Abstract
The aim of this work was to demonstrate that the use of biodegradable films activated with natamycin is effective for the preservation of regional cheeses. For this, starch and amylose films were prepared using only water as a plasticizer, gelatinizing at temperatures of 120 °C, and using two levels of antimicrobial. Moisture content, water solubility, WVP, mechanical properties, and biodegradability of all films were measured. The films obtained were ductile and homogeneous, and the active agent did not significantly change their mechanical properties, biodegradability, or solubility; however, it did reduce the WVP values, but this did not condition the applicability as a coating in any case. Visual observation, weight loss, antimicrobial migration, and microbiological analyses were carried out on cheese wrapped with films with and without natamycin and stored under different humidity and temperature conditions. Visual inspection showed the development of molds and yeasts on the surface of control cheeses and those covered with films without antimicrobial, while no developments were evident in the activated film. Weight loss was reduced by 5% for high humidity storage with the application of films, and for the levels tested, concentrations of natamycin were not detected in cheese mass. With the starch film and 10 mg/dm2 of natamycin (M6), it was possible to delay the growth of molds and yeasts (1.05 logarithmic cycle reduction) and psychrophilic bacteria for the 30 days of study, allowing a better-quality preservation of food in the storage, using an eco-friendly and easy-to-develop material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch is a material that has the property of forming solutions with remarkable filmogenic capacity. The simplest way to obtain starch films involves destructuring the native starch granules using only water as a plasticizer, but it is well known that, in order to improve the properties of the materials obtained, the use of other plasticizers in addition to water is essential. Most of the scientific background in the area of starch bioplastics proposes obtaining and characterizing films manufactured by casting, using aqueous solutions, and heating them to the gelatinization temperatures of the material (60–85 °C) (Alves Mali, Beléia, & Grossmann, 2007; Famá et al., 2005; Mukerjea & Robyt, 2010).
Johnston et al. (2011) and Päivi et al. (2002) used supra-atmospheric pressures and temperatures between 120 and 160 °C, to ensure complete starch gelatinization, while Protzman et al. (1964) found that gelatinizing in this way prevented the increase in crystallinity over time, improving the flexibility and extensibility of the materials obtained.
There are numerous studies on the development of starch films from different botanical sources and mixtures of starch with acetate starches. The vast majority of the works conclude that, although starch constitutes an interesting alternative to replace petroleum plastics due to its low cost and high availability, its high susceptibility to humidity and its limited mechanical properties conditions its application.
Regarding the improvement of mechanical properties, several authors propose the use of amylose, understanding that this last polymer presents better mechanical properties (Päivi et al., 2002; Protzman & Wagoner, 1964), and other authors use starches of high amylose obtained genetically (Bertuzzi et al., 2012; García et al., 2000; Slavutsky & Bertuzzi, 2014; Zou et al., 2021). The preponderance of amylose in starches produces stronger films (Jha, 2021; Youssef et al., 2017). Alves et al. (2007) found that the enrichment of filmogenic solutions with amylose solutions causes more tenacious and permeable films.
Another alternative represents the addition of external plasticizers such as glycerol, sorbitol, citric acid, and triacetin. These are used in order to increase the relaxation between the polymer chains and thus improve the ductility of the films obtained. This alternative is interesting, but it implies a drop in the breaking stress values and a decrease (depending on the nature of the plasticizer) in the barrier properties of the film (Bertuzzi et al., 2012; Lourdin et al., 1997; Hernández et al., 2023). Other problems are also reported by the application of plasticizers, such as the lack of compatibility between the components of the matrices, stickiness effects from certain concentrations, and acceleration in retrogradation phenomena. The water solubility of starch films also determines their use, for this reason, numerous works on acetylated starches with a high degree of substitution were carried out. The hydrophobicity of starch acetate increases as the degree of substitution and the chain length of the substituent increase. Chemically modifying starches in order to improve their physicochemical and functional properties promises potential uses in pharmaceutical applications, food industries, and other new applications (Cuenca et al., 2020; Fringant et al., 1998; López et al., 2008; Ochoa et al., 2017; Tarvainen et al., 2003).
Biopolymeric coatings (Chitosan, Sodium Alginate, Galactomannan, Soy Protein) have been tested to extend the shelf life of cheeses, partially controlling external contamination and the exchange of O2, CO2, and water vapor (Cerqueira et al., 2010; di Pierro et al., 2011; Zhong et al., 2014).
The concept of active food packaging has been innovated by the development of biodegradable or edible films with the incorporation of antimicrobial additives. Covering the cheeses with a film or active coating allows to control the dryness of the surface, the humidity and weight loss during storage and to reduce the possible microbiological deterioration, in such a way as to favor food safety and the useful life of these foods.
To improve the preservation properties of cheeses, many of the antimicrobial agents normally used in the food industry have been utilized, either directly applied to the food or incorporated into packaging coatings and films (Lucera et al., 2014).
Bacteriocins are antimicrobial agents produced by bacteria. Nisin and natamycin are two commercially produced bacteriocins used in the preparation of active films and coatings. Natamycin has been approved as a food preservative suitable for human consumption, among others, by the European Food Safety Authority (EFSA), the World Health Organization (WHO), the Food and Drug Administration (FDA), and the Argentine Food Code (CODA). Regarding the latter, the limitation in the concentration used is 1 mg/dm2 of natamycin in direct contact with the food, and it must not be detectable at 2 mm from the crust. As an antifungal, it can significantly extend the shelf life of many products, such as cheeses and cured meats. These food categories need preservation against molds and yeasts, but at the same time allow bacterial cultures to remain active.
Ollé Resa et al. (2014) added natamycin and nisin to biodegradable starch films and observed changes in their properties (Young’s modulus, WVP, color, contact angle); however, the same authors did not find significant changes in the film with the addition of natamycin (Ollé Resa et al., 2013).
Recently, some works have studied cassava starch films using glycerol as a plasticizer and incorporating other materials in order to improve the mechanical and barrier properties of the films. Pérez et al. (2021) incorporated zein into the polymeric matrix, and Berti et al. (2021) added processed rice bran to form a composite material. Both works used natamycin and nisin as active agents, testing the antimicrobial effect of the films by means of agar diffusion tests for different germs but without testing such materials on food.
