Abstract
The present study sought to evaluate the effect of basic amino acid (L-arginine (Arg), L-lysine (Lys), and L-histidine (His)) pretreatment on the quality of canned Antarctic krill. Additionally, the changes in krill meat at different pretreatment conditions and processing stages were measured. The Lys-pretreated krill meat exhibited 39.9% lower hardness, 19.8% lower chewiness, and 47.2% lower thiobarbituric acid reaction substances (TBARS) compared to the control group. The low-field nuclear magnetic resonance (LF-NMR) and the magnetic resonance imaging (MRI) analyses revealed that the Lys-pretreated krill meat showed a larger peak area of immobile water and a higher pseudo-color image brightness. The scanning electron microscopy (SEM) results showed that the microstructure of krill meat in the control group was broken and disordered, while the microstructural network of Lys-pretreated krill meat was more complete. This result indicated that Lys effectively improved the texture, water-holding capacity, and color of krill meat, protecting the microstructure and reducing the degree of oxidation. The sensory evaluation results showed that the Lys-treated canned Antarctic krill had a better flavor and texture than other canned products. Overall, Lys could be a potential regulatory strategy for effectively enhancing the quality of canned Antarctic krill.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctic krill (Euphausia superba), a small shrimp-like crustacean plankton distributed in the Antarctic Ocean, is the largest potential reservoir of animal protein on earth, with a biomass of approximately 379 million tons (Atkinson et al., 2009). Krill protein contains eight essential amino acids along with some trace minerals, vitamins, enzymes, chitin, and astaxanthin and poses a higher biological value than milk or other animal proteins (Peng et al., 2019). Antarctic krill is also rich in n-3 polyunsaturated fatty acids, especially phospholipid eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), with excellent health benefits and high nutritional value for humans. As such, Antarctic krill has received widespread attention from countries around the world.
However, the processing and utilization of Antarctic krill are difficult. Moreover, Antarctic krill exhibits autolysis, browning, and other phenomena under the action of a few enzymes, resulting in the deterioration of Antarctic krill and its quality (Chen & Jaczynski, 2007). Notably, the high fluorine content of Antarctic krill limits its available edible portion. Furthermore, the functional substances in krill, such as astaxanthin and unsaturated fatty acids, are easily decomposed by heat and oxidation during processing, leading to a decrease in their quality (Liu et al., 2018). Krill meal and krill oil are the primary by-products of Antarctic krill; krill meal is often used as a feed bait, whereas krill oil is mainly used to develop health products. So far, relatively few products have been developed using krill meat, including canned krill and krill paste. Canned food is a common method for food preservation. However, the sterilization process of canned meat can lead to food browning (color changes), flavor damage, and nutritional value reduction (Muñoz et al., 2022). Notably, the krill meat in canned krill has a rough texture and is less edible, which can be attributed to the thermal processing of krill meat, which causes severe moisture loss and forms corrugated folded microstructures, resulting in a tough texture, rough taste, and poor product quality (Sun et al., 2022). Consequently, improving the taste, flavor, and nutritional value of canned Antarctic krill is of great significance.
Phosphates are often used for processing aquatic products to improve product quality and reduce the loss of water, flavor, and nutrients (Omar et al., 2016). However, excessive phosphate intake is associated with high health and safety risks, causing calcium and phosphorus imbalance in the body and various problems, such as kidney stones and cardiovascular diseases (Mizuno et al., 2016). Recently, the application of phosphate-free additives has attracted emerging attention due to the increasing health consciousness among people (Zheng, 2017). Basic amino acids, the important components of proteins, are the essential or semi-essential amino acids for the human body with an isoelectric point greater than 7.0, such as L-arginine (Arg), L-lysine (Lys), and L-histidine (His). These amino acids can increase the pH in muscle, chelate metal ions, increase the ionic strength of proteins, promote the dissociation of actin, and inhibit the oxidation of lipids and proteins in meat products, thus improving the protein structure and functionality and regulating the quality of meat products (Zhang et al., 2022). Basic amino acids play a key role in food processing. (Lin et al., 2022) found that krill meat soaked in Lys increased the water-holding capacity and tenderness of heated krill meat. However, no studies have reported the applications of canned krill so far.
In this study, krill meat was pretreated with three basic amino acids, Arg, Lys, and His, and compared with sodium tripolyphosphate (STPP) to investigate their effects on krill meat quality during canning. Additionally, their effects on the sensory, color, texture, moisture, microstructure, and oxidation characteristics of canned Antarctic krill were evaluated. Furthermore, the effect of basic amino acid pre-impregnation on the quality of canned Antarctic krill was clarified, which could provide technical support and theoretical reference for the processing and utilization of Antarctic krill.
