Abstract
In this study, chitosan film incorporating tulsi essential oil (TEO) with good antioxidant properties was solvent casted as an antioxidant packaging film. The antioxidant films were studied for the morphological, chemical, thermal, water barrier, mechanical, and antioxidant behaviors. The presence of TEO was confirmed by infrared spectroscopy and electron microscopy. Thermal degradation study was also conducted to know the thermal stability of these films. TEO-containing films showed a decrement in moisture content, water absorption, water solubility, and water vapor transfer rate (WVTR). An increase in tensile and elongation was noted as TEO content increased, with the highest mechanical properties in T2 film (chitosan-to-oil ratio of 4:1), depicting better networking of chitosan chains and TEO components and the effect of plasticization of TEO on the chitosan. The films containing TEO showed better antioxidant activity than neat chitosan film, as confirmed by DPPH (2,2,-diphenyl-1-picrylhydrazyl) and H2O2-scavenging assays. Finally, fried potato fingers were packed in the active chitosan packaging and compared with the neat chitosan. The T2 film showed better antioxidant activity against the oxidation of oil in fried potato fingers which was confirmed by the FTIR of the packed product.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Packaging is important in protecting food from various chemical, biological, and physical hazards during different stages of the food supply chain. For the influencing factors such as temperature, humidity, light, dust, shocks, and odors which the food faces, food packaging becomes essential (Kalpana et al., 2019). The use of synthetic packaging materials has raised concerns related to the environment as they are developed from non-sustainable sources and thus are non-recyclable, non-biodegradable, or non-compostable (Ludwicka et al., 2020), thereby pushing the food packaging industry to pay attention to edible films and biopolymers in the last decade or so. Replacing conventional packaging with sustainable alternatives that can safely and efficiently degrade is an important alternative for decreasing plastic waste disposal in rivers, landfills, and marine environments (Gan & Chow, 2018).
Global packaged food market is growing at CAGR of 5.4% worldwide (Kumar et al., 2022). The role of food packaging is essential in reducing the deterioration of food quality due to different biological and chemical factors. The shelf life of packaged food can be extended by antimicrobial (Sharma et al., 2022b) or by antioxidant agents (Wang et al., 2015). After microbial growth, oxidation plays a significant role in the deterioration of food quality, resulting in modification of texture, nutritional value loss, off-flavor, and an increase in toxicity, particularly for meat products that are susceptible to lipid oxidation (Sun et al., 2013). Therefore, active antioxidant packaging (Souza et al., 2017) uses natural antioxidant sources such as rosemary and curry leaf extract (Demarco et al., 2022), seaweed extracts (Babakhani et al., 2016), and Baccharis dracunculifolia leaf powder (Zanela et al., 2021) which are now being used for preventing the oxidation of foods (Singh et al., 2021).
The factors such as abundance, film-forming ability, biodegradability, biocompatibility, and antibacterial properties make chitosan an attractive option for developing active food packaging material. Chitosan also finds its application in the biomedical and pharmaceutical fields (Lord et al., 2011). Researchers have focused on using plant extracts and essential oils instead of synthetic compounds as active natural sources that give functional properties such as antioxidant properties in biopolymer films like chitosan. In recent years, chitosan film containing essential oil of rosemary (Abdollahi et al., 2012), anise (Mahdavi et al., 2018), apricot kernel essential oil (AKE) (Priyadarshi et al., 2018), Zataria multiflora Boiss essential oil (Moradi et al., 2012), ginger essential oil (Cai & Wang, 2021), ginger and cinnamon (Wang et al., 2017), grapefruit seed extract and lemon essential oil (Bof et al., 2016), and many others has been prepared, resulting in modifications of chitosan film techno-functional property. Essential oils (EO) incorporated in films show additional functional properties such as antioxidant or antimicrobial activity (Bakkali et al., 2008; Rodrigues, 2007; Sharma et al., 2022a). Active chitosan film based on rosemary essential oil (REO) (Abdollahi et al., 2012), olive oil (Pereda et al., 2012), and anise (Mahdavi et al., 2018) showed increased tensile strength and percentage elongation, and this may be attributed due to the increased intermolecular interaction between the chitosan network and oil compounds, adding to this their addition induced plasticization effect in the film, thus improving percentage elongation. Also, the incorporation of AKE (Priyadarshi et al., 2018) in the chitosan matrix observed an increase in tensile strength, but percentage elongation showed initial increment and then decreased with increased extract concentration; this behavior was due to the plasticization effect of extract when present in lower concentration; however, at higher extract concentration, the formation of the complex network between chitosan and the extracted compounds resulted in constrained motion of polymeric chains. Different lipids have been incorporated into these hydrophilic films to lower the transfer rate of water vapor in these chitosan films. In a study, researchers showed a decrease in water vapor permeability (Priyadarshi et al., 2018) due to the covalent interaction between the chitosan network and compounds present in essential oil.
