Abstract
This study aimed to characterize edible films based on potato starch and fermented and non-fermented whey solutions by Lactobacillus rhamnosus and Lactobacillus acidophilus and applied the formulated edible films to cover Manchego-type cheese to evaluate their quality characteristics during the storage using two different secondary packages. The results indicated that among quality characteristics evaluated, thickness (0.15–0.25 mm) and tensile strength (0.19–0.30 MPa) were significantly higher (compared to control edible films) in edible films formulated with fermented whey, regardless of the lactic acid bacteria (LAB) used. After 14 days of storage, the moisture content, weight loss, and color characteristics of cheese were maintained by both edible films and linear low-density polyethylene (LLDPE) bags. The application of edible films with fermented whey increases (4.21–5.98 log CFU/g) the content of beneficial microorganisms in the cheese, which are maintained during the storage (> 7 log cycles) regardless of the conditions. Consumer judges did not notice the application of edible films based on fermented whey to the cheese at the beginning and after 14 days of storage (LLDPE packaging), showing scores between I like and I like much in all samples evaluated. This study demonstrated that the fermentation process of whey solution for film forming and the employment of LLDPE as secondary packaging maintain the quality characteristics of Manchego-type cheese during storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing demand for health-promoting compounds such as antioxidants, antimicrobials, pigments, vitamins, probiotics, and prebiotics, to improve the physical and mental performances of human beings, is matching the consumer awareness about the quite amount of food waste, environmental damages, natural resources consumption, and social and economic consequences (Baker et al., 2022; Stancu & Lähteenmäki, 2022). These trends have had such an impact that the EAT-Lancet Commission on Food, Planet, Health has developed a sustainable guideline diet, which aims at reducing the incidence of mortality related to unhealthy diets and habits while improving food system sustainability, trying to avoid malnutrition and diseases in new generation. Therefore, looking for strategies to maintain a sustainable global food system for a healthy planet and people is nowadays necessary (Willett et al., 2019).
Food packaging is the most important element for protecting food products from microbiological, chemical, and environmental contaminants, assuring their quality during the food processing chain to the consumers (Pedreiro et al., 2021). Moreover, the packaging is considered the silent salesman because various information such as name, brand, labeling, and category, can be added, affecting consumer preferences (Bou-Mitri et al., 2020). Among different packaging materials used for food products, plastic is the most used; however, it presents a serious drawback due to its non-renewable characteristic, adding harmful chemical substances to food products, and waste disposal (Kumar et al., 2022). Therefore, developing new packaging material based on renewable resources, non-toxicity, and eco-friendly characteristics is nowadays a necessity (Garavand et al., 2022). In this sense, the development of edible films is an attractive alternative to meet consumer demands for health-promoting compounds and reduce some inconvenience presented by plastic materials (Summo & De Angelis, 2022). The edible film is a preformed thin layer (0.3 mm of thickness) of edible material that is applied to food products to increase their resistance and shelf life and carry edible additives such as those demanded by consumers (Díaz-Montes & Castro-Muñoz, 2021; Iversen et al., 2022; Jafarzadeh et al., 2021). Though edible films on food products have been increasing, their application is generally as primary packaging because they cannot meet all the barrier requirements on their own (Ceylan & Atasoy, 2023). Therefore, secondary packages (groups of individual units of primary packages) are required to both enhance mechanical resistance and provide information for consumers (Kumar et al., 2022; Ncube et al., 2021).
The application of edible films containing probiotics has been studied mainly in whole and fresh-cut fruits (Ceylan & Atasoy, 2023). However, few studies have been carried out on the application of edible film containing probiotics in cheeses (Ceylan & Atasoy, 2022; Guimarães et al., 2020). In these studies, a significant reduction of lactic acid bacteria during 15 days of storage was reported (2.5 to 5 log cycles), reaching undesirable levels to exert its beneficial effect on the gastrointestinal system. In this sense, although different treatments like lyophilization and encapsulation have been explored for protecting beneficial microorganisms during film-forming, food production, distribution, and retail, as well as in the gastrointestinal system (Chen et al., 2022; Espitia et al., 2016), the use of probiotics as a starter culture for the fermentation process of whey, which may increase their resistance for subsequent treatments such as those mentioned before, has not been studied yet. Moreover, during the fermentation process of whey by LAB starter cultures, some interesting health-promoting compounds related to the nervous system (gamma-aminobutyric acid), antihypertensive (angiotensin-converting enzyme inhibitors), antidiabetic, antioxidants, and antimicrobials are produced (Daliri et al., 2018; Jitpakdee et al., 2022; Kadyan et al., 2021; Rama et al., 2019). On the other hand, Manchego-type cheese, one of the most consumed cheeses worldwide, presents a high content of fat in its composition, which can be a problem due to lipid oxidation and its affectation in the sensory acceptance (color and flavor mainly) of cheeses (Sardiñas-Valdés et al., 2021). In this aspect, one of the advantageous characteristics of bioactive edible films based on protein is its capacity to avoid oxidation reaction due to its scavenging activity (Pop et al., 2020). Therefore, this study aims to formulate edible films based on LAB-fermented whey solution and potato starch and evaluate their effectiveness in improving some quality characteristics of Manchego-type cheese during storage at 4 °C using two kinds of secondary packages.
