Table 4. The pH value of the polymer emulsions was adjusted to 8.0–9.0 with a 25% ammonia solution. The pH value of polymer emulsions is important for the stabilization of emulsions. Viscosity values were measured in the range of 100–135 cP, and since the viscosity change range is narrow, it would not be wrong to state that alkyd resin and other monomers do not have a serious effect on viscosity change.
The weight average weight (Mw), number average weight (Mn), Z-average molecular weight (Mz), and polydispersity index (PDI) of the samples were measured by GPC analysis and are indicated in Table 4. Mw’s and PDI’s were determined for HHL-4 and HHL-12 as about 3.5 × 106 and 2.1 × 106 and 4.5 and 4.0, respectively. It could be said that alkyd resin is effective in reducing Mw and also PDI. Similar results on the effect of alkyd resin on the molecular weight of alkyd-acrylic hybrid systems are also reported by Minari et al.47
The morphology and particle structure of the synthesized hydrophobic hybrid latexes were evaluated by scanning electron microscopy (FE-STEM/EDAX) and atomic force microscopy (AFM) images and dynamic light scattering (DLS) analysis.
The effects of alkyd, VTES, and TFEMA on the surface morphology of modified hybrid latex films were evaluated by the AFM technique in areas of 5 μm × 5 μm (Fig. 5). In topographical images, areas rich in acrylic polymer were associated with light colors, and areas rich in alkyd resin were associated with dark colors.48,49 When we compare the RMS roughness (Rq) values of the characterized layers with the topographical images of the hybrid polymers (without VTES and TFEMA) obtained in our previous study,36 we see that the rough surfaces and height increase in hybrid polymers modified with VTES and TFEMA. From this, it has been understood that, besides the alkyd resin, VTES and TFEMA compounds also play a significant role on the surface properties of the latexes. In addition, it has been concluded that the materials containing fluorine and silane reduce the energy of the air–film interface of the polymer films during film formation and improve the hydrophobicity of the polymers modified with these groups. In addition, the increase in Ra and Rq values in alkyd-based hybrid latex films compared to hybrid polymer films without alkyd resin suggests that the alkyd resin has an influence on the morphological properties of the Sty, BA, and AA polymers. From the images obtained, it may be said that the alkyd resin used as the binding component also reduces the interfacial energies of the films.17 Notably, HSO-based alkyd-containing coatings (HHL-9, HHL-10) exhibit a smoother and more continuous structure when compared to alkyd-free (HHL-1, HHL-2) and TOFA-based alkyd-containing (HHL-5, HHL-6) coatings. Low roughness values (Rq = 5.525 nm and Rq = 3.469 nm) clearly demonstrate this. This is also related to the chemical interaction between the HSO-based alkyd and the VTES and TFEMA compounds. Generally speaking, TFEMA-modified coatings have a rougher value. This is related to the different chemical structures of VTES and TFEMA. It has been thought that the ethoxy groups in the silicone acrylic polymers have created a more steric effect in the polymer structure compared to TFEMA. This idea was supported by the DSC curves obtained. These results showed that the presence of VTES and TFEMA and the amount of alkyd and oil content in the polymer affect the aggregation of latex particles.
STEM images of HHL-1, HHL-2, HHL-5, and HHL-10 are given in Fig. 6. From the images, it was seen that the latex particles were generally spherical, and the particle size ranged between 50 and 200 nm. HHL-1 and HHL-2 exhibited similar particle morphologies. The clearest image of sphericity was obtained from the HHL-5.
Crosslinking ratios of synthesized waterborne hydrophobic hybrid (alkyd/styrene acrylic) latexes were determined by their gel contents (Fig. 7). The gel content of the latexes was determined in the presence of acetone using the Soxhlet extractor. When Fig. 7 was examined, it is seen that as the ratio of VTES and TFEMA increases, the crosslinking ratio of latexes increases. But it could be said that the gel content in all samples is very low, below 1% and not much effect of VTES and TFEMA was observed.