The covalent bonding reduces the availability of free hydrogen for forming hydrogen bonds with water, which leads to the decreased interaction between chitosan’s film and water molecules. It was worth noting that essential oil also affected the mechanical property such as tensile strengths and percentage of elongation at break, positively or negatively. The above-mentioned chitosan-based films showed good functional properties such as antioxidant, antibacterial, or both activities.
This work aims at developing antioxidant packaging films for food packaging applications. Farming in the Indian subcontinent is one of the primary sources of occupation. If any crop can be an ingredient in the antioxidant flexible packaging market (increasing at a CAGR of 5%) (Jordan, 2022), it would be a win–win situation for both the packaging companies and farmers as both of them will get the benefit; the farmer will get sustainable packaging solutions and later will be having the scope of earning money. This work has focused on one type of crop, i.e., tulsi, in the form of its essential oil, which showed good antioxidant (Bai et al., 2022) and antimicrobial properties (Tsiraki & Savvaidis, 2013). Tulsi essential oil (TEO) contains eugenol, which has good antioxidant properties and has been previously used as a coating on yam to decrease lipid oxidation (Bai et al., 2022). Recently, the use of essential oils in packaging has increased due to many health concerns with the existing synthetic antioxidants; therefore, the incorporation of TEO in the polymer matrix is being studied. TEO obtained from the Ocimum genus is an excellent source of naturally available antioxidants. Some literature is available on basilicum (sweet basil) (da Silva et al., 2022), but there isn’t any literature discussing the effect of Tenuiflorum (holy basil, TEO) on techno-functional characteristics of chitosan film and its antioxidant effect on fried potato fingers. The essential oil used in this literary work is extracted by steam distillation of the flowering top of holy basil. The incorporation of TEO in chitosan with varying concentrations is studied for various water barriers, mechanical properties, and antioxidant properties. Lastly, the active film was compared with neat film by packaging the fried potato fingers and thus analyzing the antioxidant activity through FTIR.
Material and Method
Material
Shrimp shell chitosan (75% degree of deacetylation), glacial acetic acid (min 99.6% assay), Tween 20 (pure), methanol (min 99% assay), and H2O2 (min 30% assay) were obtained from Hi-Media Laboratories Pvt. Ltd, Mumbai. 2,2,-Diphenyl-1-picrylhydrazyl (DPPH) (min.95% assay) was acquired through Sisco Research Pvt. Ltd, Maharastra. TEO (100% pure) was acquired from RV Essential, Delhi.
Preparation of Films
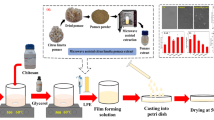
The preparation of films was done via the solvent casting method as described elsewhere (Priyadarshi et al., 2018). Briefly (Fig. 1), chitosan (1% w/v) was dissolved in 1% v/v acetic acid solution by stirring on a magnetic stirrer at 60 ℃ for 5 h. After the dissolution, the chitosan solution was filtered to remove any impurities. Tween 20 ( 0.2% v/v) and a varying amount of TEO were mixed with the filtered chitosan solution and stirred at 500 RPM for the next 10 min at 25 ℃. This solution was homogenized at 10,000 RPM with the help of IKA T25 Ultra Turrax for 5 min at ℃. The obtained final solution was poured on plastic petri plates and cast in a hot air oven at 40 ℃ for 24 h. Subsequently, before any testing, the films were placed in the desiccator at 25 °C and 50% RH. In this manner, five different films (T0, T1, T2, T3, and T4) were fabricated with varying concentrations of TEO in chitosan, as shown in Table 1.