Materials and Methods
Materials and Chemicals
Frozen Antarctic krill (Euphausia superba) was supplied by Dalian Liaoyu Fisheries Group Co., Ltd. (Dalian, China). Antarctic krill were harvested in May 2022 from the Antarctic FAO 48.1 sea areas, then shelled and peeled immediately after catching. Later, the Antarctic krill were snap frozen on-board and stored at − 30 °C. The krill blocks were shipped in heavily insulated industrial strength boxes in August 2022, with full cold chain transport to the laboratory. After arrival, the frozen krill blocks were cut into approximately 600 g of samples, vacuum-packed, and stored at − 20 °C until used. Food additives (sodium tripolyphosphate (STPP), L-arginine (Arg), L-lysine (Lys), and L-histidine (His)) were purchased from Henan Wanbang Industrial Co., Ltd. (Henan, China). Trichloroacetic acid (TCA) and 2-thiobarbituric acid were procured from Nanjing Jiancheng Co., Ltd. (Nanjing, China). All chemicals used in this study were of analytical grade and did not require further purification.
The Antarctic Krill Canning Process
The Antarctic krill canning process was performed as follows: Antarctic krill → thawing → soaking → blanching → canning → adding juice → exhausting → sealing → sterilization → cooling → product.
Preparation of Krill Meat Samples
The frozen krill meat was thawed at 4 °C for 6 h, and the foreign impurities were removed and soaked in twice the volume of soaking solution (the soaking solutions were water, 1% solution of sodium tripolyphosphate, L-arginine, L-lysine, or L-histidine, respectively) for 15 min and drained. The concentration of the soaking solution was selected to be 1%, mainly based on enterprise production practices and the previous study results (Lin et al., 2022). The sample soaked in water was used as a control, while that soaked in sodium tripolyphosphate was used as a positive control (STPP). These samples were then blanched with one time the volume of 2% salt solution (water temperature was approximately 80 °C) for 20 s, removed, and drained. Later, 74 g of canned krill meat was weighed, and 36 g of exhaust solution (water (97.5%), salt (2%), and citric acid (0.5%)) were added, which was then exhausted in boiling water for 10 min, sealed at − 0.04 MPa, and placed in a ZM-100 reverse pressure sterilizer (Guangzhou Biaoji Packaging Equipment, Guangzhou, China) at 115 °C for 60 min, cooled below 38 °C, and then removed.
The krill meat after thawing, soaking, blanching, exhausting, and sterilization were considered as samples, and the relevant indicators were measured, with the drained krill meat being used as the measurement target in each session.
Measurement of Color
The color of krill meat samples was measured based on the method described by (Ismail et al., 2019) with some modifications. The krill meat samples were taken separately, and the changes in brightness (L*), red/green (a*), and blue/yellow (b*) values were measured using a colorimeter Hunter Lab Pro (Hunter Lab Company, Reston, VA). Three parallel samples were taken each time, and each sample was measured thrice. Then, the average value was taken as the color value of the sample. The equipment was calibrated using standard white and black boards before measurement.
Texture Profile Analysis (TPA)
The TPA was performed according to the method described by (Rabeler & Feyissa, 2018) with slight modifications. The TPA values of individual krill meat samples were determined using a TA-XT plus texture analyzer (Stable Micro Systems, London, UK). The measurement conditions were as follows: 50% compression deformation using a P50 probe at a test and post-test speed of 2.0 mm/s and 15.0 mm/s, respectively, with a time interval of 5 s. The compression was performed twice. Each treatment was performed in triplicate.
Low-Field Nuclear Magnetic Resonance (LF-NMR)
The low-field NMR analysis was performed according to the method of (Chen et al., 2015) with minor modifications. Accurately weighed krill meat samples (1 g) were covered with a cling film and placed in a cylindrical glass tube with a diameter of 60 mm. The measurements were carried out using a MesoMR23-060 V-I NMR analyzer (Suzhou Niumag Analytical Instrument Corporation, Suzhou, China) with an operating magnetic field strength of 0.5 T and a permanent magnet operating temperature of 32 °C. The decay signals were then collected using a Carr-Purcell-Meiboom-Gill (CPMG). The T2 measurements were performed at a τ value of 200 μs (between 90 and 180 pulses) with two pulse lengths of 21.0 and 42.0 μs. The number of echoes (NECH) was 4500, and the number of scans was 8. The decay signal was measured using the MultiExp Inv (Suzhou Niumag Analytical Instrument Co., Suzhou, China) analysis software to perform a distributed multi-exponential fit of the CPMG decay curves. The lateral relaxation times of each process were calculated from the peak positions, and the area under each peak was determined by cumulative integration, corresponding to the proportion of water molecules exhibiting the relaxation times.
Magnetic Resonance Imaging (MRI)
The MRI was measured based on the method reported by (Xu et al., 2020) with slight modifications. Accurately weighed krill meat samples (3 g) were placed in round plastic cassettes, and each cassette was placed in a 60-mm diameter sample chamber for MRI measurements. The spatial distribution of water in the krill meat samples was analyzed using NMR. The proton density images were acquired using a multiple spin echo (MSE) imaging sequences. The MRI data were obtained using a MesoMR23-060 V-I NMR analyzer (Suzhou Niumag Analytical Instrument Corporation, Suzhou, China) equipped with 0.5 T permanent magnets on a 30-mm RF coil at 32 °C. The measurement conditions were as follows: field of view (FOV), 100 mm × 100 mm, slice width of 2.3 mm; read size, 256; and phase size, 192; the echo time (TE) and repetition time (TR) parameters were 20 and 2000 ms, respectively. The weighted imaging of the samples was obtained using these parameters, and the images were analyzed using OsiriX software (version 7.0.4, Pixmeo, Geneva, Switzerland) for pseudo-color and quantitative processing.
