Abstract
A novel hybrid polymer was developed and evaluated as a sorbent for the matrix solid-phase dispersion (MSPD) extraction of pesticides. The hybrid polymer was synthesized by the sol–gel method and by free radical polymerization. The chemical structure of the resulting hybrid polymer (SiO2–PVI) was confirmed by Fourier transform infrared spectroscopy (FT-IR). Thermal analyses suggest that the polymer consists of an organic/inorganic ratio of 28:72 wt/wt%. Scanning electron microscopy (SEM) and elemental analysis (EDS) revealed particle aggregates and a rough surface and suggested that the polymer is primarily composed of SiO2. The obtained pore size was appropriate for use in a solid-phase extraction support. X-ray photoelectron spectroscopy (XPS) was used to assess the surface composition of the hybrid polymer and indicated the presence of C, N, O, and Si. The material was tested for extraction of five selected organophosphorus pesticides (OPPs) in propolis using gas chromatography–mass spectrometry (GC/MS). In experiments performed in triplicate at 1.0 μg mL−1, pesticide recovery was in the range of 81–122 %. In addition, the sorbent hybrid polymer (SiO2–PVI) demonstrated good repeatability (RSD ≤ 11 %), on the same order as C18 (commercial sorbent) when tested under the same conditions. These results suggest that SiO2–PVI hybrid polymer is a good sorptive material that is comparable to the commercially utilized C18 and can be used in MSPD for the extraction of organophosphorus pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The determination of chemical species in a given sample is a subject of great interest in current research. Analysis can be performed using various analytical techniques; however, the direct determination of extremely low concentrations of analytes is often difficult as a result of limitations, which include matrix interference and insufficient sensitivity [1]. Extraction by matrix solid-phase dispersion (MSPD) is a relatively recently developed extraction technique for the clean-up and pre-concentration of samples; MSPD is suitable for both solid and semisolid samples. In MSPD, the components of the matrix sample are mechanically dispersed onto a sorbent, and the analytes are then selectively desorbed by the use of an appropriate solvent. This method includes disruption of the sample structure, fractionation, and a simple purification process. MSPD is a low-cost technique, as it reduces the required volume of organic solvents and provides high extraction yields and a considerable degree of selectivity [2–7]. The classical sorbents used for MSPD extraction are Florisil, octadecylsilyl silica (C18), octasilyl silica (C8), or alumina [3, 4]. The current sorbents based on organic functionalized silica have relatively low polar compound retention and low chemical stability in extreme pH conditions (pH < 2 and pH > 8). Some sorbents are moderately polar and have poor interaction with hydrophobic samples [8, 9]. The development, evaluation, and application of new sorbents for MSPD are still limited.
Over the last few years, researchers have explored alternative sorbents to replace traditional extraction phases to improve the performance of the extraction of analytes. According to current literature [10–13], these materials should have a high surface area; thus, when new materials are developed, it is important to characterize their chemical structure and morphology (i.e., specific surface area, distribution of pore diameter, particle size, etc.).
In recent years, there has been increasing interest in the development of new hybrid materials and their potential application as sorbents for the removal of various pollutants. For example, polar poly(N-vinylimidazole) (PVI) has been synthesized with divinylbenzene for the extraction of polar compounds [14], and polymethacrylic acid (PMAA) was grafted onto the surface of silica gel particles (PMAA/SiO2) for the adsorption of phenols [15]. Some researchers have reported silica-derived materials that have been successfully applied as sorbents for the removal of aromatic compounds and some heavy metal pollutants. For example, polyacrylamide (PAM) was grafted onto the surface of silica gel particles with 3-methacryloxypropyl trimethoxysilane (MPS) as the coupling agent for use in the removal of 2,4,6-trinitrotoluene (TNT). There have also been studies on the use of silica-based columns for the detection of selected heavy metals using solid-phase extraction [16].
