Abstract
The diversity of cheese varieties, dynamic microbiological and biochemical changes which undergo during cheese manufacture, ripening and marketing need special care to meet the desired consumer acceptability and satisfaction. In this area packaging plays an important role in cheese industry. Application of films or coatings to cover cheese surfaces has long been recognized as an important treatment for the protection of the quality and safety of the product. The growing environmental concern about the use of un-biodegradable materials in cheese packaging encouraged research to develop edible materials for coating and packaging of cheese. Several polysaccharides and proteins with or without plasticizers, antimicrobial agents and nanoparticles have been used as basis for the development of edible cheese coating. The present manuscript gives an overview on the development and use of edible cheese coating in relation to the cheese quality and safety.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cheese manufacture is one of the oldest food technologies carried out to concentrate and preserve nutrients for extended consumption. Nowadays cheese is one of the most important dairy products worldwide in terms of the quantity and diversity. In 2014, the recorded cheese production amounted to 19.6 thousand metric tons [38] made from about 25% of the global milk production. More than 1000 cheese varieties are produced worldwide [57], each has its unique shape, size, flavor and texture. Manufacture of cheese involves variable steps and additives leading to a product of characteristic properties. The ripening step in cheese manufacture involves a dynamic biochemical and microbiological processes that should be controlled in order to achieve the desired product. Failure to control these processes leads to variable defects in the product due to internal or external factors. Covering cheese by a film or coating before ripening or during storage may overcome most of these defects and to extend the shelf life of the product [93]. Therefore, application of films or coatings to cover cheese surfaces has long been recognized as an important treatment for the protection of the quality and safety of the product during processing and marketing until it reach the consumer. The consumer preference becomes an important factor in cheese marketability whereas the outer appearance of the product is one of its elements. In this area, selection of packaging and coating of cheese plays an important factor in determining the consumer preference for the product [20, 61].
The use of traditional (non-biodegradable) packaging and non-renewable materials such as plastics, glass, and metals in packaging applications represents a serious environmental problem worldwide. Due to the increased concern about environmental pollution there is a need for recycling or safe disposal of such waste and/or to develop environment friendly packaging materials [70, 90,91,92]. Massive amounts of packaging materials are produced every year with the purpose of use and throw [53]. Different methods applied for the disposable of wasted non-biodegradable packaging materials give rise to green house gases, a potential environmental threat affecting human health [6, 15, 78, 88].
Several efforts have been done to extend the shelf life of food products and in the same time overcome the environmental problem of handing package waste led to a growing interest in biodegradable materials particularly those from food sources namely: the edible films [26, 28, 29, 82]. Biodegradation of these films achieved by aerobic or non-aerobic fermentation with micro-organisms which excrete extracellular enzymes to hydrolyze the polymeric chains in the packaging material to small molecular weight degradation products. These products are then transferred into the microbial cells, whereas most of the biochemical changes happen and ending with the formation of environmentally safe metabolites (Fig. 1).
General mechanism of plastic biodegradation [81]
Edible coatings and films are two expressions used interchangeably in food packaging, to indicate that the surface of a food is covered by a thin layer of a food biopolymer based material. However, a film differs from a coating in that the film is prepared separately and then applied on the food as a wrapping material, whereas a coating can be a suspension or an emulsion applied directly to form a film on the cheese surface [45, 90,91,92]. In addition to its biodegradability, the use of edible coatings/films can satisfy the needs for economic and feasible way to assure and preserve the quality, safety and nutritional value of foods until it reach the consumer. Several advantages and benefits can be achieved from the application of edible films in food chain for instance protecting the food from physical damage, microbiological and chemical deterioration, lowering moisture losses and controlling gas exchange [72]. Selection of the appropriate film or coating represents a challenge to cheese manufacturers. Cheese will continue to undergo ripening changes after coating or packaging. Ideal film or coating should not interfere with the normal biochemical and microbiological changes during cheese maturation. In the meantime cheese coating should offer protection and enhancement of the shelf life of the cheese [90,91,92].
Current packaging has progressed to meet the global trends and consumer perception. Efforts have been focused on better cheese quality and safety [87] to provide consumer with essential product information and to simplify the promotion and marketability needs. The present review overviews the recent trends in the application of edible films and coatings in the preservation and extending the shelf life of different varieties of cheese.
Cheese
Cheese is a generic name of large number of milk based products of diversified flavor, texture, sizes, and origin. Cheese is made via coagulation of the casein of milk and entrapment of fat in the formed coagulum. Coagulation of milk is carried out by either rennet enzyme or by acidification [25]. Cheese is a product of concentrated proteins and fats frequently made from cows, buffalo sheep or goats.
Classification and Characteristics
Several methods have been used to classify the cheese varieties including, cheese moisture content, degree of cheese ripening, method of coagulation coupled with other factors [57]. However, the most acceptable classification is based on the texture and moisture content of the cheese whereas cheese being classified as, hard, semi-hard, and soft cheeses. From the packaging point of view, cheeses are classified into three groups; the hard and semi-hard group, the soft group, and the fresh group [67]. The fresh cheeses are considered as soft cheeses and they are characterized by high moisture content and limited shelf life.
Cheese Manufacture
The aim from cheese manufacture is to concentrate milk fat and proteins in variable volumes of the aqueous phase of milk, addition of table salt as flavor enhancer followed by ripening of the obtained green cheese through different microbiological and biochemical processes. This can be achieved through variable combinations of different steps depending on the target cheese to produce. These steps can be summarized in the following:
-
Coagulation of cheese and curd formation Enzymatic coagulation using chymosin is followed in the preparation of rennet coagulated cheeses, whereas fermentation with selected lactic acid bacteria or the addition organic acids in acid coagulated cheeses. Addition of starters is normally practiced in rennet coagulation cheese in order to enhance cheese ripening in further steps.
-
Cutting and partial removal of whey from the formed curd The formed curd is cut into cubes of variable sizes in order to enhance the exudation of whey from the curd and the exuded whey is removed by drainage.
-
Scalding The curd cubes are stirred and heated to a scolding temperature in order to obtain the desired cheese grains and to remove excess whey.
-
Molding of cheese Cheese grains are transferred to specific molds for each cheese, and then pressed to allow fusion of the cheese grains, to remove the excess whey and control the moisture content of the green cheese [36].
-
Salting of cheese Green cheeses are either surface salted with dry salt or salted in brine. In some cheeses salt is added to the curd during filling the molds.
-
Cheese coating Cheeses ripened internally with the cheese microflora are usually coated before ripening in order to control the surface dryness and moisture and weight losses during ripening. This step is not applied to surface ripened cheeses with molds or bacteria.
-
Cheese ripening Cheeses are stored at controlled conditions of temperature and relative humidity for variable time for each cheese variety to develop its desirable characteristic properties. During this period cheese constituents undergo several changes by the action of the residual enzymes and growth and activity of the starter and non-starter microorganisms. The biochemical changes that occur during ripening are regulated by the water activity, fat and salt contents, and cheese microflora and determine the flavor, aroma, and texture of the cheese [67]. The overall changes results in developing the characteristic flavor and texture of the cheese type. Failure to control the ripening conditions results in the development of several microbiological and chemical defects of which those related to cheese quality and consumer acceptability can be prevented by suitable coating and packaging of cheese.