Scanning Electron Microscopy (SEM)
The microstructure of the krill meat samples was observed using scanning electron microscopy (SEM) according to the procedure described by (Liu et al., 2020). Different krill meat samples were dried for 24 h in a freeze-dryer (Coolsafe 110–4, Labogene Corporation, Lynge, Denmark). The cross-sections of krill samples were mounted on short metal tubes, coated with gold, and observed under a scanning electron microscope (JSM-7800F; JEOL, Tokyo, Japan) with an accelerating voltage of 10 kV.
pH Value Measurements
The pH was determined according to the method of (Devi et al., 2021) with minor modifications. The individual krill meat samples (1 g) were taken in triplicate, mixed with 19 mL of distilled water, and homogenized for 1 min at 8000 rpm in an IKA Ultra Turrax T25 homogenizer (Labortechnik, Staufen, Germany), followed by centrifugation at 8000 g for 10 min at room temperature (22 °C). The pH of the supernatant was measured using a PB-10 pH meter (Sartorius Scientific Instruments Co., Ltd., Germany).
Determination of Lipid Oxidation
The thiobarbituric acid reaction substance (TBARS) values of krill meat samples were measured according to the method reported by (Wu et al., 2016) with slight modifications. Five grams of krill meat sample were homogenized with 25 mL of trichloroacetic acid solution (7.5%, v/v) at 8000 rpm for 2 min using a homogenizer and filtered through a qualitative filter paper. Later, 5 mL of the filtrate was mixed with 5 mL of 2-phenobarbitone solution (0.02 mol/L) and incubated in a water bath for 40 min at 100 °C. After cooling, the sample was centrifuged at 7470 rpm for 10 min at 4 °C. The optical density was measured at 532 nm and 600 nm using a UV–Visible Spectrophotometer Model 2100 (Unico, China). The malondialdehyde (MDA) content was calculated using the following equation:
where A532 and A600 are the absorbances of the supernatant measured at 532 nm and 600 nm, respectively; m is the sample weight in grams; 72.06 is the molar mass of malondialdehyde in g/mol; and 155 is the absorbance coefficient.
Sensory Evaluation
A total of twenty experienced experts (10 men and 10 women, aged 22–30 years) were selected to assess the sensory characteristics of canned Antarctic krill samples with different pretreatments to reach an agreement on the characterization of sensory attributes. The panel members were trained for 3 weeks according to the Chinese standard (GB/T 37,062–2018) using the sensory evaluation criteria for aquatic products. The samples were placed on a whiteboard at room temperature and randomly allocated to the panel members. The members were asked to rate each sample separately in terms of color, odor, texture, flavor, and acceptability according to the 100-point criteria listed in Table S1, and the panel members’ scores were averaged.
Statistical Analyses
All experiments were performed in triplicate, and statistical analyses were conducted using IBM SPSS Statistics 26 software (IBM Corp., Chicago, IL, USA). The significance of differences was analyzed using one-way analysis of variance (ANOVA), and multiple comparisons were performed using Duncan's multiple range test (p < 0.05 was considered statistically significant). Data were expressed as means ± standard deviation (SD). The experimental charts were prepared using Origin 2020 Pro (OriginLab Corp., Northampton, MA, USA) software.
Results and Discussion
Color Parameter Analysis
Color reflects the appearance of the food sample and is highly dependent on the food process (Choo et al., 2022). As presented in Table 1, the L* values on the surface of krill meat showed a decreasing trend when soaked in the soaking solution compared to the control. The brightness value (L*) of the meat products is negatively correlated with the moisture content (Ferrini et al., 2012). Phosphates and basic amino acids can increase the water-holding capacity of meat products, leading to a reduction in the L* value. However, the L* value on the surface of krill meat tended to increase after the blanching and exhausting processes. This might be attributed to the fact that the blanching and exhausting processes of krill meat partially inactivated the enzymes in its body and reduced the enzymatic reaction, while salt exerted a color-protective influence (Niamnuy et al., 2008). After sterilization, the L* value of krill meat decreased significantly. This might be due to the Maillard reaction caused by the sterilization process, which formed nitrogen-free or nitrogen-containing brown soluble compounds and eventually produced substances, such as melanoidin, thus decreasing the L* value of krill meat (Kong et al., 2007).
The treatment methods also affected the redness values (a*), as presented in Table 1. The a* values of krill meat increased after soaking and blanching, while they decreased significantly after the venting and sterilization processes. The krill meat soaked in phosphates and basic amino acids showed an increase in the a* value due to the increase in pH and ionic strength. After sterilization, the Lys and Arg-treated krill meat showed a higher a* value, which might be caused by the ability of Lys and Arg to chelate the metal ions and inhibit the oxidation of myoglobin/hemoglobin, thereby increasing the redness of krill meat due to the reduction in high-iron-containing myoglobin (Ning et al., 2020).