Color of Film Surface
Color is crucial in packaging and product design. The color of processed films was evaluated using a chroma meter CR-400 Minolta (Konica Minolta Co., Japan). For color analysis, “L,” “a,” and “b” values were recorded to represent developed film color. A white plate (Lstandard = 96.67, astandard = 0.07, bstandard = 0.34) was used as a reference where “L” denotes lightness, “a” denotes redness or greenness, and “b” denotes yellowness or blueness. The averages were calculated for three replications of each mixture for mean values. ∆E denotes a change in color difference and is calculated using Eq. (1):
where ∆L = (Lstandard – Ltest sample), ∆a = (astandard – atest sample), ∆b = (bstandard – btest sample).
Transparency
Film transparency was measured by Shimadzu 1800 UV–visible spectrophotometer and adopted from previous literature (Kalaycıoğlu et al., 2017). Strips of the films were kept in a spectrophotometer cell, and their transmittance was recorded from 800 to 200 nm, and transmittance values were observed at 600 nm. The blank sample here was air. Calculation of transparency was done using:
where t is film thickness (mm) and T600 is transmittance at 600 nm.
Field Emission Scanning Electron Microscopy (FE-SEM)
The morphological study of the surface and cross-section of the films was performed using FE-SEM and a TESCAN LMH Mira3 (USA) Scanning Electron Microscope at a 5 kV accelerating voltage. Cryo-fracturing in liquid nitrogen was used to acquire the cross-sections. All film samples were coated with a thin gold layer using a sputtering process via glow discharge plasma to increase picture quality, image resolution, and conductivity.
Fourier-Transform Infrared Spectroscopy
The film’s functional groups and the potential interactions of additives with the films were investigated using FTIR spectra acquired on Perkin Elmer Spectrum 2 spectrophotometer at ATR mode and in the range between 500 to 4000 cm−1 wavelengths with a 4 cm−1 resolution and analyzed after baseline correction.
Thermogravimetric Analysis (TGA)
A thermogravimetric analyzer was used to examine the heat-sensitive behavior of films (TA Instruments, USA). Film samples (3–5 mg) were heated at a 10 °C/min speed from 30 to 600 °C in a nitrogen gas atmosphere, with a 40 mL/min gas flow rate.
Moisture Content
The amount of moisture that the dry films can take up from their surrounding environment until the equilibrium between the film’s moisture content and that of the air surrounding the films defines the film’s moisture content. The films were cut into 4 cm2 area square pieces for moisture content determination and then weighed. The initially moisturized film weight was taken and named W1. The films were maintained in a vacuum oven at 105 °C until they reached a consistent weight, W2. The calculation for moisture content is done using Eq. (2):
Water Absorption
Water absorption of the films is represented in percentage, which is defined as the amount of water absorbed by the films until equilibrium is attained when it is immersed in water. The determination of water absorption is done by drying the film completed by the procedure mentioned above and naming it as Wd. Then this dry film is immersed in 30 ml of water in a beaker. The film’s weight gain was noticed until a constant weight was attained, and the water-saturated film Ws denoting the final weight was noted. The calculation for absorption of water was done using Eq. (3):
Film Solubility in Water
The determination of the solubility of films in water is expressed in percentage. It is defined as the percentage amount of dry matter of films that is water-soluble. Four cm2 area of films was kept in a vacuum oven as described in the moisture content section, and the weight of the films thereby obtained is termed as initial dry film weight Wi; these films are then immersed in a beaker containing water with continuous agitation using a magnetic stirrer for 12 h at 25 ℃. The wet films were then drawn out of the beaker and dried in a vacuum oven with the same procedure mentioned in the moisture content section. The weight of the films is then taken and termed as the final dry film weight Wf. The calculations for percentage solubility in water were done by Eq. (4):
Percentage Elongation and Tensile Strength
The mechanical characteristics, such as the film’s percentage elongation at break and tensile strength, were analyzed using the universal testing machine (UTM) INSTRON 3365, Integrated System Solutions, India. Each film strip was trimmed to a gauge length of 50 mm and a width of 10 mm. The crosshead speed is set at 10 mm/min. Mechanical characteristics such as percent elongation and tensile strength for each kind of film were determined.