Cheese Defects
Several microbiological and chemical defects can be developed in cheeses due to uncontrolled processing and ripening conditions [57]. However, discussion is limited here to those related to the cheese packaging. The consumer acceptability for cheese is related mainly to the appearance and sensory properties of the cheese. Failure to acquire the characteristic flavor or appearance results in consumer rejection of the cheese. Surface growth of molds and yeasts yields color spots that signifies the main visual appearance cheese defect [51, 93]. In addition, some of fungi represent safety concern as they can produce mycotoxins. Practically cheese manufactures use sorbate or natamycin to control surface growth of contaminating molds, but sorbates diffuses faster and more than natamycin into cheese casing undesirable changes in the cheese flavor. The use of active coating could be a better alternative for controlling the defects which get up from surface growth of microorganisms. Loss of cheese weight and drying of cheese rind represent an economic loss in addition to poor cheese quality. This defect arises from excessive water evaporation due to uncontrolled ripening conditions (high temperature and low relative humidity) and/or non-coating of cheese or the use of coating with poor moisture barrier [67]. Edible coatings and films were used to dispose of these defects.
Edible Coatings and Films
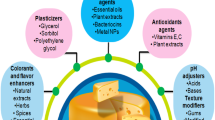
Edible coatings and films can be made from numerous food grade biopolymers as the basic film forming materials in addition to plasticizers, surfactants, antimicrobial agents and nanoparticles [59, 60, 85].
Food Grade Biopolymers
Generally, proteins, polysaccharides and lipids can be used in the preparation of edible films and coatings. They can be used in individually or as mixed blends for this purpose [26]. Each has its own advantages and limitations. The use of proteins can result in films of good mechanical stability, but has the disadvantage of low moisture and gas barrier. Films based on polysaccharides are characterized by controlled gas transfer and structural stabilities but have low moisture retention [21, 28, 29]. Waxes are the oldest material used for cheese coating. It offers the advantage of preventing moisture losses but results in almost an anaerobic fermentation due to inability to permit gas transfer [68, 89].
To attain good value and safety of foodstuffs through packing, transportation, and to extend the shelf-life of food via avoiding disapproving factors or conditions for example spoilage microorganisms, chemical impurities, light, oxygen, moisture, etc., biocomposites nanopackaging films offer physical safety and produce suitable physicochemical situations for products that are vital to gain a suitable shelf life as well as preserving food safety and quality [5, 14, 50, 77, 86]. The food package would delay gain or loss of moisture, prevent or reduce microbial contamination. Also, it acts as a barrier against permeation of water vapor, oxygen, carbon dioxide and other volatile compounds. The basic properties of packaging materials such as mechanical, optical, and thermal properties determine their capabilities to achieve these functions [30, 32, 53, 79].
Chitosan
Chitosan is a linear cationic homo polysaccharide composed of glucosamine residues in β-linkage. It is obtained from deacetylation of chitin the second most abundant polysaccharide in nature after cellulose [2]. In addition to the amino group, chitosan has reactive hydroxyl groups at C-2, C-3 and C-6 position. The chemical structure of chitosan makes it easily modified to form an immobilization support for bioactive molecules. Chitosan is not soluble in water but soluble in dilute acetic acid or dilute HCl. Chitosan is non-toxic biocompatible, biodegradable and its solutions can be casted to form a strong flexible film difficult to tear [62]. Also, chitosan (Fig. 2) exhibited antimicrobial activity [90,91,92,93].
Schematic represents the methodology of “from chitosan to nanocomposites and its antibacterial properties” [90]
Galactomannans
Galactomannans are polysaccharides found in the endosperm of numerous plants such as guar gum and locust bean gum. They are composed of a β-(1–4)-d-mannan backbone with a single d-galactose branch linked α-(1–6). The most important parameters that determine the film forming properties of galactomannans are the mannose/galactose (M/G) ratio, the average molecular weight, the fine structure, and the intrinsic viscosity [62]. The guar gum has M/G ratio of 2:1 while locust bean gum has a ratio of 3.5:1. As a general rule, gums that are cold water soluble are also freeze–thaw stable.
Alginates
Alginates are highly anionic linear, unbranched polymers containing β-(1-4)-linked d-mannuronic acid (M) and α-(1-4)-linked l-guluronic acid (G) units. Sodium alginate solutions directly form gel on addition of calcium or any divalent cation where Ca ion form salt bridges corresponding to junction zones between adjacent polymer chain. Sodium alginate forms a decent strong film, despite the negative charge on the molecule [62].
Carrageenans
They are sulfated polysaccharides made up of alternating galac to pyranosyl units dimer linked by alternating β-(1,4) and α-(1,3) glycosidic bonds.[62]. Based on the degree of sulfation carrageenans are classified into kappa (κ, low sulfation), iota (ι, medium sulfation) and (δ, high sulfation) which affect their solubility in water, δ soluble in cold water and while heating to 82 °C is needed to solubilize κ-carrageenan. Among the three carrageenan κ produces the strongest film and δ the lowest but they exhibit similar puncture force [62].
Carboxy Methyl Cellulose (CMC)
Carboxy methyl cellulose is an anionic cellulose derivative used in food applications for its viscosity. It can form films with different strength depending on the molecular weight and degree of substitution. Generally the strength of the formed films is less than that produced from alginate from solutions of similar concentration.
Starch and Starch Derivatives
Starch and starch derivatives has a long history in producing edible coatings [46]. Their low cost has been a motive behind their wide use in the coating of several foods. The use of high amylose corn starch results in films of improved properties. Chemical substitution and acid hydrolysis of amylose-containing starches improve the clarity and flexibility of coatings made from them. Addition of plasticizers is effective in improving the film properties but on expense of its barrier characteristics [46].
Proteins
Several proteins have been used in the preparation of edible films and coating such as whey protein, gelatin, zein and casein. Proteins are superior to polysaccharides as film formers exhibiting excellent gas and lipid barrier properties, [82], particularly at low relative humidity. However, protein films exhibit poor water resistance and susceptibility to cracking due to the strong cohesive energy density of the polymer. In order to improve their water resistance and mechanical properties proteins have been subjected to cross linking by means of chemical agents such as glutaraldehyde or enzymatic reaction using trans-glutaminase, or physical treatments such as heating, irradiation [82].
Whey Proteins
Whey proteins are a group of globular proteins with β-lactoglobulin and α-lactalbumin as the major constituents [1]. The commercial preparations of whey proteins include whey protein concentrates (WPC), which have variable protein contents (35–80%) and whey protein isolates (WPI) of higher protein content (>90%). These preparations have been used extensively as nanodelivery vehicles for many bioactive food ingredients. Two different methods can be used to prepare edible films from whey proteins namely: the wet and dry processing [35]. The wet or solvent casting method, is the most widely used method. In the dry process, films are produced by extrusion and compression-moulded, using the thermoplastic properties of the protein. Edible films prepared from whey were reported to possess excellent resistance to tension and puncture depending on the heat treatment received. Also, the microstructure of these films depends on the WPI concentration and pH used [69]. Whey proteins was used to replace the synthetic oxygen barrier in plastic films [8, 43, 44] and the resultant laminated films was reported to have excellent gas barrier.
Zein
Zein is the major storage protein of maize (Zea mays). It belongs to the prolamine group of proteins which is characterized by their hydrophobicity and solubility in aqueous ethanolic solutions [1, 64]. Also, it may exhibit mucoadhesive properties. It consists of consists of four major (α-β-γ- and ô-zein) of which the α-component accounts for almost 85% of the whole protein. It has been used extensively for encapsulation of hydrophobic ingredients and has been used in preparation of edible cheese coating. Zein can interact with other charged biopolymers, such as caseinate and chitosan to form hybrid particles.