The b* values of krill meat decreased after soaking and increased after the blanching and exhausting processes, while those after sterilization treatment increased significantly. After sterilization, the surface of the krill meat became dull and yellower. It could be attributed to the severe browning of krill meat caused by the Maillard reaction during the sterilization process, contributing to its yellow color. Meanwhile, lipid oxidation and myofibrillar protein denaturation also affect the meat color (Georgantelis et al., 2007). The krill meat treated with phosphate and basic amino acids after sterilization showed a significant reduction in the b* value, indicating that phosphate and basic amino acids could improve the color of krill meat.
TPA
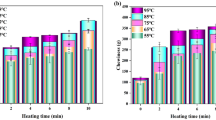
The texture is an important indicator of meat quality, and the changes in texture can characterize the tissue state, sensory quality, and physical structure of meat products. Hardness is the force with which a food sample reaches a certain deformation, and chewiness is the energy required to chew food to a state where it can be swallowed (Chen et al., 2020b). As depicted in Fig. 1a, b, the krill meat soaked in different solutions showed different degrees of decrease in hardness and chewiness. The hardness and chewiness decreased after soaking in the STPP solution, which is because phosphate might induce the dissociation of actin into myosin and actin, thereby increasing the water-holding capacity of meat and decreasing its hardness (Damasceno & Gonçalves, 2019). Soaking meat in basic amino acid solutions alters the conformation and myosin charges and its interaction with the aromatic and acidic residues of myosin, thereby enhancing the interaction between myosin and water (Zhang et al., 2020). This phenomenon might increase the water-holding capacity of krill meat, leading to a decrease in hardness and chewiness. In contrast, the hardness and chewiness were significantly reduced after cooking, especially in the samples subjected to blanching, exhausting, and sterilization processes. This could be attributed to the contraction of myofibrillar proteins and water loss due to heat treatment, resulting in a more compact muscle structure, which led to meat hardening (Gao et al., 2016). As depicted in Fig. 1a, b, the hardness and chewiness of canned Antarctic krill increased significantly in the control group and decreased more significantly in those treated with Arg and Lys.
Proton Dynamics Using LF-NMR
Water content and its status are closely related to the meat texture. The proton spin–spin relaxation time (T2) can be used to study the mobility and degrees of freedom of water molecules. As depicted in Fig. 2, three peaks were observed in the T2 relaxation curves. T21, with the shortest relaxation time from 0.1 to 10 ms, reflects the proton attached to the biomolecule, i.e., the bound water; T22, with a relaxation time between 10 and 200 ms, reflects the not readily available mobile water captured in the myofibrillar network; T23, with a relaxation time of 200–1000 ms, reflects the free water outside the myofibrils (Ezeanaka et al., 2019). As depicted in Fig. 2b, c, d and e, when krill meat was soaked in phosphate and basic amino acid solutions, T22 representing immobile water, shifted significantly to the right, indicating an increase in the interaction between water and protein, leading to an increase in proton mobility and proton freedom (Lei et al., 2016). As depicted in Fig. 2a, b and d, the relaxation time of immobile water (T22) gradually shifted towards a lower relaxation time after soaking the krill meat in water, STPP, and His during the blanching, exhausting, and sterilization processes, indicating a decrease in the mobility of immobile water. The heat treatment process induced protein denaturation and muscle contraction in krill meat, inhibiting the freedom of water molecules and reducing water mobility (Cheng et al., 2020).
Water distribution in canned Antarctic krill at different processing steps. T2 relaxation time distribution curves of canned Antarctic krill meat pretreated with different soaking solutions: a control, b STPP, c Lys, d His, and e Arg groups. The changes in the peak area of water distribution of canned Antarctic krill meat pretreated with different soaking solutions: f control, g STPP, h Lys, i His, and j Arg groups. Data are presented as means ± standard deviation (n = 3). Different lowercase letters (a, b, c) indicate significant differences (p < 0.05) between different processing steps
Figure 2f, g, h, i and j depicts the percentage of the corresponding peak areas of bound water (A21), immobile water (A22), and free water (A23) in krill meat out of the total peak area (A2), representing the relative water content. The increase in the percentage of the area of immobile water (A22) after soaking the krill meat in phosphate and basic amino acid solutions indicated an increase in the water-holding capacity of krill meat. Notably, the krill meat pretreated with the Lys and Arg solutions showed an increase in the percentage of peak area of immobile water (A22) after the sterilization process, which could be probably due to the increase in the solubility of krill proteins by Lys and Arg in the presence of NaCl. Lys possesses a better solubility effect, which can alter the protein structure of the meat. It could expose more sulfhydryl and hydrophobic groups and effectively increase the solubility of the altered protein structure (Guo et al., 2015), resulting in an increase in the percentage of peak area immobile water (A22). When krill meat underwent the exhausting and sterilization processes, the percentage of the peak area of immobile water (A22) decreased, while free water (A23) increased significantly, indicating the transformation of immobile water into free water.
