Abstract
Since alkyd resins include hazardous solvents, converting alkyds into waterborne hybrid polymers is an essential research topic. Here, alkyd/styrene acrylic waterborne hybrid polymers were synthesized in the presence of monomers, water, emulsifiers, and an initiator by using synthesized alkyd resins at 0%, 5%, 10%, and 15% ratios based on the total monomer ratio. A mini-emulsion technique and a semi-batch polymerization method were used to synthesize the latexes. Two different biosources, tall oil fatty acids (TOFA) and hemp seed oils (HSO), were used to synthesize the alkyd resins. Synthesized waterborne hybrid latexes and their films were analyzed by using FTIR, NMR, particle size, MFFT, TGA, DSC, CA, AFM, and mechanical tests. It was determined that the type and concentration of the alkyds affected the viscosity, particle size, Tg and MFFT values, and appearance. It was also identified that alkyd incorporation took place with grafting and that the alkyd concentration was particularly effective in increasing the particle size. In addition, experiments were carried out on waterborne paint systems for exterior paint by using synthesized hybrid polymer emulsions. It was observed that the alkyd content was not effective in changing the paint color in hybrid latexes. As a result, it is suggested that hybrid waterborne latexes could be used for exterior paints.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkyd resins provide physical, chemical, thermal, and mechanical resistance to coatings, and long-term tolerances against wear on the surfaces to which they are applied. For this reason, they are a functional material in coating applications. Alkyds are a type of synthetic polyester resin synthesized by oil or fatty acids, and are a general term to describe oil-based paints. Alkyd resins are a part of sustainable chemistry due to their bio-based structure, and they are preferred as a binder component in paint production facilities.1,2,3,4,5,6,7,8 Alkyd resins come to the fore especially on surfaces where water resistance is important, such as home, furniture, and metal goods. While these resins offer excellent surface protection and coatings, they also release many hazardous compounds into the environment during drying. R&D studies continue to try and prevent such negative impacts which harm human health and the environment. Recently, waterborne alkyd resins made from biomaterials have attracted significant attention, as they support the policies implemented to leave a cleaner and healthier world for future generations.9,10,11
Alkyd resin made from dried castor oil and soybean oil was synthesized by Chukwuebuk et al. According to their findings, they concluded that such oils could meet commercial standards and improve alkyd performance.12
Alkyd resin was synthesized from the oil of neem tree fruit seeds by Das et al. They reported that the alkyd resin applied to mild steel plates exhibited a superior corrosion protection, and has a great application potential in below-water marine vessel finishes against salt corrosion. The study revealed that the alkyd resin performed well in different paint and surface coating applications.13
Ifijen et al. synthesized alkyd resin from a rubber seed and soybean oil mixture. They observed that the coating properties of the alkyd were comparable to commercial polyester resins. They also reported that the coatings were highly resistant to alkalis, salt water, water, and acid.6
The physicochemical properties (acid value, viscosity, color, density, drying time, and hardness) of alkyds synthesized from Sacha inchi oil (SIO) were evaluated by Obregón et al. Fourier-transform infrared (FTIR; Spectrum Two; Perkin Elmer) spectroscopy, thermogravimetric analysis (TGA), and hydrogen-1 and carbon-13 nuclear magnetic resonance (1H-NMR and 13C-NMR; Bruker 400 NMR device in CDCl3) characterization techniques were used to identify the resin structures. SIO-based alkyd resins prepared with trimethylolpropane exhibited low viscosities that are suitable for preparing more manageable high-solids protective coatings.14
In addition, palm oil,15 tobacco seed oil,16 and linseed oil17 have been used to synthesize alkyd resins with varied properties by different authors.
Although waterborne systems have many advantages, they are insufficient in terms of properties such as adhesion and brightness compared to solvent-based systems.18,19,20 Hybrid systems have been tried in order to eliminate these defects. Combining two or more components in hybrid systems allows us to take advantage of the unique performance of each component, which contain chemical and physical bonds, to achieve superior advantages, meet consumer demands, and prevent many environmental problems.3
Styrene/acrylic alkyd waterborne polymers are one of the most common examples of hybrid alkyds. In addition, they are one of the hybrid systems preferred as binder materials in exterior and interior paints. Thanks to emulsion polymerization, alkyd structures that are perfectly compatible with acrylic compounds provide adhesion and gloss quality to paints, while acrylic structures also contribute in terms of hardness and resistance to physical and chemical conditions. When the literature is examined, it can be seen that acrylic compounds provide advantages such as higher abrasion and alkali resistance, water resistance, low yellowing tendency, high hardness, and flexibility in coating applications. In addition, vinyl compounds provide advantages such as hardness, permanent color, gloss, high drying rates, and adhesion to polymeric structures.4,21,22,23,24,25
Some studies in the literature related to alkyd hybrid polymers are also noteworthy. Ma et al. synthesized an eco-friendly waterborne alkyd resin from polyethylene terephthalate waste using Zanthoxylum bungeanum seed oil (ZSO), ZnOAc, and trimethylolpropane. It has been determined that ZSO is a potential raw material source for commercial coatings which shows superior thermal and chemical resistance in coatings.26
Assanvo et al. synthesized an oil-based hybrid alkyd acrylate latex. In the study, it was determined that Ricinodendron heudelotii oil-based alkyd resin latexes have advanced properties for producing waterborne coatings, and can be used instead of normal petroleum-based coating systems.27
Mini-emulsion polymerization is a method in which hexadecane or long chain alcohol, which are insoluble in water, are used as a co-stabilizer and can dissolve monomer and alkyd resins. This may make it possible for alkyd resins to be incorporated into styrene/acrylic emulsions. Particle size is an essential parameter of waterborne latexes which affects the stability, viscosity, film forming, and solid content.28,29,30 Wang et al. synthesized hybrid polymers by mini-emulsion polymerization using hydrophilic and hydrophobic alkyd resins, and investigated the film properties at room temperature, including the effect of functionalization with glycidyl methacrylate on the film properties.31
Uschanow et al. synthesized an alkyd-acrylate copolymer from pine oil fatty acid-based alkyd resin by the mini-emulsion technique and studied environmentally friendly waterborne binders. The research showed that it is possible to prepare stable, alkyd-acrylate hybrid copolymers with variable chemical compositions.32
Asua et al. are remarkable in the literature for their studies on acrylic-alkyd waterborne hybrid latexes. They have carried out intensive studies on the effects of alkyd resins on latex properties and morphology.33,34,35
Waterborne paints (paints based on styrene acrylics and pure acrylic latex), which are accepted by the European paint industry as offering performance advantages such as easy applicability and fast drying, are being used for both interior and exterior applications in many architectural coating markets. Since waterborne systems do not contain solvent structures that may adversely affect human health, they offer more economical and reliable possibilities in coatings.36,37,38,39,40
Waterborne alkyd paints, which contain pigments, solvents, water-soluble binder components, and additives, are water-resistant and environmentally friendly paints. The type and amounts of each component in a paint affect various factors that determine the quality of paint systems, especially in exterior and interior paints, such as water and water vapor permeability, adhesion to the substrate, and ultraviolet (UV) and alkali resistance. Long and medium oil alkyd resins are the most preferred because they form fast-drying films with high gloss, gloss resistance, and excellent durability.41,42
When some paint companies were examined, it is seen that styrene-modified alkyd-based paints provide speedy surface drying and iron-based metal surfaces in industrial sectors, such as the automotive sector, steel construction, and agricultural and industrial tools. In contrast, acrylic paints have high surface resistance exposed to outdoor weather conditions and UV rays, and are preferred in applications where chemical resistance is required. In the future, these acrylic-vinyl alkyd hybrid waterborne paints are expected to play more prominent roles in the coatings market and become more popular with consumers, manufacturers, and distributors.