Biodegradable films (chitosan, triticale flour, starches, sodium alginate) activated with natamycin and/or nisin have been tested to reduce microbiological contamination of different types of cheeses and increase their shelf life (Berti et al., 2019, 2021; Fajardo et al., 2010; Lucera et al., 2014; Ollé Resa et al., 2016).
This study seeks to demonstrate that the application of biodegradable films activated with natamycin is effective in the preservation of regionally manufactured cheeses. To verify the hypothesis, cassava starch films were prepared, using water as the only plasticizer. At the same time, the amylose fraction was separated from the native starch, and films were prepared therefrom. The physicomechanical properties that can determine the applicability of the films as coatings were studied. Finally, the effect of the film applied as a wrapper on the preservation of cheeses during storage was studied.
Experimental
Materials
Commercial cassava starch was provided by Aldema®, the regional bar-type cheese, which was purchased from Cooperativa Alto Uruguay Limitada (CAUL) (25 de Mayo, Misiones), and the water was Milli-Q ultrapure water (Millipore Corporation, Burlington, MA, USA). The n-butanol was of analytical grade and purchased from Cicarelli® Reagent S.A. (Santa Fe, Argentina). A total of 50% Natamycin in NaCl was purchased from BIOTEC S.A. (Buenos Aires, Argentina), PCA Agar, Molds and Yeasts Medium, and peptone water were purchased from Britania S.A. (Buenos Aires, Argentina), and TEFLON® molds were purchased commercially.
Film Preparation
Starch in water at a concentration of 2.6% was gelatinized for one hour at 120 °C. Once gelatinized, the filmogenic solution obtained was diluted 1/4 in water and kept at a temperature of 80 °C. A total of 100 mL of this solution were immediately poured into 20 cm diameter Teflon® molds.
The amylose solution was prepared using the Mukerjea and Robytt method (Mukerjea & Robyt, 2010), for which the starch was gelatinized in a concentration of 2.6% in water at 120 °C for 1 h, and the amylose precipitation was performed by adding 85 mL of n-butanol. The solution was stirred for 72 h and then centrifuged, resuspending the precipitate in 250 mL of water. The mixture was distilled by steam stripping to eliminate n-butanol, and the films were cast hot (80 °C) in Teflon® molds with a diameter of 20 cm.
Natamycin was incorporated by vigorous stirring at 600 rpm to the starch and amylose filmogenic solutions (at 80 °C) and hot molded. Two concentrations of natamycin were evaluated: the first is the maximum set by the Argentine Food Code for this antimicrobial, placed on the surface of the food (1 mg/dm2 of cheese surface), and the second concentration in an order higher (10 mg/dm2). Once the films were obtained, they were stabilized in a controlled atmosphere of 55% RH.
Film Characterization
Thickness
Film thickness was measured with a Fowler electron micrometer from Cole-Parmer Instruments Co. (precision 0.001 mm). The reported thickness was the average value of ten measurements taken in different areas of the film.
Moisture
The moisture of the films was determined by measuring their weight loss after being dried in an oven at 105 °C until constant weight. The samples were analyzed in triplicate and the results were expressed as a percentage (%).
where mi is the initial mass of the film and mf is the final mass of the film.
Solubility
To determine the solubility of the films, 2 × 3 cm samples were stored for 7 days in a desiccator with silica gel, then weighed and placed in beakers containing 80 mL of deionized water at 25 °C. The samples were kept under constant agitation at 200 rpm for 1 h at the corresponding temperature. Then, they were recovered by filtration and dried in an oven at 60 °C until constant weight. The total percentage of soluble matter (% solubility) was calculated as follows in Eq. (2.2):
For each condition, all samples were tested in triplicate.
Quasi-static Mechanical Properties
The tensile tests were carried out with a DY 32 model Adamel Lhomagry (France) tension/compression machine with a 1 kN load cell, with an accuracy of 0.5% (0.1% of full scale), a range of speeds from 0.01 to 999 mm/min, and with automatic recording of tension—deformation.
2.5 × 5 cm test pieces were used, recording traction (Newton)-strain (mm) and energy stored until rupture (mJ) was absorbed, and a constant speed of 0.4 mm/min was used.
Water Vapor Permeability
The water vapor permeability (WVP) of the films was evaluated with the quasi-isostatic method, already used in yerba containers (Ramallo & Albani, 2004), and following the general procedure described in the (ASTM E 96, 2016).
The cells used in the laboratory have a transfer area of 0.058 m2, and the experiences were carried out in triplicate. External relative humidity was set using saturated saline solutions (NaCl for 75% RH), and silica gel was used as 0% RH. The temperature of the test booths was kept constant in an oven with a precision of ± 1 °C.
The change in cell weight was recorded on an A and D Co. (Tokyo, Japan) brand electronic balance, with a precision of 0.001 g.
The permeability of the materials was quantified using the coefficient of permeability, according to (Robertson, 2013):
where Q is the amount of perm diffusing through a thickness x, an area A, in a time t, for partial pressures p1 and p2 on each side of the film.
Biodegradation of the Films in Vegetable Compost
The films were cut into 2 cm × 2 cm squares. Regional soil extracted randomly from the city of Posadas Misiones, whose initial humidity was 34.7 ± 2%, was sieved to remove large clumps and poured into a plastic tray to a thickness of approximately 6 cm. The samples were buried under 4 cm of soil, at room temperature (∼25 °C) and humidity conditions (70–80%). Water was sprayed twice a day to maintain the initial soil moisture, which was monitored with a thermo-hygrometer. The films were removed at different times and dried in a vacuum oven at 50 °C for 24 h. They were later photographed in order to record the changes in appearance.
Natamycin Concentration in the Film
The amount of natamycin in the film (mg/dm2) was calculated using the following Eq. (2.4):
Preparation of Cheese Samples
The cheese rind was removed with a sterile knife to approximately 5 mm depth to remove the effect of some cracks already present on the cheese surface 2.5 × 2.5 × 0.5 cm cheese cubes were covered on their entire surface by films of starch and amylose without antimicrobial and with two concentrations of natamycin (1 mg/dm2 y 10 mg/dm2).