Materials and Methods
Material and Reagents
All chemical reagents, broth, and agar used in this study were obtained from Sigma-Aldrich, Inc. (Toluca, Mexico), J.T. Baker (Mexico City, Mexico), and BD Bioxon (Mexico City, Mexico), respectively. Whey powder (10% protein, 75% carbohydrates, 1.5% fat, and 1.15% of sodium) and potato starch (0.05% protein and 80.70% carbohydrates) were obtained from Food Technologies Trading (Mexico City, Mexico) and Fabsa (Mexico City, Mexico), respectively. Information about whey powder and potato starch was provided by the manufacturer.
Fermentation of Whey Solution
L. rhamnosus (NRRL B-442) and L. acidophilus (NRRL B-4495) were obtained from the microbiological collection of the Benemerita Universidad Autonoma de Puebla (Puebla, Puebla, Mexico). LAB were cultivated in MRS broth for 24 h at 37 ± 2 °C (stationary phase) under anaerobic conditions. Then, 1 mL of each culture was added to 100 mL of formulated whey solution (10% w/v), which was selected based on preliminary assays, and incubated at 37 ± 2 °C (anaerobic condition) for 24 h to reach approximately 1–2 \(\times\) 109 (CFU/mL). Fermented whey solution was used for further filmogenic preparation.
Filmogenic Formulation and Edible Film Production
To produce the filmogenic solution based on potato starch and fermented and non-fermented whey solutions, a starch aqueous solution (10%) was made and warmed up to 90 °C for 5 min. When the temperature of the starch solution decreased to 40 °C, four filmogenic solutions were made (Table 1) by mixing 50 mL of starch solution with 50 mL of distilled water (CEF), 50 mL of non-fermented whey solution (EFNF), and 50 mL of fermented whey solution by L. rhamnosus (EFLr) or L. acidophilus (EFLa). Finally, 4 mL of glycerol was added to each formulation as a plasticizer. The filmogenic solution was set aside for 2 h at room temperature to eliminate air bubbles. The casting method was used for film production (Aparicio-Fernández et al., 2018). Briefly, to obtain an edible film (10 cm \(\times\) 10 cm), approximately 46 g of filmogenic solution was poured into wax paper and placed in a food dehydrator (model 3926, Excalibur TB, USA) at 45 °C for drying until edible films reached 10–12% of moisture (4 h approximately). Edible films were then gently withdrawn from wax paper and used for characterization or immediately applied to cover commercial Manchego-type cheese.
Physical and Mechanical Characterization of Edible Films Based on Fermented and Non-fermented Whey and Potato Starch
Moisture
The moisture content of edible films was gravimetrically quantified by oven-drying at 105 °C until a constant weight was attained (925.25 method of the AOAC, 2000). The moisture content was calculated using the following equation:
where MC is the moisture content (%) and mwet and mdry are the wet and dry weights (g), respectively.
Color
The L* (luminosity), a*(+ red to -green), and b* (+ yellow to -blue) color parameters of edible films were evaluated using a precise colorimeter reader (TCR 200, TIME High Technology, Beijing). For reading, the colorimeter sensor was placed on the top of edible films and the measurement was conducted in five different points of edible films.
Thickness
The thickness (Th) of edible films was determined using the methodology proposed by Aparicio-Fernández et al. (2018). Five random measurements of edible films were taken using a millimeter micrometer with a sensitivity of 0.01 mm (IP54, Qfun, China).