The particle sizes of the synthesized waterborne hydrophobic hybrid latexes, consisting of alkyd and styrene acrylic components, are depicted in Fig. 8. As seen in Fig. 8, the particle size of alkyd-based polymer emulsions exhibited an increase when compared to alkyd-free polymer emulsions. Notably, it was observed that the modification of polymer emulsions with VTES or TFEMA compounds has a greater effect on the particle size of the synthesized latex with the addition of alkyd. The varying particle size has been mostly related to the chemical interaction between alkyd and hydrophobic monomers (VTES and TFEMA). In addition, it is worth noting that while there is a unimodal distribution in hybrid latex modified with TFEMA, a multimodal distribution was observed in hybrid latex modified with VTES. This phenomenon may be interpreted as VTES increasing the homogeneous nucleation number of the latex particle in the polymer system.21 As a result, it was observed that the particle size of hybrid latexes increased with the addition of alkyd, TFEMA, and VTES. In our previous study,36 using 1.5% anionic (Exosel 073) and 1.8% nonionic surfactant (span 80), particle sizes were determined to be 400 nm in latex containing 10% alkyd. Contrary to our previous work, here, the increase in the amount of anionic surfactant allowed to reach the desired particle size (250 nm).
As a result, when STEM and AFM images and DLS results are examined, the chemical interaction of alkyd oil content with hydrophobic monomers is important for parameters such as particle size, latex morphology, and surface morphology.
The zeta potential values of the synthesized waterborne hydrophobic hybrid (alkyd/styrene acrylic) latexes are shown in Fig. 9. When we look at the graph in general, there is a decrease in zeta potential values due to the increase in VTES and TFEMA content. This shows that VTES and TFEMA affect the electrolyte properties of latex structures according to the prediction of the Gouy-Chapman model.50,51
The thermal properties of hydrophobic hybrid latexes were determined by DSC and TGAs. When the glass transition temperatures (Tg) values obtained from DSC analyses (Fig. S3 and values presented in Table 4) were examined, the glass transition temperatures of the polymer latexes varied in the range of 7–17°C. This Tg value differs from the homopolymers of Sty (100°C) and BA (− 54°C), which directly confirms the successful preparation of the latex given the Fox equation21. When DSC curves are compared, we can say that there are many parameters (alkyd oil content, alkyd content, VTES, and TFEMA contents) affecting Tg values. Firstly, when examining the curves, it is evident that alkyd resins contribute to enhancing the flexibility of hybrid polymers. This is primarily observed through a reduction in the glass transition temperature (Tg) values in alkyd-based hybrid polymers compared to those without alkyd. It can be observed that these reductions are more pronounced in TOFA-based alkyd resin and are not contingent on the quantity of alkyd used. This phenomenon is believed to be associated with the fatty acid composition of TOFA (C18:1) and HSO (C18:2). Additionally, it is suggested that TOFA-based alkyd resin demonstrates a more plasticizer-like behavior in comparison with HSO-based alkyd resin. This view is supported by Limousin et al.49. If we evaluate the curves from a different perspective, the effect of the crosslinking abilities of VTES and TFEMA compounds (Fig. 7) on Tg values is also important. It is seen that the VTES compound slightly reduces the Tg values of polymer latexes compared to polymer latexes containing the TFEMA compound. This is related to the chemical structures of VTES and TFEMA. It is thought that the VTES compound causes less restriction in the movement of the polymer segment and creates more free volume in the polymer structure compared to TFEMA. The reactive ethoxy groups in the structure of VTES cause more branching in the polymer structure, and the volume increases; thus, the density decreases. This causes the decrease in Tg value of latex films modified with VTES to be greater than TFEMA. It can be argued that TFEMA causes more network structure in polymer structures. In addition, it was assumed that random copolymerization occurred and an amorphous structure was formed based on the detection of a single Tg point in the DSC curves in the investigated temperature range and the absence of crystallization and melting points.11,27,28,52,53,54
