Abstract
A coating of poly(dimethylsiloxane) (PDMS) on a stir bar is typically used for sorptive extraction and preconcentration of nonpolar organic analytes from aqueous matrices. Here, we used sol–gel to synthesize three new coatings based on hydroxy-terminated PDMS crosslinked with 3-cyanopropyltriethoxysilane (CN) and phenyltriethoxysilane (PH) with three molar ratios of CN:PH (2:1, 1:1, and 1:2). The water contact angle of the coatings was below 90°. Cyanopropyl and phenyl groups were identified by infrared spectroscopy. The coatings were thermally stable up to 200°C. Also, they were stable to organic solvents and aqueous solutions at different pH. Glass stir bars were covered with the coatings to explore its potential application in stir-bar sorptive extraction. The test included polar antibiotics (sulfathiazole, sulfamethoxazole, trimethoprim, and chloramphenicol) dissolved in spiked water (pH 4.5) and organic solvent. Different experimental conditions were used to explore the capacity of the coatings to extract and preconcentrate the antibiotics. Unsatisfactory results of enrichment effect were obtained in aqueous solutions of antibiotics. In organic medium, the antibiotics were extracted and preconcentrated with the three coatings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis and application of sorbent coatings have played a central role in analytical chemistry. In sample preparation, sorbent coatings have contributed to diminish (or avoid) the use of organic solvents, simplify procedures, and miniaturize the analytical devices.1 Thus, coatings have led to the development of environmentally friendly sample preparation techniques such as solid-phase microextraction (SPME) and stir-bar sorptive extraction (SBSE).2
In SBSE, a stir bar typically coated with poly(dimethylsiloxane) (PDMS) extracts and preconcentrates organic analytes from aqueous matrices after the stirring of the sample for a certain time. The stir bar is withdrawn, and the analytes are released from the coating by thermal (TD) or liquid desorption (LD). TD is compatible with gas chromatography (GC) and LD with either GC or liquid chromatography.3
SBSE has a great enrichment capacity. It requires a relatively low sample volume, and it can operate overnight without any special requirements. However, the main weakness of SBSE is the scarce availability of commercial coatings with different chemical composition. Thus, the lipophilic nature of PDMS restricts the SBSE applications to nonpolar compounds. However, the introduction of stir bars coated with PDMS/polyethylene glycol (PEG) helped to expand the applications to polar analytes.4
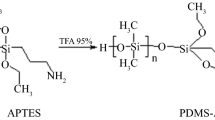
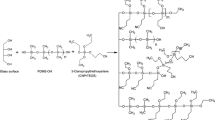
A typical approach to obtain new polymeric hybrid coatings is sol–gel technology.3,4 It uses an acid (or base) to catalyze simultaneous reactions of hydrolysis and condensation of organoalkoxysilanes at room temperature with simple experimental setup.5 If a glass support is present during the synthesis, the polymer can be chemically bound to the bare hydroxyl groups on the glass’ surface. This feature provides mechanical, thermal, and chemical stability to the polymer.6 These properties are crucial in SBSE, during stirring, TD, and LD. Another advantage is the wide variety of polar monomers to obtain tailor-made coatings for SBSE.
The compounds introduced in the PDMS network by sol–gel include β-cyclodextrin, divinylbenzene, poly(vinyl alcohol), cyanopropyltriethoxysilane, and amino-modified carbon nanotubes.4 The modified PDMS coatings were tested by extraction of organophosphorus pesticides, organic sulfur compounds, nonsteroidal antiinflammatory drugs, brominated flame-retardants, steroid hormones, and phenols. However, there are no reports of PDMS modified with two functional organic moieties at different ratios and its interactions with polar organic analytes.
In this paper, three molar ratios of cyanopropyl and phenyl groups were introduced to the PDMS network by sol–gel process. The coating characterization included water contact angle, infrared spectroscopy, and thermogravimetric analysis (TGA). The stability under LD conditions was evaluated by submerging the coating into organic solvents, acid, and basic aqueous solutions. In contrast with published reports in which the probe analytes have a value of the logarithm of octanol–water partition coefficient (log Kow) higher than two, the potential application of the coatings was tested for the extraction and preconcentration of four polar antibiotics (0< log Kow< 2) from aqueous and organic media. These antibiotics are associated with environmental and toxicological concerns, and they could be present in ecosystems, foods, and beverages.7,8
Materials and methods
Reagents and dissolutions
Hydroxy-terminated poly(dimethylsiloxane) (PDMS-OH, Mn~550), 3-cyanopropyltriethoxysilane (CN), phenyltriethoxysilane (PH), and isooctane were from Sigma-Aldrich (Saint Louis, MO, USA). Trifluoroacetic acid (TFA), methanol (MeOH), acetone, sodium chloride (NaCl), sodium hydroxide (NaOH), formic acid (HFor), hydrochloric acid (HCl), methylene chloride (DCM), nitric acid (HNO3), ethyl acetate (EtOAc), and acetonitrile (ACN) were acquired from J.T Baker (Phillipsburg, NJ, USA) and used without further purification.