Pine fatty acid, also known as tall oil fatty acid (TOFA), is a by-product of the kraft process of wood pulp manufacture when pulping mainly coniferous trees. It is used in many fields, such as the petroleum and textile industries, alkyd resin production, and biodiesel, and in surface coating material production, thanks to the 90–99% C16–20 chain length saturated and unsaturated fatty acids it contains. The Cannabis sativa plant, also known as industrial cannabis, is utilized in many agro-industrial fields, such as the construction, biofuels, cosmetics, and pharmaceutical industries. Its seeds generally contain saturated, unsaturated, and polyunsaturated fatty acids with a C16–20 chain length (hemp seed oil; HSO), similar to TOFA.17,43
The use of bio-based and waterborne polymers to ensure sustainability and reduce carbon footprints is a popular subject nowadays in both industry and academia. Although there have been studies on alkyd-based hybrid waterborne latexes, there is no literature on the combined use of TOFA and HSO and a comparison of their properties, nor is there any information on the use of waterborne latexes made from these biosources for painting. In this study, firstly, besides evaluating sustainable industrial by-products such as TOFA and HSO, aims to add a new dimension by evaluating HSO for synthesizing alkyd resin. Secondly, this research has resulted in a generation of stable polymer dispersions of alkyd resins produced in waterborne systems employing TOFA and HSO, as well as the successful application of these waterborne dispersions in the production of exterior paint. More environmentally friendly aqueous latexes and paints have been synthesized by incorporating bio-based alkyd resin while utilizing less synthetic polymers.
Experimental
Materials
TOFA was kindly supplied by Alfa Kimya, Turkey. Hemp seeds were supplied within the borders of Yozgat Province, and HSO was obtained from the seeds by cold-pressing and bleached using clay.
Pentaerythritol (PENTA), phthalic anhydride (PA), xylene, ortho-phosphoric acid (o-PA), lithium stearate (LS), potassium hydroxide (KOH), ethanol, phenolphthalein, potassium hydrogen phthalate (KHP), styrene (Sty), butyl acrylate (BA), acrylic acid (AA), Span 80, Exosel 073, hexadecane (HD), potassium persulfate (KPS), Lopon P, Tylose H 100.000 YP2, Xanthan Gum TGRD, AMP 90, Tego Foamex 8050, Genapol ED 3060, Rhodafac NS 200-S25E, Ti-Pure R-706, and Turkcarb 75 X were used as received in the synthesis of alkyd resins, hybrid latexes, and paints. All these chemicals are listed in Table 1. Deionized water (DIW) was used throughout the studies (MES MP MINIpure, Turkey).
TOFA-based alkyd resin synthesis
TOFA is a fatty acid-based oil and its composition and some of its properties are given in Tables S1 and S2. The K constant, the R-value (ratio of the total -OH groups to total -COOH groups) and oil length were calculated as about 1.02, 1.27, and 60.76 (%), respectively. A TOFA-based alkyd resin was synthesized using long fatty alkyd resin formulation by the fatty acid method (Fig. 1a). Polycondensation was carried out under an inert atmosphere, in an azeotropic environment, and at 240–245 °C, with the help of a Dean–Stark apparatus, a mantle heater, a thermometer, and a mechanical stirrer, by adding the chemicals formulated as indicated in Table 1. The reaction was terminated when the acid number fell below 20 mg/KOH.12,17 Xylene was used as the azeotropic solvent. The temperature of the reaction medium was quickly lowered to 150–180 °C to prevent gelling.
HSO-based alkyd resin synthesis
HSO is a triglyceride-based seed oil, and its composition and some properties are given in Tables S1 and S2. The K constant, the R-value (ratio of the total -OH groups to total -COOH groups) and oil length were calculated as about 1.03, 0.76, and 64.34 (%), respectively. The synthesis of the long oil alkyd resin was carried out in two stages by using the monoglyceride (alcoholysis) method (Fig. 1b). Polyester alcoholysis was formulated as indicated in Table 2. Chemicals were added to a three-necked flask, and it was carried out under an inert atmosphere, at 240–245 °C, in an azeotropic environment with the help of a Dean–Stark apparatus, a mantle heater, a thermometer, and a mechanical stirrer.
The first stage was the alcoholysis stage which was carried out at 240–245 °C in the presence of hemp seed oil, pentaerythritol, and lithium stearate. The alcoholysis process was checked by a methanol test.15 The reaction was terminated when a clear solution was obtained with the sample and methanol mixed in a 1:3 volume ratio.
After the positive monoglyceride test, the reaction flask was cooled to 150 °C. After adding phthalic anhydride and xylene, the temperature was increased to 240–245 °C, and the reaction was continued in an azeotropic environment. The reaction was terminated when the acid number fell below 20 mg/KOH.12,13
Synthesis of alkyd/styrene acrylic waterborne hybrid polymers
The polymer emulsions were synthesized in a three-necked reactor using monomers and initiator, feed pumps, and mixing equipment, in a temperature-controlled, inert atmosphere with a mini-emulsion technique semi-batch polymerization in the amounts (wt%) indicated in Table 3. As the amount of alkyd increased in the formulation, the amount of Sty and BA monomers decreased. However, the amounts of DIW, AA, HD, KPS, and surfactants were kept constant throughout the formulation. Before using the alkyd resin for waterborne emulsion synthesis, the xylene was removed by a rotary evaporator under vacuum distillation. In the first stage, the alkyd resins were dissolved in a mixture of the determined monomers, HD and Span 80. Then, a pre-emulsion was prepared by adding DIW, Exosel 073, and a comonomer (AA) to the mixture prepared in the first stage, and homogenized at 10,000 rpm. After keeping a constant temperature of 78 °C for the reaction, some part of the DIW, initiator-I, and a quarter of the prepared pre-emulsion were added to the reactor. Half an hour later, the remaining pre-emulsion was slowly added to the polymerization medium over 3 h, using a dosing pump. After the monomer addition was completed, the emulsion was stirred for more than 30 min. Later, the initiator solution-II was added to prevent free monomers, and the reaction temperature was increased to 85 °C and stirred again for more than an hour. Polymerization was terminated by cooling the latex to room temperature. Finally, the pH was adjusted to 8–9 with an ammonia solution.