The synthesis of novel materials with improved properties and performance is an interesting goal of chemistry and materials science. The development of hybrid polymers and modification of silica particles have improved the absorption of various analytes [17–20]. In particular, the inclusion of well-defined polymers in inorganic substrates is of significance because the functionality, composition, and dimensions of these macromolecules enable the resulting hybrids to be designed with specific properties. Well-defined organic polymers have been attached to inorganic (co)polymers, particles, surfaces, glassy networks, and interpenetrating polymer networks to prepare organic/inorganic hybrids [17–21].
The aim of this study was to synthesize, characterize, and evaluate a new hybrid polymer based on SiO2 particles grafted with PVI as a sorbent in MSPD. The hybrid polymer was analyzed using FT-IR, and the thermal stability was assessed using thermogravimetric analysis (TGA). To characterize the surface type and porosity, the polymer was evaluated by scanning electron microscopy (SEM), elemental analysis (EDS), and X-ray photoelectron spectroscopy (XPS). Finally, the hybrid polymer was evaluated as a sorbent in MSPD for the extraction of five pesticides from propolis. The MSPD extracts were analyzed using gas chromatography coupled to mass spectrometry (GC/MS). There were no scientific reports about the MSPD extraction of organophosphorus pesticides from propolis with the application of this hybrid polymer as a sorbent.
Materials and methods
Materials
Monomers for SiO2–PVI hybrid polymer preparation, tetraethylorthosilicate (TEOS), triethoxyvinylsilane (VTEOS), and N-vinylimidazole (N-VI), were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Hydrochloric acid (HCl), acetone, hexane, dimethyl acetamide (DMAc), ethanol (EtOH), ethyl acetate (EtOAc), ammonium hydroxide (NH4OH), 2,2-azobisisobutyronitrile (AIBN), acetonitrile (ACN), dichloromethane (DCM), and isooctane were purchased from J.T. Baker (Phillipsburg, NJ, USA) and used as received and without further purification. Octadecylsilyl silica (C18) (specific surface area 550 m2 g−1, pore size 60 Å), dichlorvos (DCV), diazinon (DZN), methyl parathion (MPT), malathion (MLT), and coumaphos (CMF) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). The propolis sample was obtained from the Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias (INIFAP) in Yucatán, Mexico.
Synthesis of SiO2–PVI hybrid polymer
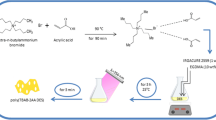
Sol–gel solution was prepared by mixing HCl, water, TEOS and EtOH (1:8:1:8 mol) under vigorous magnetic stirring for 2 h. Next, NH4OH (0.42 mol) was added, and the solution was stirred for 24 h. The SiO2 particles obtained were washed several times with EtOH and acetone and then dried under vacuum at 65 °C for 24 h (Fig. 1a). For functionality, dried SiO2 particles were mixed with water, NH4OH and ethanol under vigorous magnetic stirring for 1 h. Immediately, VTEOS was added to the solution, which was then stirred for 24 h. The functionalized particles were washed several times with EtOH and acetone and dried under vacuum at 65 °C for 24 h (Fig. 1b).
A mixture (1:1 wt/wt) of vinyl functionalized particles and N-vinylimidazole (N-VI) monomer was placed into a three-necked flask. Next, 80 mL of dimethylacetamide (DMAc) and AIBN (1 wt% relative to the monomer, N-VI) were added. The free radical polymerization was conducted by heating and stirring this mixture at reflux (110 °C) under nitrogen atmosphere for 24 h. Next, the ungrafted PVI was removed via Soxhlet extraction for 24 h with DMAc as the solvent (Fig. 1c).
Characterization of SiO2–PVI hybrid polymer
The structure of the resulting hybrid polymer was analyzed by attenuated total reflectance (ATR) Fourier transform infrared spectroscopy (FT-IR) in a Bruker Tensor 27 (Billerica, Massachusetts, USA), taking an average of 64 scans per sample between 400 and 4,000 cm−1.
The thermal stability of the hybrid polymer was determined by TGA using a TA Instruments SDT Q600 (NewCastle, DE, USA) under nitrogen atmosphere. The scans for thermal decomposition were taken between 50 and 800 °C at a heating rate of 10 °C min−1.