TGAs were performed to determine the effect of TFEMA and VTES contents on the thermal stability of hybrid polymers (Fig. 10). Upon examining the curves, it was evident that there was poor thermal degradation in the temperature range of 120–300°C, primarily attributed to the presence of small molecules like water. The principal thermal decomposition took place in the range of 350–480°C, signifying the degradation of the main chain of the hybrid polymer (alkyd/styrene acrylic) during this second decomposition step.11 In general, it was clearly seen that all polymers showed a stable thermal decomposition process, and the synthesized polymer films had good thermal stability.27 In addition, it was seen from the curves that the materials do not show complete deterioration and a weight loss of 90–95% around 350–450°C. Observation of 5% by weight residue at 500°C has shown that VTES and TFEMA compounds create a steric effect in the polymer structure, and the network structure increases due to hydrogen bond interaction. It was observed that this 5% rate increases up to 10% in alkyd-based hybrid polymers with the addition of alkyd resin. We may say that alkyd is in chemical interaction with VTES and TFEMA compounds. This is also supported by the increased gel percentage contents in Fig. 7. In addition, it was observed that the decomposition temperature of latex films containing VTES and TFEMA slightly increased compared to latex without. This showed that the thermal stability of the latex film containing fluorine and silane group is good. This can be attributed to the higher bond energies of C–F (485 kJ/mol) and Si–O (451 kJ/mol) than the bond energies of C–C (356 kJ/mol) and C–O (336 kJ/mol).21,55 Similar thermal degradation curves have been observed in the literature.28
The hydrophobic and hydrophilic properties of latex films were determined by water contact angle analysis. Contact angles (CA) were calculated by dropping water onto the coatings and measuring the angle between phases. The contact angle results of each film are shown in Fig. 11. As indicated in Fig. 11, the contact angle degrees of the synthesized polymer latex were calculated in the range of 30–90°. The contact angle values of the latexes are related to the surface energies of the latexes and thus depend on the particle morphology at equilibrium. When examining the contact angle values, it becomes evident that the alkyd content increases the hydrophobicity of the polymer latex. This shows that the hydrophobic nature of the alkyd resin also imparts hydrophobic properties to the latex films.37 Moreover, the effect of the chemical interaction of alkyd with hydrophobic monomers on particle morphology has been indicated in AFM (Fig. 4) and STEM (Fig. 5) images. In addition, it was concluded that the materials containing fluorine and silane reduce the energy of the air–film interface of the polymer films during film formation and improve the hydrophobicity of the polymers modified with these groups. The contact angle values of latex films remained below 90°. But it has been seen that the CA values are good enough when compared to hybrid latex films modified with silane and fluorine agents given in the literature. CA values lower than 90° have been associated with AA and emulsifiers in the medium during polymerization. Considering the hydrophilic properties of the emulsifiers (EXOSEL073 and Span80) used during polymerization, such a result was inevitable. The obtained latex films were washed with distilled water to remove excess surfactant from the film's surface. Subsequently, the contact angle (CA) test was conducted again, revealing that the emulsifiers within the structure decreased the hydrophobicity of the films. In addition, the experiment was repeated for HHL-11 to prove the effect of emulsifiers and AA on the hydrophobicity of latex films. Initially, the amount of emulsifiers used was increased by 25%, leading to a significant reduction in the contact angle value of the resulting latex film (by 50°). AA, known for its hydrophilic properties, is commonly used to enhance the pigment binding capacity of latex in industrial coating production, typically at a 2% ratio. To assess the effect of AA on the hydrophobicity of the synthesized latex films, the experiment was repeated for HHL-11 without the inclusion of AA. This time, it was observed that the contact angle of the resulting latex film increased to 92°.