All the latexes were passed through a 100-µm polyester fabric sieve and the colloidal parts formed in the reaction medium were removed. The filtered latex was stored in a closed container in the dark. The latex synthesized using Sty/BA/AA without alkyd was called HL-1.
Preparation of paints
Opaque (O) and transparent base paint (T) production was carried out based on the previous study of Eren and Can21 (Table 4), with the help of a conventional dye dispersant (Dissolver Dispermat CN). To explain briefly, the first portion of the thickeners (Tylose H 100.000 YP2, Xanthan Gum TGRD), the pH regulator (AMP 90), the defoamer (Tego Foamex 8050), and the water put into the dispenser were all blended at 750 rpm for 5 min. Dispersing agents (Lopon P, Rhodafac NS 200-S25E), a wetting agent (Genapol ED 3060), a defoamer (Tego Foamex 8050), titanium dioxide (Ti-Pure R-706) (for opaque paints), various fillers (Turkcarb 75 X, Esen Kalsit A-3, Coattun S.PR.C5), and an additional amount of thickener (Coatex BR 100 P) were then added to the mixture and mixed for 10–15 min at 3000 rpm to achieve a ground size of less than 40 μm. Finally, during the discharge stage, a number of additives were introduced to the grinding process and combined for 5 min at 750 rpm, including a binder (Polymer Emulsion-HL-O,…HL-6), hydrophobic agent (Tegophobe 1401), co-solvent (Dowanol DPnB), biocide (Acticide MBS), acrylic thickener (Betapol BT-10), and defoamer (Tego Foamex 8050). The paints obtained from each hybrid latex (HL-1–HL-7) were called HL-1P–HL-7P.
Tests and Measurements
Analysis for TOFA and HSO
The fatty acid compositions of TOFA and HSO used in the alkyd synthesis were determined by gas chromatography/mass spectrometry based on literature.13 The physicochemical properties of TOFA and HSO were determined using standard methods.
Analyzes of alkyd/styrene acrylic waterborne hybrid polymers
The synthesized polymer emulsions were poured into silicone molds and left to dry in a controlled manner at 20–25 °C for 7 days. Thus, latex films were obtained with about 1 mm thickness. The solid content (SC) of the polymer emulsions was measured with a Shimadzu MOC63u moisture analyzer at 120 °C. Kinematic viscosity values were meaured with Fungilab brand viscometer at 20 °C, 100 rpm with R2 spindle. pH measurements were made with a Hanna brand pH meter. Particle size was determined with the Malvern Mastersizer 2000 particle size analyzer. Particle size of the emulsion that diluted 1:10 with distilled water was measured by dynamic light scattering technique with a light source of 488 nm wavelength at 25 °C. The chemical structure of the alkyd resins and latex films were determined by FTIR, 1H-NMR, and 13C-NMR. The surface morphology of the latex films obtained on glass plates with a 200-µm applicator was determined with the model atomic force microscope (AFM; Multimode 8; Veeco). The root mean square (Rq) of roughness and the arithmetic mean (Ra) of roughness were obtained from 5 × 5 µm2 areas. Minimum film forming temperature (MFFT) was determined with Rhopoint MFFT Bar 60 according to the ASTM D 2354 and ISO 2115 standards. The glass transition temperature (Tg) of the latex films were determined with a Perkin Elmer 6000 DSC with an internal cooling system at a heating and cooling rate of 10 °C/min from − 40 to 110 °C under a nitrogen atmosphere. Thermal analyses were performed with a Perkin Elmer Diamond model instrument under a nitrogen atmosphere with a 10 °C/min heating rate. Static water contact angle (CA) measurements were made using an optical tensiometer system (Attension, Theta Lite) equipped with a goniometer and 5 µL of deionized water according to the standard sessile drop method. The contact angle values of each coating were measured from three different places, and the values were placed in the tables by taking the averages. Tensile testing of the films was carried out with a Shimadzu AGXD 50 kN device. The latex film of about 10 × 20 mm2 and 1 mm thickness was placed in the grips of the tensile testing machine at a speed of 50 mm/min, an applied F load, and an elongation (ΔL) of the material.
Analysis of paints
The polymer characterization results of the binder samples were compared with a standard pure acrylic binder. The experiments were carried out by making laboratory-scale paint studies based on the amounts and the MFFT values of 7 different binders synthesized instead of the 100% pure acrylic binder used in semi-gloss waterborne exterior paint. In addition, the paints were subjected to full paint performance tests. The measurement results are given in comparison with standard product results.
The hiding power of paints was determined using hiding test cards and class 4.2.5 of the TS 5808 standards.
The paint adhesion test (cross-cut) is a method for testing the adhesion of the paint on different substrates. This test was performed by using Tesa 4657 (an aggressive tape used in the automotive industry) on the samples, which were applied with a 200-μm applicator to the sanded metal surface and cured for 1 week, and their adhesion was tested. The results are classified according to the EN ISO 2409. The König pendulum hardness of paints was determined according to the EN ISO 1522 standard. Paint samples were applied with a 200-μm applicator to the sanded metal surface and cured for 1 week.
The cross-cut test was performed using Tesa 4657 on the samples, which were applied with a 200-μm applicator to the sanded metal surface and cured for 1 week, and their adhesion was tested. The results were classified according to ISO and ASTM. In addition, the hardness test was applied with a 200-μm applicator to the sanded metal surface and cured for 1 week.
The rubbing fastness of the paints was carried out by rubbing the test sample placed in a crock meter at 20 °C and the accompanying cloth against each other. For the rubbing fastness test, the paint samples were colored with 5% (w/w) Betek Tint 25. The colored samples were applied as 300 µm to black standard panels, and the coated panels were allowed to dry under standard conditions (23 ± 2 °C, 50 ± 5% relative humidity) for 7 days. After conditioning, the panels were cut into specified sizes for the device and the brightness values of the samples measured before rubbing. The panels were rubbed during 40 cycles with a crockmeter device adopted from the textile industry. The rubbing process was performed with a standard cloth as dry and wet. After rubbing, the color change on the cloths was measured with a spectrophotometer and given as the ΔE value. At the same time, the brightness values were measured again to determine the gloss change (Δgloss) of the samples. The staining of the accompanying cloth at the end of the test was evaluated with a gray scale according to the TS423-3EN20105-A03 standard.
The elasticity, adhesion, and elongation tests of the paint were evaluated in accordance with the TS EN ISO 1519 standard. The samples were applied to the sanded and dusted metal surfaces with a 200-μm applicator and kept in a conditioned room for 1 week. The bending test was applied to the specimens cured for 1 week.
For QUV accelerated weathering tests, the paint was prepared by applying 200-µm wet films on glass plates with automatic film applicators and conditioned in a similar way. QUV tests were performed on a UV Accelerated Weathering Tester manufactured by Q-Lab (QUV-condensation model, UVA-340 Lamp). For this, 4-h UV exposures at 85 ± 2 °C and 4-h condensation cycles at 50 ± 2 °C were applied for up to 1000 h.