The nitrogen adsorption–desorption isotherms of the SiO2–PVI sorbent were determined in a Quantachrome Autosorb iQ device (Boynton Beach, FL, USA). Prior to the experiments, the samples were degassed at 700 K in a vacuum for 16 h. The volume of adsorbed N2 was normalized to a standard temperature and pressure. The specific surface area of the hybrid material was determined using the Brunauer–Emmett–Teller (BET) multipoint method, and the pore size distribution was obtained using the Barret–Joyner–Halenda (BJH) method.
Field emission scanning electron microscopy (SEM) analyses were performed using a Nova NanoSEM 200 FEI microscope (Hillsboro, OR, USA). Elemental characterization was performed using an energy-dispersive X-ray spectroscope attached to the FEI microscope equipped with an INCA X-Sight Oxford detector (Concord, MA, USA). Samples were fixed on a support with a copper film and sputter-coated with gold in a Denton Desk IV (Moorestown, NJ, USA) sputtering chamber.
XPS analysis was performed using a Jeol JPS-9200 (Akishima, Japan) equipped with a Mg source (1,253.5 eV) and operating at 200 W under a vacuum of 1 × 10−8 torr; for all samples, the analysis area was 1 mm2. SpecSurf™ software was used to analyze the experimental results. Charge correction was based on the adventitious carbon signal (C1s) at 285.5 eV. The Shirley method was used for background adjustment, and the Gauss–Lorentz method was utilized for curve fitting.
Fortification procedure
Fortified propolis samples were prepared by adding 100 μL of a standard solution containing five organophosphorus pesticides, dichlorvos (DCV), diazinon (DZN), methyl parathion (MPT), malathion (MLT), and coumaphos (CMF) (10 μg mL−1), to 1 g of propolis to obtain a sample concentration of 1 μg g−1. The fortified propolis samples were left to stand (40 min) to allow absorption of pesticides into the matrix sample. The tests were conducted in triplicate. The chemical structures of the organophosphorus pesticides evaluated are presented in Table 1.
MSPD extraction
For the MSPD extraction, each fortified propolis sample (1 g) was solubilized in 10 mL of hexane. A 1-mL aliquot of this propolis solution was added to 1 g of C18 in a glass mortar. Next, SiO2–PVI hybrid polymer and the propolis solution were homogenized using a pestle. The obtained mixture was packed into a polypropylene column (85 mm × 15 mm i.d.) and gently compressed to eliminate air pockets. Subsequently, the pesticides were eluted with 8 mL of acetonitrile/dichloromethane (25:75 v/v). The extracts were collected in a graduated vial and then evaporated until dry with a gentle air flow. The extract was reconstituted in isooctane (1 mL) and frozen (<10 °C) for at least 2 h to precipitate high molecular weight compounds. Finally, the extracts were centrifuged for 45 s (10,000 rpm), and then the supernatants were placed in vials for analysis using GC/MS in SIM mode.
Chromatographic conditions
GC/MS analyses were performed using an Agilent Technologies (Santa Clara, CA, USA) 6890N gas chromatograph coupled to a mass spectrometer 5973N (MSD) and a bonded fused-silica capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness of 5 % phenyl/ 95 % dimethylpolysiloxane) supplied by Supelco (Bellefonte, PA, USA). Helium (99.999 % purity) was used as the carrier gas at 1.0 mL min−1. One microliter of sample was introduced into the GC inlet (splittess, 280 °C). The oven temperature program was as follows: 100 °C (3 min) → 20 °C (min−1) → 300 °C (3 min).
Recovery evaluation
Pesticide recovery was evaluated with external calibration curves. Standard solutions for calibration (0.025, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5 μg mL−1) were prepared by dilution of the pesticide stock solution (1 mg mL−1) with an appropriate volume of unfortified propolis extracts obtained by MSPD. The slope and intercept values and their standard deviations were determined using regression analysis.
Results and discussion
Synthesis of SiO2–PVI hybrid polymer
SiO2 particles were functionalized with VTEOS (coupling agent) using the sol–gel method. The functionalized particles were organically modified with N-VI via free radical polymerization to obtain PVI on the surface of the SiO2 particles.