Plasticizers
The use of food grade biopolymers only in the preparation of edible coatings/films yields brittle and stiff films due to the excessive interactions between the polymer molecules [74]. Plasticizers are hydrophilic molecules, such as polyols, supplementary to the film forming materials with the purpose of develop the physical and mechanical properties of the film [82]. The interaction between the plasticizer and the biopolymer molecules reduces the intermolecular forces as well raise the mobility of polymeric chains [74] which results in improving the elastic modulus and mechanical properties of the film. In addition the use of plasticizers increases the resistance of the coating/film to permeation of vapors and gases. Glycerol considers the greatest utilized plasticizer but sorbitol and ethylene glycol have been used as plasticizers.
Surfactants and Lipids
Surfactants are amphoteric molecules characterized by opposite hydrophobicity of its two ends. They are conservatively added to improve the stability of emulsions and could be combined in the formulation of the coating to reduce its surface tension and improve its wettability [95].
Antimicrobial Agents
Great numbers of antimicrobial agents have been used to improve antimicrobial properties of coatings and films. They should be a practical approach only when effective levels of these additives come in direct contact with food [12]. Based on how these materials exhibit their activities, they are classified into two categories, the first category those which can exhibit its action by migrating low concentration to the food surface and other category those that could prevent microbial growth on the food surface without significant migration of the preservative to the food [12]. Organic acids and their salts such as sorbates, benzoates and propionates have been used in the first generation of antimicrobial coatings and films. Lysozyme, a single peptide enzyme, has been used as antimicrobial agent against Gram negative and Gram positive microorganisms. It has the ability to inhibit these microorganisms by lysis of their cell wall [89].
Bacteriocins are antimicrobial agents produced by lactic acid bacteria. Nisin and natamycin (or primaricin) are the two commercially produced bacteriocins used in the preparation of antimicrobial coatings and films. Nisin is a 3.5 kDa peptide composed of 34 amino acid residue which has antibacterial activity against Gram negative microorganisms and has high surface activity. It has the ability to be adsorbed on solid surfaces and to kill microorganisms subsequently adhered to these surfaces [80]. Natamycin is a polyene antifungal agent widely used to control mold growth of the surfaces of many cheeses.
Essential oils and some of its active constituents have been used as antimicrobial and antifungal agents in the preparation of active coatings and films [47,48,49]. In addition to its GRAS grade, essential oils exhibit wide spectrum effects on harmful microorganisms and some has been approved as food additives particularly as flavoring agent [27].
Historically silver has been used to prevent the transmission and infection of diseases. Silver zeolite (silver zinc alumino silicate) has been approved in European Union for contact to foods subjecting that the maximum content in polymer should not exceed 10% (w/w) by silver zeolite a containing ≤5% silver, and limits of silver and total migration of 0.05 mg Ag and 60 mg (kg food)–1 respectively [67]. Silver zeolite mixed with poly (vinyl acetate) has been used as nonedible cheese coating which was reported to reduce the viable microorganisms of 99% for Escherichia coli, 80% for Staphylococcus aureus, and 98% for Aspergillus niger after 30 min of application.
The use of nanomaterial in food packaging has largely increased in the past decade [9, 33, 37]. The global market of nano-enabled packaging materials for food as well as the drink amounted to 4.13 billion US dollars in 2008 and was predicted to reach 7.3 billion by 2014 with an annual growth rate of 11.65% [83]. Several metal nanoparticles exhibit biocidal properties including Cu, Zn, Au, Ti, and Ag [84]. However, silver nanoparticles (Ag-NPs) exhibit the most effective bactericidal properties against a wide range of pathogenic microorganisms [73]. In addition Ag-NPs showed better antimicrobial properties compared to metallic silver which can be attributed to their large surface area providing a better contact with the microorganisms [84]. The actual application of Ag-NPs in food packaging is regulated by EU and USA food safety authorities in a prudent way, due to the inability to make conclusive statements about their toxicity [10]. Moreover, the addition of nanomaterials enhances the physical and barrier properties of the polymer films.
The addition of the antimicrobial agents in the formulation of the edible coating and films results in variable changes in the physicochemical properties of the formed film depending on the type and concentration used. The incorporation of nisin and natamycin decreased the resistance and elongation of cellulose polymer films and caused changes in their molecular conformation [76]. After modification of the concentration of the biopolymer used should be considered in order to retain the characteristic properties of the film.
Methods of Cheese Coating
Coating of cheese can be done with one of the following methods:
-
1.
Dipping is the most common lab-scale way in coating of cheese due to its simplicity, low cost, and good coverage on uneven food surface. However, dipping method leads to dilution of the coating-solution, residual of high quantity of coating materials, and microorganism growth in the dipping tank [94].
-
2.
Brushing In order to avoid the disadvantage of dipping, coating solution is applied on the surfaces of cheese using a brush. The method is suitable for lab-scale and small scale cheese production [41].
-
3.
Enrobing technology is prevalent in chocolate and meat industries whereas the coating solution flows vertically to the treated food items, and the products are coated by viscous and gravitational forces. Coating solution viscosity is a key parameter for even coat coverage, good product quality and accurate weight control. Also, the surface of food should better be flat [65].
-
4.
Spraying is widely used method to apply coatings. It offers uniform coating, thickness control, and the possibility of successive applications without contaminating the coating solution [4].
-
5.
Electrostatic spraying which has the advantages of controlling the droplet size, increase the droplet coverage and deposition, produce homogenous distribution, and reduce wastage [19].
Zhong et al. [94] evaluated solutions of (2% chitosan and 0.5% glycerol in 1% acetic acid), (1% sodium alginate and 0.25% glycerol in deionized water), and (5% soy protein isolate and 1.25% glycerol in deionized water) as coating materials for Mozzarella cheese. The cheese was coated by these solutions by dipping, enrobing, spraying and electrostatic spraying. Sodium alginate solution was the most viscous (η = 0.155 Pa s) and had better spread ability on cheese. Film thickness displayed obvious differences based on the coating methods (ranging from 30.6 to 83.3 mm). Coating of the cheese by the two spraying methods resulted in thinner coating film. Sodium alginate coated cheese possessed the best overall physicochemical properties during storage whereas no significant differences were found in the preservation of cheese between the four coating methods.
Effect of Coating on Cheese Quality
Coating with Biopolymer Solutions
Kampf and Nussinovitch [41] coated cubes of semi-hard and dry white brined cheeses by immersion in 0.2% solutions of k-carrageenan, alginate and gellan solutions. The coated cheese cubes were then immersed in KCl solution in case of k-carrageenan and Ca lactate solution in case of alginate and gellan for cross-linking of the fashioned films followed by air drying for better adherence of the coating on the cheese cubes. The coated cheeses were stored at 48 °C and at a relative humidity of 73% and weight loss, gloss, roughness of surface area, changes in mechanical properties, peel-bond strength of the coating film from the cheese and sensory evaluation were followed during storage. The coated semi-hard cheese reduced weight loss through 46 days of storage without slightly significant differences between the used coatings. Coating resulted in improved color and gloss of cheeses and reduced the roughness of its surfaces. The coated cheeses were characterized by a desirable softer and a less brittle texture and sensory evaluation indicated that the coated cheeses had better quality than the non-coated cheeses.
