Abstract
A new tetraethoxysilane-polydimethylsiloxane (TEOS-PDMS) for use as sorbent of stir bar sorptive extraction (SBSE) towards two selected organophosphorus pesticides (OPPs) namely chlorpyrifos and malathion was successfully synthesized through sol–gel technology. Four different molar ratios of TEOS:PDMS (1:1, 2:1, 3:1 and 4:1) sol solutions were prepared and dipped coated onto the surface of a glass-encased stir bar. Extraction efficiency of the prepared coatings towards the two selected OPPs were compared. A number of factors have been found to greatly affect the characteristics and properties of a particular sol–gel coating. Hence, in this study, several sol–gel coating conditions have been optimized using the optimized molar ratio 3:1 TEOS:PDMS to obtain the best coating as the stationary phase for SBSE. The raw OH-TPDMS and TEOS were characterized using Fourier Transform Infrared Spectroscopy (FT-IR) and compared with spectra of the four different molar ratios of TEOS:PDMS. The FT-IR spectrum of TEOS:PDMS showed the co-polymerization between PDMS and hydrolyzed TEOS molecules demonstrating the formation of the hybrid network in the sol–gel hybrid material. Surface morphology of hybrid sol–gel TEOS-PDMS with optimized molar ratio of 3:1 TEOS:PDMS were examined using FE-SEM. The surface of the sol–gel coating seems to be rough and homogeneous. The more rough structure formed by the 3:1 molar ratio TEOS:PDMS provides enhanced surface area which in turn improved sample capacity or adsorption process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
To the best of our knowledge, polydimethylsiloxane (PDMS) is the only commercially available coating material for used in stir bar sportive extraction (SBSE) method [1–5]. They have been used to extract many types of analytes from different sample matrix. The type of sorbent, its structure and its interactions with the solute are clearly related to the efficiency of the extraction process. However, PDMS is a non-polar phase. It fails in the extraction of polar analytes. There are a few recent studies that modified the coating of the stir bar for extraction purpose to increase the versatility of SBSE technique. Besides using commercial PDMS stir bar, Guan et al. [3] prepared a novel stationary phase (poly(phthalizine ether sulfone ketone)) for SBSE of organochlorine pesticides (OCPs) and organophosphorus pesticides (OPPs) by immersion precipitation technique. Preparation of PDMS/β-CD/DVB coated “dumbbell-shaped” stir bar has been done by Yu and Hu [6] for the analysis of polycyclic aromatic hydrocarbons and polycyclic aromatic sulphur heterocycles compounds. The “dumbbell-shaped” stir bar was proposed to prevent the friction loss of coating during the stirring process and thus prolonged the lifetime of stir bars [6].
The development of polyurethane foams as polymeric phase of SBSE has been introduced by Neng et al. [7] to extract atrazine, 2,3,4,5-tetrachlorophenol and fluorene and by Portugal et al. [8] to extract seven triazine herbicides. Stir bar coating based on poly(vinylpyrrolidone and divinylbenzene) (poly(VPL-DVB)) monolithic material (SBSEVD) was designed and prepared to extract non-polar and polar organic compound and heavy metal ions [9]. Melo et al. [10] has developed polymeric coating consisting of a dual-phase, PDMS and PPY (polypyrrole) for SBSE of antidepressant from plasma sample. A hydrophilic poly(vinylimidazole-divinylbenzene) (VIDB) monolithic material was prepared and acted as SBSE coating for determination of strongly polar aromatic amines (PAAs) by Huang et al. [11]. However, there is no study on the use of tetraethoxysilane-polydimethylsiloxane (TEOS-PDMS) as SBSE sorbent to extract non-polar and polar organophosphorus pesticides (OPPs). TEOS-PDMS has been used by Pliego and Schiavon [12] as a supporting polymeric membrane to overcome a problem in catalysis, mainly related to recovery of the catalyst. In the TEOS-PDMS network, tetraethoxysilane (TEOS) was used to generate a silica cluster, which crosslinked the PDMS linear chains, generating a three-dimensional structure. This heterogenization process enables the exclusive recovery of the hydrophobic oxidation product in the organic phase [12].