This study has determined that the hydrophobicity of polymer latexes is affected by many factors, such as the VTES/TFEMA content of polymer emulsions, the alkyd ratio, alkyd content, emulsifier content, and acrylic acid content. It is also thought that acrylic acid and emulsifiers increase the energy of the air–film interface during film formation.28
Conclusions
In this study, monomers containing silane and fluorine groups were successfully incorporated into the waterborne hybrid (alkyd/styrene acrylic) polymer latex system. The effects of these fluorine and silane agents on the physical and chemical properties of the functional coating materials were discussed. The synthesized waterborne hybrid latexes and films were characterized by FTIR, NMR, particle size, zeta potential, gel content, AFM, STEM, EDAX, TGA, DSC, and CA tests. The structures of waterborne hybrid (alkyd/styrene acrylic) polymer latex modified with VTES and TFEMA synthesized from FTIR and NMR results were confirmed. The tests (AFM, STEM, EDAX, CA, DSC, and TGA) showed that, in addition to the alkyd content, silane and fluorine groups also contribute positively to the synthesized films. A clearer understanding of the advantages of fluorine and silane groups, especially in polymer emulsions containing alkyd (HHL-5 and HHL-12), was gained. In addition, improvements in thermal properties were observed depending on the monomers including silane and fluorine. The results of the DSC and TGA supporting each other were the biggest proof of this. The observation of a single Tg point was an important parameter in determining that copolymerization had taken place. The observations showed that waterborne hybrid (alkyd/styrene acrylic) polymer latex modified with VTES and TFEMA could form a smooth coating film. While the use of silane and fluorine-containing monomers did not significantly affect the gel content and viscosity changes of alkyd resin latexes, it was determined that these monomers and alkyd resin were effective in particle size changes. As a result of zeta potential analysis and observations conducted in the laboratory, it was concluded that the latexes were stable. As a result, it is expected to obtain composite materials with advantages and to expand the application range with the modification of silane and fluorine agents in addition to the copolymerization of three materials with high complementarity (alkyd-styrene-acrylate). It could be suggested that these synthesized alkyd-based hybrid latexes will increase their potential as protective coatings. Furthermore, it is believed that the utilization of plant-based sources such as TOFA and HSO in the synthesis of alkyd-based hybrid latex, with regard to sustainability and environmental friendliness, will serve as the foundation for more dependable and environmentally friendly coating applications in the future.
References
Kotlík, P, Doubravová, K, Horálek, J, Kubác, L, Akrman, J, “Acrylic Copolymer Coatings for Protection Against UV Rays.” J. Cultural Herit., https://doi.org/10.1016/j.culher.2013.01.002 (2013)
Grigsby, WJ, “Photooxidative Stability Provided by Condensed Tannin Additives in Acrylic-Based Surface Coatings on Exterior Exposure.” J. Coat. Technol. Res., https://doi.org/10.1007/s11998-018-0086-z (2018)
Zhang, F, Jing, C, Yan, Z, Ge, S, Liu, P, Maganti, S, Xu, BB, Mahmoud, KH, El-Bahy, ZM, Huang, M, Guo, Z, “Fluorinated Acrylic Monomer Modified Core-Shell Polyacrylate Latex Particles: Preparation, Properties and Characterizations.” Polymer, 247 124783. https://doi.org/10.1016/j.polymer.2022.124783 (2022)