Fior di latte cheese was made from milk with or without the addition of 0.012% chitosan [16]. The cheese samples were dipped in 8% sodium alginate solution followed by dipping in solution containing 0.25 mg ml−1 lysozyme and 50 mM of ethylenediaminetetraacetic acid, disodium salt (Na2–EDTA) and then in 5% calcium chloride solution to crosslink the polymer matrix. The cheese was then packaged in modified atmosphere (MAP). The combination between chitosan active packaging and MAP improved the shelf life of the cheese.
Regional (semi-hard) cheese was coated with solutions of chitosan (0.5% chitosan, 2% glycerol/sorbitol), and galactomannan extracted from Gleditsia triacanthos seeds (1.5% galactomannan, 2.0% of glycerol and 0.5% of corn oil) stored at different temperature and the rate of gas exchanges were followed [11]. Cheese coated with galactomannan showed the highest decrease in gas exchange, decreased moisture content (2.5 and 1.9%) and weight losses (3.8 and 3.1% at storage temperature of 4 and 20 °C, respectively. Also, coating decreased the cheese hardness and color changes.
Ricotta cheese was packaged using a chitosan/whey protein edible coating/film and kept under modified atmosphere at 4 °C [17]. The oxygen and carbon dioxide permeability of chitosan/whey protein film were 35 and 21% lower, respectively, and about three times higher in water vapor permeability than film fabricated using pure chitosan. Above a thirty day storage period, no changes were observed in the pH of control and treated Ricotta cheeses. Although the titratable acidity of the control raised linearly for the period of the first 2 weeks and kept constant for the rest of the storage period, the consistent values for coated Ricotta cheese did not change meaningfully through the first 21 days and gotten the acidity level (0.34 ± 0.02 meq/100 g) of the control only on day 30. The possible numbers of lactic acid bacteria, mesophilic and psychrotrophic microorganisms were considerably lower (p < 0.05) in the chitosan/whey protein coated cheese, compared to the control, at every storage time. These findings suggest that chitosan/whey protein coatings can be used to prolong fresh dairy product shelf-life.
Low moisture Mozzarella cheese was dipped in a solution containing 2% sodium alginate and 1% potassium sorbate, the cheese was immersed in 5% calcium chloride for 1 min and removed, then air dried for 2 min [54]. The coated cheese was stored under modified atmosphere packaging (MAP) at different temperatures. The combined coating and MAP extended markedly the shelf life of the cheese up to 100, 40 and 11 days at 4, 8 and 14 °C, respectively.
Pena Serna & Lopes Filho [65] developed biodegradable films by casting emulsions prepared by dissolving 20 g zein (Z) in 100 ethanol and addition of 2 g glycerol, 1 g emulsifier and 14 g oleic acid (OA) with the addition of 0.05 g xanthan gum (Z–OA–XG) and without xanthan (Z–OA). The Z–OA–XG film showed higher water solubility (13.09%) and opacity (8.49 AU/mm) than the Z–OA film (10.80% and 5.19 AU/mm, respectively). However, the Z–OA film had greater flexibility but less resistant to tension than the Z–OA–XG film. Cheese was coated with three layers of the emulsions used in preparing these films and stored for 50 days [66]. The coated cheese exhibited 30% reduced weight losses and shelf life of 50 days compared to 21 days for the uncoated cheese.
Ras cheese was coated by chitosan solutions of concentrations ranging from 0.5 to 2% [23]. Coated cheese retained significantly higher moisture content and ripening indices, viability of lactic acid bacteria was three folds higher in cheese coated with 2% chitosan, fungal growth reduced by log 1.5 after 120 days of ripening as compared to uncoated cheese. Also, cheese coated with 2% chitosan was ranked highest scores for organoleptic properties.
Coating with Biopolymer Solutions Containing Bacteriocins
Films were produced by a cellulose polymeric base integrated with natamycin by casting process [63]. The films were 33 and 95 mm in thickness and an average coating weight of 43.7 and 126.1 g/m2, respectively. The tested natamycin concentrations ranged from 0.2 to 4% of the cellulose flake weight used. Gorgonzola cheese was coated with films prepared with different natamycin concentration to evaluate its efficacy against Penicillium roqueforti on the cheese surface. Films with 2 and 4% natamycin exhibited acceptable inhibitory effect on the fungus. Also, the quantity of natamycin released to the cheese were below the permitted limits by the legislation.
Antimicrobial films were prepared by inclusion of nisin (NI), natamycin (NA) or a mixture of both (NI + NA) into cellulose polymer [76]. The inhibitory effects of those films were assessed in vitro against Staphylococcus aureus, Listeria monocytogenes, Penicillium sp. and Geotrichum sp. Inhibition of molds and yeasts, Staphylococcus sp. and psychrotrophic bacteria was similarly determined effects on sliced mozzarella cheese coated with prepared films. Also, the mechanical and microscopic properties of the prepared films as well as the migration of the antimicrobial agents from the film to the cheese were also assessed. Films containing NI displayed an antimicrobial influence against S. aureus and L. monocytogenes, whereas films containing NA were effective against Penicillium sp. and Geotrichum sp. During the 9 day of storage at 12 ± 2 °C, the count of yeasts and molds on cheese enclosed with films containing NA was reduced by 2 log10 colony forming units (CFU) as compared with the count on cheese covered with control films. NI film did not display any effect against Staphylococcus sp., but it was active against psychrotrophic bacteria. The incorporation of antimicrobial compounds decreased the resistance and elongation of the films and produced changes in their molecular conformation. No diffusion of NI from the films to the cheese was detected. However, time-dependent diffusion of NA from the film containing NI + NA to the cheese was found. The combination of NI and NA together in the films did not demonstrate any influence. The film containing NA can be used as smart food packaging for sliced mozzarella cheese.
Fajardo et al. [24] used solutions containing 0.5% chitosan, 0.2% Tween 80 and 0.5 glycerol with and without the addition of 0.50 mg ml−1 of natamycin for coating surfaces of Saloio semi-hard cheese. Cheeses with natamycin containing coat showed a decrease in molds/yeasts count of 1.1 CFU g−1 compared to control after 27 days of storage. Addition of natamycin also increased the permeability of O2 and CO2 from 7.12 to 7.68 × 10−15 g (Pa s m)−1, and from 10.69 to 64.58 × 10−14 g (Pa s m)−1, respectively. Diffusion of natamycin from the film showed coefficient values to phosphate buffered, saline solution and to the cheese of 3.60 × 10−10 and 1.29 × 10−12 cm2 s−1, respectively. Chitosan-based coating/films can be used to extend the shelf life of cheese.
Edible cheese coating was prepared from sheep WPC containing natamycin by heat treatment with or without UV polymerization [34]. UV polymerization in combination with thermal treatment (HD + UV) developed coatings that showed good mechanical performance. Also, these coatings prevented growth of Staphylococcus spp., Pseudomonas spp., Enterobacteriaceae, yeasts and molds assuring the safety of cheese.
Lucera et al. [52] added 0.1% potassium sorbate (PS), 1.5% sodium benzoate (SB), 0.5% calcium lactate (CL) and 0.5% calcium ascorbate (CA) separately to sodium alginate solution (2%). Cubes of Mozzarella cheese were coated by dipping in the prepared solutions, and then immersed in 5% calcium chloride and coated cubes were stored at 8 ± 1 °C. Coating with CL and CA based coat did not improve the quality of the cheese whereas the sodium alginate solution which contains PS and BS gave better results in quality of cheese. Cheese coated with 3% PS coat showed inhibitory effect on cheese microflora and doubled the shelf life Mozzarella cheese compared to the uncoated one.