MRI Analysis
MRI can provide information on water distribution and the changes in the internal structure to be analyzed during processing. Figure 3 depicts the pseudo-color images of proton-weighted imaging and corresponding relative signal intensities during the processing of canned Antarctic krill. The signal intensity in different areas of the sample is proportional to the hydrogen proton content; the brighter the image, the stronger the hydrogen proton signals in this area, indicating a higher water content of the krill meat (Lv et al., 2021). The red and blue regions represent the hydrogen protons with high and low signals, respectively. As depicted in Fig. 3a, the raw krill meat had a high density of hydrogen protons, with the area of the red region continuing to increase after soaking, indicating a significant increase in the intensity of the hydrogen proton signal. These results elucidate that phosphate and basic amino acids have a beneficial effect on the water holding capacity of krill meat. However, the red areas were significantly reduced after the blanching, exhausting, and sterilization processes of krill meat, and the hydrogen proton density reduced, indicating a significant loss of water from krill meat during processing. This might be due to the transfer of water from inside to the interstices of the myofibrils with gradual dissipation from the surface of the krill meat (Chumngoen et al., 2016). As depicted in Fig. 3e, the signal intensity of krill meat pretreated with Lys and Arg during sterilization was higher, indicating a significant effect of Lys and Arg on holding water. The MRI signal intensity map was consistent with the pseudo-color images, visually showing the contribution of Lys and Arg towards holding water in krill meat.
a Proton density-weighted magnetic resonance of different processing steps of canned Antarctic krill. Corresponding histograms of the signal intensity of magnetic resonance images (MRI) of canned Antarctic krill meat samples pretreated with different soaking solutions during b soaking, c blanching, d exhausting, and e sterilization processes. Data are presented as means ± standard deviation (n = 3). Different uppercase letters (A, B, C) indicate significant differences (p < 0.05) between the different soaking solutions
Simplified Model of Hydrodynamics in the Processing of Canned Antarctic Krill Meat
As depicted in Fig. 4, a simplified model of water mobility and distribution kinetics was developed to interpret the above LF-NMR and MRI results. This schematic diagram could provide a better understanding of water dynamics and distribution during the processing of canned Antarctic krill. As for the Lys-pretreated canned Antarctic krill, the bound water and free water content showed a decreasing trend after the Lys soaking process, while the immobile water content increased. This result indicated the successful transformation of free water into immobile water, and the Lys immersion process increased the water-holding capacity of krill meat. The free water content of krill meat decreased, and the bound water content increased after the blanching process. After the exhausting process of krill meat, the immobile water content decreased, while the free water increased. In sterilized krill meat, the free water content decreased, while the immobile water content increased, indicating the successful transformation of the water status of the canned Antarctic krill during processing.
Microstructure Analysis
The water status and textural properties of meat products highly depend on their microstructures (Guo et al., 2020). The microstructures of different processing steps of canned Antarctic krill pretreated with different basic amino acids are depicted in Fig. 5. Compared to the raw samples, the microstructure of krill meat soaked in STPP and basic amino acids showed a more homogeneous three-dimensional network structure, predicting an increase in the water-holding capacity and a decrease in hardness. After the blanching process, some disruption in the network structure of krill meat was observed with a broken bridge structure. After the exhausting and sterilization processes, the network structure of krill meat appeared to be aggregated and disrupted and eventually led to a disordered structure, especially in the control group, where the microstructure was severely affected. In contrast, the Lys-treated group was better at maintaining the micro-network structure, being more regular and compact, reflecting a better water-holding capacity. This result suggested that the processing of canned Antarctic krill changed the microstructure, which might be closely related to the changes in proton density and water distribution.
pH Value Analysis
As depicted in Fig. 6, the pH of the krill meat increased after soaking in phosphate and basic amino acid solutions. It is reported that an increase in the pH can improve meat quality (Joseph et al., 1997). The pH value influences the protein conformation. The isoelectric point of the protein deviates, and the net negative charge of the protein molecules increases with the increase in the pH value. This phenomenon could increase the electrostatic repulsion among the molecules, introduce a large gap between actin and myosin, and enhance the interaction between protein and water, thereby reducing water loss during the cooking process to decrease the hardness of meat (Lorenzetti et al., 2015). This result was consistent with the hardness results of the present study. The pH values were higher after Lys and Arg pretreatment, while the pH of krill meat was significantly lower after sterilization than after blanching. It might be attributed to the fact that protein undergoes irreversible denaturation when heated under high-temperature conditions, causing a decrease in the protein solubility with hydrolysis (Tamilmani & Pandey, 2016). Furthermore, the pH change at high temperatures might be closely related to lipid oxidation (Domiszewski et al., 2020).