Zhong, S, Qin, K, Hou, Y, Xu, T, Cai, Y, Yi, L, “Waterborne Corrosion-Resistant Hydrophobic Alkyd Resin Composite Coatings Modified with Fluorinated Acrylate–Siloxane and Submicron-Sheet Zinc Phosphate Pigment.” J. Coat. Technol. Res., 18 (5) 1309–1320. https://doi.org/10.1007/s11998-021-00493-x (2021)
Yu, Z, Yan, Z, Zhang, F, Wang, J, Shao, Q, Murugadoss, V, Alhadhrami, A, Mersal, GAM, Ibrahim, MM, El-Bahy, ZM, Li, Y, Huang, M, Guo, Z, “Waterborne Acrylic Resin Co-Modified by Itaconic Acid and γ-Methacryloxypropyl Triisopropoxidesilane for Improved Mechanical Properties, Thermal Stability, and Corrosion Resistance.” Prog. Org. Coat., 168 106875. https://doi.org/10.1016/j.porgcoat.2022.106875 (2022)
Wang, G, Wen, S, Qian, S, Wang, J, Wang, C, Chen, Y, “Synthesis of Novel Nano Hyperbranched Polymer Resin and Its Corrosion Resistance in Coatings.” Prog. Org. Coat., 140 105496. https://doi.org/10.1016/j.porgcoat.2019.105496 (2020)
Nanvaee, AA, Yahya, R, Gan, SN, “Alkyd Resins are Still of Major Important Bınders in Organic Coatings.” Malaysia Polymer International Conference (2009)
Kausar, A, “Polymer Coating Technology for High Performance Applications: Fundamentals and Advances.” J. Macromol. Sci. A Pure Appl. Chem., https://doi.org/10.1080/10601325.2018.1453266 (2018)
Dong, W, Zhou, L, Guo, Y, Tang, Y, Pan, R, Liu, M, He, D, “Modification of Styrene-Acrylic Emulsion by Organic UV Absorber in Synergy with Fluorine and Silicon Monomers for Weatherable Coatings.” J. Coat. Technol. Res., 19 (2) 607–616. https://doi.org/10.1007/s11998-021-00550-5 (2022)
Wang, P, Zhang, D, Lu, Z, “Advantage of Super-Hydrophobic Surface as a Barrier Against Atmospheric Corrosion Induced by Salt Deliquescence.” Corros. Sci., https://doi.org/10.1016/j.corsci.2014.09.001 (2014)
Zheng, B, Ge, S, Wang, S, Shao, Q, Jiao, C, Liu, M, Das, R, Dong, B, Guo, Z, “Effect of γ-Aminopropyltriethoxysilane on the Properties of Cellulose Acetate Butyrate Modified Acrylic Waterborne Coatings.” React. Funct. Polym., 154 104657. https://doi.org/10.1016/j.reactfunctpolym.2020.104657 (2020)
Machotova, J, Černošková, E, Honzíček, J, Šňupárek, J, “Water Sensitivity of Fluorine-Containing Polyacrylate Latex Coatings: Effects of Crosslinking and Ambient Drying Conditions.” Prog. Org. Coat., https://doi.org/10.1016/j.porgcoat.2018.03.016 (2018)
Zhang, P, Lv, FY, “A Review of the Recent Advances in Superhydrophobic Surfaces and the Emerging Energy-Related Applications.” Energy, https://doi.org/10.1016/j.energy.2015.01.061 (2015)
Vazirinasab, E, Jafari, R, Momen, G, “Application of Superhydrophobic Coatings as a Corrosion Barrier: A Review.” Surf. Coat. Technol., 341 40–56. https://doi.org/10.1016/j.surfcoat.2017.11.053 (2018)
Chardon, F, Denis, M, Negrell, C, Caillol, S, “Hybrid Alkyds, the Glowing Route to Reach Cutting-Edge Properties?” Prog. Org. Coat., 151 106025. https://doi.org/10.1016/j.porgcoat.2020.106025 (2021)
Huang, CM, Wang, HY, Fang, SY, Yang, WD, “Influence of Fluorine-Containing Monomer Content on the Hydrophobic and Transparent Properties of Nanohybrid Silica Polyacrylate Coating Materials.” Materials, 14 4261. https://doi.org/10.3390/ma14154261 (2021)
Bogdanowicz, KA, Dutkiewicz, M, Maciejewski, H, Nowicki, M, Przybył, W, Plebankiewicz, I, Iwan, A, “Siloxane Resins as Hydrophobic Self-Cleaning Layers for Silicon and Dye-Sensitized Solar Cells: Material and Application Aspects.” RSC Adv., 12 19154. https://doi.org/10.1039/d2ra02698h (2022)