Tensile Strength and Elongation at Break
Tensile strength (TS) and elongation at break (EAB) were studied using a texture analyzer (EZ-test, EZ-SX, Shimadzu corp., Japan). Film rectangles of 3.5 cm \(\times\) 6.0 cm were held tightly between mechanical grips at initial distance of 3 cm. The force (N) and deformation (mm) were recorded during extension at 60 mm/min. Mechanical properties were determined using the interface software TRAPEZIUM X Material Testing Operation Software V 1.4.0. TS and EAB were calculated following the ASTM D882 method (1995).
Water Barrier Property
The water vapor permeability (WVP) of edible films was gravimetrically measured using the ASTM E96 method (1980). Vessel sealed with edible films containing distilled water was placed at 4 ± 1 °C (45 ± 2% RH) and periodically weighted (1 h) for 10 h. The WVP was calculated using the next equation:
where WVTR is the water vapor transmission rate (g/h m2), Th is the thickness (mm), and ∆P is the difference between partial water vapor pressure (kPa).
Effect of Storage Condition on Some Quality Characteristics of Manchego-Type Cheese With and Without Edible Films
Edible Films’ Application on Manchego-Type Cheese and Storage Conditions
Edible films were superficially applied to squares (9 cm2) of commercial Manchego-type cheese slices (22% protein, 26% total fat, 1.2% carbohydrates, information provided by the manufacturer), trying to cover them completely (Fig. 1). Cheeses (4 pieces) with and without edible films were placed in two kinds of packages, polyethylene terephthalate (PET) container (15 cm \(\times\) 15 cm \(\times\) 10 cm) and LLDPE bags (17.7 cm \(\times\) 18.8 cm). Both packages were kept at 4 ± 2 °C (45 ± 5% RH) for 14 days. After this time, moisture, weight loss, color, LAB counts before and after gastrointestinal simulation, and sensory acceptance were evaluated.
Applied edible film (EF) to Manchego-type cheese for consumer acceptance test. Cheese + EFLa: cheese with edible film with fermented whey by L. acidophilus. Cheese + EFLr: cheese with edible film with fermented whey by L. rhamnosus. Cheese + EFNF: cheese with edible film with non-fermented whey. Cheese + CEF: cheese with control edible film
Moisture Content, Color, and Weight Loss of Manchego-Type Cheese With and Without Edible Films During Storage
The moisture content was evaluated according to the methodology described before for edible films. Weight loss was evaluated gravimetrically by recording the weight of treatments at the beginning and after storage time. Total color change (∆E) of cheese with and without edible films were evaluated following the next equation:
where \({L}^{*}\), \({a}^{*}\), and \({b}^{*}\), and \({L}_{0}^{*}\), \({a}_{0}^{*}\), and \({b}_{0}^{*}\) are the color parameters at the beginning and at the end of storage, respectively.
LAB Analysis Before and After Gastrointestinal Simulation
LAB were quantified in cheese with and without fermented edible films during the storage and before and after the gastrointestinal process following the methodology proposed by He et al. (2021) with some modifications. For LAB survival, 1 g of cheese with and without edible film was placed in a sterilized bag containing 9 mL of peptone water (0.1% w/v) to be processed with stomacher equipment (model 400, Seward, West Sussex, UK) for 5 min. For the gastrointestinal process, 1 g of cheese with and without edible film was submitted to a 25-mL gastric solution (2 h), formulated by solubilizing 3.2 g of pepsin and 2 g of NaCl in 1 L of sterile distilled water (pH = 2.0), adjusted with HCl. After 2 h of the gastric simulation, the sample was centrifuged (Premiere XC-2450, TX, USA) at 5000 rpm for 30 min. The precipitate was collected and mixed with 25 mL of intestinal solution (10 g of pancreatin and 6.8 g of K2HPO4 in 1 L of sterile distilled water, 7.0 pH adjusted with NaOH). After 3-h intestinal process (30 min at 5000 rpm), the precipitate was taken and mixed with 10 mL of distilled water for LAB survival analysis. LAB analysis in samples before and after the gastrointestinal simulation was performed by placing 1 mL of processed sample with 9 mL of peptone water until an appropriate count (30–300 CFU/g) was obtained. Samples were plated on MRS agar and stored at 37 ± 1 °C under an anaerobic environment. A microbial count was performed after 24 h of incubation.