Water Vapor Transmission Rate (WVTR)
The WVTR of the fabricated film wvtr was evaluated using gravimetric analysis following the ASTM E96 (ASTM, 2017). The permeability cups were filled with 8 g of silica gel, and the film sample was attached to the top of the permeability cup. The cups were placed in a laboratory desiccator chamber with a saturated salt solution to maintain 75% RH and 25 ℃. Periodically, the weight of the cups was noted, thereby calculating the gain in weight of each cup. Water vapor transfer rate (g/m2 day) was calculated using the linear slopes between the weight gain and time.
Antioxidant Activity
2,2-Diphenyl-1-Picrylhydrazyl Radical Scavenging Assay
UV–visible spectrophotometer evaluated the scavenging rate of DPPH radicals of chitosan control and chitosan-blended films by following a previously reported method (Kaya et al., 2018) with slight changes. Firstly, each sample of films weighing 30 mg was dissolved in DPPH solution (0.1 mM) with a volume of 3 ml. The incubation of the mixtures was done in a dark condition for 30 min, and after that, absorbance at 517 nm was noted. Three readings were taken following the above procedure for computing the antioxidant activities of each sample using Eq. (5):
where:
H2O2 Scavenging Assay
The hydrogen peroxide (H2O2) radical scavenging experiment was performed to test the films’ antioxidant properties, which was adapted from the past literature (Priyadarshi et al., 2018) with slight modification. Each film’s extract was created by combining a 300 mg film piece with 9 ml methanol to make the extract. The mixture was then ultrasonicated for 2 h, followed by 6000 rpm centrifugation for 10 min, and the supernatant was collected. A 40 mM solution of hydrogen peroxide was separately prepared with a buffer solution of phosphate (pH 7.4). The extract (500 l) was mixed with an H2O2 solution (4 ml) and incubated at 37 °C for 30 min. After that, Shimadzu 1800 UV–visible spectrophotometer was used to measure absorbance at 230 nm. The blank solution for this test was phosphate buffer not having H2O2. The antioxidant activity was determined using Eq. (5) described in the above section, where DPPH is replaced with H2O2.
Oxidative Stability Test of Fried Potato Finger in Chitosan–TEO Package
The application of this antioxidant packaging film with fried potato fingers was tested with the help of FTIR. The fried potato fingers were packed in neat chitosan and TEO-containing chitosan. The films were packed in LDPE pouches to resemble pouch-in-pouch type packaging. These packages were kept in a hot air oven for 7 days at 60 °C, as the rancidity developed at 60 °C in lipids and oils. The FTIR of fried potato fingers was done after 7 days, and the obtained spectrums were analyzed to check the oxidation of products.
Statistics Analysis
All described experiments were statistically analyzed using IBM SPSS Statistics (version 28.0.0.0, USA), and the analysis of variance (ANOVA) was performed using a post hoc Tukey test with a 95% confidence interval (significance level, p < 0.05). For the stated characterization, the measurements were done in triplicate and recorded as mean standard deviation.
Results and Discussion
Color of Film Surface
From the consumer’s point of view, the color of packaging plays an essential role in the acceptance of the product packed in it. The color qualities were displayed as “L,” “a,” “b,” and ∆E values, where the higher “L” value denotes brightness. The lower “L” value suggests darkness; “ + a” is for redness, while “–a” is for greenness; “ + b” is for yellowness, while “–b” is for blueness; and ∆E represents color change. Table 2 shows the “L,” “a,” “b,” and ∆E values for chitosan and chitosan–TEO (at various TEO concentrations)-based films. The lightness of the film increased for the T1 film, whereas the films’ color was darker with the increase in oil concentration (T2, T3, T4). This is probably due to the rise of the phenolic compounds, due to an increase in the concentration of TEO, thus resulting in increased scattering and light reflections going through the films (Ramakanth et al., 2022; Tanwar et al., 2021). The “a” values didn’t changed significantly, and the T2 film showed the highest greenness, whereas the T4 film was reddish. The yellowness “b” value of modified chitosan films didn’t changed significantly at lower levels of oil (T1 and T2). However, the yellowness of films increased at higher TEO content (T3 and T4). The ∆E value, which signifies color change, did not change significantly with increased essential oil concentration in films.