A mixture of 4.0 g of triticale flour and 1.2 g glycerol/100 ml water, pH was adjusted to 10.7 and then heated at 75 °C/15 min [75]. Natamycin was added to aliquots of the prepared solution at the ratios of 0.02, 0.04 and 0.08 mg/100 ml, respectively and then casted to form the active films. Addition of natamycin reduced the solubility value and water permeability and increased the color parameters of the film, whereas it did not significantly affect its puncture force or moisture content. Packaging of cheese in the formed films prevented the formation of visual mold growth on the cheese surfaces during storage for 14 days at 4 °C or at room temperature whereas uncovered cheeses or cheeses covered with the films without added natamycin showed mold growth.
Coating with Biopolymer Solutions Containing Essential Oils
Acevedo-Fani et al. [3] compared the mechanical and antimicrobial properties of edible films prepared from nanoemulsions containing alginate and essential oils [thyme (TH-EO), lemongrass (LG-EO) or sage (SG-EO)]. Coarse emulsions were prepared from sodium alginate dispersion containing 2% glycerol and 3% Tween 80 with or without the addition of 1% TH-EO, LG-EO or SG-EO and then microfluidized to obtain nanoemulsions. Films were prepared from the obtained nanoemulsions and its mechanical and antimicrobial properties were tested. The average droplet size of nanoemulsions ranged from 82 ± 3 nm for TH-EO to 35 ± 7 nm for SG-EO and exhibiting multimodal size distributions. The z-potentials of nanoemulsions were between −41 mV for LG-EO and −70 mV for SG-EO. The lowest whiteness index was found in SG-EO nanoemulsions, whereas those containing TH-EO showed the highest value. Films formed from SG-EO nanoemulsions exhibited higher transparency, water vapor resistance and flexibility than films formed from TH-EO or LG-EO. Edible films containing TH-EO showed the strongest antimicrobial effect against Escherichia coli, achieving up to 4.71 Log reductions after 12 h.
Edible coatings were prepared from 1.5% (w/v) sorbitol + 5% (w/v) whey protein isolate (WPI) + 0.5% (w/v) alginate with or without the addition of 1.5% (v/v) ginger (G) essential oil [42]. Kashar cheese samples were artificially contaminated with Escherichia coli O157:H7 and Staphylococcus aureus at a level of 106 cfu/ml and then divided into three groups the 1st was left uncoated and the 2nd and 3rd groups were coated solutions without or with G addition, respectively, and all samples were stored at 4 °C for 30 days. The formed films were found to have good water barrier properties, which was further improved with the addition of G to the coating. Films containing G exhibited antimicrobial effects. During storage, Escherichia coli O157:H7 and Staphylococcus aureus levels increased in the control samples, while they decreased in samples coated with G containing films.
Coating with Biopolymer Solutions Containing Nanoparticles
Silver–montmorillonite (Ag-MMT) antimicrobial nanoparticles were obtained by replacing the Na+ of natural montmorillonite with silver ions and reducing it by thermal treatment [13, 39]. The Ag-MMT nanoparticles were embedded in agar, zein, and poly(ε-caprolactone) polymer matrices. The antimicrobial effectiveness of these nanocomposites were tested in vitro against a three-strain mixture of Pseudomonas spp. Out of the tested nanocomposites only the Ag-MMT nanoparticles embedded into agar may have antimicrobial activity against selected spoilage microorganisms. The water content of the polymeric matrix was the key parameter associated with antimicrobial effectiveness of this active system intended for food packaging applications.
Gammariello et al. [31] evaluated the combined effects of a bio-based coating containing silver-montmorillonite (Ag-MMT) nanoparticles and modified-atmosphere packaging (MAP) on microbial and sensory quality decay of Fior di latte cheese. Ag-MMT nanoparticles were prepared by ion exchange reaction between Na-montmorillonite and silver nitrate solution. Different concentrations of the prepared AG-MMT nanoparticles (0.25, 0.50, and 1.00 mg/ml) were dispersed in a sodium alginate solution (8% w/v) before coating the cheese followed by hardening in calcium chloride solution. Modified-atmosphere packaging was made up of 30% CO2, 5% O2, and 65% N2. The combination of coating AG-MMT nanoparticles and MAP enhanced the shelf life of Fior di latte cheese. The cheese stored in the traditional packaging showed a shelf life of about 3 days, whereas coated cheese stored under MAP reached a shelf life of more than 5 days, regardless of the concentration of silver nanoparticles which can extend the marketability of the product.
Fiord latté cheese was coated by dipped in a solution of 2% sodium alginate containing 0.25 mg/ml of silver nanoparticles, followed by immersion in 5% calcium chloride and air dried for 2 min [55]. The coated cheese was stored in modified atmosphere packaging (MAP) at 8 °C with and without the traditional covering liquid. The combination of active coating and MAP enhanced Fiord latte shelf life up to 10 days when packaged in the brine in comparison to 4 days for the control.
Bionanocomposite Films
Films based on chitosan/poly(vinyl alcohol)/titanium nanoparticles (CS/PVA/TiO2 nanocomposite) were prepared and used as packaging materials for soft white cheese [90,91,92]. The films were prepared by mixing 2% CS solution and 1% PVA solution (1:1), 1% glutaraldehyde was added to crosslink the polymers. NanoTiO2 suspension (1 mg/ml) was added at the ratio of 2, 4 and 8% to the polymer solution, poured in a Teflon dish and left to harden within 72 h. The films exhibited good mechanical and antimicrobial properties. White soft cheese was packaged in the prepared film and stored in brine at 7 °C for 30 days. Cheese packaged in the prepared film showed good textural, microbiological and sensory properties compared to unpackaged cheese. Youssef et al. [93] prepared a novel bionanocomposite films by casting a mixture (1:1) of 2% chitosan and 1% carboxymethyl cellulose containing 2, 4 and 8 ml/100 ml of zinc oxide nanoparticle suspension (1 mg/ml−1). The film had a thickness of about 0.95 µm and was characterized by good mechanical properties and displayed good antibacterial activity against gram positive (Staphylococcus aureus), gram negative (Pseudomonas aeruginosa and Escherichia coli) bacteria and fungi (Candida albicans). Packaging of white soft cheese in the formed film was found to improve the microbiological quality of the cheese and extended its shelf life during storage in brine at 7 °C for 30 days.
Meira et al. [58] studied the combined effect of adding nano clay and nisin on the properties of starch films and its use in packaging of cheese. They prepared a nanocomposite film in two steps. In the first step 75 g of corn starch were mixed with 25 g glycerol, 3 g (H3) and 6 g of the nanoclay (halloysite), respectively. Then 2 or 6 g of nisin were added to the prepared mixture, respectively. The mixture was kept at 40 °C/48 h at room temperature. In the second step the blend was extruded twice at 115–120 and 135–140 °C, respectively. Homogenous dispersion of the halloysite (HNT) in the starch matrix was observed in all samples. Aggregates were denoted in the film surface on the high addition of nisin. The mechanical properties of the film were improved by the addition of HNT, whereas it decreased when nisin was added. Also, the thermal stability of the film decreased with added nisin. These films were applied on Minas Frescal cheese surface previously inoculated with L. monocytogenes. After 4 days, antimicrobial nanocomposite films with 2 g/100 g nisin significantly reduced the initial counts of L. monocytogenes and those with 6 g/100 g nisin completely inhibited it. These results suggested that nisin embedded in starch/halloysite films can be an active and useful barrier to control food contamination.