Sensory Acceptance
To evaluate the sensory acceptance of Manchego-type cheese covered with and without edible films, sandwiches containing ham and cheese were made. The consumer acceptance was evaluated through a 7-point hedonic scale, where 1 means dislike very much and 7 means like very much (Greis et al., 2020). A square (9 cm2) of sandwich (at room temperature) was provided to fifty untrained judges who frequently consume a sandwich with cheese. The odor, flavor, texture, and overall acceptance of the sandwich were evaluated at the beginning and after 14 days of edible films storage (4 ± 1 °C).
Statistical Analysis
Formulation and application of edible films were performed in triplicate, and each test was performed in duplicate. Results were analyzed by comparison of means (α = 0.05) through analysis of variance (ANOVA) using Tukey´s test of Minitab 15 software (Minitab Inc., State College, USA).
Results and Discussions
Characterization of Edible Films Based on Fermented and Non-fermented Whey and Potato Starch
Physical and mechanical characteristics of edible films based on fermented and non-fermented whey and potato starch are presented in Table 2. As observed, the moisture content, L*, a*, b*, WVP, Th, and EAB, was not affected (p > 0.05) by the formulation composition of edible films showing values in the range of 11.37 to 13.93, 77.51 to 79.42, − 1.36 to 1.44, − 0.83 to 2.34, 8.82 to 34.40, 0.40 to 0.65, and 32.93 to 40.87, respectively. The moisture content of formulated edible films were in the range of the values reported by Kanmani and Lim (2013) who informed moisture values from 9.14 to 12.23% in edible films formulated with pullulan and potato starch with and without probiotic microorganisms such as L. plantarum, L. acidophilus, and L. reuteri. Moreover, as color parameters did not change due to the addition of whey regardless of the fermentation process, therefore, it is possible to indicate that the color characteristics of edible films are due to potato starch and glycerol. In this sense, Basiak et al. (2017) informed that the color parameters of edible films based on potato starch are 92.35 ± 0.87, 1.32 ± 0.14, and − 1.51 ± 0.39 for L*, a*, and b*, respectively. As is observed, luminosity is higher than those obtained in this study, which can be attributed to the glycerol content (0.8:1 glycerol:starch ratio against 0.3:1 glycerol:starch ratio), which reduces the luminosity as its concentration increased in the edible film formulations (Basiak et al., 2018). On the other hand, the results obtained in this study about Th, TS, and EAB are comparable to those results obtained by Guimarães et al. (2020), who indicated values of 0.17–0.23 mm, 0.5–0.6 MPa, and 48.1 to 53.8% for the Th, TS, and EAB, respectively, in edible films formulated with whey (10% protein) with and without L. buchneri. Moreover, as is observed, both Th and TS were significantly affected by the addition of fermented whey solution in the edible film formulation showing an increase of 0.15–0.25 mm and 0.19–0.30 MPa, compared to the control edible film. The interaction of protein from whey and starch reduced the intermolecular spacing via hydrogen bonds (Sogut et al., 2022). In this sense, a higher cross-linking degree (caused by the protein content) increases the density and strength of the film networks, parameters related to the Th and TS (Farajpour et al., 2020).
Effect of Storage Condition on the Quality Characteristics of Manchego-Type Cheese With and Without Edible Films
The moisture content and weight loss of cheese with and without edible films are shown in Fig. 2. As is observed, the moisture content of Manchego-type cheese (34.39 ± 0.67%) was lower when formulated edible films were applied for covering, reducing from 34.39 ± 0.67% to values in the range of 16.48–21.80%, which is due to edible films having low moisture content (Table 2, 11.37–13.93%). After 14 days of storage, cheeses stored using PET containers and LLDPE bags as secondary packages maintained their moisture content during the storage, while cheese without edible film and stored with PET significantly reduced their moisture content (10.6 ± 2.0%), which can be due to the higher headspace of PET container. In the same way, the higher weight loss is quantified in cheese without edible film (67.05 ± 3.8%) and stored in PET, whereas cheeses with edible films and stored in the same package showed a weight loss in the range of 72.01 to 82.01% (p > 0.05). It is noted that cheese with edible films formulated with fermented whey showed (p > 0.05) a lower weight loss (77.63–82.01%), probably caused by the high intermolecular-binding capacity of proteins, which improves the cross-linking between starch and proteins, enhancing the mechanical properties and reduces dehydration process of edible films (Kumar et al., 2022; Ribeiro et al., 2021). On the other hand, cheese with edible film stored in LLDPE bags did not present weight loss during storage, which is associated with its impermeable capacity of LLDPE lower headspace.