Sulfathiazole (STZ), sulfamethoxazole (SMX), chloramphenicol (CRF), and trimethoprim (TMP) standards were from Sigma-Aldrich (Saint Louis, MO, USA).
The standard solutions of antibiotics were diluted in MeOH and formic buffer (0.10% v/v, pH 4.5); the MeOH content was 40% v/v in each standard solution.
Spiked water was prepared daily with working solutions of antibiotics (100 mg L−1 in MeOH). However, isooctane is not miscible with water or MeOH. Therefore, standard solutions of antibiotics were prepared in acetone to spike isooctane.
Preparation of stir bars with hybrid coating
A magnet of 10 mm was enclosed in a borosilicate glass. The glass stir bars were placed in NaOH (1 mol L−1) for 3 h to expose the silanol groups on the glass surface. The stir bars were cleaned with water and HCl (0.10 mol L−1, 3h) to neutralize the excess of NaOH. Then, they were rinsed three times with water and dried 2 h at 110°C in a convection oven (Techni-Lab model HCM-D82).9,10
The composition of polymerization reaction solutions to prepare the coatings by sol–gel is shown in Table 1. The sol solutions were prepared in an Eppendorf tube of 2 mL by mixing DCM with PDMS-OH on a vortex (Genie-2). The procedure was repeated after the addition of PH and CN. Finally, aqueous TFA solution (95 v/v %) was added to the solution. The sol was mixed with a vortex (2 min) at maximum speed, sonicated (5 min), and aged (15–16 h) at room temperature (25°C) in a desiccator.
Each sol mixture was poured into polyvinyl chloride (PVC) molds (3 mm ID × 25 mm) that contained the treated glass stir bar. The coated stir bar was released from the PVC cast after seven days.9,10
After that, the coated stir bars were placed in a vacuum oven (<4 in Hg) to produce a xerogel. The vacuum oven (Barnstead Lab Line) was held for 24 h at 40°C; then, the temperature was increased to 60°C and it was held for 24 h; after that, the temperature was raised to 100°C and it was maintained for 24 h; finally, the temperature was increased to 200°C with a holding time of 4 h. After that, the vacuum oven was turned off and it was left to reach room temperature before depressurizing. The length of the coated stir bars was adjusted to 15 mm.9,10
Physicochemical characterization of the coatings
A spectrometer Thermo-Nicolet Nexus 670 was used for DRIFTS analysis. The surface of the coatings was abraded with silicon carbide disks to obtain debris. They were analyzed with an average of 64 scans per sample. Spectra were taken between 4000 and 400 cm−1 with a resolution of 4 cm−1.
The coated bars were weighted before the chemical stability tests. The stir bars were immersed in 1 mL of water, MeOH, ACN, isooctane, acetone, or EtOAc at room temperature. Then, they were exposed to ultrasound (40 MHz, Branson model 1510) for 30 and 60 min. In addition, they were agitated in vortex at maximum power (15 and 30 min). The bars were removed, placed on tissue paper, and weighed to obtain the average weight difference (n = 3).
The experiments of pH stability were done with a Jenway pH meter (model 3510) and Milli-Q water (18.2 MΩcm Millipore, USA). The coated bars were weighed and immersed (24 or 48 h) into aqueous solutions at different pH (1, 3, 6, 9, and 12) adjusted with HCl, HNO3, or NaOH. Then, the coated bars were removed, wiped with tissue paper, and weighed to get the average weight difference (n = 3).
The wettability of the coating was assessed by measuring the contact angle with a camera and the Motic Images Plus 2.0 software. The sessile drop method was used to measure the contact angle of de-ionized water (20 µL) on the dried coating surfaces at 25°C. The average and standard deviation of water contact angle were reported (n = 10).