Paint color parameter measurements were performed under an illuminator (D65) with a standard observation angle of 10° by using a Datacolor 600 spectrophotometer according to CIELab color measurement system standards. The L, a, and b parameters were determined before and after the QUV aging tests, and the 85° gloss values were determined using a Haze-Gloss BYK Gardner.
The pH and viscosity measurements of the waterborne paints were carried out using the TS 5808 standard. The density, hiding, whiteness, gloss, adhesion, König pendulum hardness, rubbing fastness, and friction fastness tests were determined according to QUV accelerated weathering tests, TS EN ISO 2811-1, TS EN ISO 6504-3, TS 7169, TS EN ISO 2813, EN ISO 2409, EN ISO 1522, and TS423-3EN20105-A03 standards, respectively.
Results and Discussion
Evaluation of TOFA and HSO
The oil content and species in the resin affect the hardness, drying rate, gloss, and other performance properties of coatings.3,44 It has been observed that the viscosity, particle size, MFFT values, and appearance vary depending on the alkyd content and fatty acid/oil species.
Considering the fatty acid composition (Table S1), TOFA and HSO contain 90–99% saturated, unsaturated, and polyunsaturated fatty acids with C16–20 chain lengths in their structures, as well as oleic acid (C18:1) and linoleic acid (C18:2). In addition, the acid number and iodine index values (Table S2) evaluated according to the ASTM standard show that TOFA and HSO are usable bioresources for coating applications.
Evaluation of alkyd resins and alkyd/styrene acrylic waterborne hybrid polymers
The acid number of the alkyds during the reaction was determined volumetrically according to ASTM D-1639 standards. Since the growth of molecules will continue as the reaction progresses and the number of acids will decrease, the reaction process was terminated when the desired acid number in the resin was reached. For our situation, the acid number was kept at around 20 mg KOH/g to prevent gelation, and also to increase the interaction between the alkyd resin and styrene acrylic polymer. For both TOFA- and HSO-based alkyd resin production, the 20 mg KOH/g value was attained after about 6 h of reaction time (Fig. S1).
The structures of the synthesized alkyd resins and hybrid polymers were elucidated by FTIR, 1H-NMR and 13C-NMR analyses.
The IR spectra of the alkyd resins containing TOFA and HSO and polymers containing 0% (pure Sty-BA-AA latex film), 5%, 10%, and 15% alkyd resin based on the total monomer ratio are shown in Figs. 2 and 3.
When Fig. 2 is examined, characteristic bands of Sty-BA and TOFA resin are seen. While peaks of alkyd resin are observed in the spectrum of Fig. 2-b, peaks originating from both the alkyd resin and styrene acrylic latex are clearly seen in the spectra of c, d, and e. In particular, the sharp peak of the C–O–C stretching vibration of the ester around 1100–1200 cm−1 belonging to a, c, d, and e indicates polyesterification. An increase in the intensity of the out-of-plane aromatic C–H bending vibration around 750 cm−1, contrary to the b spectrum, indicates that the aromatic structure originating from styrene increases in latex structures. Moreover, while olefinic (–C=C–H) stretching vibrations of the alkyd resin are observed at 3006 cm−1 (another band at 3030 cm−1 belongs to the aromatic C=C–H of the styrene) in the spectrum (b), it is seen that this band disappears in the spectra of hybrid latex (c, d, and e). This indicates that monomeric units are most likely grafted onto the alkyd backbone. Finally, the presence of the hydroxyl group (–OH) is also seen in the adsorption band at 3500 and 1060 cm−1. When Fig. 3 is examined, it can be seen that the spectra of the HSO resin and latex films are similar to Fig. 2.45,46,47,48
The chemical shift values of the 1H-NMR and 13C-NMR spectra (Fig. S2) of the TOFA- and HSO-based alkyd resins and hybrid polymers were evaluated. Deuterated chloroform (CDCl3) was the solvent for all the experiments. When the spectra were examined, similar chemical shift values were observed when the alkyd resins containing TOFA and HSO were compared with the literature. It was observed that the characteristic peak around δ 5.30 ppm belonging to the protons of the unsaturated carbons in the fatty acid chains disappeared in the spectra of the polymers. It was observed that the peaks of aromatic protons observed in the spectra shifted lower in polymer structures (δ 7.00 ppm) than in alkyd resins (δ 8.00 ppm) with the effect of styrene. In addition, the increase in the intensity of this peak in the spectra of hybrid polymers compared with alkyd resins indicates that the number of aromatic rings in polymer structures increases: CH3 and CH2 protons at 0.90 ppm; –CH=CHCH2CH3 protons at 1.00 ppm; protons of all internal (CH2)n groups in fatty acid chains at 1.30 ppm; –CH2CH2COO– protons at 1.60 ppm; at 2.05 ppm we see CH2CH=CH– and at 2.30 ppm –CH2COO– protons. As seen from the 13C-NMR spectra, the carbonyl groups of the ester at 175 ppm stand out. This also confirmed its structure with chemical shifts of 145, 128, and 61–66 ppm, corresponding to C=O, C=C, and C–O, respectively.10,46,47,49, 50
Hybrid latexes were synthesized as uniform and stable emulsions with minimum coagulation. The amount of free monomers assessed by GC–MS was within the limit values, and all polymerizations had conversion rates of almost 100%. In addition, it was observed that polymer emulsions maintain their stability for at least 6 months. Physicochemical properties of polymer emulsions such as SC, pH, viscosity, particle size (PS), MFFT, Tg, CA, and emulsion appearance (EA) are presented at Table 5. The color of the alkyd-free latex (HL-1) and its film is milky and transparent, respectively. However, the color of the latexes and their films containing TOFA- (HL-2, HL-3, HL-4) and HSO (HL-5, HL-6, HL-7)-based alkyd changed to yellowish-brownish tones depending on the alkyd resin amount and species due to the characteristic alkyd resin color. The varying colors of the waterborne latexes affect the color and transparency of the films.
The variation of the particle size distribution of the emulsions according to the amount of alkyd resin is shown in Table 5, from which it can be seen that the particle size distribution of the emulsions varies between approximately 150 and 1600 nm. It is clearly seen that this change is not dependent on the alkyd resin type. Particle sizes exhibit significant increases for both HL-4 and HL-7 hybrid emulsions and bimodal particle size distribution. For the HL-4 and HL-7 hybrid latexes, the significant increase in particle size with increasing alkyd resin content can be explained by the alkyd unit’s aggregation and dispersion in the reaction media rather than the micelles.51,52 Also, it means that the used surfactant ratio is inadequate for the HL-4 and HL-7 alkyd resin ratio. Even though more emulsifiers are used than commercial latexes (1.5% of the monomer content is used here), such an increase in particle size requires the use of more emulsifiers. This is also undesirable for other applications such as paint. Due to the stability of the latex for more than 6 months, the use of more emulsifiers is not preferred. Goikoetxea et al. synthesized latexes using 6% emulsifier with respect to the organic phase (the alkyd/acrylic ratio was 50/50 wt/wt%), and low particle size (88–135 nm) latexes were obtained.