Transparency
The oxidation of food products can be affected by the presence of light; therefore, a package’s light barrier can reduce the food products’ photooxidation (Dadex, 2016). Therefore, the transparency of the films was tested to study the light barrier properties. The transparency of neat chitosan film T0 was 3.36 and was reduced by 4.16% to 3.22 for T4. However, the change in transparency was not significant, which might be due to similar optical properties of TEO and chitosan or by better dispersion of TEO in the chitosan matrix.
Field Emission Scanning Electron Microscopy
Figure 2 shows the FE-SEM images of chitosan with and without TEO; neat chitosan film (T0) (Fig. 2a, d) exhibited plane and a smooth surface without many fissures and discontinuities. As the TEO concentration in the films increased, an observable change in the microstructure of chitosan film was noticed. Oil droplets seem to increase with the increased oil concentration, as seen in the cross-section images. The number and size of droplets also increased with increased concentration.
As observed in the transverse image, an increase in roughness shows the oil phase present in the T4 film in an almost continuous manner, thus leading to the formation of a structure like a bi-layer. We can see that oil has covered most of the chitosan in T4 film at higher magnification. T0 and T1 seem similar on the surface, with a slight increase in particles. In T4, the oil particles increased, and almost a non-uniform morphology of the surface was noted. The uniformness in the film’s topography is lost as the concentration of TEO increases, which was in line with the past literature when orange peel extract was incorporated (Alparslan & Baygar, 2017). The observation of bigger oil droplets was made probably due to the rise in the number of collisions for oil droplets attributing to agglomeration similar to previous studies (Priyadarshi et al., 2018).
Fourier-Transform Infrared Spectroscopy
Figure 3 shows FTIR spectra of the chitosan films with and without TEO. Characteristic bands of Chitosan can be noted for T0 film; a broad band from 4000 to 3000 cm−1 represents O–H bond stretching (Queiroz et al., 2015); the peaks at 2928 cm−1 and 2874 cm−1 are due to symmetric and asymmetric vibrations of CH2 respectively, while the peak at 1538 cm−1 is due to N–H bending of amide II; the bands at 1064 cm−1 and 1019 cm−1 corresponds to C-O stretching. The presence of residual N-acetyl groups was assured due to the bands at around 1631 cm−1 (C = O stretching of amide I). In contrast, the absorption band at 1152 cm−1 can be attributed to asymmetric stretching C–O–C bridge. For TEO, the peaks can denote the presence of eugenol, methyl chavicol, and linalool, as stated in the past literature (Sharma et al., 2022a). A peak at 2848 cm−1, specific to methylene groups (Hemalatha et al., 2017), was observed. The spectrum at 3070 cm−1 was assigned to the asymmetric C–H stretching vibrations in double-bond C = C. The bands observed at 2927 cm−1 and 2859 cm−1 indicated the asymmetric and symmetric C–H stretching vibrations in –CH2–. Very small peaks at 1640 cm−1, 1599 cm−1, 1580 cm−1, and 1511 cm − 1 are assigned to the alkene/aromatic double-bond (− C = C −) stretching vibrations, and the scissor C–H bending vibrations in double-bond CH2 appeared at 1462 cm − 1; similar observations were reported in the past literature (Sutaphanit & Chitprasert, 2014). A peak at 1726 cm−1 representing C = O bond, denoting ketone group present in methyl chavicol, 1269 cm−1 intense C-O stretching for aryl- alkyl ether, was observed denoting eugenol presence, 3466 cm−1 for O–H bond denoting hydroxyl compound present in eugenol and linalool, and 2957 cm−1 for C-H stretching of linalool as was seen by (Xiao et al., 2017).
Mixing of chitosan and TEO is depicted with peaks at 3269 cm−1 as O–H band intensity increased, denoting mixing of both the compounds (OH in chitosan and eugenol + linalool). Some essential oil might have evaporated during mixing or film casting due to its volatile nature; hence ketonic group present due to methyl chavicol at (1726 cm−1) had its intensity decreased. Also, the same phenomenon was observed at 2957 cm−1, denoting some loss of linalool. Moreover, the 1269 cm−1 peak was also reduced, denoting some loss of eugenol.