A thermogravimetric analyzer Perkin Elmer model 8000 allowed the evaluation of thermal stability by heating from 50 to 800°C at 10°C min−1 under inert N2 atmosphere.
SBSE tests with coated stir bars
First, 10 mL of aqueous buffer of HFor 0.10 % v/v (pH 4.5) were spiked with STZ, TMP, SMX, and CRF at 1 mg L−1. The solution was stirred at room temperature with a coated stir bar. The stir bar was removed and dried with lintless paper. The stir bar was placed in an Eppendorf vial filled with 500 µL of mixture 40%MeOH–60% aqueous HFor buffer (pH 4.5) with the aid of vortex-assisted LD. The performance of the coatings CN:PH 2:1, 1:1, and 1:2 was compared under the same extraction (120 min, 250 rpm) and LD conditions (40 min, 40% MeOH–60% aqueous HFor buffer pH 4.5).
Isooctane (10 mL) was spiked with antibiotics at 1 mg L−1. The solution was stirred at room temperature with a coated stir bar (250 rpm, 120 min). The stir bar was retired, and the residues of isooctane were removed with a lintless tissue. The stir bar was placed in a 600 µL Eppendorf vial filled with 500 µL of mixture 40% MeOH–60% aqueous HFor buffer (pH 4.5) with the aid of vortex-assisted LD.
Experimental design and statistical analysis
The experimental conditions that affect the extraction of antibiotics from water were explored with the coating CN:PH 2:1. A 24 experimental design was carried out with the factors and levels based on literature.11 The significance was evaluated using ANOVA after satisfying the statistical assumptions. The effect of a main factor or interaction was significant at p value ≤ 0.05. (Table 2)
An analysis of variance (ANOVA) with a design of two-way fixed effects12 was done with Statgraphics 5.113 software to establish the effect of materials and antibiotics in the sum of peak area of all antibiotics. Then, the multiple comparison of minimum significant difference (MSD) was applied.12
Because the sum of peak area did not show requirements of ANOVA, the peak areas were square-root transformed (SRArea) to fulfill those requirements.
Significance level of all statistic tests was α = 0.05.
Chromatographic analysis
Aliquots of 20 µL were injected in a HPLC (Agilent 1100) equipped with a quaternary pump, degasser, and DAD. The separation was realized at 25°C with a Kinetex C18 column (150 × 4.2 mm DI × mm × 5 μm). The mobile phase A was an HFor aqueous buffer (0.10% v/v, pH 4.5) with 10% of MeOH, and the mobile phase B was MeOH solution of HFor (0.10% v/v). A binary gradient at 1.00 mL min−1 was applied as follows: started at 20% B and ramped to 50% B at 10 min, isocratic for 3 min, and then back to the initial conditions within 2 min. A post time of 8 min was set between sample runs for the column re-equilibrium. The analytical and reference wavelengths were 278 nm and 360 nm, respectively.
The peak area after the LD was used to evaluate the enrichment effect. The preconcentration factors were calculated as a ratio of the peak area of each antibiotic after the LD step between the peak areas of the antibiotic in a standard solution of the antibiotics at 1 mg L−1. Based on the sample volume for the extraction (10 mL) and the final volume of LD (500 µL), the expected preconcentration factor was 20.
Results and discussion
CN and PH have a value of log Kow lower than 2.5. Specifically, CN is polar, and it has an unshared electron pair in the nitrile nitrogen which may interact through hydrogen bonds with the amino or –OH groups present in antibiotics.14 Also, CN and PH have pi-electrons which are polarizable.15 Thus, CN and PH in the coating may interact by dispersive interactions with the aromatic rings present in the antibiotic molecules. Therefore, it was expected that the combination of CN in different molar ratios helped to improve the adsorption due to dipole–dipole and dispersion interactions between the coating and the antibiotics.
Sol–gel synthesis of coatings
Different molar ratios of PDMS:(CN:PH) were done in preliminary experiments. Experiments with an increment of CN yielded brittle and rigid materials. The molar ratio PDMS:(CN:PH) of 1:3 with CN:PH 2:1, 1:1, 1:2 yielded a clear homogeneous-derived hybrid sol. This ensured the reaction of organic and inorganic components during sol–gel process. Furthermore, homogeneity was crucial prior the coating process to produce homogeneous and stable coatings.