The obtained material was a fine white powder. The introduction of organic functional groups onto the silica surfaces provides a partial conversion of the silanol surface to a new organofunctional surface that acquires organophilic properties. Thus, ligand-grafted silica imparts particular properties to the surface that differ considerably from those of the original matrix. The proposed reactions are shown in Fig. 1.
Characterization of the SiO2–PVI hybrid polymer
Figure 2 displays the FT-IR spectra of the synthesized polymer. There are several bands present in the spectra that are expected for the polymerization of the hybrid material. The characteristic bands for the imidazole ring group appear at 1,630, ca. 1,500, 1,400, and 660 cm−1 due to C=C (ring) stretching, C–C and C=N (ring) stretching, C–H (ring) in the plane and C–N (ring) stretching vibrations, and C=N torsion stretching, respectively, similar to previous literature reports [22, 23].
Peaks at 1,086, 778, and 464 cm−1 were attributed to Si–O–Si stretching, bending, and rocking modes, respectively, according to observations by Bange et al. [24]. According to the technique used here, these results indicate that PVI is present on the surface of the SiO2 particles.
The thermal behavior and the composition of the organic/inorganic hybrid polymer were studied via thermogravimetric analysis. The thermogram (TG) and DTG curves are shown in Fig. 3. The TG and DTG curves exhibited an initial weight loss (5 wt%) at 100 °C that is associated with the loss of absorbed water or residual solvent. A second weight loss (13 wt%) at 320 °C is related to oligomers in the sample. A third decomposition occurs at 404 °C (13 wt%), which is attributed to thermal decomposition of PVI, similar to previous reports [22, 25, 26]. These results suggest that the polymer is composed of an organic/inorganic ratio of 28:72 wt/wt%, considering that, at 400 °C, only the organic part of the hybrid polymer was lost and that the SiO2 particles do not exhibit any significant weight loss up to 700 °C [27].
The pore size distribution was obtained from the N2 adsorption–desorption isotherms using the BJH method. The nitrogen adsorption–desorption isotherm is shown in Fig. 4. According to the IUPAC classification, the isotherm of SiO2–PVI hybrid polymer is a type IV isotherm. A type IV isotherm is indicative of a material with micropores (≤2 nm) and mesopores (2–50 nm) [28]. The specific total pore volume was 0.997 cm3 g−1, and the specific surface area (BET) was 234.8 m2 g−1.
According to the literature, sorbents with surface area greater than 100 m2 g−1 are suitable for solid-phase extraction. The organically modified silica materials offer advantages that are similar to those of commercial silica gel, such as a large specific surface area (S > 200 m2 g−1) and pore volume (V p > 0.2 cm3 g−1) [29]. The C18 commercial sorbent utilized here has a specific surface area of 550 m2 g−1 according to the supplier.
Surface morphology and elemental analyses were utilized to determine the structure and composition of the SiO2–PVI hybrid polymer (Figs. 5 and 6, respectively). The surface of the SiO2–PVI hybrid polymer displayed round-shaped, porous particle aggregates. These images show that the particles have a rough surface. The surface roughness should be considered as a factor in increasing surface area. Material surface area increases with increased surface roughness, which is related to the adsorption characteristics [30]. Energy dispersive X-ray elemental mapping was used to estimate the chemical composition and homogeneity of the hybrid polymer. The images in Fig. 6 clearly show a homogeneous and uniform distribution of chemical elements on the surface of the hybrid polymer. According to these results, the material is composed of C (25.14 %), O (45.77 %), Si (14.35 %), and N (13.86 %). As expected, these results suggested that the polymer is primarily composed of SiO2.
XPS measurements were used to further investigate the polymer surface composition. The signals identified in the wide scan XPS spectra of the SiO2–PVI hybrid polymer correspond to C1s (38.4 %), N1s (13.3 %), O1s (29.1 %), and Si2p (19.2 %). Each of these peaks was analyzed and deconvoluted (curve fitted) to identify the chemical species and their possible interactions (Fig. 7). Note that the quantities of C, O, Si, and N were relatively consistent with those measured via EDS analysis. The differences in some of the obtained values from these two methods can be explained by their different excitation depths [31].