Visual Assessment
Cheese pieces coated with starch films and amylose films (without natamycin) and uncoated pieces (control) were tested in triplicate. The samples were stored for 7 days at 25 °C and 50% RH (a condition that simulates a cool and dry environment) and 20 days at 4 °C with 92% RH (a condition that simulates storage in a domestic refrigerator). The samples were photographed at the end of the storage period.
Evaluation of Natamycin Content in Cheese
Since it is essential to evaluate the diffusion of the antimicrobial in the food, 2.5 × 2.5 × 2.5 cm cubes of cheese coated with the active films were placed in plastic bags and vacuum sealed, storing them for 30 days at 4 °C. Once this time had elapsed, the amount of disseminated natamycin was analyzed, following the methodology reported by González-Forte et al., (2019). To do this, the active film was removed and the first 2 mm of each face of the cube were discarded, homogenizing. The remaining mass in a 2:1 solution of methanol and water. Analysis of natamycin concentrations was performed by UV spectrophotometry, evaluating the peaks at 311 and 329 nm.
Weight Loss
The weight loss of 2.5 × 2.5 × 0.5 cm cubes of cheese was determined after being stored refrigerated at 4 °C at 35, 75, and 92% RH. The samples were analyzed in duplicate, at times: 0; 3; 6; 9; 12, and 15 days. Mass loss was reported with a precision of 0.001 g and calculated using the following Eq. (2.5):
where w0 is the initial weight of the sample and wi is the weight of the sample at the time i.
Microbiological Tests
Changes in psychrophilic bacteria and total yeasts and molds, microbial groups that commonly cause cheese spoilage, were measured during storage. For this, the cubes of cheese covered with “films” of starch and amylose, prepared in the most sterile conditions possible, were placed in sterile containers and then incubated for up to 30 days at 4 °C and 92% RH to reproduce typical cheese storage conditions.
Sample Denomination: M0: control cheese (without coating); M1: amylose-coated cheese (without antimicrobial); M2: starch-coated cheese (without antimicrobial); M3: cheese coated with amylose with 1 mg/dm2 of antimicrobial; M4: cheese coated with amylose with 10 mg/dm2 of antimicrobial; M5: cheese coated with starch with 1 mg/dm2 of antimicrobial; M6: cheese coated with starch with 10 mg/dm2 of antimicrobial.
The tests were carried out for M1–M6 at 10, 20, and 30 days, while the uncoated cheese M0 (control) was analyzed at time 0 and on day 30. The cheese samples to be analyzed were stripped of their coating; they were weighed and homogenized in water with peptone (1:10) in sterile sampling bags, and a dilution volume (20 μL) was placed in the appropriate media to determine the number of CFU/mL of each microorganism. Yeasts and molds were enumerated on plates containing molds and yeasts medium and incubated at 28 °C for 72 h. Psychrophilic bacteria were determined using plate count agar (PCA) after incubation for 5 days at 4 ± 1 °C. Colony enumeration was performed, and the growth of microorganisms was expressed as log CFU/g. The determinations were performed by triplicate.
Statistical Analysis
All the tests were performed at least in triplicate, and results were reported as means ± standard errors. The analysis of variance (ANOVA) was performed, and the means were compared by Tukey’s test at a 5% significance level using the “statsmodels” library in Python language.
Results
Film Appearance
The starch and amylose films obtained were homogeneous, flexible, transparent, and could be easily separated from the cast plates. The film thickness was controlled by the solid concentration and the amount of solution cast on the support (Wolff et al., 1951). From the solutions prepared following the methodology proposed for each polymer matrix, film thicknesses of 0.030 ± 0.005 mm (amylose and native starch) were obtained, and no statistically significant differences were observed with respect to the thicknesses of the films prepared with natamycin. It is important to mention that the films obtained were continuous and of sufficient thickness despite the low concentration of polymer with which they were prepared. Liu (2005) and López et al. (2008) maintain that below a concentration of 4% w/w of starch, it is difficult to obtain a film with these characteristics for gelatinized starches at 90 °C for relatively short times. The authors of this work attribute this different behavior (obtaining uniform and thin films from very dilute solutions) to the heat treatment used in the starch gelatinization process, to the casting temperature, and to the drying process of the film. Figure 1a shows the amylose films, and Fig. 1b shows the amylose + 10 mg/dm2 of natamycin films.
Moisture Content, Solubility, and Water Vapor Permeability
Moisture content values are lower in the case of starch films compared to those of amylose, but these differences are small and not significant. Incorporation of natamycin in the selected concentrations to the films of both polymers did not significantly affect the moisture content values in relation to the films without antimicrobial; this behavior was also reported by Ollé Resa et al. (2014a) and Romero et al. (2016).
The water solubility of the films constitutes an important property since it determines their applicability, especially if the film is used as a container or coating for cheeses with high moisture content. The amylose films were insoluble in water for the conditions tested, a result similar to that reported for this polymer in solution (Mukerjea & Robyt, 2010) making it an interesting material for packaging or coating of food. Regarding the addition of antimicrobial, there were no statistically significant changes in solubility for any of the films studied, a result similar to that obtained by Ollé Resa et al. (2013). On the contrary, Basch et al. (2013) found differences between cassava starch films with and without nisin, as active agents.
Water vapor permeability obtained for starch films was lower than for those of amylose; similar results have been reported by Alves et al. (2007), who attribute this effect to the greater availability of free hydroxyls plausible to interact with water in the amylose molecule. Mali et al. (2006) reported similar trends when they analyzed starch films from different botanical sources, finding that those polymeric matrices with lower amylose content in their composition had lower WVP. With the addition of natamycin, statistically significant changes were registered with respect to the films without antimicrobial. This trend was also seen by other authors in similar polymer coatings, attributing this effect to the hydrophobic nature of natamycin (Ollé Resa et al., 2014a, b; Romero et al., 2016). Although the decrease in the water vapor permeability of the films from the aggregate is statistically significant of natamycin, this change is not important enough to impact the applicability of the coatings.