Kanai, T, Mahato, TK, Kumar, D, “Synthesis and Characterization of Novel Silicone Acrylate–Soya Alkyd Resin as Binder For Long Life Exterior Coatings.” Prog. Org. Coat., 58 259–264. https://doi.org/10.1016/j.porgcoat.2006.11.002 (2007)
Zhong, S, Li, J, Yi, L, Cai, Y, Zhou, W, “Cross-Linked Waterborne Alkyd Hybrid Resin Coatings Modified by Fluorinated Acrylate-Siloxane with High Waterproof and Anticorrosive Performance.” Polym. Adv. Technol., 30 (2) 292–303. https://doi.org/10.1002/pat.4464 (2019)
Huang, K, Liu, Y, Wu, D, “Synthesis and Characterization of Polyacrylate Modifiedby Polysiloxane Latexes and Films.” Prog. Org. Coat., 77 1774–1779. https://doi.org/10.1016/j.porgcoat.2014.06.001 (2014)
Bao, Z, Li, W, Xu, T, Fu, Z, Chen, L, “Preparation and Thermal Stability of Phosphorus Acrylic Latex Modified with Organic Silicon.” J. Polym. Mater., 32 (4) 483–490 (2015)
Lü, T, Qi, D, Zhang, D, Liu, Q, Zhao, H, “Fabrication of Self-Cross-Linking Fluorinated Polyacrylate Latex Particles with Core-Shell Structure and Film Properties.” React. Funct. Polym., 104 9–14. https://doi.org/10.1016/j.reactfunctpolym.2016.04.020 (2016)
Zhao, H, Gao, L, Gao, S, Shi, J, “Synthesis and Polymerization Kinetics of Copolymer Styrenevinyltrimethoxysilane by Emulsifier-Free Emulsion Polymerization.” Adv. Mater. Res., 150–151 1537–1540. https://doi.org/10.4028/www.scientific.net/amr.150-151.1537 (2011)
Zhong, S, Li, J, Cai, Y, Yi, L, “Novel Surfactant-Free Waterborne Acrylic-Silicone Modified Alkyd Hybrid Resin Coatings Containing Nano-Silica for the Corrosion Protection of Carbon Steel.” Polym. Plast. Technol. Mater., 58 (8) 866–878. https://doi.org/10.1080/03602559.2018.1542711 (2019)
Gao, Y, Liu, S, Wang, Q, Wang, G, “Preparation of Melamine–Formaldehyde Resin Grafted by (3-Aminopropyl) Triethoxysilane for High-Performance Hydrophobic Materials.” J. Appl. Polym. Sci., 48664 1–10. https://doi.org/10.1002/app.48664 (2019)
Xu, Y, Li, M, Liu, M, “Corrosion and Fouling Behaviors of Phosphatized Q235 Carbon Steel Coated with Fluorinated Polysiloxane Coating.” Prog. Org. Coat., 134 177–188. https://doi.org/10.1016/j.porgcoat.2019.04.079 (2019)
Zhu, B, Liu, Z, Liu, J, Yang, Y, Meng, Y, Yu, F, Jiang, L, Wei, G, Zhang, Z, “Preparation of Fluorinated/Silanized Polyacrylates Amphiphilic Polymers and Their Anticorrosion and Antifouling Performance.” Prog. Org. Coat., 140 105510. https://doi.org/10.1016/j.porgcoat.2019.105510 (2020)
Wu, Y, Zhu, C, Yanchen, Z, Qiu, H, Ma, H, Gao, C, Liu, Y, “A Type of Silicone Modified Styrene-Acrylate Latex for Weatherable Coatings with Improved Mechanical Strength and Anticorrosive Properties.” React. Funct. Polym., 148 104484. https://doi.org/10.1016/j.reactfunctpolym.2020.104484 (2020)
Wang, X, Cui, Y, Wang, Y, Ban, T, Zhang, Y, Zhang, J, Zhu, X, “Preparation and Characteristics of Crosslinked Fluorinated Acrylate Modified Waterborne Polyurethane for Metal Protection Coating.” Prog. Org. Coat., 158 106371. https://doi.org/10.1016/j.porgcoat.2021.106371 (2021)
Wu, J, Wang, C, Lin, W, Ngai, T, “A Facile and Effective Approach for the Synthesis of Fluorinated Waterborne Polyurethanes with Good Hydrophobicity and Antifouling Properties.” Prog. Org. Coat., 159 106405. https://doi.org/10.1016/j.porgcoat.2021.106405 (2021)
Yilgör, E, Yilgör, İ, “Silicone Containing Copolymers: Synthesis, Properties and Applications.” Prog. Polym. Sci., 848 31. https://doi.org/10.1016/j.progpolymsci.2013.11.003 (2013)