Figure 7a shows the peak of Si2p, which was fit with two signals. The first peak located at 102.3 eV contributes 52.5 % of the total peak area. This peak shift is consistent with an interaction between silicon and carbon. The second peak, located at 103.5 eV, represents 47.5 % of the total peak area and can be assigned to the Si–O bond present in the SiO2 molecule [32–34].
The peak of O1s was also fit with two signals, as shown in Fig. 7b. One peak at 531.5 eV contributes 70 % of the total peak area and is assigned to O attached to Si in the SiO2. The other signal, at 532.2 eV, accounted for the remaining 30 % and is assigned to atmospheric oxygen that was adsorbed onto the material surface.
Figure 7c shows the peak of N1s, which was fit with two peak components that have binding energies of 398.7 eV and 400.7 eV corresponding to C–N and C=N interactions, respectively.
The XPS spectra of C1s is shown in Fig. 7d. The binding energy results are correlated with C–N and C–C bonds at 284.2 eV and C=C and C=N at 285.5 eV, which contribute 40 and 60 %, respectively, of the total of the fitted area. These assignments are supported by the intensity ratio between these two components and by literature reports [32–34].
In summary, the results of the different characterization techniques of TGA, FT-IR, EDS, and XPS confirm that the PVI was grafted successfully onto the SiO2 particles.
Extraction procedure
The performance of the SiO2–PVI hybrid polymer as a sorbent material for MSPD extraction was evaluated and compared with C18, a commercial adsorbent typically used in MSPD. Both materials were used for the MSPD extraction of organophosphorus pesticides from propolis eluted with 8 mL of ACN/DCM (25:75 v/v).
Propolis is a complex and sticky mixture of different plant exudates that is collected, modified, and used by honey bees to build and adapt their hive; among the types of chemical substances found in propolis are waxes, resins, essential oils, organic acids, alcohols, pigments, pollen, and other organic matter [35].
Two sets of matrix-matched calibration solutions, one for each material, were prepared for pesticide recovery evaluation. Parameters obtained for calibration curves by linear regression are presented in Table 2. In general, the slopes and intercepts were of the same order of magnitude. All pesticide calibration curves exhibited determination coefficients greater than 0.99.
Although the specific surface area of the SiO2–PVI hybrid polymer was lower than that of C18, similar recoveries and precision were obtained on two different days for both materials. In particular, the pesticide recoveries and precisions were acceptable for diazinon, methyl parathion, malathion, and coumaphos (Table 3), with C18 recoveries ranging from 83 to 126 % (RSD < 12 %) and SiO2–PVI recoveries ranging from 81 to 122 % (RSD ≤ 11 %). Dichlorvos was the exception, as it presented recoveries below 65 %. This result was attributed to its loss during the evaporation step.
These results demonstrate that SiO2–PVI hybrid polymer has the potential to be applied as a sorbent material for MSPD extraction of pesticides from complex samples such as propolis. In comparison to hydrocarbon chains attached to SiO2 in C18, the polymer attached to the surface of SiO2 particles in SiO2–PVI presents more interactions, namely (i) hydrophobic interactions due to the presence of the –[CH2–CH2] n – group, (ii) π–π interactions, and iii) hydrogen bonding dipole–dipole interactions due to the imidazole ring.
Conclusions
A hybrid polymer with PVI grafted to SiO2 particles was prepared successfully. The polymer exhibited suitable characteristics for use as a sorbent in solid-phase extraction. The surface of the hybrid polymer exhibited a rough surface with a specific total pore volume of 0.997 cm3 g−1 and a specific surface area of 234.8 m2 g−1.
The synthesized hybrid polymer exhibited good performance for the MSPD extraction of organophosphorus pesticides. The pesticide recoveries and relative standard deviations obtained with SiO2–PVI hybrid polymer were similar to those of commercial C18. Therefore, SiO2–PVI has the potential to be applied for the MSPD extraction of other samples and analytes.