In order to prepare the coating on the surface of stir bar, sol–gel technique is commonly used recently instead of direct usage or simple physical deposition [13–15]. In general, the sol–gel process, involves the evolution of inorganic networks through the formation of a colloidal suspension (sol) and gelation of the sol to form a network in a continuous liquid phase (gel). TEOS is a common precursor used in sol gel process as it is non-toxic and the cost is cheaper than other precursors such as tetramethoxysilane (TMOS). However, when using TEOS, cracking of the coating occurs and cause the peak area of analytes extracted to decrease. Therefore, this study focused on the optimization of parameters affecting production of this new sol–gel hybrid in order to reduce the cracking occurred on the coating and enhance the extraction efficiency of two selected OPPs as test analytes namely, chlorpyrifos (non-polar) and malathion (mid-polar). A number of parameters affecting the coating process of the sol–gel TEOS-PDMS on the surface of glass-encased stir bar namely etching solution (sodium hydroxide) concentrations, etching times, coating times, dipping times, heating temperatures and drying times were optimized using the classical one variable at a time (OVAT) methodology [16] for the extraction of the two selected OPPs. The TEOS:PDMS sol–gel material was characterized by using Fourier transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FESEM).
2 Experimental
2.1 Materials
Methanol (HPLC reagent grade) as the solvent was obtained from Merck (Darmstadt, Germany). Sodium hydroxide (NaOH) and hydrochloric acid (HCl) were purchased from Fluka Chemika (Buchs, Switzerland). The OPPs used as test analytes are malathion (mid-polar) and chlorpyrifos (non-polar). Both OPPs were purchased from Fluka Sigma–Aldrich (Missouri, USA). A 1,000 ppm pesticide stock solutions were prepared using methanol and diluted as needed. The solutions were then stored under 4 °C in the refrigerator when they were not in use. The concentration of malathion and chlorpyrifos used in the optimization of the coating process is 1 ppm (spiked into distilled water). The structure of malathion and chlorpyrifos are given in Fig. 1. The most likely force of interaction between the OPPs and the sol–gel coating is through van Der Waals force on adsorption sites of the sol–gel material.
Chemicals used for sol solution namely hydroxy-terminated polydimethylsiloxane (OH-TPDMS), tetraethoxysilane (TEOS) and trifluoroacetic acid (TFA 95%) were all purchased from Fluka Sigma–Aldrich (USA) while poly(methylhydrosiloxane) (PMHS) was purchased from Sigma–Aldrich (USA). The glass used for stir bar coating was of borosilicate type and magnetic bar (10 mm × 2 mm) were purchased from Cole-Palmer (Illinois, USA).
2.2 Preparation of stir bar and sol solution
The dimension of the stir bar is 10 mm × 2 mm × 0.4 mm i.d.. It was jacketed with a home-made glass tube with a dimension of 20 mm × 4 mm × 0.5 mm i.d.. The glass-encased stir bar was initially cleaned with 5 mL water, followed by pretreatment with 1.5 M NaOH solution for 90 min. Next, it was placed in 0.1 M HCl solution for 30 min before rinsed with 5 mL distilled water. Then, it was dried at 100 °C for 2 h in the oven. The glass encased stir bar was immersed vertically into the sol solution for 90 min. Then, it was dried for 7 h before been cleaned using ethanol in ultrasonic bath for 5 min before used for extraction.
The sol–gel solution was prepared in a 2 mL polyethylene bullet-shape tube; 80 μL TEOS, 155 μL OH-PDMS, 25 μL PMHS and 200 μL 95% TFA were mixed and vortex for 2 min before undergo centrifugation at 12,000 rpm for 5 min. The mixture was allowed to react at room temperature for 30 min. The preparation steps were repeated using 160, 240 and 320 μL TEOS. A clear solution was obtained for all the sol–gel solution and used in dipped coating process of the glass-encased stir bar. The parameters studied in the optimization of the coating process are given in Table 1.
2.3 Stir bar sorptive extraction process and liquid chromatography conditions
In SBSE, organic analytes are enriched from aqueous samples by sorption onto a thick film of sorbent such as polydimethylsiloxane (PDMS) coated onto a glass-enveloped magnet. Solute extraction from the aqueous phase into the extraction phase is controlled by the partitioning coefficient of the solute between the silicone phase and the aqueous phase. Recent studies have correlated this partition coefficient with the octanol–water distribution coefficient (Ko/w). The amount of analyte extracted is proportional to the volume of the extraction phase.