Phase separation of components and precipitation are common problems in sol–gel process using alkoxysilanes.16 Thus, the use of TFA as catalyst mixed with 5% of water avoided the formation of aqueous phase. As a result, the stir bars had a translucid coating regardless the molar ratio of CN and PH. Its volume and thickness average were 61.50±0.88 µL and 1.11±0.04 mm (n = 30), respectively.
Characterization of hybrid coatings
Infrared analysis
Figure 1 shows the bands at 1200–950 cm−1 that correspond to Si–O–Si stretching, bending, and rocking modes. According to the literature,17,18,19 the band at 1090 cm−1 confirms the formation of a network structure. The band at 2980 corresponds to the stretching of C–H bonds on methyl group from PDMS. The typical band of –CN appears at 2240 cm−1.16 The presence of phenyl in the coatings is demonstrated by the aromatic ring C–H stretching signals (3080–3010 cm−1), the C=C bond stretching (1590 cm−1), and the overtones (2000–1650 cm−1) associated with monosubstitution of a phenyl aromatic ring.20 The broad signal at 3400 cm−1 is typical of residual silanols (Si–OH).21
Stability of coatings against solvents
The coated stir bars were exposed for 15 min and 30 min to polar solvents (H2O, MeOH, and ACN) under vortex agitation. The weight losses were lower than 1% as is shown in Online Resource 1. After isooctane exposition, the weight losses were below 4%. In contrast, acetone and EtOAc produced weight losses from 11% to 48% after 15 min and 30 min of vortex shaking.
The application of ultrasound provoked relatively higher weight losses than vortex shaking (see Online Resource 2). The materials had more stability against isooctane and polar solvents, but the weight losses reached 11%. In contrast, acetone and EtOAc produced weight losses from 47% to 51% regardless of the time of ultrasound application.
In brief, the three coatings can be applied in SBSE using MeOH or ACN as desorption liquids. Those solvents or their aqueous mixtures can be used for LD with vortex assistance up to 30 min. Aqueous mixtures of MeOH or ACN could be used with ultrasonic assistance to minimize the coating damage.
Stability against pH conditions
In general, the polymeric coatings were stable in the pH range 1–12 (see Online Resource 3). This occurs even with aqueous media prepared with oxidant acids such as HNO3. The greatest weight loss was 0.28 wt. % at pH 12 after 48 h for CN:PH 1:2. This implies that the three coatings can be useful when pH values need to be set to extract ionizable analytes.
Determination of water contact angle
The contact angle characterizes the hydrophobicity of surfaces. It is related to the interfacial tensions of the contacting phases involved (air, water, and substrate) through the Young equation. The contact angle of PDMS deposited on a glass substrate varies from 50° to 60°, but after the curing process, the values of contact angle of the PDMS become higher than 100° because of the hydrophobic nature of the polymer.22 The average value of water contact angle (n=10) for coatings CN:PH 2:1, 1:1, and 1:2 was 77.44±1.16°, 80.76±1.78°, and 85.09±1.94°, respectively. Burgos-Tan et al.9 reported a lower value of contact angle (75.04° ± 1.78°) for a PDMS-CN hybrid coating. Then, it was evident that the hydrophobicity of the coating surface decreases as the CN content increases due to polar cyano moiety and residual –Si–OH.
Thermogravimetric analysis
The thermograms of the three coatings showed similar profiles (Fig. 2). The initial weight loss is related to PDMS chain degradation. It is observed than when the amount of PH increases, the percentage of residual mass is higher. This is attributed to the presence of phenyl groups in the hybrid polymer. Near 700°C, the residual mass of the coating is 22, 36 and 50 wt. %. The three hybrid coatings were thermally stable up to 200°C. Wan Ibrahim et al.16 reported a similar thermal stability for PDMS-CN. However, there are reports of similar coatings with relatively high thermal stability such as PDMS-CN (approx. 450°C)9 and PDMS-PH (up to 420°C).10
SBSE tests with coated stir bars
In this work, SBSE tests involve the extraction of the antibiotics with the coated stir bars followed by its desorption with a relatively small volume of solvent or aqueous solution.
Preliminary extractions were run with stir bars coated with PDMS:(CN:PH) (2:1, 1:1, and 1:2). Stir bars coated with PDMS:CN9 and PDMS:PH10 were included for comparison.