References
Lemos VA, Gómez-Teixeira LS, Almeida Bezerra M, Spínola-Costa AC, Teixeira-Castro J, Martins-Cardoso LA, Santiago de Jesús D, Souza-Santos E, Xavier-Baliza P, Novaes-Santos L (2008) New materials for solid-phase extraction of trace elements. Appl Spectrosc Rev 1217:303–334
Capriotti AL, Cavaliere C, Giansanti P, Gubbiotti R, Samperi R, Laganá A (2010) Recent developments in matrix solid-phase dispersion extraction. J Chromatogr A 16:2521–2532
Barker SA (2007) Matrix solid-phase dispersion (MSPD). J Biochem Biophys Methods 70:151–162
Lozowicka B, Jankowska M, Rutkowska E, Kaczynski P, Hrynko I (2012) Comparison of extraction techniques by matrix solid phase dispersion and liquid-liquid for screening 150 pesticides from soil, and determination by gas chromatography. Pol J Environ Stud 21:973–992
Bogialli S, Di Corcia A (2007) Matrix solid-phase dispersion as a valuable tool for extracting contaminants from foodstuffs. J Biochem Biophys Methods 70:163–179
Zhang L, Liu S, Cui X, Pan C, Zhang A, Chen F (2012) A review of sample preparation methods for the pesticide residue analysis in foods. Cent Eur J Chem 10:900–925
Souza S, Bolzan CM, Jaime E, Venquiaruti AL, Gaspar CM, Bianchini A, Primel EG (2013) A vortex-assisted MSPD method for the extraction of pesticides residues from fish liver and crab hepatopancreas with determination by GC-MS. Talanta 112:63–68
García M, Canosa P, Rodríguez I (2008) Trends and recent applications of matrix solid phase dispersion. Anal Bioanal Chem 391:963–974
Gilar M, Bouvier E, Compton B (2001) Advances in sample preparation in electromigration, chromatographic and mass spectrometric separation methods. J Chromatogr A 909:111–135
Fontanals N, Galiá M, Marcé RM, Borrull F (2004) Comparison of hydrophilic polymeric sorbents for on-line solid-phase extraction of polar compounds from aqueous samples. Chromatographia 60:511–515
Fontanals N, Marcé RM, Borrull F (2007) New materials in sorptive extraction techniques for polar compounds. J Chromatogr A 1152:14–31
Żwir-Ferenc A, Biziuk M (2006) Solid phase extraction technique–trends, opportunities and applications. Pol J Environ Stud 15:677–690
Toribio L, Moyano E, Puignou, Galceran MT (2000) Comparison of different commercial solid-phase extraction cartridges used to extract heterocyclic amines from a lyophilized meat extract. J Chromatogr A 880:101–112
Fontanals N, Marcé R, Galiá M, Borull F (2004) Synthesis of hydrophilic sorbents from N-vinylimidazole/divinylbenzene and the evaluation of their sorption properties in the solid-phase extraction of polar compounds. J Polym Sci Pol Chem 42:2019–2025
An F, Feng X, Gao B (2009) Adsorption mechanism and property of a novel adsorption material PAM/SiO2 towards 2,4,6-trinitrotoluene. J Hazard Mater 168:352–357
An F, Gao B, Feng X (2009) Adsorption mechanism and property of novel composite material PMAA/SiO2 towards phenol chemical. Chem Eng J 153:108–113
Sun M, Ma X, Wang J, Wang W, Wu Q, Wang C, Wang Z (2013) Graphene grafted silica-coated Fe3O4 nanocomposite as absorbent for enrichment of carbamates from cucumbers and pears prior to HPLC. J Sep Sci 36:1478–1485
Mei M, Wan A, Abdala AE (2014) Sol–gel hybrid methyltrimethoxysilane–tetraethoxysilane as a new dispersive solid-phase extraction material for acrylamide determination in food with direct gas chromatography–mass spectrometry analysis. Food Chem 158:302–309