Influence of Bionanocomposites on Human Health
The safety of films and coating made only from edible polymer is fully guaranteed, safety of films and coatings containing nanomaterial still questionable. Although the advantageous properties of polymer nanocomposite materials are well known, its prospective (eco-) toxicological impact and its influence on human health have not been fully established. The major problem arises from using the nanocomposites are the high speed of diffusion of the nano materials to the consumer products convey. This requires better understanding for the possible effects which nanoparticles may have on biological systems [7, 22]. Due to their high surface-to-volume ratio, nanomaterials may be more sensitive and more toxic than normal sized materials. Nanomaterials are highly reactive and they can cooperate with other materials throughout removal and recycling of nanoparticle-bearing composites. However, doubts have been raised about special toxicological effects of on the biological systems [18]. On the other hand anxieties that using those nanomaterials may develop new allergens, different toxic strains, as well as raised rates of nanoparticles absorption have been suggested [40]. Exposure to nanomaterials existent in food packaging materials can happen mainly from direct ingestion of nanoparticles migrated to food [71]. McCracken et al. [56] critically reviewed studies on the toxicity of ingested nanoparticles. Differences in toxicity were found between the different nanoparticles. They concluded that ingested silica or TiO2-NPs at estimated quantities of human consumption unlikely to impose significant acute toxicity upon tissues of the GI tract or other organs while ingestion of ZnO-NPs and Ag-NPs demand a greater level of caution. Currently available data on the balance of post-ingestion NPs retention and elimination in feces and urine are unclear. This raises the problem of potential accumulation of these NPs over decades to levels that may be harmful to tissues or organs. Although the available data provide valuable information they suggested the need data generated large, long-term, prospective human epidemiologic investigation correlating dietary intake with health status could potentially provide definitive answers to questions regarding the safety/hazards of NPs ingestion in foods. Until such information is available, common sense will have to suffice as a guide to dietary consumption. In the light of these information, the low concentration of NPs in nanocomposite packaging material and their limited migration if any from the packaging material to cheese, and low per capita consumption of cheese it is unlikely that the use of nanocomposite film and coating on cheese to pose health threat.
Future Trends and Conclusion
Previous studies demonstrated that edible films and coatings can be prepared from several polysaccharides and proteins aiming to preserve cheese. Generally, these coatings had no significant effect on the normal biochemical changes during cheese maturation, decreased moisture and weight losses of cheese during storage and extended the shelf life of cheese. Addition of bacteriocins, essential oils and nanoparticles enhanced variably the antimicrobial properties of the cheese coat.
Large number and new edible biopolymers of variable film forming properties are available as base materials for the formation of edible films/coatings for coating and packaging. Formulation of coating solution from more than one polymer may lead to more suitable coatings for cheese than those based on a single biopolymer. Interaction of polysaccharides and proteins via Maillard reaction results of conjugates with novel functional properties. To our knowledge this trend has not been explored for the development of new edible food package materials. With the existence of many polysaccharides and proteins unlimited combination of polysaccharides-protein conjugates can be developed and studies in this area should be encouraged. Waxes have been the tradition cheese coating and inclusion of these waxes in coating emulsions based on polysaccharides and protein can result in new generation of edible coating for cheeses.
Nanotechnology will certainly has growing role in different food areas particularly food packaging. The current review has highlighted some aspect of the greatest importance in food packaging applications. Inclusion of nanoclay and nanoinorganic material is a new trend for the production of edible coating for cheese. However, more research is needed to verify the migration of the added nanomaterials in cheese in relation to the safety regulations. As a conclusion the area of edible coating of cheese still in need of research and development in order to improve the shelf life and marketability of different cheese varieties.
References
Abd El-Salam MH, El-Shibiny S (2016) Natural biopolymer as nanocarrier for bioactive ingredients used in food industries. In: Grumezescu A (ed) Nanotechnology in agri-food industry, vol 2. Encapsulation. Academic Press, Cambridge, pp 793–830. ISBN: 978-0-12-8043307-3
Abdelrahman MA, El-Naggar ME, Hudson SM, Orlando J (2017) Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. Int J Biol Macromol 94:96–105
Acevedo-Fani A, Salvia-Trujillo L, Rojas-Graü MA, Martín-Belloso O (2015) Edible films from essential-oil-loaded nanoemulsions: physicochemical characterization and antimicrobial properties. Food Hydrocoll 47:168–177
Andrade RD, Olivier S, Osorio FA (2012) Atomizing spray systems for application of edible coatings. Compr Rev Food Sci Food Saf 11(3):323–337
Arunvisut S, Phummanee S, Somwangthanaroj A (2007) Effect of clay on mechanical and gas barrier properties of blown film LDPE/clay nanocomposites. J Appl Polym Sci 106:2210–2217
Borm PJA, Kreyling W (2004) Toxicological hazards of inhaled nanoparticles potential implications for drug delivery. J Nanosci Nanotechnol 4:521–531
Bouwmeester H, Dekkers S, Noordam MY, Hagens WI et al (2009) Review of health safety aspects of nanotechnologies in food production. Regul Toxicol Pharmacol 53:52–62
Bugnicourt E, Schmid M, Nerney OM, Wildner J, Smykala L, Lazzeri A, Cinelli P (2013) Processing and validation of whey-protein-coated films and laminates at semi-industrial scale as novel recyclable food packaging materials with excellent barrier properties. Adv Mater Sci Eng. doi:10.1155/2013/496207 (Article ID 496207)
Bumbudsanpharoke N, Ko S (2015) Nano-food packaging: an overview of market, migration research and safety regulations. J Food Sci 80:R910–R923
Carbone M, Donia DT, Sabbatella G, Antiochia R (2016) Silver nanoparticles in polymeric matrices for fresh food packaging. J King Saud Univ Sci 28:273–279
Cerqueira MA, Sousa-Gallagher MJ, Macedo I, Rodriguez-Aguilera R et al (2010) Use of galactomannan edible coating application and storage temperature for prolonging shelf-life of ‘‘Regional” cheese. J Food Eng 9:87–94
Cha DSC, Chinnan MS (2004) Biopolymer-based antimicrobial packaging: a review. Crit Rev Food Sci Nutr 44:223–237