Table 3 shows the color parameters of cheese with and without edible films stored in different packages. The Manchego-type cheese without edible film presented a characteristic very light shade of yellow color (L* = 95.01, a* = − 0.94, and b* = 16.24), which changed to a light shade of yellow in cheese stored with PET, whereas cheese stored in LLDPE bags did not change its color characteristics after 14 days of storage. On the other hand, the application of edible film to cheese significantly affected the L* (23.4–25.2 units) and a* (4.7–6.5 units) color parameters of the samples, while b* was non-affected by the edible film application. In this aspect, despite raw materials (potato starch and whey) may affect the edible film color, it seems that as the edible film alone, the color characteristics are associated with potato starch since all edible films presented the same reduction in L* and a* color parameters. Similar behavior was reported by Mahcene et al. (2021) when home-made cheese was covered with an edible film based on sodium alginate; they informed a reduction of L* and a* color parameters while b* remained constant. On the other hand, in general, cheeses stored with LLDPE bags were not affected (p > 0.05) in their color characteristics, regardless of the use of edible films. However, cheeses with the edible films in PET containers presented significant (p < 0.05) changes in edible film color parameters during storage, associated with the oxygen contained in the headspace of the PET container, which led to oxidation processes. This effect is evident with the ∆E, where the storage in PET presented a higher color change (12.92–17.05), while cheeses with and without edible films stored in LLDPE did not show a significant effect on the color change ranging from 3.21 to 10.25. The ∆E found in this study are in the range of those obtained by Ramos et al. (2012), who informed values between 5–19 units of color change in semihard cheeses coated with edible solution based on whey protein isolate. However, higher ∆E (17–22 units) were reported in “regional” cheese with edible film stored at 4 and 20 °C for 15 days (Cerqueira et al., 2010). Both authors concluded that the color change observed in cheese is caused by the oxidation and dehydration processes.
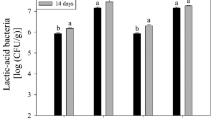
Table 4 presents the LAB survival in cheese with and without edible films before and after gastrointestinal simulation at the beginning and after 14 days of storage. Manchego-type cheese contains an initial amount of LAB (3.77 log CFU/g), which is maintained during storage regardless of the packages. The application of both edible films based on fermented whey with L. acidophilus and L. rhamnosus significantly increased the LAB count in 3.97 and 5.1 log cycles, respectively. The increase in probiotic microorganisms is of paramount importance because they can exert their health benefits (improve the intestinal microbial balance and the immune system, reduce pathogen infection, alleviate inflammatory diseases, etc.) in the gastrointestinal system at a count higher than 106–7 CFU/mL or g (Misra et al., 2022). On the other hand, during storage, it is informed that the LAB load added to the edible films was reduced by 20–35% and 25 to 90% after 20 days of storage at room temperature and 4 °C, respectively, in different edible films based on pullulan/potato starch and pullulan/tapioca starch combinations (Kanmani & Lim, 2013). In this study, the LAB concentration at the end of the storage was higher than 7.17 log cycles, which is approximately 90% of the initial microbial load, regardless of the storage packaging. This advantage may be associated with the use of probiotics as starter cultures for the fermentation process of whey, which may induce a stress-tolerance due to luxS gene biosynthesis, which appears to have a clear role in acidic stress response in probiotic lactobacilli (Mohammadi et al., 2012). In addition, the use of protein in edible film formulation resulted in higher viability of the probiotic strains because proteins can scavenge free radicals and supply micronutrients essential for the survival of the probiotic (Pop et al., 2020). Interestingly, a “higher” microbial count was observed when the gastrointestinal fluids were used, probably caused by the degradation process of the edible film and the consequence liberation of fermentation induced resistance LAB. In this sense, when L. rhamnosus was added without protection in the formulation of goat cheese, a microbial count reduction of 4.2 and 2.3 log cycles were observed after gastric and intestinal simulations, respectively, which confirm the relevance of the application of edible films as protecting agent of probiotic microorganisms (Martins et al., 2018).