On the other hand, permeability values obtained in this work are lower (in at least one order) than those obtained by other authors with cassava starch films. This discrepancy is probably due to the fact that the films tested here do not have the addition of external plasticizers such as glycerol, whose known effect is to increase the solubility, moisture content of the films, and water vapor permeability, given their hydrophilic nature (Berti et al., 2021; Bertuzzi et al., 2007; Mohamed et al., 2020; Ollé Resa et al., 2014a, b).
Regarding the mechanical properties, the amylose films had a higher elongation than the starch films (Table 1). The values obtained are characteristic of polymeric materials without the addition of external plasticizers. Despite the reported low elongation values, films were very flexible, probably due to the low polymer concentration in the film-forming solutions and the film preparation technique (gelatinization at 120 °C and 2 atm, hot molding, and slow drying at room temperature) (Päivi et al., 2002). This allowed to achieve films with small thicknesses (0.030 ± 0.005 mm). Alves et al. (2007), using cassava starch enriched with amylose, obtain very brittle films that are difficult to handle when glycerol is not used. In the same sense, Pérez et al. (2021) reported that at low concentrations of glycerol (less than 0.5% p/p), the films they obtained were brittle, and the declared thicknesses were significantly higher than those reported in this work (0.162 ± 0.002 mm). Berti et al. (2021) managed to improve the mechanical properties of cassava starch films by incorporating PRB (processed rice bran) as reinforcement into the matrix; however, due to the fact that brittleness forces it to use glycerol as plasticizers, the breaking stresses are below of 2 MPa.
Starch films were 5% more tenacious than the amylose ones, an expected effect given the amylopectin content; however, films of both polymers had adequate mechanical properties to be applied as a coating (Table 1).
On the other hand, natamycin will not increase a notable change both in the breaking stress and in the elongation of the films for both polymers, and this behavior could be due to the low concentrations of the antimicrobial used. Sirisha Nallan Chakravartula et al. (2020), who report loss of ductility in the films, used high concentrations of natamycin in the polymeric matrices. Table 1 summarizes the main properties of the starch and amylose films (control) and those that incorporate the two levels of natamycin.
Biodegradation of the Films in Vegetable Compost
Figure 2 shows the macroscopic appearance of the films as a function of the burial time in vegetable compost. Biodegradability tests were performed both for films with and without antimicrobial, evaluating the highest level of natamycin (10 mg/dm2), but the effect of the antimicrobial did not show significant differences for starch. As could be observed, the starch films experienced slight changes in their hue as well as breaks, showing the beginning of degradation on the third day of composting, and they were the first to degrade, ending the test after 9 days. This result agrees and correlates with the solubility of said film; the humidity of the compost facilitates the said process. In soil, water diffuses into the polymer sample causing swelling and enhancing biodegradation due to increased microbial growth (González Seligra et al., 2016). The films that took the longest to degrade were those with amylose and natamycin, with the trial ending at 15 days. Although the incorporation of natamycin should lead to a decrease in the probability of attack by microorganisms, this situation only delayed the degradation of amylose films, with respect to the film without antimicrobial. There is a discrepancy in the bibliography, around the mean degradation periods in soil for starch films. For example, Xiong et al. (2008) observed that the rates of biodegradation of starch films occurred in 100 days, while Medina Jaramillo et al. (2016) declare an average of complete degradation of their materials of 12 days, similar to those reported in this study.
Cheese Analysis
Visual Assessment
Two storage conditions were tested in order to monitor the changes in the appearance of the cheeses, evaluating the effects of two different polymers with two levels of natamycin. Figure 3 shows cheeses covered with starch films stored at 25 °C in an environment of 50% RH for a period of 7 days.
Effect of storage in a 50% RH environment, 25 °C, for seven days of: a amylose film-coated cheese; b amylose film-coated cheese + 1 mg/dm2 of natamycin; c amylose film-coated cheese + 10 mg/dm2 of natamycin; d starch film-coated cheese; e starch film-coated cheese + 1 mg/dm2 of natamycin; f cheese coated with starch film + 10 mg/dm2 of natamycin; g uncoated cheese
Although moisture loss is observed in uncoated cheeses, no development of fungal colonies is observed despite the storage temperature. This is due to the fact that most of the microorganisms present in cheese need aw values in the order of 0.6 to 1 (Marcos, 1993). In both tests, it can be observed that the uncoated cheese undergoes a greater color change than the coated cheese. This is due to the fact that the presence of the film restricts the availability of oxygen for the lipid oxidation reactions of the cheeses.
On the other hand, when the cheeses are stored under conditions of 92% RH at 4 °C for 20 days, the development of microorganisms was evident for some samples (Fig. 4).
Effect of storage in a 92% RH environment at 4 °C, for 20 days of: a uncoated cheese (M0); b amylose film-coated cheese (M1); c starch film-coated cheese (M2); d amylose film-coated cheese + 1 mg/dm2 of natamycin (M3); e starch film-coated cheese + 1 mg/dm2 of natamycin (M4); f amylose film-coated cheese + 10 mg/dm2 of natamycin (M5); g cheese coated with starch film + 10 mg/dm.2 of natamycin (M6)
The growth of microorganisms, particularly molds, was as expected due to the high percentage of humidity in the storage environment. Uncoated cheese showed visible mold growth. Regarding the samples coated with amylose (M1) and starch (M2), it could be observed that the presence of the film did not prevent the development of microorganisms, neither on the surface of the coating nor on the surface of the food; therefore, these films are not suitable to act as a barrier against external contamination. It was also found that the M1 sample presented greater microbial development than the sample covered with starch films and the uncoated cheese. The antifungal activity of the films containing natamycin was evidenced since no sample with antimicrobial exhibited evident development, neither on the surface of the film nor on the cheese. The lowest concentration of natamycin proved to be adequate to prevent the visible development of molds, for the test conditions studied. Fajardo et al. (2010) found that the minimum concentration of natamycin capable of inhibiting the development of molds in Saloio cheese is 0.2 mg/mL of natamycin, while in the present study, inhibition has been obtained in the growth of molds and yeasts using a concentration of 0.027 mg/mL of natamycin equivalent to 1 mg/dm2 of film.