Lipid Oxidation
The sterilization process is often accompanied by lipid oxidation, and the main indicator of lipid oxidation, TBARS, directly reflects the number of secondary oxidation products produced by lipid oxidation (Husein & Abdullah, 2021). Figure 7 depicts the TBARS formed by different canning process steps. The krill meat soaked in phosphate and basic amino acids had significantly lower TBARS values than before soaking. Phosphates and basic amino acids pose antioxidant properties and can inhibit lipid oxidation (Kılıç et al., 2014; Zhang et al., 2015). Phosphates can chelate metal ions (e.g., Ca, Mg, and Fe) and prevent and reduce oxidation during processing, whereas Lys, Arg, and His inhibit lipid oxidation in meat products mainly by chelating iron ions and removing hydroxyl radicals (Xu et al., 2018; Zhang et al., 2015). In contrast, the TBARS value of krill meat increased after blanching due to the acceleration of lipid oxidation by heating and salting (Chen et al., 2020a). The increased TBARS value of krill meat after the exhausting and sterilization processes showed the accelerated oxidation of krill meat by high temperatures. The control group had the most significant increase in TBARS, while the STPP- and Lys-treated groups exhibited lower TBARS values, indicating that Lys and STPP could significantly prevent lipid oxidation.
Sensory Evaluation Analysis
The sensory qualities of the products after sterilizing with different soaking solution pretreatments are shown in Fig. 8a, b and Table 2. The control group showed the lowest sensory score, while the canned Antarctic krill treated with phosphate and basic amino acids significantly improved the flavor, texture, and acceptability of the product. Based on the overall acceptability, the product obtained after the Lys treatment was of the best quality, providing a unique meaty note of krill without the ammonia-like flavor with enhanced taste and higher acceptability, making it moderately hard.
Conclusion
In this study, the effect of basic amino acid pretreatment on the quality of canned Antarctic krill was comprehensively studied. As the canning process proceeded, the water content, the brightness (L*) value, and the pH of the krill meat in the control group decreased while the hardness and TBARS values increased. Furthermore, the microstructure broke down severely, and the morphology was incomplete, while the sensory scores were the lowest. The quality of the canned Antarctic krill pretreated with STPP and basic amino acids in different processing steps was significantly improved. Compared with the control group, the Lys-pretreated krill meat had a larger peak area of immobile water, a higher pseudo-color image brightness of krill meat, a 39.9% decrease in hardness, a 19.8% decrease in chewiness, and a 47.2% decrease in oxidation. It also showed a more complete microstructure of the network, with a significant increase in the sensory scores in terms of taste and flavor. It is well known that the basic amino acids reduce the destruction of texture, color, and sensory quality during the canning process, significantly improving the physicochemical properties, inhibiting the oxidation ability, and enhancing the microstructure and sensory quality of the meat products, thereby avoiding the deterioration of quality caused by the canning process. Overall, these results showed that basic amino acid pretreatment improved the quality of canned Antarctic krill even better than the commonly used quality-improving agent, such as STPP. Notably, the Lys pretreatment improved the quality of canned Antarctic krill; however, the mechanism involved in improving the quality of canned krill meat still needs further exploration. The present study results provide a basis for broadening the application of basic amino acids in other canned or processed meat products.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article. The data that support the findings of this study are available upon reasonable request.
References
Atkinson, A., Siegel, V., Pakhomov, E. A., Jessopp, M. J., & Loeb, V. (2009). A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Research Part I: Oceanographic Research Papers., 56(5), 727–740.
Chen, Y.-W, Cai, W.-Q, Shi, Y.-G, Dong, X.-P, Bai, F., Shen, S.-K, Jiao, R., X-Y, Z., & Zhu, X. (2020a). Effects of different salt concentrations and vacuum packaging on the shelf-stability of Russian sturgeon (Acipenser gueldenstaedti) stored at 4 °C. Food Control, 109, 106865.
Chen, Y. C., & Jaczynski, J. (2007). Gelation of protein recovered from whole Antarctic krill (Euphausia superba) by isoelectric solubilization/precipitation as affected by functional additives. Journal of Agricultural and Food Chemistry., 55(5), 1814–1822.
Chen, Y., Xu, A., Yang, R., Jia, R., Zhang, J., Xu, D., & Yang, W. (2020b). Myofibrillar protein structure and gel properties of Trichiurus haumela surimi subjected to high pressure or high pressure synergistic heat. Food and Bioprocess Technology., 13(4), 589–598.
Chen, L., Zou, G.-H., & Zhang, W.-G. (2015). Effects of high oxygen packaging on tenderness and water holding capacity of pork through protein oxidation. Food and Bioprocess Technology., 8(11), 2287–2297.
Cheng, S., Wang, X., Yang, H., Lin, R., Wang, H., & Tan, M. (2020). Characterization of moisture migration of beef during refrigeration storage by low-field NMR and its relationship to beef quality. Journal of the Science of Food and Agriculture., 100(5), 1940–1948.
Choo, K., Ramachandran, R. P., Sopiwnyk, E., & Paliwal, J. (2022). Effects of seed moisture conditioning and mechanical scouring pre-treatments on roller-milled green lentil (Lens culinaris) and Chickpea (Cicer arietinum) Flours. Food and Bioprocess Technology., 15(6), 1311–1326.
Chumngoen, W., Chen, H.-Y., & Tan, F.-J. (2016). Validation of feasibility and quality of chicken breast meat cooked under various water-cooking conditions. Animal Science Journal., 87(12), 1536–1544.
Damasceno, Md. S. P., & Gonçalves, A. A. (2019). The effect of the food grade additive phosphate pre-treatment prior to the industrial cooking process in the quality of cooked peeled shrimp (Litopenaeus vannamei). Journal of the Science of Food and Agriculture., 99(7), 3299–3306.