Chuayjuljit S, Thongraar R, Saravari O (2008) Preparation and properties of PVC/EVA/organo modified montmorillonite nanocomposites. J Reinf Plast Compos 27:431–442
Dadbin S, Noferesti M, Frounchi M (2008) Oxygen barrier LDPE/LLDPE/organoclay nanocomposite films for food packaging. Macromol Symp 274:22–27
Davis G, Song J (2006) Biodegradable packaging based on raw materials from crops and their impact on waste management. Ind Crops Prod 23:147–161
Del Nobile MA, Gammariello D, Conte A, Attanasio M (2009) A combination of chitosan, coating and modified atmosphere packaging for prolonging Fior di latte cheese shelf life. Carbohydr Polym 78:151–156
Di Pierro P, Sorrentino A, Mariniello L, Giosafatto CVL, Porta R (2011) Chitosan/whey protein film as active coating to extend Ricotta cheese shelf-life. LWT Food Sci Technol 44:2324–2327
Donaldson K, Seaton A (2007) The Janus faces of nanoparticles. J Nanosci Nanotechnol 7:4607–4611
Edward Law S (2001) Agricultural electrostatic spray application. A review of significant research and development during the 20th century. J Electrost 51–52:25–42
El-desouky A, Mesías FJ, Elghannam A, Gaspar P, Escribano M (2016) Are packaging and presentation format key attributes for cheese consumers. Int Dairy J 61:245–249
El-Feky GS, El-Rafie MH, El-Sheikh MA, El-Naggar ME (2015) Utilization of crosslinked starch nanoparticles as a carrier for indomethacin and acyclovir drugs. Nanomed Nanotechnol 6:254
El-Naggar ME, El-Rafie MH, El-sheikh MA, El-Feky GS, Hebeish A (2015) Synthesis, characterization, release kinetics and toxicity profile of drug-loaded starch nanoparticles. Int J Biol Macromol 81:718–772
El-Sisi AS, Gapr AM, Kamaly KM (2015) Use of chitosan as an edible coating in RAS cheese. Biolife 3:564–570
Fajardo P, Martins JT, Fuciños C, Pastrana L, Teixeira JA, Vicente AA (2010) Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J Food Eng 101:349–356
Fankhauser DB (2007) Fankhauser’s cheese page. Retrieved 23 Sept 2007
Follain NG, Belbekhouche S, Bras J, Siqueira G, Marais SP, Dufresne A (2013) Water transport properties of bionanocomposites reinforced by Luffa cylindrica cellulose nanocrystals. J Membr Sci 427:218–229
Food and Drug Administration (2005) FDA code of federal regulations, title 21-food and drugs, 2005 revised as of April 1, 2005, pp 479–585
Fortunati E, Peltzer M, Armentano I, Jimenez A, Kenny JM (2013) Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nanobiocomposites. J Food Eng 118:117–124
Fortunati E, Puglia D, Luzi F, Santulli C, Kenny JM, Torre L (2013) Binary PVA bio-nanocomposites containing cellulose nanocrystals extracted from different natural sources: part I. Carbohydr Polym 97:825–836
Frounchi M, Dadbin S, Salehpour Z, Noferesti M (2006) Gas barrier properties of PP/EPDM blend nanocomposites. J Membr Sci 282:142–148
Gammariello D, Conte A, Buocore GG, Del Nobile MA (2011) Bio-based nanocomposite coating to preserve quality of Fior di latte cheese. J Dairy Sci 94:5298–5304
Ghanbari A, Heuzey M-C, Carreau PJ, Ton-That M-T (2013) Morphological and rheological properties of PET/clay nanocomposites. Rheol Acta 52:59–74
Hemati F, Garmabi H (2011) Compatibilised LDPE/LLDPE/nanoclay nanocomposites: I. Structural, mechanical, and thermal properties. Can J Chem Eng 89:187–196
Henriques M, Santos G, Rodrigues A, Gomes D, Pereira C, Gil M (2013) Replacement of conventional cheese coatings by natural whey protein edible coatings with antimicrobial activity. J Hyg Eng Des 3:34–47
Henriques M, Gomes D, Pereira C (2016) Whey protein edible coatings: recent developments and applications. In Nedović V et al. (ed) Emerging and traditional technologies for safe, healthy and quality food. Food engineering series. pp 177–179. doi:10.1007/978-3-319-24040-4_10
Huth PJ, Park KM (2012) Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr 13:266–285
Hyun K, Chong W, Koo M, Chung IJ (2003) Physical properties of polyethylene/silicate nanocomposite blown films. J Appl Polym Sci 89:2131–2136
IDF (2015) The world dairy situation 2015. Bulletin No. 481/2015. International Dairy Federation, Brussels, Belgium
Incoronato AL, Buonocore GG, Conte A, Lavorgna M, Nobile MA (2010) Active systems based on silver-montmorillonite nanoparticles embedded into bio-based polymer matrices for packaging applications. J Food Prot 73:2256–2262
Jonathan HS, Jared MB (2015) Engineered nanomaterial exposure and the risk of allergic disease. Curr Opin Allergy Clin Immunol 14:95–99
Kampf N, Nussinovitch A (2000) Hydrocolloid coating of cheeses. Food Hydrocoll 14:531–537
Kavas N, Kavas G, Saygili D (2016) Use of ginger essential oil-fortified edible coatings in Kashar cheese and its effects on Escherichia coli O157:H7 and Staphylococcus aureus. CyTA J Food Sci Nutr 14:317–323
Khosravi R, Hashemi SA, Sabet SA, Rezadoust AM (2013) Thermal, dynamic mechanical, and barrier studies of potassium permanganate–LDPE nanocomposites. Polym Plast Technol Eng 52:126–132
Kim Y, Chang J-H (2013) Colorless and transparent polyimide nanocomposites: thermo-optical properties, morphology, and gas permeation. Macromol Res 21:228–233
Klaine SJ, Koelmans AA, Horne N, Carley S, Handy RD, Kapustka L (2012) Paradigms to assess the environmental impact of manufactured nanomaterials. Environ Toxicol Chem 31:3–14
Kramer ME (2009) Structure and function of starch-based edible films and coatings. In: Embuscado ME, Huber KC (eds) Edible films and coatings for food applications. Springer Science + Business Media, LLC, pp 113–134. doi:10.1007/978-0-387-92824-1_3
Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW (2011) Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J Food Sci 76:R164–R177
Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW (2011) Antimicrobial activity of natural agents against Saccharomyces cervisiae. Packag Technol Sci 24:299–307
Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW (2014) Evaluation of antifungal activity of antimicrobial agents on Cheddar cheese. Packag Technol Sci 27:49–58
Lagaron JM, Cabedo L, Cava D, Feijoo JL, Gavara R, Gimenez E (2005) Improving packaged food quality and safety. Part 2: nanocomposites. Food Addit Contam 22:994–998
Ledenbach LH, Marshall RT (2009) Microbiological spoilage of dairy products. In: Sperber WH, Doyle MP (eds) Compendium of the microbiological spoilage of foods and beverages. Food microbiology and food safety. Springer Science + Business Media, LLC, pp 41–68. doi:10.1007/978-1-4419-0826-1-2
Lucera A, Mastromatteo M, Conte A, Zambrini AV, Faccia M, Del Nobile MA (2014) Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Packag Shelf Life 1:25–29
Marsh K, Bugusu B (2007) Food packaging—roles, materials, and environmental issues. J Food Sci 72(3):R39–R55
Mastromatteo M, Conte A, Faccia M, Del Nobile MA (2014) Combined effect of active coating and modified atmosphere packaging on prolonging the shelf life of low-moisture Mozzarella cheese. J Dairy Sci 97:36–45
Mastromatteo M, Conte A, Lucera A, Saccotelli MA, Buonocore GG, Zambrini AV, Del Nobile MA (2015) Packaging solutions to prolong the shelf life of Fiordilatte cheese: bio-based nanocomposite coating and modified atmosphere packaging. LWT Food Sci Technol 60:230–237