Sensory Acceptance
Based on the results presented above, sensory acceptance of edible films stored with LLDPE bags using commercial box bread and ham as a carrier was conducted (Fig. 1). As is observed in Fig. 3, the scores obtained for odor, flavor, texture, and overall acceptance of cheese and cheese with the edible film were similar (p > 0.05), with values in the range of 5–6, which means I like–I like much, at the beginning and at the end of the storage (14 days). Therefore, the consumers did not notice the addition of edible film with LAB to the cheese, which may be due to both products having the same food lactic origin. In this sense, Papadopoulou et al. (2022) indicated that commercial Gouda cheese (a similar lactic acid flavor to Manchego cheese) was not affected by the addition of edible films added with L. acidophilus due to among all acids produced by L. acidophilus during the fermentation process, lactic acid is the most predominant acid. Moreover, Zoghi et al. (2020) indicated that edible films containing probiotics do not alter the sensory characteristics of the applied food product. Mozzarella cheese coated based on alginic acid added with different concentrations of potassium sorbate was sensory evaluated using a 7-points hedonic scale. Results indicated that mozzarella cheeses with and without edible films were well-accepted (> 4 on the hedonic scale) during the first 3-days storage using polypropylene trays; after that, cheeses were refused by the judges (Lucera et al., 2014).
Conclusions
Edible films based on potato starch and non-fermented and fermented whey by LAB were developed and applied to Manchego-type cheese to evaluate their effect during the storage using two kinds of packages. In general, the addition of whey regardless of fermentation process on the edible film formulation does not affect the physical and mechanical characteristics of edible films, being TS the factors that was improved by the addition of fermented whey. On the other hand, the application of edible films based on potato starch and fermented whey by LAB onto cheese and the effect of storage packaging was studied. Results indicated that LLDPE bags maintained the moisture content, weight, and color characteristics of cheese during the storage time. Moreover, the edible film significantly increased the probiotic content of cheese without affectation by the packages and gastrointestinal simulation, maintaining a viable count of both L. acidophilus and L. rhamnosus (> 7 log cycles) after 14 days of storage. Cheese with edible films was well-accepted by the consumers without affectation of the storage time and the beneficial starter fermentation microorganisms. This study indicated that LAB as a starter culture for fermentation is useful for improving their resistance to subsequent processes and maintaining an adequate microbial load in edible films during storage using both LLDPE and PET packages.
Data Availability
The datasets obtained during the current study are available from the corresponding author upon reasonable request.
References
AOAC. (2000). Official methods of analysis of the association of official analytical chemists, 20th ed.; AOAC International: Washington, DC, USA.
Aparicio-Fernández, X., Vega-Ahuatzin, A., Ochoa-Velasco, C. E., Cid-Pérez, S., Hernández-Carranza, P., & Ávila-Sosa, R. (2018). Physical and antioxidant characterization of edible films added with red prickly pear (Opuntia ficus-indica L.) cv. San Martín peel and/or its aqueous extracts. Food and Bioprocess Technology, 11, 368–379.
ASTM. (1995). Standard test method for tensile properties of thin plastic sheeting; method D882-American Society for testing and materials; ASTM: West Conshohocken. PA.
ASTM. (1980). Standard test method for water vapor transmission of materials; method E96-American Society for testing and materials; ASTM: West Conshohocken. PA.
Baker, M., Lu, P., Parrella, J., & Leggette, H. (2022). Consumer acceptance toward functional foods: A scoping review. International Journal of Environmental Research and Public Health, 19, 1217.
Basiak, E., Lenart, A., & Debeaufort, F. (2017). Effect of starch type on the physico-chemical properties of edible films. International Journal of Biological Macromolecules, 98, 348–359.
Basiak, E., Lenart, A., & Debeaufort, F. (2018). How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers, 10, 412.
Bou-Mitri, C., Abdessater, M., Zgheib, H., & Akiki, Z. (2020). Food packaging design and consumer perception of the product quality, safety, healthiness and preference. Nutrition and Food Science, 51, 71–86.
Cerqueira, M., Sousa-Gallagher, M. J., Macedo, I., Rodríguez-Aguilera, R., Souza, B., Teixeira, J., & Vicente, A. (2010). Use of galactomannan edible coating application and storage temperature for prolonging shelf-life of “Regional” cheese. Journal of Food Engineering, 97, 87–94.
Ceylan, H. G., & Atasoy, A. F. (2022). Optimization and characterization of prebiotic concentration of edible films containing Bifidobacterium animalis subs. lactis BB-12® and its application to block type processed cheese. International Dairy Journal, 134, 105443.