Evaluation of Natamycin Content in Cheese
The results obtained by UV spectrophotometry did not show the presence of detectable natamycin in the cheese mass. This found result is expected given the low concentrations of natamycin used and the fact that it is not directly deposited on the surface of the cheese, but rather in the matrix of the film. De Oliveira et al. (2007) obtain similar results (it does not detect) when they analyze the migration of natamycin in Gorgonzola cheese for concentrations of 2% (45 mg/dm2) of coated cheese, even considering that the cheese area analyzed by this author was the crust and not the mass.
González-Forte et al. (2019), however, reported the finding in the mass of Tybo cheese of 3.5 mg of natamycin per kg of cheese during the ripening process, but it should be noted that the concentration used was more than one order larger than the one used in this work.
Laurindo et al. (2019), after studying the presence of natamycin in the mass of cheeses that are currently marketed in Brazil and Argentina, found that 50% of them did not comply with current legislation in both countries; however, in all the cases analyzed, the natamycin was incorporated directly into the cheese rind. Vierikova et al. (2013) and Paseiro-Cerrato et al. (2013) found similar results. The latter highlights the importance of having a polymeric support as a container for natamycin.
Weight Loss
Moisture loss, expressed as percent weight loss (Δw %), in cheese samples uncoated (M0), coated with an amylose film (M1), and coated with a starch film (M2), stored at 4 °C and 92% RH, can be seen in Fig. 5a. The weight loss was higher in the uncoated samples, which after 15 days lost 23% of their initial weight. On the other hand, the coated samples did not show significant differences among themselves, and in both cases, they reached weight losses of 18% for the mentioned time. The weight loss data found for the coated cheeses were similar to those reported by Zhong et al. (2014) for mozzarella cheese coated with various materials (chitosan, sodium alginate, soy protein) and exposed to 4 °C and 85% RH, although unlike this work, Zhong found less weight loss in uncoated cheese. A lower moisture loss, like the one obtained in this work, was also reported by Cerqueira et al. (2010) who for “regional” semi-hard cheese coated with chitosan, found a 2.5% lower weight loss in coated cheeses compared to untreated ones, for storage of 21 days at 4 °C, 92% HR.
Figure 5b presents the results of experiments on similar samples stored at the same temperature 4 °C, but exposed to 75% RH. It could be observed that the weight loss was also higher in the uncoated cheese, in comparison with those coated with starch or amylose films. The moisture loss at 15 days was 30%. In the case of the coated samples, although the data had some dispersion, it could be seen that they were not linear, and at 15 days, the moisture loss was 23–25%.
The results of experiments at 4 °C and 35% RH are shown in Fig. 5c. The samples exposed to these conditions (which simulate those of a no-frost refrigerator), as expected, showed a greater loss of moisture over time, but in this case, they all turned out to be similar. The graph presented two zones with different speeds, high at the beginning and then a lower, practically linear speed, with a moisture loss of 32% for the 13 days.
This behavior could be due to the fact that when the superficial humidity of the cheese decreases, an area of difficult transport of water is formed from the interior of the food to the surface, which implies an additional barrier to that of the film, which reduces the speed of drying (0.36%/day).
Weight loss experiences with the addition of the active component to the films were also carried out for the three storage humidity conditions. The weight loss trends were similar to those previously exposed to films without antimicrobial, and in both cases (starch and amylose), the addition of natamycin did not affect weight loss, regardless of the concentration considered. In Fig. 5d, this effect is shown for amylose films exposed to conditions of 4 °C and 92% RH, including M0 in the graph.
Microbiological Tests
The count of molds and yeasts is shown in Fig. 6. The tests showed significant differences between the uncoated cheese samples and those coated with starch and 10 mg/dm2 of natamycin (M6) for a storage time of 30 days (p > 0.05) achieving a logarithmic cycle reduction of 1.09, respectively.
In analyses carried out after 10 days of storage, a lower growth was verified in the cheeses coated with starch in the two antimicrobial levels tested (M5 and M6) as well as for those coated with amylose and natamycin (M3 and M4), compared with the developments for cheeses coated with the corresponding polymer and without antimicrobial (M1 and M2), which showed greater development. At this storage time, an effect of the concentration of the active agent is also appreciated for the starch films, where the best performance was obtained by the sample M6, which showed a greater fungicidal effect.
For day 20, a similar trend was found regarding the effect of natamycin in both polymers, showing greater microbial development at a lower concentration of the active agent. When the effect of the polymer was analyzed, it was observed that, for all antimicrobial levels, the starch films behaved better than those of amylose. When the combined effect of both factors was analyzed, it was found that at high concentrations of natamycin, the predominant effect is that of the antimicrobial, and at low concentrations of natamycin, the predominant effect is that of the polymer.
After 30 days of storage, for the effect of the polymer, it was observed that, at high levels and without natamycin, starch generates less development than amylose. While at 1 mg/dm2, no significant difference is observed. If the effect of the concentration of natamycin in starch films is analyzed, significant differences were only observed for 10 mg/dm2. However, for amylose, the significance was appreciated in the coatings with and without natamycin.
The combined effect showed that the starch film with 10 mg of antimicrobial (M6) had the best performance, and the greatest development of molds and yeasts was for the amylose film (M1). The latter correlated perfectly with visual observation (Fig. 4). The behavior described in this last paragraph is still valid when the comparison is made with uncoated cheese, with no statistical significance between M0, M2, M4, and M5. Fajardo et al. (2010) found no significant differences (P < 0.05) in microbiological counts between coated and uncoated cheeses without inoculation at refrigerated temperatures, for chitosan and natamycin coatings. However, Ollé Resa et al. (2014a, b, 2016) found that cassava starch coatings containing natamycin and nisin as antimicrobials were effective in preventing the growth of molds and yeasts.
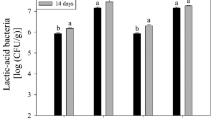
Ananalysis of total psychrophilic bacteria counts is shown in Fig. 7.