Jiang, W, Dai, A, Zhou, T, Xie, H, “Hybrid Polysiloxane/Polyacrylate/Nano-SiO2 Emulsion for Waterborne Polyurethane Coatings.” Polym. Test., 80 106110. https://doi.org/10.1016/j.polymertesting.2019.106110 (2019)
Ifijen, IH, Odi, HD, Maliki, M, Omorogbe, SO, Aigbodion, AI, Ikhuoria, EU, “Correlative Studies on the Properties of Rubber Seed and Soybean Oil-Based Alkyd Resins and Their Blends.” J. Coat. Technol. Res., 18 (2) 459–467. https://doi.org/10.1007/s11998-020-00416-2 (2021)
Lyu, B, Li, X, Liu, H, Gao, D, Ma, J, Zhang, M, “Preparation of an Amphiphilic Janus SiO2/Fluorinated Polyacrylate Latex Film and Its Application as a Hydrophobic Fabric Agent.” J. Colloid Interface Sci., 599 88–99. https://doi.org/10.1016/j.jcis.2021.04.061 (2021)
Lei, H, He, D, Hu, J, Li, P, Huang, H, “A Fluorine–Silicone Acrylic Resin Modified with UV-Absorbing Monomers and a Free Radical Scavenger.” J. Coat. Technol. Res., https://doi.org/10.1007/s11998-018-0078-z (2018)
Kartaloğlu, N, Akçin, SE, Eren, M, Delibaş, A, “Waterborne Hybrid (Alkyd/Styrene Acrylic) Emulsion Polymers and Exterior Paint Applications.” J. Coat. Technol. Res., https://doi.org/10.1007/s11998-023-00767-6 (2023)
Yun, X, Xin-yi, Y, Dun-hong, G, Yong-bo, D, Liang, S, “Preparation and Characterization of Waterborne Alkyd-Amino Baking Coatings Based on Waste Polyethylene Terephthalate.” R. Soc. Open Sci., 7 191447. https://doi.org/10.1098/rsos.191447 (2020)
Angın, N, Ertaş, M, “Farklı Çözücü Türlerinin Ekstraksiyon Reçinesinin Verimi Ve Kimyasal Özellikleri Üzerine Etkisi.” Turk. J. For., 22 (4) 439–443. https://doi.org/10.18182/tjf.960674 (2021)
Saravari, O, Phapant, P, Pimpan, V, “Synthesis of Water-Reducible Acrylic–Alkyd Resins Based on Modified Palm Oil.” J. Appl. Polym. Sci., 96 (4) 1170–1175. https://doi.org/10.1002/app.21009 (2005)
Muhammad, A, Abbas, S, Shafeeq, A, Al-Turaif, HA, Taimoor, AA, Ali, AM, Deshannavar, UB, “Synthesis and Characterization of Pentaerythritol Phthalic Anhydride Resin from Soybean Oil.” Asian J. Chem., 30 572–574 (2018)
Hadzich, A, Gross, GA, Leimbach, M, Ispas, A, Bund, A, Flores, S, “Characterization of Plukenetia volubilis L. Fatty Acid-Based Alkyd Resins.” Polym. Test., 82 106296. https://doi.org/10.1016/j.polymertesting.2019.106296 (2020)
Assanvo, EF, Gogoi, P, Dolui, SK, Baruah, SD, “Synthesis, Characterization, and Performance Characteristics of Alkyd Resins Based on Ricinodendron heudelotii Oil and Their Blending with Epoxy Resins.” Ind. Crops Prod., 65 293–302. https://doi.org/10.1016/j.indcrop.2014.11.049 (2015)
Tiwari, S, Saxena, M, Tiwari, S, “Preparation and Characterization of Penta Alkyds Based on Mahua Oil.” J. Sci. Ind. Res., 61 110–116 (2002)
Li, H, Zhou, J, Zhao, J, Li, Y, Lu, K, “Synthesis of Cellulose Nanocrystals-Armored Fluorinated Polyacrylate Latexes via Pickering Emulsion Polymerization and Their Film Properties.” Colloids Surf. B Biointerfaces, 192 111071. https://doi.org/10.1016/j.colsurfb.2020.111071 (2020)
He, S, Liu, W, Yang, M, Liu, C, Jiang, C, Wang, Z, “Fluorinated Polyacrylates Containing Amino Side Chains for the Surface Modification of Waterborne Epoxy Resin.” J. Appl. Polym. Sci., https://doi.org/10.1002/APP.47091 (2018)
Irska, I, Paszkiewicz, S, Goracy, K, Linares, A, Ezquerra, TA, Jedrzejewski, R, Rosłaniec, Z, Piesowicz, E, “Poly(Butylene Terephthalate)/Polylactic Acid Based Copolyesters and Blends: Miscibility-Structure-Property Relationship.” Express Polym. Lett., 14 (1) 26–47. https://doi.org/10.3144/expresspolymlett.2020 (2020)