The first stage of SBSE was run with 10 mL of spiked water at pH 4.5 to get the neutral form of the antibiotics (1 mg L−1). After the extraction (250 rpm, 120 min), the residual antibiotics in water were analyzed by HPLC. The analytical signal of the antibiotics decreased in the water after the extraction with stir bars coated with CN:PH 2:1 and PDMS:CN. This suggests that the cyanopropyl moiety in the coating promoted polar interactions with the analytes. This result agrees with the increment of the polarity of the coating’s surface (contact angle tests).
The liquid desorption step (LD) was done with 500 µL of MeOH shaking with vortex for 20 min. The MeOH was diluted to 1 mL with buffer (pH 4.5) and analyzed by HPLC. The peaks in the chromatogram revealed the desorption of the antibiotics previously extracted with the coated stir bar. However, the enrichment effect was not observed.
The preconcentration capacity of the coating was assessed under different LD conditions using the coating CN:PH 2:1 with the lowest contact angle (77.44±1.16°). The extractions were done with 10 mL of spiked water (pH 4.5), 250 rpm, and 120 min. Then, 500 mL of water (pH 4.5), MeOH, or its mixtures (20%, 40%, 60%, and 80%, v/v) were tested to get a preconcentration factor of 20. The desorption was assisted with vortex or ultrasonic bath (UB) during 20 min or 40 min.
The results revealed that RSArea was statistically different among LD desorption time (F = 130.11, p < 0.0001), MeOH amount (F = 49.66, p < 0.0001), and the interaction MeOH amount-LD assistance (F = 4.26, p < 0.0039). Forty minutes of LD increased the RSArea. This result suggests that antibiotics diffused slowly from the coating to the desorption liquid. In general, high content of MeOH caused relatively high RSArea. However, there was no statistical difference between the RSArea obtained with water (pH 4.5) and MeOH mixtures in combination vortex and UB. Only pure MeOH assisted by UB yielded an RSArea statistically higher than vortex. Thus, the antibiotics were desorbed with 500 µL of 40% MeOH (v/v) during 40 min with the assistance of vortex.
After we explore and establish the desorption conditions, the effect of extraction time, stirring speed, and percentage of NaCl and ACN on the total area of chromatographic peaks were evaluated at room temperature (25°C). The experimental design (Table 2) showed that 120 min of extraction time, 750 rpm, and 15% NaCl without ACN increased the total area of the antibiotics. In particular, the area of the relatively nonpolar TMP and CRF improved with 20% ACN. However, the preconcentration factor was lower than one for all compounds despite the cyanopropyl groups increasing the wettability of the coating. A chromatogram after extraction and desorption of the analytes with stir bars coated with CN:PH 2:1 is displayed in Fig. 3b.
The aim of the coated stir bars for SBSE is to extract and preconcentrate the analytes from aqueous samples. The result of experiments with the coating CN:PH 2:1 under different conditions suggests that the thickness and hydrophilicity of the coating could influence the extraction and enrichment effect. Both impeded the diffusion of the antibiotics into the coating. Therefore, the organic moieties inside the coating were not accessible to interact with the antibiotics.
The impact of diffusion on the interaction coating-antibiotic was evaluated with spiked isooctane (1 mg L−1 of each antibiotic). To prevent coating damage, the extraction step was done for 120 min and 250 rpm followed by an LD with 500 µL of MeOH 40% v/v assisted with vortex.
The antibiotics were preconcentrated from twofold to fivefold in SBSE extracts (Table 3 and Fig. 4). This result relates to the swelling of the coating due to the diffusion of isooctane into it. The diffusion promotes the interaction of the antibiotics with the moiety –(CH2)2C≡N: in the chemical structure of the coatings. Therefore, the higher preconcentration factors were reached with coatings CN, CN:PH 2:1, 1:1, and 1:2.
The results showed that the root square of the sum of antibiotic peaks area (SRArea) differed among materials (F=13.02, p<0.0001) and antibiotics (F=129.03, p<0.0001). In other words, the coating composition is important for the interaction with the antibiotics. Thus, regardless of the antibiotic, the coatings with CN had SRArea significantly higher than PH (Fig. 5a).
With respect to the antibiotics, the response (SRArea) of STZ and SMX was significantly higher than CRF and TMP independently of the coating composition (Fig. 5b). STZ and SMX have a higher polarity than the other two antibiotics. Therefore, the interactions of STZ and SMX with isooctane are not favorable and both molecules interact with chemical groups in the coating such as –(CH2)2C≡N:, –Si-O-Si–, and –Si-OH. This confirms the change of hydrophobicity of the coatings due to –(CH2)2C≡N: moiety in the contact angle test.