Zhu Y, Yang S, Chen G, Xing J (2014) Single “click” synthesis of a mixed-mode silica sorbent and application in matrix solid-phase dispersion extraction of β-agonistsfrom porcine liver. J Chromatogr A 1354:101–108
Wen Y, Chen L, Li J, Liu D, Chen L (2014) Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. Trac-Trends Anal Chem 59:26–41
Pyun J, Matyjaszewski K (2001) Synthesis of nanocomposite organic/inorganic hybrid materials using controlled/“living” radical polymerization. Chem Mater 13:3436–3448
Pekel N, Guven O (2002) Synthesis and characterization of poly(N-vinylimidazole) hydrogels crosslinked by gamma irradiation. Polym Int 51:1404–1410
Li J, Zhang Y, Ping Z, Li M, Zhang Q (2011) Synthesis and endotoxin removal properties of a novel affinity sorbent with poly(1-vinylimidazole) as the ligand. Process Biochem 46:1462–1468
Bange JP, Patil LS, Gautam DK (2008) Growth and characterization of SiO2 films deposited by flame hydrolysis deposition system for photonic device application. Prog Electromagn Res 3:165–175
Wang H, Meng S, Xu P, Zhong W, Du Q (2007) Effect of traces of inorganic content on thermal stability of poly(methyl methacrylate) nanocomposites. Polym Eng Sci 47:302–307
Strat M, Vasiliu S, Strat G, Luca C, Grecu I, Gurlui S, Stratulat SI (2006) Spectral and thermogravimetric analysis of some poly(carboxybetaine)s polymers. J Optoelectron Adv Mater 8:181–184
Park JT, Seo JA, Ahn SH, Kim JH, Kang SW (2010) Surface modification of silica nanoparticles with hydrophilic polymers. J Ind Eng Chem 17:517–522
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl Chem 57:603–619
Walkarius A, Collinson M (2009) Analytical chemistry with silica sol-gels: traditional routes to new materials for chemical analysis. Ann Rev Anal Chem 2:121–143
Osmana B, Kara A, Uzunb L, Besirli N, Denizli (2005) A vinyl imidazole carrying metal-chelated beads for reversible use in yeast invertase adsorption. J Mol Catal B 37:88–94
Fibikar S, Rinke MT, Schafer A, De Cola L (2010) Quantification of cation-exchange zeolites by XPS and EDS: a comparative study. Micro Meso Mater 132:296–299
Zhang H, Lei X, Su Z, Liu P (2007) A novel method of surface-initiate atom transfer radical polymerization of styrene from silica nanoparticles for preparation of monodispersed core-shell hybrid nanospheres. J Polym Res 14:253–260
Benne D, Maccallini E, Rudolf P, Sooambar C, Prato M (2006) X-ray photoemission spectroscopy study on the effects of functionalization in fulleropyrrolidine and pyrrolidine derivatives. Carbon 44:2896–2903
Zhang H, Li C, Guo J, Zang L, Luo J (2012) In situ synthesis of poly(methyl methacrylate)/SiO2 hybrid nanocomposites via “grafting onto” strategy based on UV irradiation in the presence of iron aqueous solution. J Nanomater 2012:1–9
Walker P, Crane E (1987) Constituents of propolis. Apidologie 18:327–334
Acknowledgments
This work was supported by Consejo Nacional de Ciencia y Tecnología, CONACyT (83390). The authors are grateful to M. C. Lizbeth Triana Cruz and M. C. Alejandra Núñez Pineda for their technical support in the FT-IR measurements and to L.Q.I. Nayely Pineda Aguilar and M. C. Alberto Toxqui Teran for their technical assistance in the structural analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medina-Dzul, K., Carrera-Figueiras, C., Pérez-Padilla, Y. et al. SiO2/polyvinylimidazole hybrid polymer as a sorbent for extraction by matrix solid-phase dispersion (MSPD): synthesis, characterization, and evaluation. J Polym Res 22, 45 (2015). https://doi.org/10.1007/s10965-015-0677-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0677-7