With storage time, an increase in the number of total psychrophilic bacteria was observed. For a storage of 20 days, significant differences were found between the samples coated with films with natamycin and the films without antimicrobial; there was no difference between the treatments with different concentrations of the same. For the maximum storage time (30 days), the M6 coating showed the best performance in controlling the growth of psychrophilic bacteria. This trend was shared, but to a lesser extent, by M4. Although natamycin is known to have antifungal properties, this study found that coatings containing the antimicrobial, preferably in concentrations of 10 mg/dm2, demonstrated moderate inhibitory activity in controlling bacterial growth. The authors of this work consider that this effect could be a consequence of the sodium chloride (NaCl) content with which natamycin is marketed, which for the films resulted in a surface area of 10 mg/dm2, just like the antimicrobial. NaCl inhibits the microbial growth by restriction of the available water (i.e., lowers aw) in the food products. Sallam and Samejima (2004) significantly delay the proliferation of psychrophilic when using NaCl and sodium lactate (NaL) in meats, managing to double the shelf life of the food. Azhdari and Moradi (2022) find a similar control result in the growth of psychrophiles in active CMC and natamycin films, but they attribute this effect to the protective activity of the film and not to the antifungal concentrations.
Conclusions
Based on the findings presented in this study, it can be concluded that it was possible to obtain ductile films of homogeneous thickness of cassava starch and amylose in low polymer concentrations, using only water as a plasticizer, by gelatinization at high temperature.
At high relative humidity storage, the coating acts as a protective barrier that decreases weight loss by up to 5% compared to untreated cheese. This effect is not observed at low storage RH.
The incorporation of natamycin into starch and amylose films will improve their antimicrobial properties and potentially extend the quality of the tested products. Visual inspection showed the development of molds and yeasts on the surface of control cheeses and those covered with films without antimicrobial, while no developments were evident in the activated film. The results showed that cassava starch films containing natamycin were effective in controlling the growth of molds, yeasts, and psychrophilic bacteria, with starch films plus 10 mg/dm2 of natamycin (M6) showing the best performance in this sense; however, the presence of natamycin was not found significantly in the cheese mass.
In addition, the study highlights that natamycin in the concentrations used does not affect the biodegradability of the films studied.
As a corollary, this study provides valuable insight into the potential of natamycin-containing starch-based films as a sustainable and effective solution to extend the food quality of products, while also considering the environmental impact of such materials. Further research in this area may help optimize the formulation and performance of antimicrobial films and explore their potential applications in a variety of industries.
Data Availability
The data are available from the corresponding author upon suitable request.
Abbreviations
- CS:
-
Cassava starch
- CMC:
-
Carboxymethyl cellulose
- M0:
-
Control cheese (without coating)
- M1:
-
Amylose-coated cheese (without antimicrobial)
- M2:
-
Starch-coated cheese (without antimicrobial)
- M3:
-
Cheese coated with amylose with 1 mg/dm2 of antimicrobial
- M4:
-
Cheese coated with amylose with 10 mg/dm2 of antimicrobial
- M5:
-
Cheese coated with starch with 1 mg/dm2 of antimicrobial
- M6:
-
Cheese coated with starch with 10 mg/dm2 of antimicrobial
- NaCl:
-
Sodium chloride
- NaL:
-
Sodium lactate
- WVP:
-
Water vapor permeability
- RH:
-
Relative humidity
- PRB:
-
Processed rice bran
References
Alves, V. D., Mali, S., Beléia, A., & Grossmann, M. V. E. (2007). Effect of glycerol and amylose enrichment on cassava starch film properties. Journal of Food Engineering, 78(3), 941–946. https://doi.org/10.1016/j.jfoodeng.2005.12.007
ASTM E 96. (2016). Standard test methods for water vapor transmission of materials, E 96/E 96M - 05. ASTM International, (October), 1–8.
Azhdari, S., & Moradi, M. (2022). Application of antimicrobial coating based on carboxymethyl cellulose and natamycin in active packaging of cheese. International Journal of Biological Macromolecules, 209(PB), 2042–2049. https://doi.org/10.1016/j.ijbiomac.2022.04.185
Basch, C. Y., Jagus, R. J., & Flores, S. K. (2013). Physical and antimicrobial properties of tapioca starch-HPMC edible films incorporated with nisin and/or potassium sorbate. Food and Bioprocess Technology, 6(9), 2419–2428. https://doi.org/10.1007/s11947-012-0860-3
Berti, S., Jagus, R. J., & Flores, S. K. (2021). Effect of rice bran addition on physical properties of antimicrobial biocomposite films based on starch. Food and Bioprocess Technology, 14(9), 1700–1711. https://doi.org/10.1007/s11947-021-02669-0
Berti, S., Ollé Resa, C. P., Basanta, F., Gerschenson, L. N., & Jagus, R. J. (2019). Edible coatings on Gouda cheese as a barrier against external contamination during ripening. Food Bioscience, 31(August), 100447. https://doi.org/10.1016/j.fbio.2019.100447
Bertuzzi, M. A., Castro Vidaurre, E. F., Armada, M., & Gottifredi, J. C. (2007). Water vapor permeability of edible starch based films. Journal of Food Engineering, 80(3), 972–978. https://doi.org/10.1016/j.jfoodeng.2006.07.016
Bertuzzi, M. A., Gottifredi, J. C., & Armada, M. (2012). Mechanical properties of a high amylose content corn starch based film, gelatinized at low temperature. Brazilian Journal of Food Technology, 15(3), 219–227. https://doi.org/10.1590/s1981-67232012005000015