Devi, A. F., Au, X. N., Weerakkody, R., Sanguansri, P., Swiergon, P., Singh, T., Ng, S., & V. Gamage T,. (2021). Microwave pasteurised pear snack: Quality and microbiological stability. Food and Bioprocess Technology., 14(9), 1615–1630.
Domiszewski, Z., Duszyńska, K., & Stachowska, E. (2020). Influence of different heat treatments on the lipid quality of African Catfish (Clarias gariepinus). Journal of Aquatic Food Product Technology., 29(9), 886–900.
Ezeanaka, M. C., Nsor-Atindana, J., & Zhang, M. (2019). Online low-field nuclear magnetic resonance (LF-NMR) and magnetic resonance imaging (MRI) for food quality optimization in food processing. Food and Bioprocess Technology., 12(9), 1435–1451.
Ferrini, G., Comaposada, J., Arnau, J., & Gou, P. (2012). Colour modification in a cured meat model dried by Quick-Dry-Slice process® and high pressure processed as a function of NaCl, KCl, K-lactate and water contents. Innovative Food Science & Emerging Technologies., 13, 69–74.
Gao, R., Feng, X., Li, W., Yuan, L., Ge, J., Lu, D., Chen, B., & Yu, G. (2016). Changes in properties of white shrimp (Litopenaeus vannamei) protein during thermal denaturation. Food Science and Biotechnology., 25(1), 21–26.
Georgantelis, D., Blekas, G., Katikou, P., Ambrosiadis, I., & Fletouris, D. J. (2007). Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Science., 75(2), 256–264.
Guo, X. Y., Peng, Z. Q., Zhang, Y. W., Liu, B., & Cui, Y. Q. (2015). The solubility and conformational characteristics of porcine myosin as affected by the presence of l-lysine and l-histidine. Food Chemistry., 170, 212–217.
Guo, X., Tao, S., Pan, J., Lin, X., Ji, C., Liang, H., Dong, X., & Li, S. (2020). Effects of l-Lysine on the physiochemical properties and sensory characteristics of salt-reduced reconstructed ham. Meat Science., 166, 108133.
Husein, R., & Abdullah, N. (2021). Effect of temperature and long storage against profile amino acid and value protein carbonyls fish snapper (lutjanus sp). IOP Conference Series: Earth and Environmental Science., 681(1), 012012.
Ismail, I., Hwang, Y.-H., & Joo, S.-T. (2019). Effect of different temperature and time combinations on quality characteristics of sous-vide cooked goat gluteus medius and biceps femoris. Food and Bioprocess Technology., 12(6), 1000–1009.
Joseph, J. K., Awosanya, B., Adeniran, A. T., & Otagba, U. M. (1997). The effects of end-point internal cooking temperatures on the meat quality attributes of selected Nigerian poultry meats. Food Quality and Preference., 8(1), 57–61.
Kılıç, B., Şimşek, A., Claus, J. R., & Atılgan, E. (2014). Encapsulated phosphates reduce lipid oxidation in both ground chicken and ground beef during raw and cooked meat storage with some influence on color, pH, and cooking loss. Meat Science., 97(1), 93–103.
Kong, F., Tang, J., Rasco, B., & Crapo, C. (2007). Kinetics of salmon quality changes during thermal processing. Journal of Food Engineering., 83(4), 510–520.
Lei, Z., Fu, Y., Xu, P., Zheng, Y., & Zhou, C. (2016). Effects of l-arginine on the physicochemical and gel properties of chicken actomyosin. International Journal of Biological Macromolecules., 92, 1258–1265.
Lin, J., Zhang, Y., Li, Y., Sun, P., Ren, X., & Li, D. (2022). Improving the texture properties and protein thermal stability of Antarctic krill (Euphausia superba) by L-lysine marination. Journal of the Science of Food and Agriculture., 102(9), 3916–3924.
Liu, Y., Cong, P., Li, B., Song, Y., Liu, Y., Xu, J., & Xue, C. (2018). Effect of thermal processing towards lipid oxidation and non-enzymatic browning reactions of Antarctic krill (Euphausia superba) meal. Journal of the Science of Food and Agriculture., 98(14), 5257–5268.
Liu, Y., Zeng, Y., Hu, X., & Sun, X. (2020). Effect of ultrasonic power on water removal kinetics and moisture migration of kiwifruit slices during contact ultrasound intensified heat pump drying. Food and Bioprocess Technology., 13(3), 430–441.
Lorenzetti, E., Soares, J., Treichel, H., Cansian, R. L., Steffens, C., & Valduga, E. (2015). Brine absorption in seasoned chicken pieces. Journal Für Verbraucherschutz Und Lebensmittelsicherheit., 10(4), 331–340.
Lv, Y., Chu, Y., Zhou, P., Mei, J., & Xie, J. (2021). Effects of different freezing methods on water distribution, microstructure and protein properties of cuttlefish during the frozen storage. Applied Sciences., 11(15), 6866.