Viscosity values were measured in the range of 100–130 cP, and it was observed that these values increased partially proportionally with the amount of alkyd. This is considered to be associated with an increase in particle size.53
MFFT and Tg values are considerable parameters that allow the production of formulations that will ensure the correct curing of the products under the specified application conditions. Tg values were measured in the range of 8.5–13.5 °C for hybrid polymers. The observation of a single Tg point from the DSC curves (Fig. S3) was considered as a copolymerization or graft copolymerization in the hybrid polymer structures. The results from both the DSC and FTIR spectra support graft copolymerization. Wu et al. determined three different Tg values in their study and reported these values as Tg values of alkyd, acrylic, and hybrid resins.54 Also, Yousefi et al. reported similar results.55 In addition, considering the 13.5 °C Tg value of the Sty/BA/AA (Alkyd-0) film without alkyd addition, it is noteworthy that there is a decrease in the Tg values. However, it can be said that these decreases are more in the TOFA-based alkyd resin and are not dependent on the amount of alkyd. This is related to the fatty acid composition of TOFA (C18:1) and HSO (C18:2). Moreover, it is thought that the TOFA-based alkyd resin exhibits more plasticizer-like behavior than the HSO-based resin. Moreover, Assua et al. reported that alkyd resin has a plasticizing effect and thus determines the equilibrium morphology of the particles in alkyd/acrylic latex.33 The MFFT values were also determined to be close to that of HL-1 latex. In general, it has been observed that soft alkyd resin reduces MFFT and Tg values in alkyd/styrene acrylic latexes compared to alkyd-free styrene acrylic latex. It was concluded that the measured Tg and MMFT values were suitable for exterior paint production.
On the other hand, the CA values of the latexes are related to the surface energies of the latexes; thus, they depend on the particle morphology in equilibrium.34 The CA measurement results are important in determining the hydrophobic properties of the coatings. The CAs were measured by dripping water, a polar solvent, onto the coatings. The degree of CA depends upon the attractive (cohesion) forces between the molecules of the liquid and the size of the attraction (adhesion) forces between the liquid and the solid. The CA results of each film are shown in Table 5. Although the CAs are measured below 90°, a more or less significant increase is observed in the CAs with increasing alkyd content. This is interpreted as the effect of the alkyd resins, which are partially hydrophobic (Fig. S4). The most serious factor in CA values below 90 is the use of hydrophilic AA and also the surfactants used. AA is used industrially to increase the pigment-binding capacity of the latex used in paint production, and this ratio is generally 2%, as we prefer. In addition, the increase in CAs of alkyd-based latex films compared to alkyd-free latex films indicates that the alkyd used is hydrophobic. Furthermore, it is understood that the CA values are good enough compared to those of siloxane-doped hybrid latex films given in the literature.49,56
The effect of alkyd resin on the thermal properties of hybrid polymers was investigated by TGA as expressed in Fig. 4. Overall, it is clear from Fig. 4 that all the hybrid latex films showed two degradation stages (main chain degradation of polystyrene and polyacrylate). While the hybrid latex decomposes quite stably between room temperature and 300 °C, a decrease in the weight of the hybrid polymers was observed between 300 and 400 °C, due to the loss of water and the decomposition of the side groups in the acrylate and ester part of the polymer structure, with 20% weight loss. It was observed that the thermogram of the samples decreased rapidly in the temperature range of 400–500 °C, and that the degradation rates of the polymers increased up to 500 °C. It has been determined that the polymer structures lose mass at rates of almost 100% in this temperature range. As a result, TGA research revealed that all the hybrid polymers degraded completely when the temperature reached 500 °C.57,58
The surface morphology of hybrid latex films produced by semi-batch emulsion polymerization is shown in Fig. 5 using AFM pictures. When the height maps and 3D diagrams of the hybrid latex films obtained by AFM analysis were examined, it was determined that the hybrid polymer films contained multiple convex structures in various sizes and shapes. This result demonstrates how the unequal distribution of hybrid nanoparticles leads to the formation of irregular brilliant spots and convex forms. In addition, the increase in Ra and Rq values in alkyd-based hybrid latex films compared to the hybrid polymer film without alkyd resin suggests that alkyd resin affects the form of the STY/BA/AA polymer.21,59 The areas that are light in color correlate to those that are rich in acrylic polymer, and the parts that are dark in color correspond to those rich in alkyd resin, according to information from the literature.33,34 In particular, it is observed that the dark (red) color distribution, which is thought to be soft regions, is high in the HL-4 and HL-7 hybrid polymers with a high alkyd ratio. As the alkyd ratio increases, the increased flexibility and softness also coincide with the results obtained from the paint tests. Together, the results of the PS, CA, DSC, MFFT, and AFM tests demonstrate that the data are consistent with the bimodal behavior of latexes with increasing alkyd ratios.
Mechanical analysis, such as tensile testing, is one of the critical parameter determining polymeric materials' usability. Table 6 displays the tensile test outcomes for the hybrid latex-containing films as the average of three measurements. As seen in Table 6, the tensile strengths of the films exhibit an interesting and disproportionate behavior. A 20% increase in tensile strength was observed for HL-3 and HL-4 compared to HL-1. In contrast, a 20% reduction in tensile strength was observed for HL-6.
Evaluation of paint samples prepared with hybrid latexes
The classification of paint adhesion test results according to ASTM is given in Table 7, from which we can see that the adhesion of HL-2P, HL-6P, and HL-7P paints is one click better than paint containing standard acrylic binders (Fig. S5).
The hiding power in paint is the ability of the paint to close and cover the applied surface. How good a paint is at hiding depends on how many microns cover the surface. Hiding power problems can be caused by many reasons, such as the viscosity of the paint, the quality of the pigments, the application method of the paint, and the quality of the application surface. Hiding power values were determined according to the paint class (Table 7) 4.2.5 of the TS 5808 standard. When the results are evaluated by this standard, the paint samples prepared with hybrid latexes are in the 2nd class.
The brightness values are lower compared to the standard acrylic binder. Similarly, the colors of the paints are adversely affected by the increasing alkyd ratio. The increase in particle size (bimodal behavior) with increasing alkyd ratio was evaluated as effective in decreasing the brightness of the paints.