For extraction process, the different molar ratios TEOS-PDMS coated stir bar was added into separate beakers each containing 2 mL sample spiked with 1 ppm of each OPPs, stirred at 450 rpm for 10 min at room temperature before it was removed carefully and rinsed with a small amount of deionized water (Milipore, France). Then, it was patted dry with a clean tissue and placed in a Hettich Zentrifugen ultrasonic assisted system (Germany) using 3 mL ethyl acetate as desorption solvent before HPLC–UV analysis. The amount of OPPS extracted is based on the detector response observed from the chromatogram and the best TEOS:PDMS compositions were determined for the two OPPs extracted and analyzed using an Agilent 1100 series high performance liquid chromatography (Agilent Technologies, USA). For coating process optimization, the 3:1 molar ratio TEOS:PDMS was used. Extraction condition used were: 360 rpm stirring rate, 5 min extraction time, 5 min desorption time, desorption using 3 mL ethyl acetate and no addition of NaCl salt.
The HPLC system consists of a vacuum degasser, a quaternary pump, manual injector with a 20 μL loop and a variable wavelength detector. The separation was performed on a reverse phase C18 HPLC column (5 μm, 4.6 × 150 mm Eclipse XDB-C18, Agilent Technologies, USA). The mobile phase used was methanol and water (70:30, v/v) at a flow rate of 1.0 mL min−1 with UV detection at 210 nm.
2.4 Characterization of sol–gel coatings
Field Emission Scanning Electron Microscopy (FE-SEM) model JSM-6710F from Japan was used to evaluate the morphology of the sol–gel samples, while it was characterized for functional groups using a Perkin Elmer 1600 Series Fourier Transformed Infrared Spectroscopy (FT-IR) from Massachusetts, USA.
3 Results and discussion
3.1 Mechanism of sol–gel coating process
TEOS-PDMS sol–gel involved the processes of hydrolysis and polycondensation of a sol–gel precursor (TEOS) and polycondensation of its hydrolysis products between themselves and with other sol–gel active organic intermediates (OH-TPDMS). The preparation of sol solution through sol–gel method [17] can be summarized as follows:
-
1.
Catalytic hydrolysis of TEOS by TFA
-
2.
Polycondensation reaction of hydrolyzed TEOS
-
3.
Condensation of the hydrolyzed TEOS with OH-TPDMS
-
4.
Chemical bonding of TEOS-PDMS on the glass surface
-
5.
Deactivation of bonded TEOS-PDMS with PMHS
The initial reaction was the catalytic hydrolysis of TEOS as shown in Fig. 2. TFA was added to decrease the water reaction content in the sol which allowed the two stationary phases to bond together easily. Polycondensation reaction of hydrolyzed TEOS occurred after the hydrolysis reaction to produce a longer polymer network as shown in Fig. 3. This is followed by condensation of OH-TPDMS to the evolving sol–gel network (Fig. 4). OH-TPDMS acted as coating materials. Being hydroxyl terminated, this polymer is sol–gel active. Selection of this polymer aimed at chemically binding the PDMS stationary phase to the growing silica network. The last step is the bonding process between sol–gel network and the silanol group of the glass surface. The silanol groups on the glass surface take part in the condensation reactions and it provides chemical anchorage to the polymeric network. Fig. 5 represents the bonding of TEOS-PDMS sol–gel in the vicinity of the glass surface. Fig. 6 shows the deactivation of sol-gel TEOS-PDMS bonded surface with PMHS, the deactivation agent.
3.2 TEOS-PDMS sol–gel
Investigation on how the TEOS:PDMS molar ratio affects the characteristic of sol–gel coating as the SBSE sorbent was carried out by using four different molar ratios of TEOS:PDMS (1:1, 2:1, 3:1 and 4:1). The molar ratios of the other sol ingredients (polymethylhydrosiloxane, PMHS and 95% trifluoroacetic acid, TFA as acid catalyst) were kept constant at 0.05 and 2.5 molar ratio respectively. PMHS is used as a deactivation agent. The sol–gel solution of molar ratio 3:1 was chosen as the best compositions for coating and to be used in further analysis as it exhibit the highest ability to extract the selected OPPs (Fig. 7). Introduction of more TEOS amount lengthened the silica network in the sol–gel making it more hydrophobic in nature. A longer time was taken for gelation to occur as the concentration of TEOS added was increased (60 min for 1:1, 75 min for 2:1, 180 min for 3:1 and 275 min for 4:1 TEOS:PDMS). Gelation time is the duration after the preparation of sol in which it seizes to move even when the test tube is tilted or inverted. The overall sol–gel process involved catalytic hydrolysis of sol–gel precursor (TEOS) by TFA, polycondensation reaction of hydrolyzed TEOS (SiOH4), condensation of the hydrolyzed TEOS with OH-TPDMS, and chemical bonding of TEOS-PDMS on the glass surface followed by a deactivation with PMHS.