Ceylan, H. G., & Atasoy, A. F. (2023). New bioactive edible packing systems/symbiotic edible films/coatings as carries of probiotics and prebiotics. In Press.
Chen, W., Ma, S., Wang, Q., McClements, D. J., Liu, X., Ngai, T., & Liu, F. (2022). Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Critical Reviews in Food Science and Nutrition, 62, 5029–5055.
Daliri, E. B. -M., Lee, B., Park, B. -J., Kim, S. -H., & Oh, D. -H. (2018). Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Science and Biotechnology, 27, 1781–1789.
Díaz-Montes, E., & Castro-Muñoz, R. (2021). Edible films and coatings as food-quality preservers: An overview. Foods, 10, 249.
Espitia, P. J. P., Batista, R. A., Azeredo, H., & Otoni, C. G. (2016). Probiotics and their potential applications in active edible films and coatings. Food Research International, 90, 42–52.
Farajpour, R., Emam, Z., Moeini, S., Tavañolipour, H., & Safayan, S. (2020). Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with zein nanoparticles. International Journal of Biological Macromolecules, 149, 941–950.
Garavand, F., Khodaei, D., Mahmud, N., Islam, J., Khan, I., Jafarzadeh, S., Tahergorabi, R., & Cacciotti, I. (2022). Recent progress in using zein nanoparticles-loaded nanocomposites for food packaging applications. Critical Reviews in Food Science and Nutrition, 12, 1–21.
Greis, M., Sainio, T., Katina, K., Nolden, A., Partanen, R., & Seppä, L. (2020). Dynamic textura perception in plant-based yogurt alternatives: Identifying temporal drivers of liking by TDS. Food Quality and Preference, 86, 104019.
Guimarães, A., Ramos, O., Cerqueira, M., Venãncio, A., & Abrunhosa, L. (2020). Active whey protein edible films and coatings incorporating Lactobacillus buchneri for Penicillium nordicum control in cheese. Food and Bioprocess Technology, 13, 1074–1086.
He, C., Sampers, I., & Raes, K. (2021). Dietary fiber concentrates recovered from agro-industrial by-products: Functional properties and application as physical carriers for probiotics. Food Hydrocolloids, 111, 106175.
Iversen, L., Rovina, K., Vonnie, J., Matanjun, P., Erna, K., ‘Aqilah, N., Felicia, W., & Funk, A. A. (2022). The emergence of edible and food-application coatings for food packaging: A review. Molecules, 27, 5604.
Jafarzadeh, S., Salehabadi, A., Mohammadi, A., Oladzadabbasabadi, N., & Jafari, S. M. (2021). Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends in Food Science & Technology, 116, 218–231.
Jitpakdee, J., Kantachote, D., Kanzaki, H., & Nitoda, T. (2022). Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting. LWT-Food Science and Technology, 160, 113269.
Kadyan, S., Rashmi, H. M., Pradhan, D., Kumari, A., Chaudhari, A., & Deshwal, G. K. (2021). Effect of lactic acid bacteria and yeast fermentation on antimicrobial, antioxidative and metabolomic profile of naturally carbonated probiotic whey drink. LWT-Food Science and Technology, 142, 111059.
Kanmani, P., & Lim, S. T. (2013). Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chemistry, 141, 1041–1049.
Kumar, L., Ramakanth, D., Akhila, K., & Gaikwad, K. (2022). Edible films and coatings for food packaging applications: A review. Environmental Chemistry Letters, 20, 875–900.
Lucera, A., Mastromatteo, M., Conte, A., Zambrini, A. V., Faccia, M., & Del Nobile, M. A. (2014). Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Packaging and Shelf Life, 1, 25–29.
Mahcene, Z., Khelil, A., Hasni, S., Bozkurt, F., Bilal, M., & Tornuk, F. (2021). Home-made cheese preservation using sodium alginate based on edible film incorporating essential oils. Journal of Food Science and Technology, 58, 2406–2419.
Martins, J. B. A., Deliza, R., dos Santos, K. M. O., Walter, E. H. M., Martins, J. M., & Rosenthal, A. (2018). Viability of probiotics in goat cheese during storage and under simulated gastrointestinal conditions. Food and Bioprocess Technology, 11, 853–863.