Conclusions
Three hybrid coatings based on hydroxy-terminated poly(dimethylsiloxane) (PDMS-OH), 3-cyanopropyltriethoxysilane (CN), and phenyltriethoxysilane (PH) were synthesized by acid catalyzed sol–gel reactions. The coatings based on PDMS had three ratios of CN:PH, 2:1, 1:1, and 1:2. The coatings were thermally stable up to 200°C and showed resistance against different pH and solvents (MeOH, ACN, water, and isooctane). In general, the increase in CN content was associated with relatively low superficial water contact angles.
The capacity of the coating for its potential application in SBSE was evaluated with glass stir bars covered with the developed materials. In spiked water, the coating CN:PH 2:1 behaved like PDMS-CN. In the extraction step of SBSE, the increase in the extraction time, stirring speed, NaCl, and ACN induced a slight increment of the antibiotics total area. The increment of the liquid desorption time and the amount of MeOH contributed to the release of the antibiotics from the coating but without a preconcentration effect.
The diffusion of isooctane into the coatings (CN:PH 2:1, 1:1, 1:2) promoted the extraction and preconcentration of sulfonamides, TMP, and CRF.
References
Lorenzo-Parodi, N, Kaziur, W, Stojanović, N, Jochmann, MA, Schmidt, TC, ‘‘Solventless Microextraction Techniques for Water Analysis.’’ TrAC Trends Anal. Chem., 113 321–331 (2019)
Medina-Dzul, K, Carrera-Figueiras, C, Pérez-Padilla, Y, Vilchis-Nestor, RA, López-Téllez, G, Sánchez, M, Muñoz-Rodríguez, D, ‘‘SiO2 Polyvinylimidazole Hybrid Polymer as a Sorbent for Extraction by Matrix Solid-Phase Dispersion (MSPD): Synthesis, Characterization, and Evaluation.’’ J. Polym. Res., 22 1–9 (2015)
David, F, Ochiai, N, Sandra, P, ‘‘Two Decades of Stir Bar Sorptive Extraction: A Retrospective and Future Outlook.’’ TrAC Trends Anal. Chem., 112 102–111 (2019)
Gilart, N, Marcé, RM, Borrull, F, Fontanals, N, ‘‘New Coatings for Stir-Bar Sorptive Extraction of Polar Emerging Organic Contaminants.’’ TrAC Trends Anal. Chem., 54 11–23 (2014)
Kloskowski, A, Pilarczyk, M, Chrzanowski, W, Namieśnik, J, ‘‘Sol-Gel Technique—A Versatile Tool for Adsorbent Preparation.’’ Crit. Rev. Anal. Chem., 40 172–186 (2010)
Collinson, MM, ‘‘Recent Trends in Analytical Applications of Organically Modified Silicate Materials.’’ TrAC Trends Anal. Chem., 21 31–39 (2002)
Baran, W, Adamek, E, Ziemiańska, J, Sobczak, A, ‘‘Effects of the Presence of Sulfonamides in the Environment and Their Influence on Human Health.’’ J. Hazard. Mater., 196 1–15 (2011)
Dmitrienko, SG, Kochuk, EV, Apyari, VV, Tolmacheva, VV, Zolotov, YA, ‘‘Recent Advances in Sample Preparation Techniques and Methods of Sulfonamides Detection—A Review.’’ Anal. Chim. Acta, 850 6–25 (2014)
Burgos-Tan, MJ, Pérez-Padilla, Y, Avila-Ortega, A, Barrón-Zambrano, JA, Vilchis-Néstor, AR, Carrera-Figueiras, C, Muñoz-Rodríguez, D, ‘‘Preparation, Characterization and Evaluation of a Hybrid Polymeric Coating with Sorbent Properties.’’ Chem. Pap., 71 1205–1215 (2017)
Pérez-Padilla, Y, Cetina, SAM, Ávila-Ortega, A, Barrón-Zambrano, JA, Vilchis-Néstor, AR, Carrera-Figueiras, C, Muñoz-Rodríguez, D, Evaluation of ‘‘Polydimethylsiloxane-Phenylsiloxane as a Coating for Stir Bar Sorptive Extraction.’’ J. Mexican Chem. Soc., 62 348–357 (2018)
David, F, Sandra, P, ‘‘Stir Bar Sorptive Extraction for Trace Analysis.’’ J. Chromatogr. A, 1142 54–69 (2007)
Montgomery, DC, Design and Analysis of Experiments, 8th edn. Wiley, New Jersey. (2013)