Effect of different molar ratios of TEOS:PDMS used in the extraction of the selected OPPs. Extraction conditions: 360 rpm stirring rate, 5 min extraction time, 5 min desorption time, desorption using 3 mL ethyl acetate and no addition of NaCl salt. Sol–gel coating conditions: 30 min etching time, 1 h coating time, 100 °C heating temperature, 1× dipping and 7 h drying time
3.3 Optimization of sol–gel coating conditions
In order to obtain stronger and more porous coating on supporting material (glass surface), effect of etching time, etching solution (NaOH) concentration, coating time, drying time and heating temperature were investigated using the optimized 3:1 TEOS:PDMS molar ratio. These effects were also optimized using the classical one variable at a time (OVAT) methodology [16, 18].
3.3.1 Etching solution concentration
The influence of etching solution concentration was evaluated using three different concentrations of sodium hydroxide (NaOH) from 1 to 2 M. Sol–gel coating condition was fixed at 30 min etching time, 1 h coating time, 100 °C heating temperature, 1× dipping and 7 h drying time. Etching solution concentration effect is the phenomenon where the alkaline solution, sodium hydroxide (or potassium hydroxide) lightly etches the glass surface. Its etching of the glass ensures the removal of surface residue, resulting in a clean surface [19]. As shown in Fig. 8, it was found that the detector response for both OPPs were highest at 1.5 M concentration of NaOH. According to Aegerter and Mennig [19], this optimum concentration of NaOH is believed to produce stronger and more porous coating on glass surface and also to expose the maximum number of silanol groups on the surface of the glass.
Effect of etching solution concentrations (NaOH) on the extraction efficiency of OPPs using optimized 3:1 molar ratio of TEOS-PDMS. Extraction conditions: 360 rpm stirring rate, 5 min extraction time, 5 min desorption time, desorption using 3 mL ethyl acetate and no addition of NaCl salt. Sol–gel coating conditions: 30 min etching time, 1 h coating time, 100 °C heating temperature, 1× dipping and 7 h drying time
3.3.2 Etching time
Etching time is important to determine the number of exposed silanol groups on glass. Sol–gel coating conditions were fixed at 1.5 M NaOH concentration, 1 h coating time, 100 °C heating temperature, 1 time dipping and 7 h drying time. Etching of the glass ensures the removal of surface residue, resulting in a clean surface [19]. Etching time may significantly increase the physical roughness of glass surfaces [19]. The etching time effect on extraction efficiency was evaluated at three different levels from 30 to 90 min at 30 min interval. From Fig. 9, 60 min etching time produces the highest peak area of both extracted OPPs. Therefore, it was chosen in the sol–gel coating process to increase the physical roughness of glass surfaces.
Effect of etching times on the extraction efficiency of OPPS using optimized 3:1 molar ratio TEOS-PDMS. Extraction conditions: 360 rpm stirring rate, 5 min extraction time, 5 min desorption time, desorption using 3 mL ethyl acetate and no addition of NaCl salt. Sol–gel coating conditions: 1.5 M NaOH concentration, 1 h coating time, 100 °C heating temperature, 1× dipping and 7 h drying time
3.3.3 Coating time
Three different coating times were evaluated: 30, 60 and 120 min to show the effect of coating time on the extraction efficiency of the TEOS-PDMS sol–gel coating towards the two OPPs (Fig. 10). Sol–gel coating condition was fixed at 1.5 M NaOH concentration, 1 h etching time, 100 °C drying temperature, 1 time dipping and 7 h drying time. The cleaning procedures have two separate categories, the first dealing with particle removal, and the second with exposing the active silanol sites at the glass surface. The glass should be coated with a non-wetting layer; this exposure (coating) may need to be longer for the solution to attack the glass under the non-wetting layer. From Fig. 10, 1 h coating time was selected as the optimum coating time for the sol–gel TEOS-PDMS coated stir bar of both analytes. Inadequate wetting ability of glass substrate (insufficient hydroxyl group) for deposition of sol liquid coating during dip coating process will cause the coating material (TEOS-PDMS) not to be chemically bonded to the surface of the glass [19].