Compared to the standard acrylic binder, the Haake viscosity values are low. Because additional thickener was not used to keep the ratio of the ingredients, the Haake viscosity values were lower (standard binder is 31 and others are 4–9) and the HP values decreased with little change from 98.8 to 98.3. The W (%) values show a slightly decrease due to the alkyd resin, a colored component. Although RF tests are usually used for interior paints, they were carried out to show the behavior of the hybrid paints. Due to the low viscosity of the paint products made with hybrid binders, the wet and dry film thickness decreases and, as a result, lower RF values are found. The difference in RF ratios increases with increasing alkyd content in hybrid paints. This situation is interpreted as the increase in the softness and elasticity of the paints with the increase in the amount of alkyd. The changes in RF-∆E fabric (after wipe–wet), RF-∆E fabric (after wipe–dry) and RF-∆g gloss (85°) were consistent with the decrease in Tg, the MFFT results, and the König test result. The RF results showed that hybrid paints are suitable for both interior and exterior paints, but more suitable for exteriors. For the CC results in Table 7, 0 indicates the worst and 5 is the best. As the adhesion properties of alkyd-containing hybrid paints are improved with modification, an increase in the CC values is observed. When the bending test (BT) results of the films (Table 7) were examined, it was observed that all the samples were flexible, and that there was no cracking. The BT results are the same for standard acrylic paint and no negativity has been observed. Also, it could be said that the adhesion was improved with the increasing alkyd ratio. As expected, no corrosion was observed in any of the products (Fig. S6). Both the gloss test and the wipe test results (before and after) revealed that there is a partial worsening of the glossing and wiping with the increasing alkyd ratio. It is thought that this situation is related to the decrease in the Tg and MFFT values of the soft alkyd resin.
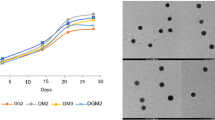
The effect of QUV exposure on color parameters of hybrid polymer films was investigated and results were presented in Table 8. Time-dependent whiteness monitoring, color change, and gloss values are also shown in Figs. S6, S7, and S8. Especially, the 1000-h QUV results are an essential parameter for the evaluation of paints. After 1000 h, ΔEs were found to be 1.07, 0.59, 1.13, and 1.07 for HL-1P, HL-2P, and HL-3P, and HL-4P, and 0.98, 1.30, and 1.07 for HL-5P, HL-6P, and HL-7P, respectively, and the lower differences in ΔE show that the films are stable against color change. From the results, it is understood that the QUV resistance of hybrid latexes (when the a, b, c, and ΔE values are compared collectively) is almost the same compared with the alkyd-free styrene acrylic (HL-1P) binder, and remains white without changing color. In addition, it can be said that, as the alkyd ratio increases, there are partial improvements in color changes. This can be explained by the fact that the unsaturated bonds in alkyd resins are not free and participate in the polymerization reaction, as expressed in the FTIR results section. Minor changes in ΔE were considered usual, both due to the aromatic monomer styrene and partially due to the alkyd resin containing unsaturated bonds. When the ΔE values of both the TOFA- and HSO-sourced alkyd-containing hybrid paints were compared, QUV resistance was comparable to standard styrene acrylic paints for all the ratios (5, 10, and 15%).
In addition to excellent adhesion, flexibility, and impact resistance, the hardness of the paint film is also important. A hardness test was applied to the samples cured for 1 week and 2 weeks. The results of the tests are given in Table 9. Because the alkyd copolymer ratio increased, the hardness partially decreased, as expected from the effect of the alkyd resins. However, an increase in the hardness values was observed in the two measurements made 1 week apart.
Conclusions
Alkyd resins from two different bioresources, TOFA and HSO, were synthesized and the resins were successfully incorporated into the waterborne STY/BA/AA latex system. Synthesized waterborne hybrid latexes and their films were characterized using FTIR, NMR, particle size, MFFT, TGA, DSC, CA, AFM, and mechanical tests. The structures of the alkyd and STY/BA/AA resins were confirmed from the FTIR and NMR results. The effect of the alkyd resins on particle sizes and viscosity for waterborne hybrid latexes were determined, and an increase with the increasing alkyd ratio was seen. It was determined that alkyd resins did not significantly affect the MFFT in hybrid waterborne polymers. It was generally seen that the CA values were enhanced with the alkyd ratio, and that alkyd resins increased the hydrophobicity of the hybrid waterborne latexes. The observation of a single Tg point was considered to form copolymers of alkyd and STY/BA/AA polymers. The TGA thermograms show that the hybrid polymer films almost completely degrade at 500 °C. It was determined from the AFM images that the morphologies of the hybrid polymers were different from the HL-1 and that alkyd resins affected the morphology. Tensile strength measurements revealed that the TOFA-based alkyd resin has more effect in increasing the tensile properties than the HSO-based alkyd resin. Moreover, it was observed that polymer emulsions maintain their stability for a minimum of 6 months. It was understood that waterborne hybrid polymers have good storage stability. From the paint tests, it can be said that commercially available paints with similar UV resistance to the styrene acrylic paint binder, better adhesion to metal surfaces, and showing oil paint behavior as an application were obtained. Analyses were performed for both exterior and interior paints, and, especially, the 1000-h QUV results showed that hybrid paints containing up to 15% alkyd resin gave good results comparable to standard styrene acrylic paints. The collective results obtained from the paint tests indicated that the paints synthesized with bio-based hybrid latexes are especially suitable for exterior applications. In terms of sustainability and being more environmentally friendly, it can be recommended to use alkyd-based hybrid latexes for paint applications. In future studies, it is planned to obtain better polymers and paints in terms of brightness, whiteness, and color changes with a similar study using more sustainable polymers.