McCracken C, Dutta PK, Waldman WJ (2016) Critical assessment of toxicological effects of ingested nanoparticles. Environ Sci: Nano 3:256–282
Mc-Sweeney PLH (2007) Principle families of cheese. In: Mc Sweeney PLH (ed) Cheese problems solved. Woodhead Publishing Ltd., Cambridge, pp 176–188
Meira SMM, Zehetmeyer G, Scheibel JM, Werner JO, Brandelli A (2016) Starch-halloysite nanocomposites containing nisin: characterization and inhibition of Listeria monocytogenes in soft cheese. LWT Food Sci Technol 68:226–234
Mingliang GE, Demin J (2008) Influence of organoclay prepared by solid state method on the morphology and properties of polyvinyl chloride/organoclay nanocomposites. J Elast Plast 40:223–235
Mondal D, Mollick MMR, Bhowmick B, Maity D et al (2013) Effect of poly (vinyl pyrrolidone) on the morphology and physical properties of poly (vinyl alcohol)/sodium montmorillonite nanocomposite films. Prog Nat Sci Mater Int 23:579–587
Murray JM, Delahunty CM (2000) Mapping consumer preference for the sensory and packaging attributes of Cheddar cheese. Food Qual Prefer 11:419–435
Nieto MB (2009) Structure and function of polysaccharide gum-based edible films and coatings. In: Embuscado ME, Huber KC (eds) Edible films and coatings for food applications. Springer Science + Business Media, LLC, pp 57–112. doi:10.1007/978-0-387-92824-1-3
Oliveira TM, Soares NFF, Pereira RM, Fraga KF (2007) Development and evaluation of antimicrobial natamycin-incorporated film in Gorgonzola cheese conservation. Packag Technol Sci 20:147–153
Ozcalik O, Tihminlioglu F (2013) Barrier properties of corn zein nanocomposite coated polypropylene films for food packaging applications. J Food Eng 114:505–513
Pena Serna C, Lopes Filho JF (2015) Biodegradable zein-based blend films: structural, mechanical and barrier properties. Food Technol Biotechnol 53:348–353
Pena-Serna C, Penna ALB, Filho JFL (2016) Zein-based blend coatings: impact on the quality of a model cheese of short ripening period. J Food Eng 171:206–213
Poças MF, Pintado M (2009) Food packaging and shelf life. In: Robertson GL (eds) Packaging and the shelf life of cheese, Chap 6, CRC Press, Boca Raton, 2009, pp 103–125. https://doi.org/10.1201/9781420078459-c6
Reddy MM, Vivekanandhan S, Misra M, Bhatia SK, Mohanty AK (2013) Biobased plastics and bionanocomposites: current status and future opportunities. Prog Polym Sci 38:1653–1689
Regalado C, Pérez-Pérez C, Lara-Cortés E, García-Almendarez B (2006) Whey protein based edible food packaging films and coatings. In Guevara-González RG, Torres-Pacheco I (eds) Advances in agricultural and food biotechnology. Research Signpost, Trivandrum, pp 237–261. ISBN: 81-7736-269-0
Rehim MA, Youssef AM, Ghanem A (2015) Polystyrene/hydrophobic TiO2 nanobelts as a novel packaging material. Polym Bull 72:2353–2362
Restuccia D, Spizzirri UG, Parisi OI, Cirillo G et al (2010) New EU regulation aspects and global market of active and intelligent packaging for food applications. Food Control 21:1425–1435
Rhim J-R, Park H-M, Ha C-S (2013) Bio-nanocomposites for food packaging applications. Prog Polym Sci 38:1629–1652
Rhim J-W, Wang L-F, Lee Y, Hong S-I (2014) Preparation and characterization of bio-nanocomposite films of agar and silver nanoparticles: laser ablation method. Carbohydr Polym 103:456–465
Rivero S, García MA, Pinnoti A (2010) Correlations between structural, barrier, thermal and mechanical properties of plasticized gelatin films. Innov Food Sci Emerg Technol 11:369–375
Romero V, Borneoa R, Passalacqua N, Aguirre A (2016) Biodegradable films obtained from triticale (x Triticosecale Wittmack) flour activated with natamycin for cheese packaging. Food Packag Shelf Life 10:54–59
Santos Pires AC, Soares NFF, Andrade NJ, Silva LHM et al (2008) Development and evaluation of active packaging for sliced Mozzarella preservation. Packag Technol Sci 21:375–383
Sanuja S, Agalya A, Umapathy MJ (2014) Studies on magnesium oxide reinforced chitosan bionanocomposite incorporated with clove oil for active food packaging International. J Polym Mater Polym Biomater 63:733–740
Scott G (2000) ‘Green’ polymers. Polym Degrad Stab 68:1–7
Singhet RK, Singh N (2005) Quality of packaged food. In: Han JH (ed) innovations in food packaging. Elsevier Academic Press, San Diego, pp 24–44
Sobrino-Lopez A, Martin-Belloso O (2008) Use of nisin and other bacteriocins for preservation of dairy products. Int Dairy J 18:329–343
Souza VGL (2015) Thesis plan proposal—development of a novel bionanocomposite based on Chitosan/Montmorilonite with antioxidant activity for food appliances. Universidade Nova De Lisboa 46
Šuput DZ, Lazić VL, Popović SZ, Hromiš NM (2015) Edible films and coatings—sources, properties and applications. Food Feed Res 42:11–22
Duncan TV (2011) Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J Colloid Interface Sci 363:1–24
Toker RD, Kayaman-Apohan N, Kahraman MV (2013) UV-curablenano-silver containing polyurethane based organic–inorganic hybrid coatings. Prog Org Coat 76:1243–1250
Wu CL, Zhang MQ, Rong MZ, Friedrich K (2005) Silica nanoparticles filled polypropylene: effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos Sci Technol 65:635–645
Xie L, Lv X-Y, Han Z-J, Ci J-H, Fang C-Q, Ren P-G (2012) Preparation and performance of high-barrier low density polyethylene/organic montmorillonite nanocomposite. Polym Plast Technol Eng 51:1251–1257
Yam KL, Takhistov PT, Miltz J (2005) Intelligent packaging: concepts and applications. J Food Sci 70:R1–R10
Youssef AM (2013) Polymer nanocomposites as a new trend for packaging applications. Polym Plast Technol Eng 52:635–660
Youssef AM, El-Nahrawy AM, Abou Hammad AB (2017) Sol–gel synthesis and characterizations of hybrid chitosan-PEG/calcium silicate nanocomposite modified with ZnO-NPs and (E102) for optical and antibacterial applications. Int J Biol Macromol 97:561–567
Youssef AM, Abou-Yousef H, El-Sayed SM, Kamel S (2015) Mechanical and antibacterial properties of novel high performance chitosan/nanocomposite films. Int J Biol Macromol 76:25–32
Youssef AM, El-Gendy A, Kamel S (2015) Evaluation of corn husk fibers reinforced recycled low density polyethylene composites. Mater Chem Phys 152:26–33
Youssef AM, El-Sayed SM, Salama HH, El-Sayed HS, Dufresne A (2015) Evaluation of bionanocomposites as packaging material on properties of soft white cheese during storage period. Carbohydr Polym 132:274–285
Youssef AM, El-Sayed SM, Salama HH, El-Sayed HS, Dufresne A (2016) Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr Polym 151:9–19
Zhong Y, Cavender G, Zhao Y (2014) Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT Food Sci Technol 56:1–8
Ziani K, Oses J, Coma V, Maté JI (2008) Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT Food Sci Technol 41:2159–2165
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Youssef, A.M., Assem, F.M., El-Sayed, S.M. et al. Utilization of Edible Films and Coatings as Packaging Materials for Preservation of Cheeses. J Package Technol Res 1, 87–99 (2017). https://doi.org/10.1007/s41783-017-0012-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41783-017-0012-3