Mizuno, M., Mitchell, J. H., Crawford, S., Huang, C.-L., Maalouf, N., Hu, M.-C., Moe, O. W., Smith, S. A., & Vongpatanasin, W. (2016). High dietary phosphate intake induces hypertension and augments exercise pressor reflex function in rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology., 311(1), R39–R48.
Muñoz, I., De Sousa, D. A. B., Guardia, M. D., Rodriguez, C. J., Nunes, M. L., Oliveira, H., Cunha, S. C., Casal, S., Marques, A., & Cabado, A. G. (2022). Comparison of different technologies (conventional thermal processing, radiofrequency heating and high-pressure processing) in combination with thermal solar energy for high quality and sustainable fish soup pasteurization. Food and Bioprocess Technology., 15(4), 795–805.
Niamnuy, C., Devahastin, S., & Soponronnarit, S. (2008). Changes in protein compositions and their effects on physical changes of shrimp during boiling in salt solution. Food Chemistry., 108(1), 165–175.
Ning, C., Bao, P., Zhang, D., Li, L., Chen, L., Fang, H., Tang, Y., & Zhou, C. (2020). Reduction and coordination properties of l-Lysine/l-arginine/l-cysteine for the improvement of the color of cured sausage. Food Chemistry., 312, 126122.
Omar, S. D., Yang, J. E., Oh, S. C., Kim, D. W., & Lee, Y. B. (2016). Physiochemical changes and optimization of phosphate-treated shrimp (Litopenaeus vannamei ) using response surface methodology. Preventive Nutrition and Food Science., 21(1), 44–51.
Peng, Y., Ji, W., Zhang, D., Ji, H., & Liu, S. (2019). Composition and content analysis of fluoride in inorganic salts of the integument of Antarctic krill (Euphausia superba). Science and Reports, 9(1), 7853.
Rabeler, F., & Feyissa, A. H. (2018). Kinetic modeling of texture and color changes during thermal treatment of chicken breast meat. Food and Bioprocess Technology., 11(8), 1495–1504.
Sun, P., Lin, J., Ren, X., Zhang, B., Liu, J., Zhao, Y., & Li, D. (2022). Effect of heating on protein denaturation, water state, microstructure, and textural properties of Antarctic krill (Euphausia superba) meat. Food and Bioprocess Technology., 15(10), 2313–2326.
Tamilmani, P., & Pandey, M. C. (2016). Thermal analysis of meat and meat products. Journal of Thermal Analysis and Calorimetry., 123(3), 1899–1917.
Wu, C., Fu, S., Xiang, Y., Yuan, C., Hu, Y., Chen, S., Liu, D., & Ye, X. (2016). Effect of chitosan gallate coating on the quality maintenance of refrigerated (4 °C) silver pomfret (Pampus argentus). Food and Bioprocess Technology., 9(11), 1835–1843.
Xu, D., Yang, X., Wang, Y., & Sun, L. (2020). Cascading mechanism triggering the activation of polyphenol oxidase zymogen in shrimp Litopenaeus vannamei after postmortem and the correlation with melanosis development. Food and Bioprocess Technology., 13(7), 1131–1145.
Xu, P., Zheng, Y., Zhu, X., Li, S., & Zhou, C. (2018). L-lysine and L-arginine inhibit the oxidation of lipids and proteins of emulsion sausage by chelating iron ion and scavenging radical. Asian-Australas Journal Animal Science., 31(6), 905–913.
Zhang, Y., Guo, X., Peng, Z., & Jamali, M. A. (2022). A review of recent progress in reducing NaCl content in meat and fish products using basic amino acids. Trends in Food Science & Technology., 119, 215–226.
Zhang, Y., Zhang, D., Huang, Y., Chen, L., Bao, P., Fang, H., Xu, B., & Zhou, C. (2020). Effects of basic amino acid on the tenderness, water binding capacity and texture of cooked marinated chicken breast. LWT., 129, 109524.
Zhang, Y. W., Zhang, L., Hui, T., Guo, X. Y., & Peng, Z. Q. (2015). Influence of partial replacement of NaCl by KCl, l-histidine and l-lysine on the lipase activity and lipid oxidation in dry-cured loin process. LWT - Food Science and Technology., 64(2), 966–973.
Zheng, Y. (2017). Effects of L-lysine/L-arginine on the physicochemical properties and quality of sodium-reduced and phosphate-free pork sausage. International Journal of Nutrition and Food Sciences., 6(1), 12–18.
Funding
This work was supported by the National Key Research and Development Project of Dalian (Grant No. 2022YF16SN033).
Author information
Authors and Affiliations
Contributions
Peizi Sun: conceptualization, methodology, investigation, and writing—original draft. Xuedi Zhang: methodology and investigation. Xiang Ren: investigation. Zhiqi Cao: investigation and methodology. Yanfen Zhao: investigation. Hao Man: investigation and methodology. Dongmei Li: conceptualization, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, P., Zhang, X., Ren, X. et al. Effect of Basic Amino Acid Pretreatment on the Quality of Canned Antarctic Krill. Food Bioprocess Technol 16, 1690–1702 (2023). https://doi.org/10.1007/s11947-023-03027-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03027-y