References
Deyab, MA, “Anticorrosion Properties of Nanocomposites Coatings: A Critical Review.” J. Mol. Liq., 313 113533 (2020)
Kausar, A, “Polymer Coating Technology for High Performance Applications: Fundamentals and Advances.” J. Macromol. Sci. Part A, 55 (5) 440–448 (2018)
Chardon, F, Denis, M, Negrell, C, Caillol, S, “Hybrid Alkyds, The Glowing Route to Reach Cutting-Edge Properties?” Prog. Org. Coat., 151 106025 (2021)
Jiao, C, Sun, L, Shao, Q, Song, J, Hu, Q, Naik, N, Guo, Z, “Advances in Waterborne Acrylic Resins: Synthesis Principle, Modification Strategies, and Their Applications.” ACS Omega, 6 (4) 2443–2449 (2021)
Mukhtar, A, Ullah, H, Mukhtar, H, “Fatty Acid Composition of Tobacco Seed Oil and Synthesis of Alkyd Resin.” Chin. J. Chem., 25 (5) 705–708 (2007)
Ifijen, IH, Odi, HD, Maliki, M, Omorogbe, SO, Aigbodion, AI, Ikhuoria, EU, “Correlative Studies on the Properties of Rubber Seed and Soybean Oil-Based Alkyd Resins and Their Blends.” J. Coat. Technol. Res., 18 (2) 459–467 (2021)
Büyükyonga, ÖN, Akgün, N, Acar, I, Güçlü, G, “Synthesis of Four-Component Acrylic-Modified Water-Reducible Alkyd Resin: Investigation of Dilution Ratio Effect on Film Properties and Thermal Behaviors.” J. Coat. Technol. Res., 14 (1) 117–128 (2017)
Wang, H, Li, Y, Fei, G, Sun, L, Wang, Y, Liu, X, Wang, M, Rang, N, “Comparison Study on Chelated and Non-chelated Titanate Functionalized Graphene Nanosheets for Enhancement of Waterborne Alkyd Anticorrosion Coating.” Prog. Org. Coat., 150 105961 (2021)
Pathan, S, Ahmad, S, “Green and Sustainable Anticorrosive Coating Derived from Waterborne Linseed Alkyd Using Organic–Inorganic Hybrid Cross Linker.” Prog. Org. Coat., 122 189–198 (2018)
Hadzich, A, Gross, GA, Leimbach, M, Ispas, A, Bund, A, Flores, S, “Effect of Polyalcohols on the Anticorrosive Behaviour of Alkyd Coatings Prepared with Drying Oils.” Prog. Org. Coat., 145 105671 (2020)
Wang, H, Hu, G, Liu, X, Guo, L, Li, X, Guo, R, Li, Y, “Concurrent Alkylation and Crosslinking of Polyaniline for Enhanced Anticorrosive Performance of Waterborne Alkyd Coating.” Prog. Org. Coat., 168 106865 (2022)
Chukwuebuka, AS, Samuel, EO, Asadu, CO, Onoghwarite, OE, Celestine, O, Ezema, CA, “Optimal Analysis of the Effects of Process Conditions on the Yield of Alkyd Resins from Castor and Soybean Seed Oils Using Response Surface Methodology.” Chem. Eng. J. Adv., 9 100217 (2022)
Das, P, Sharma, N, Puzari, A, Kakati, DK, Devi, N, “Synthesis and Characterization of Neem (Azadirachta indica) Seed Oil-Based Alkyd Resins for Efficient Anticorrosive Coating Application.” Polym. Bull., 78 (1) 457–479 (2021)
Obregón, D, Toledo, C, Hadzich, A, Flores, S, “Low Viscosity Alkyd Resins Based on Trimethylolpropane and Peruvian Oil.” J. Polym. Res., 28 (6) 203 (2021)
Saravari, O, Phapant, P, Pimpan, V, “Synthesis of Water-Reducible Acrylic–Alkyd Resins Based on Modified Palm Oil.” J. Appl. Polym. Sci., 96 (4) 1170–1175 (2005)
Ogunniyi, DS, Odetoye, TE, “Preparation and Evaluation of Tobacco Seed Oil-Modified Alkyd Resins.” Bioresour. Technol., 99 (5) 1300–1304 (2008)
Odetoye, TE, Ogunniyi, DS, Olatunji, GA, “Preparation and Evaluation of Jatropha curcas Linneaus Seed Oil Alkyd Resins.” Ind. Crops Prod., 32 (3) 225–230 (2010)
Eren, M, Akbulut, G, Senler, S, Kayaoğlu, BK, “Synthesis of Core–Shell-Type Styrene Acrylic Latexes with Low NMA Content and Their Application in Pigment Printing Pastes.” J. Coat. Technol. Res., 15 (1) 121–129 (2018)
Delibaş, A, Yıldız, U, Tauer, K, “Composite Latex Production with High Solid Content.” J. Appl. Polym. Sci., 136 (18) 47423 (2019)
Delibaş, A, “Synthesis of (Sty-co-BA-AA) Latexes Including ZnO and TiO2 Nanoparticle by Miniemulsion Polymerization.” J. Coat. Technol. Res., 18 (3) 717–727 (2021)
Eren, M, Can, HK, “Preparation of Zinc Methacrylate-Methylmethacrylate-Butyl Acrylate Emulsions and Their Application in Exterior Paints.” Prog. Org. Coat., 135 424–437 (2019)
Lovell, PA, Schork, FJ, “Fundamentals of Emulsion Polymerization.” Biomacromolecules, 21 (11) 4396–4441 (2020)
Akgün, N, Büyükyonga, ÖN, Acar, I, Güçlü, G, “Synthesis of Novel Acrylic Modified Water Reducible Alkyd Resin: Investigation of Acrylic Copolymer Ratio Effect on Film Properties and Thermal Behaviors.” Polym. Eng. Sci., 56 (8) 947–954 (2016)
Elrebii, M, Ben Mabrouk, A, Boufi, S, “Synthesis and Properties of Hybrid Alkyd–Acrylic Dispersions and Their Use in VOC-Free Waterborne Coatings.” Prog. Org. Coat., 77 (4) 757–764 (2014)
Nabuurs, T, Baijards, RA, German, AL, “Alkyd-Acrylic Hybrid Systems for Use as Binders in Waterborne Paints.” Prog. Org. Coat., 27 (1) 163–172 (1996)
Ma, Y, Lei, R, Yang, X, Yang, F, “Eco-friendly Waterborne Alkyd Resin from Polyethylene Terephthalate Waste.” J. Polym. Environ., 28 (3) 1083–1094 (2020)
Assanvo, EF, Baruah, SD, “Synthesis and Properties of Ricinodendron heudelotii Oil Based Hybrid Alkyd–Acrylate Latexes via Miniemulsion Polymerization.” Prog. Org. Coat., 86 25–32 (2015)
Guyot, A, Landfester, K, Joseph Schork, F, Wang, C, “Hybrid Polymer Latexes.” Prog. Polym. Sci., 32 (12) 1439–1461 (2007)
Landfester, K, Schork, FJ, Kusuma, VA, “Particle Size Distribution in Mini-Emulsion Polymerization.” Comptes Rendus Chimie, 6 (11) 1337–1342 (2003)
Vale, HM, McKenna, TF, “Modeling Particle Size Distribution in Emulsion Polymerization Reactors.” Prog. Polym. Sci., 30 (10) 1019–1048 (2005)
Wang, T, de las Heras Alarcón, C, Goikoetxea, M, Beristain, I, Paulis, M, Barandiaran, MJ, Asua, JM, Keddie, JL, “Cross-linked Network Development in Compatibilized Alkyd/Acrylic Hybrid Latex Films for the Creation of Hard Coatings.” Langmuir, 26 (17) 14323–14333 (2010)
Uschanov, P, Heiskanen, N, Mononen, P, Maunu, SL, Koskimies, S, “Synthesis and Characterization of Tall Oil Fatty Acids-based Alkyd Resins and Alkyd–acrylate Copolymers.” Prog. Org. Coat., 63 (1) 92–99 (2008)