Effect of coating time on the extraction efficiency of OPPs using optimized 3:1 molar ratio TEOS:PDMS. Extraction conditions: 360 rpm stirring rate, 5 min extraction time, 5 min desorption time, desorption using 3 mL ethyl acetate and no addition of NaCl salt. Sol–gel coating conditions: 1.5 M NaOH concentration, 1 h etching time 100 °C heating temperature, 1× dipping and 7 h drying time
3.3.4 Heating temperature
The effect of heating temperature was studied to increase the extraction efficiency of sol–gel hybrid TEOS-PDMS as coating phase for SBSE of OPPs and also to minimize the cracking problem occurred in previous study. All extractions were analyzed using the same HPLC–UV conditions. The influence of heating temperature was evaluated using three different temperatures from 50 to 100 °C. The temperature ramp was also conducted because according to previous study, it can avoid the degradation of organic groups bound to the silica reticulate [20]. Sol–gel coating condition: 1.5 M NaOH concentration, 1 h etching time, 1 h coating time, 1 time dipping and 7 h drying time.
An increase in heating temperature leads to an increase of extraction efficiency of selected OPPs. However, at high temperature, analyte concentration decreases and the sol–gel hybrid stationary phase (TEOS-PDMS) begin to lose its ability to adsorb analytes. This happened may be because the sol–gel hybrid stationary phase, TEOS-PDMS cannot withstand high temperature, thus cracking occurred. The cracking problem leads to low extraction efficiency of OPPs. As shown in Fig. 11, it was found that the peak area for both OPPs were highest at heating temperature of 80 °C.
Effect of heating temperature on the extraction efficiencies of OPPs using optimized 3:1 molar ratio TEOS:PDMS. Extraction conditions: 450 rpm stirring rate, 10 min extraction time, 10 min desorption time, desorption using 3 mL ethyl acetate and no addition of NaCl salt. Sol–gel coating conditions: 1.5 M NaOH concentration, 1 h etching time, 1 h coating time, 1× dipping and 7 h drying time
3.3.5 Dipping time
Dipping time is different with coating time. Dipping time (number of dipping) is the number of times the glass jacketed stir bar was dipped (manually) in the sol solution, while coating time is how long the glass jacketed stir bar was dipped in the sol solution. Three different dipping times were evaluated: 1×, 2×, and 3×. Sol–gel coating conditions was fixed at 1.5 M NaOH concentration, 1 h etching time, 1 h coating time, 80 °C heating temperature and 7 h drying time. The effect of number of dipping times on the extraction efficiency of OPPS extracted by sol–gel hybrid TEOS-PDMS sorbent (measured in terms of area extracted) is displayed in Fig. 12.
Effect of number of dipping of on extraction efficiency of OPPs using optimized 3:1 molar ratio TEOS:PDMS for used as sorbent in SBSE. Extraction conditions: 450 rpm stirring rate, 10 min extraction time, 10 min desorption time, desorption using 3 mL ethyl acetate with no addition of NaCl salt. Sol–gel coating conditions: 1.5 M NaOH etching solution, 1 h etching time, 1 h coating time, 80 °C heating temperature and 7 h drying time
There was an increased of the OPPs extracted when the dipping time was increased from 1 to 2 times. However, when the number of dipping was increased further to three times, a decreased in peak areas of OPPs extracted was noted. The decrease in the extraction efficiency is probably due to the many times dipping that increase the coating thickness, causing slower equilibration time [21] and finally decrease in extraction efficiency of selected OPPs. Thus, two times dipping of glass jacketed stir bar into the sol solution was chosen as the optimum dipping number for coating as it exhibit the highest ability to extract OPPs.
3.3.6 Drying time
Drying times were evaluated as follows: 3, 4, 5, 6 and 7 h. Other sol–gel coating conditions was fixed at 1.5 M NaOH, 1 h etching time, 1 h coating time, 80 °C heating temperature and 2 times dipping. The peak areas of malathion and chlorpyrifos obtained from HPLC/UV at different drying time for sol–gel hybrid TEOS-PDMS is shown in Fig. 13. Extraction was successfully achieved for both OPPs at this drying time range.