Thermogravimetric Analysis
The thermal stability of the film can be used to assess processability at high temperatures and give an idea about the heat resistivity of the film. TGA provides quantifiable data that may be interpreted to determine the thermal stability of the film. Figure 4 shows the heat degradation behavior of the produced active films. Weight loss at lower temperatures up to 120 °C is due to the evaporation of the solvent traces such as acetic acid and water (Altiok et al., 2010). Further weight loss from 200 to 400 °C indicates the degradation of chitosan due to the decomposition of the amines group in chitosan. Above 400 °C, weight loss may be due to the degradation of the glucopyranose of chitosan (Susilowati et al., 2016). All the TEO films had similar degradation patterns as chitosan, except T4, which shows a high weight loss near 175 to 200 °C. This might be due to the vaporization of TEO, as the flashpoint of TEO is also near 175 °C.
Moisture Content, Water Absorption, and Water Solubility
Table 3 shows the water solubility, moisture content, and water absorption values of the neat and TEO-incorporated chitosan films. The film’s water absorption and moisture content decreased by 37% and 32%, respectively, as TEO content increased from T0 to T4 film. In the case of moisture content and water absorption, the decrease was monotonic for all the films. The moisture level of the film rose to lower concentrations of grapefruit seed extract due to its hydrophilic character (Bof et al., 2016), but our study used essential oil, which is non-polar, thus lowering the moisture content, which was comparable to the use of lemon essential oil in the past literature (Bof et al., 2016). Probably, this is because of the formation of the covalent bond between chitosan polymer and functional groups present in oil as TEO is incorporated in chitosan films, thus reducing the availability of hydroxyl groups and free amine of chitosan for water and therefore limiting interaction in polysaccharide water (Hafsa et al., 2016). This reduction in affinity of water with chitosan films also shows a noticeable effect on the film solubility in water. The strong polymer networking of chitosan films was evident as all the films were almost intact after 24 h of stirring in water. Dry mass reduction in films suggests solubility to some extent. Around 26.32% solubility in water for neat chitosan film, i.e., T0 film, reduced to around 11% for T4 films, thus showing a notable reduction as the TEO percentage in chitosan increased. However, previous research indicates that water solubility reduces with increasing concentrations of ginger essential oil (Cai & Wang, 2021) at lower concentrations (≤ 1.5 wt%) and increases at higher concentrations (≥ 2 wt%). Furthermore, previous literature has demonstrated that there is no influence on water solubility at lower concentrations (≤ 1wt%) of essential oil (Wang et al., 2017). The addition of TEO at a higher ratio ((≥ 12.5 wt%) was studied in this paper, and it was discovered that the water solubility could be reduced to 10% by reducing the availability of hydroxyl groups and also due to the non-polar nature of the TEO, thus reducing the literature gap of increased essential oil in the chitosan films.
Mechanical Properties
Mechanical property is one of the critical factors that need to be considered for food packaging material. The material used for packaging applications should be able to handle mechanical stresses while maintaining its integrity. The mechanical properties of the mentioned chitosan-based films are given in Table 4. Chitosan film, when incorporated with TEO, showed an increase in tensile strength and percentage elongation from 14.95 (for T0 film) to 31.27 MPa (for T2 film) and 8.26 (for T0 film) to 16.45% (for T2 film), respectively. The increase in tensile strength with the addition of TEO was somehow different when compared with ginger essential oil (Cai & Wang, 2021), grapefruit seed extract, and lemon essential oil (Bof et al., 2016), which decreased the tensile strength of the chitosan composite film due to formation of heterogeneous structure. However, in our study, the increase in tensile strength was visible, probably due to the increase in chemical bonding, similar to previous literature (Siripatrawan & Harte, 2010; Wang et al., 2015). The increased chemical bonding can be due to higher intermolecular interaction between the chitosan network and essential oil compounds. Moreover, the TEO addition induced a plasticization in the film, thus improving percentage elongation. However, these values decreased at higher oil concentrations, as depicted in Table 4, probably due to development of structural discontinuity, decrease in crystalline structure, and intermolecular hydrogen bonding in chitosan matrix, along with the reduction in molecular mobility with the addition of TEO (Moradi et al., 2012; Peng et al., 2013; Sun et al., 2017).