Misra, S., Pandey, P., Dalbhagat, C. G., & Mishra, H. N. (2022). Emerging technologies and coating materials for improved probiotication in food products: A review. Food and Bioprocess Technology, 15, 998–1039.
Mohammadi, R., Sohrabvandi, S., & Mohammad, A. (2012). The starter culture characteristics of probiotic microorganisms in fermented milks. Engineering in Life Sciences, 4, 399–409.
Ncube, L., Ude, A., Ogunmuyiwa, E., Zulkifli, R., & Beas, I. (2021). An overview of plastic waste generation and management in food packaging industries. Recycling, 6, 12.
Papadopoulou, O. S., Argyri, A. A., Bikouli, V. C., Lambrinea, E., & Chorianopoulos, N. (2022). Evaluating the quality of cheese slices packaged with Na-alginate edible films supplemented with functional lactic acid bacteria cultures after high-pressure processing. Foods, 11, 2855.
Pedreiro, S., Figueirinha, A., Sanches, A., & Ramos, F. (2021). Bioactive edible films and coatings based in gums and starch: Phenolic enrichment and foods application. Coatings, 11, 1393.
Pop, O., Pop, C., Dufrechou, M., Vodnar, D., Socaci, S., Dulf, F., Minervini, F., & Suharoschi, R. (2020). Edible films and coating functionalization by probiotic incorporation: A review. Polymers, 12, 12.
Rama, G., Kuhn, D., Beux, S., Maciel, M., & de Souza, C. F. (2019). Potential applications of dairy whey for the production of lactic acid bacteria cultures. International Dairy Journal, 98, 25–37.
Ramos, Ó., Pereira, J., Silva, S., Fernandes, J., Franco, M., Lopes-da-Silva, J., Pintadoo, M. E., & Malcata, F. (2012). Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. Journal of Dairy Science, 95, 6282–6292.
Ribeiro, A. M., Estevinho, B. M., & Rocha, F. (2021). Preparation and incorporation of functional ingredients in edible films and coatings. Food and Bioprocess Technology, 14, 209–231.
Sardiñas-Valdés, M., García-Galindo, S., Chay-Canul, A. J., Velázquez-Martínez, J. R., Hernández-Becerra, J. A., & Ochoa-Flores, A. A. (2021). Ripening changes of the chemical composition, proteolysis, and lipolysis of a hair sheep milk Mexican Manchego-style cheese: Effect of nano-emulsified curcumin. Foods, 10, 1579.
Sogut, E., Filiz, E., & Seydim, A. C. (2022). Whey protein isolate- and carrageenan-based edible films as carriers of different probiotic bacteria. Journal of Dairy Science, 105, 4829–4842.
Stancu, V., & Lähteenmäki, L. (2022). Consumer-related antecedents of food provisioning behaviors that promote food waste. Food Policy, 108, 102236.
Summo, C., & De Angelis, D. (2022). The importance of edible films and coatings for sustainable food development. Foods, 11, 3221.
Willett, W., Rockström, J., Loken, B., Springmann, M., Lang, T., Vermeulen, S., Garnett, T., Tilman, D., DeClerck, F., Wood, A., Jonell, M., Clark, M., Gordon, L., Fanzo, J., Hawkes, C., Zurayk, R., Rivera, J., De Vries, W., Sibanda, L., … & Murray, C. (2019). Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. The Lancet Commissions, 393, 447–492.
Zoghi, A., Khosravi-Darai, K., & Mohammadi, R. (2020). Application of edible films containing probiotics in food products. Journal of Consumer Protection and Food Safety, 15, 307–320.
Funding
This study was financed by the Vicerrectoría de Investigación y Estudios de Posgrado of the Benemérita Universidad Autónoma de Puebla (Project number: 000128-VIEP2022).
Author information
Authors and Affiliations
Contributions
Fierro-Corona, Guadalupe: Conceptualization, data curation. Ruiz-López, Irving Israel: Supervision, validation, visualization, writing—original draft. Hernández-Carranza, Paola: Investigation, methodology, project administration. Ochoa-Velasco, Carlos Enrique: Conceptualization, formal analysis, funding acquisition, writing—original draft.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guadalupe, FC., Israel, RL.I., Enrique, OV.C. et al. Effect of Edible Films’ Application on the Quality Characteristics of Manchego-Type Cheese During Storage. Food Bioprocess Technol 16, 2910–2920 (2023). https://doi.org/10.1007/s11947-023-03120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03120-2