Effect of drying times of optimized 3:1 molar ratio TEOS:PDMS on the extraction efficiency of OPPs. Extraction conditions: 450 rpm stirring rate, 10 min extraction time, 10 min desorption time, desorption using 3 mL ethyl acetate and no addition of NaCl salt. Sol–gel coating condition: 1.5 M NaOH, 1 h etching time, 1 h coating time, 80 °C heating temperature and 2× dipping
Based on this data, optimum drying time was chosen as 5 h. Although there is slightly increase in peak area of chlorpyrifos at 6 h drying time, but it is not significant. Five hours drying time was employed in all subsequent investigations. Five hours drying time was suitable for the loss of water and other volatile component present in the TEOS-PDMS sol–gel network [22]. Cracking on the TEOS-PDMS stationary phase may occur if the drying time keep increased above 5 h and caused the peak area of OPPs extracted to decrease.
3.4 Characterization
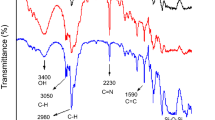
The raw OH-TPDMS and TEOS spectra were characterized using Fourier Transform Infrared Spectroscopy, FT-IR spectrum, while only the optimum 3:1 molar ratio TEOS:PDMS was characterized using FE-SEM. The OH-TPDMS and TEOS were compared with sol–gel TEOS-PDMS spectrum as shown in Fig. 14 a, b and c respectively. They were recorded in the range between 4,000 and 400 cm−1 using NaCl plate as the samples is in liquid form.
The characteristic absorption of the Si–OH at 3,368 cm−1 does not appear in the spectrum of the hybrid sample, which means that the most of the terminal Si–OH groups have been removed during the reaction process. The absorption bands at 3,390 and 1,675 cm−1 correspond to O–H bond stretching and bending, respectively, which suggests the presence of absorbed water in silica network. The band located at 908 cm−1 corresponds to the co-polymerization between PDMS and hydrolyzed TEOS molecules [23], demonstrating the formation of the hybrid network in the hybrid material. The Si–O-Si network stretching band of 1,023 cm−1 in the PDMS shifted to higher wave number in comparison with that of 1,087 cm−1 in the hybrid. This band intensity increases with increasing of TEOS content. Therefore, the hydrolyzed TEOS already condensed to form the three-dimensional Si–O-Si network.
Comparison between different TEOS:PDMS molar ratio infrared spectra also have been observed (Fig. 15). In general, from the comparison of different TEOS:PDMS molar ratio infrared spectra, the shape of the spectra looks exactly the same except for the intensity of broad peak around 3,400 cm−1 which is attributed to—OH functional group. It became more intense with the increase of TEOS:PDMS molar ratio (Fig. 15). This is attributed to the introduction of more TEOS which lengthened the silica network and provide more attachment point for the coating material, PDMS which enriched the polar—OH functional group.
The surface morphology of 3:1 molar ratio of hybrid sol–gel TEOS-PDMS was characterized using FE-SEM under different magnifications as shown in Fig. 16. The surface of the 3:1 molar ratio TEOS:PDMS sol–gel coating seems to be rough, and homogenous (uniform). The roughness increased with increasing TEOS content (micrographs not shown). The thickness of the coating is ~2.8 μm. The porous structure formed by 3:1 molar ratio TEOS:PDMS provides enhanced surface area which in turn improved sample capacity or adsorption process.
It is widely accepted that acid synthesis results in the formation of polymers that are short branched linear type, which interpenetrate easily during drying to form highly dense structures with low surface areas [24]. This is also supported by Menon et al. [25] that if non-branching existed, then interpenetration of the polymers during drying would have collapsed the xerogel microstructure (and the micropores) resulting in high densities and low surface areas. In the current study, the surface area of the TEOS:PDMS sol–gel hybrid materials were all less than 3 m2/g.
4 Conclusions
The TEOS-PDMS for use as stir bar sorptive extraction (SBSE) coating was successfully synthesized via sol–gel technique with TEOS as the precursor, PDMS as the coating material and 95% TFA as acid catalyst. The new sol–gel SBSE coating with composition of 3:1 molar ratio TEOS:PDMS showed highest extraction performance towards the two selected OPPs, chlorpyrifos and malathion. Coating process parameters affecting the extraction performance of the coating produced were optimized as follows: 1.5 M NaOH as etching solvent, 60 min etching time, 60 min coating time, 80 °C heating temperature, 2× dip coating and 5 h drying time. The thickness of the coating produced is ~2.8 μm.