Cerqueira, M. A., Sousa-Gallagher, M. J., Macedo, I., Rodriguez-Aguilera, R., Souza, B. W. S., Teixeira, J. A., & Vicente, A. A. (2010). Use of galactomannan edible coating application and storage temperature for prolonging shelf-life of “Regional” cheese. Journal of Food Engineering, 97(1), 87–94. https://doi.org/10.1016/j.jfoodeng.2009.09.019
Cuenca, P., Ferrero, S., & Albani, O. (2020). Preparation and characterization of cassava starch acetate with high substitution degree. Food Hydrocolloids, 100(August 2019), 105430. https://doi.org/10.1016/j.foodhyd.2019.105430
de Oliveira, T. M., & de Fátima Ferreira Soares, N., Pereira, R. M., & de Freitas Fraga, K. (2007). Development and evaluation of antimicrobial natamycin-incorporated film in gorgonzola cheese conservation. Packaging Technology and Science, 20(2), 147–153. https://doi.org/10.1002/pts.756
di Pierro, P., Sorrentino, A., Mariniello, L., Giosafatto, C. V. L., & Porta, R. (2011). Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT - Food Science and Technology, 44(10), 2324–2327. https://doi.org/10.1016/j.lwt.2010.11.031
Fajardo, P., Martins, J. T., Fuciños, C., Pastrana, L., Teixeira, J. A., & Vicente, A. A. (2010). Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. Journal of Food Engineering, 101(4), 349–356. https://doi.org/10.1016/j.jfoodeng.2010.06.029
Famá, L., Rojas, A. M., Goyanes, S., & Gerschenson, L. (2005). Mechanical properties of tapioca-starch edible films containing sorbates. LWT - Food Science and Technology. https://doi.org/10.1016/j.lwt.2004.07.024
Fringant, C., Rinaudo, M., Foray, M. F., & Bardet, M. (1998). Preparation of mixed esters of starch or use of an external plasticizer: Two different ways to change the properties of starch acetate films. Carbohydrate Polymers, 35(1–2), 97–106. https://doi.org/10.1016/S0144-8617(97)00250-6
García, M. A., Martino, M. N., & Zaritzky, N. E. (2000). Microstructural characterization of plasticized starch-based films. Starch/staerke. https://doi.org/10.1002/1521-379X(200006)52:4%3c118::AID-STAR118%3e3.0.CO;2-0
González-Forte, L. del S., Amalvy, J. I., & Bertola, N. (2019). Corn starch-based coating enriched with natamycin as an active compound to control mold contamination on semi-hard cheese during ripening. Heliyon, 5(6). https://doi.org/10.1016/j.heliyon.2019.e01957
González Seligra, P., Eloy Moura, L., Famá, L., Druzian, J. I., & Goyanes, S. (2016). Influence of incorporation of starch nanoparticles in PBAT/TPS composite films. Polymer International, 65(8), 938–945. https://doi.org/10.1002/pi.5127
Hernández, M. S., Ludueña, L. N., & Flores, S. K. (2023). Citric acid, chitosan and oregano essential oil impact on physical and antimicrobial properties of cassava starch films. Carbohydrate Polymer Technologies and Applications, 5. https://doi.org/10.1016/j.carpta.2023.100307
Jha, P. (2021). Functional properties of starch-chitosan blend bionanocomposite films for food packaging: The influence of amylose-amylopectin ratios. Journal of Food Science and Technology, 58(9), 3368–3378. https://doi.org/10.1007/s13197-020-04908-2
Johnston, D. A., Mukerjea, R., & Robyt, J. F. (2011). Preparation and characterization of new and improved soluble-starches, -amylose, and -amylopectin by reaction with benzaldehyde/zinc chloride. Carbohydrate Research, 346(17), 2777–2784. https://doi.org/10.1016/j.carres.2011.10.003
Laurindo, J., & do Prado, N. V., Morés, S., & Tonial, I. B. (2019). Quantification, migration and decline of natamycin in blue cheeses. Brazilian Journal of Food Technology, 22, 1–8. https://doi.org/10.1590/1981-6723.07718
Liu, Z. (2005). Edible films and coatings from starches. In Innovations in Food Packaging. https://doi.org/10.1016/B978-012311632-1/50051-6
López, O., & v., García, M. A., & Zaritzky, N. E. (2008). Film forming capacity of chemically modified corn starches. Carbohydrate Polymers, 73(4), 573–581. https://doi.org/10.1016/j.carbpol.2007.12.023
Lourdin, D., Coignard, L., Bizot, H., & Colonna, P. (1997). Influence of equilibrium relative humidity and plasticizer concentration on the water content and glass transition of starch materials. Polymer. https://doi.org/10.1016/S0032-3861(97)00082-7
Lucera, A., Mastromatteo, M., Conte, A., Zambrini, A., & v., Faccia, M., & del Nobile, M. A. (2014). Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Packaging and Shelf Life, 1(1), 25–29. https://doi.org/10.1016/j.fpsl.2013.10.002
Mali, S., Grossmann, M. V. E., García, M. A., Martino, M. N., & Zaritzky, N. E. (2006). Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. Journal of Food Engineering, 75(4), 453–460. https://doi.org/10.1016/j.jfoodeng.2005.04.031
Marcos, A. (1993). Water activity in cheese in relation to composition, stability and safety. Cheese: Chemistry, Physics and Microbiology, 439–469. https://doi.org/10.1007/978-1-4615-2650-6_11
Medina Jaramillo, C., Gutiérrez, T. J., Goyanes, S., Bernal, C., & Famá, L. (2016). Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydrate Polymers, 151, 150–159. https://doi.org/10.1016/j.carbpol.2016.05.025
Mohamed, S. A. A., El-Sakhawy, M., & El-Sakhawy, M. A. M. (2020). Polysaccharides, protein and lipid -based natural edible films in food packaging: A review. Carbohydrate Polymers, 238(February), 116178. https://doi.org/10.1016/j.carbpol.2020.116178
Mukerjea, R., & Robyt, J. F. (2010). Isolation, structure, and characterization of the putative soluble amyloses from potato, wheat, and rice starches. Carbohydrate Research, 345(3), 449–451. https://doi.org/10.1016/j.carres.2009.11.021
Ochoa, T. A., García-Almendárez, B. E., Reyes, A. A., Pastrana, D. M. R., López, G. F. G., Belloso, O. M., & González, C. R. (2017). Design and characterization of corn starch edible films including beeswax and natural antimicrobials. Food and Bioprocess Technology, 10(1), 103–114. https://doi.org/10.1007/s11947-016-1800-4