STATGRAPHICS Centurion XVIII, Statgraphics Technologies Inc. 2018
Kulkarni, S, Fang, L, Alhooshani, K, Malik, A, ‘‘Sol–Gel Immobilized Cyano-Polydimethylsiloxane Coating for Capillary Microextraction of Aqueous Trace Analytes Ranging from Polycyclic Aromatic Hydrocarbons to Free Fatty Acids.’’ J. Chromatogr. A, 1124 205–216 (2006)
Gura, S, Tarifa, A, Mulloor, J, Torres, MN, Almirall, JR, ‘‘Capillary Microextraction of Volatiles Device for Enhanced BTEX Vapors Sampling Based on a Phenyl Modified PDMS Sol–Gel Adsorption Phase.’’ Anal. Chim. Acta, 1014 27–40 (2018)
Wan Ibrahim, WA, Abdul Keyon, AS, Prastomo, N, Matsuda, A, ‘‘Synthesis and Characterization of Polydimethylsiloxane-Cyanopropyltriethoxysilane-Derived Hybrid Coating for Stir Bar Sorptive Extraction.’’ J. Sol-Gel Sci. Technol., 59 128–134 (2011)
Cai, D, Neyer, A, Kuckuk, R, Heise, HM, ‘‘Raman, Mid-Infrared, Near-Infrared and Ultraviolet-Visible Spectroscopy of PDMS Silicone Rubber for Characterization of Polymer Optical Waveguide Materials.’’ J. Mol. Struct., 976 274–281 (2010)
Bange, JP, Patil, LS, Gautam, DK, ‘‘Growth and Characterization of SiO2 Films Deposited by Flame Hydrolysis Deposition System for Photonic Device Application.’’ Prog. Electromagn. Res. M, 3 165–175 (2008)
Rao, AV, Gurav, AB, Latthe, SS, Vhatkar, RS, Imai, H, Kappenstein, C, Wagh, PB, Gupta, SC, ‘‘Water Repellent Porous Silica Films by Sol-Gel Dip Coating Method.’’ J. Colloid Interface Sci., 352 30–35 (2010)
Li, Y-S, Wang, Y, Ceesay, S, ‘‘Vibrational Spectra of Phenyltriethoxysilane Phenyltrimethoxysilane and Their Sol-Gels.’’ Spectrochim. Acta Part A Mol. Biomol. Spectrosc., 71 1819–1824 (2009)
Wan Ibrahim, WA, Wan Ismail, WN, Abdul Keyon, AS, Sanagi, MM, ‘‘Preparation and Characterization of a New Sol-Gel Hybrid Based Tetraethoxysilane-Polydimethylsiloxane as a Stir Bar Extraction Sorbent Materials.’’ J. Sol-Gel Sci. Technol., 58 602–611 (2011)
Bouteau, M, Cantin, S, Fichet, O, Perrot, F, ‘‘Teyssié D Contribution Toward Comprehension of Contact Angle Values on Single Polydimethylsiloxane and Poly(Ethylene Oxide) Polymer Networks.’’ Langmuir, 26 17427–17434 (2010)
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología, CONACyT (CB-167800). We thank Dr. José Antonio Azamar Barrios from Centro de Investigación y de Estudios Avanzados of IPN for letting us run the FTIR spectra of the coatings in his laboratory. In addition, we thank M.Sc. Maria Isabel Loría Bastarrachea from Centro de Investigación Científica de Yucatán for helping us with the thermogravimetric analysis.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. MAÁ-M was involved in investigation, visualization, and writing—original draft. YP-P helped in supervision, methodology, and validation. SM-P contributed to formal analysis and visualization. AÁ-O was involved in visualization. DM-R helped in conceptualization, supervision, methodology, and funding acquisition. All authors commented on previous versions of the manuscript, read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the Supplementary Information.
Rights and permissions
About this article
Cite this article
Ávila-Martínez, M.A., Pérez-Padilla, Y., Medina-Peralta, S. et al. Preparation and characterization of polydimethylsiloxane containing cyano/phenyl groups for potential use in sorptive extraction. J Coat Technol Res 18, 1087–1094 (2021). https://doi.org/10.1007/s11998-021-00462-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-021-00462-4