The co-polymerization between PDMS and hydrolyzed TEOS molecules is demonstrated by the band located at 908 cm−1. The Si–O–Si network stretching band of 1,023 cm−1 in the PDMS increased to 1,087 cm−1 in the sol–gel TEOS-PDMS as the TEOS content increase, demonstrating the hydrolyzed TEOS already condensed to form the three-dimensional Si–O–Si network. The rougher and homogenous surface of the 3:1 TEOS:PDMS sol–gel coating as compared to the other compositions improved the sample capacity for the OPPs. More work is in progress to study other factors such as acid catalyst types, curing temperature, precursor types, water content, etc. affecting sol–gel coating process in an attempt to further improve the performance of the sorbent material for SBSE use.
References
Baltussen E, Sandra P, David F, Cramers C (1999) J Microcol Sep 11:737
Blasco C, Fernández M, Picó Y, Font G (2004) J Chromatogr A 1030:77
Guan W, Wang Y, Xu F, Guan Y (2008) J Chromatogr A 1177:28
Liu W, Wang H, Guan Y (2004) J Chromatogr A 1045:15
Richter P, Leiva C, Choque C, Giordano A, Sepúlveda B (2009) J Chromatogr A 1216:8598
Yu C, Hu B (2009) J Sep Sci. 32:147
Neng NR, Pinto ML, Pires J, Marcos PM, Nogueira JMF (2007) J Chromatogr A 1171:8
Portugal FCM, Pinto ML, Nogueira JMF (2008) Talanta 77:765
Pico Y, Fernandez M, Ruiz MJ, Font G (2007) J Biochem Biophys Methods 70:117
Melo LP, Nogueira AM, Lanças FM, Queiroz MEC (2009) Anal Chim Acta 633:57
Huang X, Qiu N, Yuan D, Lin Q (2009) J Chromatogr A 1216:4354
Pliego JR, Schiavon MA (2008) J Phys Chem C 112:14830
Luo Y-B, Ma Q, Feng Y-Q (2010) J Chromatogr A 1217:3583
Huang X, Qiu N, Yuan D (2008) J Chromatogr A 1194:134
Wan Ibrahim WA, Farhani H, Sanagi MM, Aboul-Enein HY (2010) J Chromatogr A 1217:4890
Khan BA, Farid A, Asi MR, Shah H, Badshah AK (2009) Food Chem 114:286
Kim GD, Lee DA, Moon JW, Kim JD, Park JA (1999) Appl Organomet Chem 13:361
Rodil R, Moeder MJ (2008) J Chromatogr A 1178:9
Aegerter MA, Mennig M (2004) Sol-gel Technologies for Glass Producers and Users. Kluwer Academic Publishers, USA
Biajoli AFP, Augusto F (2008) Anal Sci Chem 24:1141
Varinder K, Ashok KM, Neelam V (2006) Wiley Inter Sci 29:333
Ghosh S, Peterson DG, Coupland JN (2006) J Agric Food Chem 54:1829
Babonneau F, Thorne K, Mackenzie JD (1989) Chem Mater 1:554
Nair N, Elferink WJ, Keizer K, Verweij H (1996) J Colloid Interf Sci 178:565
Menon VC, Komarneni S, Park M, Schmucker M, Schneider H (1998) J Sol-gel Sci Tech 11:7
Acknowledgments
The authors gratefully acknowledged the grant provided by Ministry of Higher Education, Malaysia (MOHE) under the Fundamental Research Grant Scheme (FRGS) vote number 78519. W. N. Wan Ismail also would like to thank Universiti Teknologi Malaysia (UTM) for the Zamalah Scholarship received.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wan Ibrahim, W.A., Wan Ismail, W.N., Abdul Keyon, A.S. et al. Preparation and characterization of a new sol–gel hybrid based tetraethoxysilane-polydimethylsiloxane as a stir bar extraction sorbent materials. J Sol-Gel Sci Technol 58, 602–611 (2011). https://doi.org/10.1007/s10971-011-2434-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2434-7