Ollé Resa, C. P., Gerschenson, L. N., & Jagus, R. J. (2013). Effect of natamycin on physical properties of starch edible films and their effect on saccharomyces cerevisiae activity. Food and Bioprocess Technology, 6(11), 3124–3133. https://doi.org/10.1007/s11947-012-0960-0
Ollé Resa, C. P., Gerschenson, L. N., & Jagus, R. J. (2016). Starch edible film supporting natamycin and nisin for improving microbiological stability of refrigerated argentinian Port Salut cheese. Food Control. https://doi.org/10.1016/j.foodcont.2015.06.056
Ollé Resa, C. P., Jagus, R. J., & Gerschenson, L. N. (2014a). Effect of natamycin, nisin and glycerol on the physicochemical properties, roughness and hydrophobicity of tapioca starch edible films. Materials Science and Engineering C, 40, 281–287. https://doi.org/10.1016/j.msec.2014.04.005
Ollé Resa, C. P., Jagus, R. J., & Gerschenson, L. N. (2014b). Natamycin efficiency for controlling yeast growth in models systems and on cheese surfaces. Food Control, 35(1), 101–108. https://doi.org/10.1016/j.foodcont.2013.06.049
Päivi, M., Riitta, P., Jukka, S., & Pirkko, F. (2002). Effect of glycerol on behaviour of amylose and amylopectin films. Carbohydrate Polymers, 50(4), 355–361. https://doi.org/10.1016/S0144-8617(02)00042-5
Paseiro-Cerrato, R., Otero-Pazos, P., Rodriguez-Bernaldo de Quirós, A., Sendón, R., Angulo, I., & Paseiro-Losada, P. (2013). Rapid method to determine natamycin by HPLC-DAD in food samples for compliance with EU food legislation. Food Control, 33(1), 262–267. https://doi.org/10.1016/j.foodcont.2013.03.006
Pérez, P. F., Ollé Resa, C. P., Gerschenson, L. N., & Jagus, R. J. (2021). Addition of zein for the improvement of physicochemical properties of antimicrobial tapioca starch edible film. Food and Bioprocess Technology, 14(2), 262–271. https://doi.org/10.1007/s11947-020-02565-z
Protzman, T., & Wagoner, J. (1964). |Gelatinizad starch products.
Ramallo, L. A., & Albani, O. A. (2004). Prediction and determination of water uptake in packaged yerba mate. Food Science and Technology International, 10(1), 35–40. https://doi.org/10.1177/1082013204041877
Robertson, G. L. (2013). Food packaging: principles and practice, third edition. In Taylor & Francis Group.
Romero, V., Borneo, R., Passalacqua, N., & Aguirre, A. (2016). Biodegradable films obtained from triticale (x Triticosecale Wittmack) flour activated with natamycin for cheese packaging. Food Packaging and Shelf Life, 10, 54–59. https://doi.org/10.1016/j.fpsl.2016.09.003
Sallam, K. I., & Samejima, K. (2004). Microbiological and chemical quality of ground beef treated with sodium lactate and sodium chloride during refrigerated storage. Lwt, 37(8), 865–871. https://doi.org/10.1016/j.lwt.2004.04.003
Sirisha Nallan Chakravartula, S., Lourenço, R. V., Balestra, F., Quinta Barbosa Bittante, A. M., Sobral, P. J. do A., & Dalla Rosa, M. (2020). Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packaging and Shelf Life, 24(February), 100498. https://doi.org/10.1016/j.fpsl.2020.100498
Slavutsky, A. M., & Bertuzzi, M. A. (2014). Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydrate Polymers, 110, 53–61. https://doi.org/10.1016/j.carbpol.2014.03.049
Tarvainen, M., Sutinen, R., Peltonen, S., Mikkonen, H., Maunus, J., Vähä-Heikkilä, K., Lehto, V. P., & Paronen, P. (2003). Enhanced film-forming properties for ethyl cellulose and starch acetate using n-alkenyl succinic anhydrides as novel plasticizers. European Journal of Pharmaceutical Sciences, 19(5), 363–371. https://doi.org/10.1016/S0928-0987(03)00137-4
Vierikova, M., Hrnciarikova, E., & Lehotay, J. (2013). Determination of natamycin content in cheese using ultra performance liquid chromatography-mass spectrometry. Journal of Liquid Chromatography & Related Technologies, 36(20), 2933–2943. https://doi.org/10.1080/10826076.2012.731671
Wolff, I. A., Davis, H. A., Cluskey, J. E., Gundrum, L. J., & Rist, C. E. (1951). Preparation of films from amylose. Industrial & Engineering Chemistry, 43(4), 915–919. https://doi.org/10.1021/ie50496a039
Xiong, H. G., Tang, S. W., Tang, H. L., & Zou, P. (2008). The structure and properties of a starch-based biodegradable film. Carbohydrate Polymers, 71(2), 263–268. https://doi.org/10.1016/j.carbpol.2007.05.035
Youssef, A. M., Assem, F. M., El-Sayed, S. M., Salama, H., & Abd El-Salam, M. H. (2017). Utilization of edible films and coatings as packaging materials for preservation of cheeses. Journal of Packaging Technology and Research, 1(2), 87–99. https://doi.org/10.1007/s41783-017-0012-3
Zhong, Y., Cavender, G., & Zhao, Y. (2014). Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT - Food Science and Technology, 56(1), 1–8. https://doi.org/10.1016/j.lwt.2013.11.006
Zou, Y., Yuan, C., Cui, B., Liu, P., Wu, Z., & Zhao, H. (2021). Formation of high amylose corn starch/konjac glucomannan composite film with improved mechanical and barrier properties. Carbohydrate Polymers, 251. https://doi.org/10.1016/j.carbpol.2020.117039
Acknowledgements
The authors are grateful to Laboratory BLB INGENIERIA INDUSTRIAL, for their assistance with the microbiological measurements.
Funding
Financial support for this study came from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) through Internal Doctoral Scholarship National Resolution No. 4823 awarded to Pamela Cuenca.
Author information
Authors and Affiliations
Contributions
Pamela Cuenca: methodology and experimental development, writing, image editing, statistical treatment, review. Oscar Albani: methodology and experimental development, writing, review.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cuenca, P., Albani, O. Biodegradable Active Films to Improve the Preservation of Regional Cheese During Refrigerated Storage. Food Bioprocess Technol 17, 217–230 (2024). https://doi.org/10.1007/s11947-023-03131-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03131-z