Goikoetxea, M, Reyes, Y, de las Heras Alarcón, CM, Minari, RJ, Beristain, I, Paulis, M, Barandiaran, MJ, Keddie, JL, Asua, JM, “Transformation of Waterborne Hybrid Polymer Particles into Films: Morphology Development and Modeling.” Polymer, 53 (5) 1098–1108 (2012)
Limousin, E, González, E, Martínez-Tong, DE, Ballard, N, Asua, JM, “Modelling the Dynamic Development of the Curing Process and Film Morphology of Films Cast from Waterborne Acrylic-Alkyd Hybrids.” Chem. Eng. J., 400 125891 (2020)
Limousin, E, Martinez-Tong, DE, Ballard, N, Asua, JM, “Cure-Dependent Morphology of Acrylic/Alkyd Hybrid Latex Films via Nanomechanical Mapping.” ACS Appl. Polym. Mater., 1 (8) 2213–2223 (2019)
Liz Manning, DOM, Hamacek, K, Opportunities for the Alkyd Resin Market to Create a Sustainable Future for the European Coatings Industry. Paint Coatings Industry (2019)
Elrebii, M, Kamoun, A, Boufi, S, “Waterborne Hybrid Alkyd–Acrylic Dispersion: Optimization of the Composition Using Mixture Experimental Designs.” Prog. Org. Coat., 87 222–231 (2015)
Athawale, VD, Nimbalkar, RV, “Waterborne Coatings Based on Renewable Oil Resources: An Overview.” J. Am. Oil Chem. Soc., 88 (2) 159–185 (2011)
Fei, G, Sun, L, Wang, H, Gohar, F, Ma, Y, Kang, Y-M, “Rational Design of Phosphorylated Poly(vinyl alcohol) Grafted Polyaniline for Waterborne Bio-based Alkyd Nanocomposites with High Performance.” Prog. Org. Coat., 140 105484 (2020)
Hofland, A, “Alkyd Resins: From Down and Out to Alive and Kicking.” Prog. Org. Coat., 73 (4) 274–282 (2012)
Somtürk, SM, Emek, İY, Senler, S, Eren, M, Kurt, SZ, Orbay, M, “Effect of Wollastonite Extender on the Properties of Exterior Acrylic Paints.” Prog. Org. Coat., 93 34–40 (2016)
Lewarchik, R, Basics of Alkyd Resin Technology. Prospector Knowledge Center (2016)
Siano, F, Moccia, S, Picariello, G, Russo, GL, Sorrentino, G, Di Stasio, M, La Cara, F, Volpe, MG, “Comparative Study of Chemical, Biochemical Characteristic and ATR-FTIR Analysis of Seeds, Oil and Flour of the Edible Fedora Cultivar Hemp (Cannabis sativa L.).” Molecules, 24 (1) 83 (2019)
Abd El-Wahab, H, Abd El-Hai, F, Abd El-Fattah, M, Lin, L, “Effect of Short Oil-Length Alkyd Additive on the Properties of Coal Tar Binder.” Pigment Resin Technol., 40 (5) 305–310 (2011)
Muhammad, A, Abbas, S, Shafeeq, A, Al-Turaif, HA, Taimoor, AA, Ali, AM, Deshannavar, UB, “Synthesis and Characterization of Pentaerythritol Phthalic Anhydride Resin from Soybean Oil.” Asian J. Chem., 30 572–574 (2018)
Hadzich, A, Gross, GA, Leimbach, M, Ispas, A, Bund, A, Flores, S, “Characterization of Plukenetia volubilis L. Fatty Acid-Based Alkyd Resins.” Polym. Test., 82 106296 (2020)
Assanvo, EF, Gogoi, P, Dolui, SK, Baruah, SD, “Synthesis, Characterization, and Performance Characteristics of Alkyd Resins Based on Ricinodendron heudelotii Oil and Their Blending with Epoxy Resins.” Ind. Crops Prod., 65 293–302 (2015)
Tiwari, S, Saxena, M, Tiwari, S, “Preparation and Characterization of Penta Alkyds Based on Mahua Oil.” J. Sci. Ind. Res., 61 110–116 (2002)
Neelambaram, P, Shankar, A, Sykam, K, Kumar, DBR, Chakrabarty, A, Narayan, R, “Siloxane-Based High Solid Acrylic Latex by Mini-Emulsion Polymerization for Coatings with Improved Water Resistance.” Prog. Org. Coat., 171 107011 (2022)
Iizuka, Y, Yamamoto, Y, Kawahara, S, “Latex-State 13C-NMR Spectroscopy for Poly(butyl acrylate).” Colloid Polym. Sci., 297 (1) 133–139 (2019)
Zhu, X, Jiang, X, Zhang, Z, Kong, XZ, “Influence of Ingredients in Water-Based Polyurethane–Acrylic Hybrid Latexes on Latex Properties.” Prog. Org. Coat., 62 (3) 251–257 (2008)
Wu, L, You, B, Li, D, “Synthesis and Characterization of Urethane/Acrylate Composite Latex.” J. Appl. Polym. Sci., 84 (8) 1620–1628 (2002)
Do Amaral, M, Van Es, S, Asua, JM, “Effect of the Particle Size Distribution on the Low Shear Viscosity of High-Solid-Content Latexes.” J. Polym. Sci. Part A Polym. Chem., 42 (16) 3936–3946 (2004)
Wu, XQ, Schork, FJ, Gooch, JW, “Hybrid Miniemulsion Polymerization of Acrylic/Alkyd Systems and Characterization of the Resulting Polymers.” J. Polym. Sci. Part A Polym. Chem., 37 (22) 4159–4168 (1999)
Yousefi, AA, Pishvaei, M, Yousefi, A, “Preparation of Water-Based Alkyd/Acrylic Hybrid Resins.” Prog. Color Colorants Coat., 4 (1) 15–25 (2011)
Li, H, Yuan, J, Qian, H, Wu, L, “Synthesis and Properties of SiO2/P(MMA-BA) Core–Shell Structural Latex with Siloxanes.” Prog. Org. Coat., 97 65–73 (2016)
Wei, Z, Ling, H, Junyan, L, Gang, C, Na, W, “Preparation and Properties of Core–Shell Nanosilica/Poly(methyl methacrylate–butyl acrylate–2,2,2-trifluoroethyl methacrylate) Latex.” J. Appl. Polym. Sci., 120 (2) 1152–1161 (2011)
Ifijen, IH, Ikhuoria, EU, Omorogbe, SO, Aigbodion, AI, “Ordered Colloidal Crystals Fabrication and Studies on the Properties of Poly (Styrene–Butyl Acrylate–Acrylic Acid) and Polystyrene Latexes.” Proc. Nanocomposites VI: Nanoscience and Nanotechnology in Advanced Composites, Cham, 2019
Zhenqian, Z, Bo, X, Pei, W, Ninyyi, Y, “Hybrid Latex Particles Preparation with Seeded Semibatch Emulsion Polymerization.” Colloids Surf. A Physicochem. Eng. Asp., 482 422–430 (2015)
Acknowledgments
This study was supported by Yozgat Bozok University Scientific Research Projects Unit with code 6602c-FEN/21-459. We also thank Betek Boya ve Kimya San. A.Ş. for their support of the analyses and studies.
Author information
Authors and Affiliations
Contributions
NK: Investigation, Visualization, Methodology, Formal analysis, Validation, Writing. SEA: Investigation, Formal Analysis, Validation. ME: Resources, Methodology, Writing, Review and Editing. AD: Project administration, Writing—review and editing, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kartaloğlu, N., Akçin, S.E., Eren, M. et al. Waterborne hybrid (alkyd/styrene acrylic) emulsion polymers and exterior paint applications. J Coat Technol Res 20, 1621–1637 (2023). https://doi.org/10.1007/s11998-023-00767-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-023-00767-6