Minari, RJ, Goikoetxea, M, Beristain, I, Paulis, M, Barandiaran, MJ, Asua, JM, “Molecular Characterization of Alkyd/Acrylic Latexes Prepared by Miniemulsion Polymerization.” J. Appl. Polym. Sci., 114 3143–3151. https://doi.org/10.1002/app.30866 (2009)
Goikoetxea, M, Reyes, Y, Heras Alarcón, CM, Minari, RJ, Beristain, I, Paulis, M, Barandiaran, MJ, Keddie, JL, Asua, JM, “Transformation of Waterborne Hybrid Polymer Particles Into Films: Morphology Development and Modeling.” Polymer, 53 (5) 1098–1108. https://doi.org/10.1016/j.polymer.2012.01.021 (2012)
Limousin, E, González, E, Martínez-Tong, DE, Ballard, N, Asua, JM, “Modelling the Dynamic Development of the Curing Process and Film Morphology of Films Cast from Waterborne Acrylic-Alkyd Hybrids.” Chem. Eng. J., 400 125891 (2020)
El-Gholabzouri, O, Cabrerizo-Vílchez, MA, Hidalgo-Alvarez, R, “Zeta-Potential of Polystyrene Latex Determined Using Different Electrokinetic Techniques in Binary Liquid Mixtures.” Colloids Surf. A Physicochem. Eng. Aspects, 291 (1–3) 30–37. https://doi.org/10.1016/j.colsurfa.2006.05.017 (2006)
García-Salinas, MJ, Romero-Cano, MS, Nieves, FJ, “Zeta Potential Study of a Polystyrene Latex with Variable Surface Charge: Influence on the Electroviscous Coefficient.” Trends Colloid Interface Sci., 115 112–116. https://doi.org/10.1007/3-540-46545-6_23 (2000)
Yousefi, AA, Pishvaei, M, Yousefi, A, “Preparation of Water-Based Alkyd/Acrylic Hybrid Resins.” Prog. Color Color. Coat., 4 (1) 15–25 (2011)
Wei, Z, Ling, H, Junyan, L, Gang, C, Na, W, “Preparation and Properties of Core–Shell Nanosilica/Poly(Methyl Methacrylate–Butyl Acrylate–2,2,2-Trifluoroethyl Methacrylate) Latex.” J. Appl. Polym. Sci., 120 (2) 1152–1161. https://doi.org/10.1002/app (2011)
Wu, XQ, Schork, FJ, Gooch, JW, “Hybrid Miniemulsion Polymerization of Acrylic/Alkyd Systems and Characterization of the Resulting Polymers.” J. Polym. Sci. A Polym. Chem., 37 (22) 4159–4168 (1999)
Eduok, U, Faye, O, Szpunar, J, “Recent Developments and Applications of Protective Silicone Coatings: A Review of PDMS Functional Materials.” Prog. Org. Coat., 111 124–163. https://doi.org/10.1016/j.porgcoat.2017.05.012 (2017)
Acknowledgments
This study was supported by the Yozgat Bozok University Scientific Research Projects Unit with code 6602c-FEN/21-459. We would like to thank Betek Paint and Chemical Industry Inc and Dr. Mesut Eren and Özlem Yılmaz Adıgüzel for helping in the supply of monomers and performing some analysis.
Author information
Authors and Affiliations
Contributions
NK helped in investigation, visualization, methodology, formal analysis, validation, writing. AD contributed to project administration, writing—review and editing, funding acquisition, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The article has been written by the stated authors, who are all aware of its content and approve its submission. This paper has not been previously published elsewhere, nor is it being considered by another journal. There is no conflict of interest. If accepted, the article will not be published elsewhere in the same form or in any language without the written consent of the publisher.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kartaloğlu, N., Delibaş, A. Investigation of the effect of fluorine and silane-containing monomers on waterborne hybrid (alkyd/styrene acrylic) latexes. J Coat Technol Res 21, 1067–1084 (2024). https://doi.org/10.1007/s11998-023-00873-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-023-00873-5