Water Vapor Transmission Rate (WVTR)
The WVTR values of all the chitosan-based films are listed in Table 5. It was noted that, as oil content is increased in the chitosan films, the water vapor transmission rate ranged from 45.68 to 43.96 g/m2/day without any significant difference, as depicted in Table 5. The plasticization effect of TEO was expected to play a decremental role in water barrier as seen in the past literatures (Rodríguez-Núñez et al., 2014; Sharma et al., 2022b), rather the barrier didn’t changed which could be explained due to the hydrophobic nature of TEO. It is known that due to the addition of oils in films having a “hydrophilic” polymer matrix nature, the “hydrophobic” constituents in the film get increased, thus enhancing the films barrier property (Atarés & Chiralt, 2016).
The incorporation of Citrus limonia (Filho et al., 2020), apricot kernel (Priyadarshi et al., 2018), and anise (Mahdavi et al., 2018) essential oils in chitosan has shown similar trends probably due to the essential oil’s hydrophobicity, which resulted in lowering the water affinity. However, since these essential oils have different chemical constituents, there is variation in hydrophobicity for different essential oils; thus, the efficiency in lowering the transfer rate of water vapors is affected (Atarés & Chiralt, 2016).
Antioxidant Activity
The primary reason for the spoilage of foods and loss in their nutritional value is oxidation, as oxygen in the atmosphere reacts with the nutritional parts of food, thus causing its structural degradation. Recently, the focus has been on using antioxidant sources in the food package instead of using them directly in the food itself. TEO, a good source of antioxidants, shows good antioxidant properties when incorporated in packaging films based on chitosan, which was assured by tests like DPPH-scavenging and H2O2 (hydrogen peroxide) assays. The activities of both the assays for all mentioned chitosan-based films were noted and are shown in Fig. 5. It is noted that with an increase in essential oil concentration, the film antioxidant activity increased against both assays, which was in a similar trend with ginger essential oil when incorporated in the chitosan composite films (Cai & Wang, 2021). In both the assays, T0 film showed some antioxidant activity mainly because of the reaction between the free unbound –NH2 groups and the free radicals present in chitosan, thus forming stable radicals (Priyadarshi et al., 2018; Yen et al., 2008). However, the films except the T0 film show antioxidant activity primarily because of methyl chavicol, linalool, and eugenol, the major constituents in TEO (see table). The T0 films showed 20.4% DPPH-scavenging activity, which increased from 43.9 to 88.2% with the increase in TEO content. Similar results were obtained in previous studies (Hromiš et al., 2015; Moradi et al., 2012; Priyadarshi et al., 2018; Souza et al., 2019), where chitosan films were incorporated with the essential oils of Zataria multiflora Boiss, apricot kernel, and caraway, respectively. H2O2-scavenging activity of the T0 film was 7.9%, elevating from 13.7 to 47.6% as the TEO ratio increased from 0.125 to 1 with respect to CS (chitosan) as depicted in Fig. 5; a similar pattern can be found in the past literature (Priyadarshi et al., 2018).
Oxidation Study of Fried Potato Finger
The product (fried potato finger) was packed in T0 and T2 films, and their oxidation was comparatively analyzed by FTIR spectroscopy. However, the antioxidant study in the past literature are done by DPPH (Cai & Wang, 2021; Wang et al., 2017), Trolox (Bof et al., 2016; Li et al., 2022), ABTS, and FRAP methods (de Souza et al., 2020) which do not involve food products. The oxidative stability of food products is also done by thiobarbituric acid (TBA) analysis (Y. Wang et al., 2017), which is an extensive and complex study. However, in this study, we have used FTIR, a facile approach to study the oxidative stability of food products. As discussed in previous sections, T2 was selected among all the active films due to its better mechanical properties. The product was packed in a pouch-in-pouch packaging system, as depicted in Fig. 6. Products packed in the T0 and T2 films are shown in Fig. 6a–d. The FTIR spectra of the products packed in T0 and T2 film on day 7 are analyzed for oxidative analysis. As shown in Fig. 7, at 3005 cm−1, the band was assigned to C-H groups of cis double, showing the isomerization of oil in the fried potato finger. Therefore, a decrease in intensity indicates more oxidation (Durazzo et al., 2018). This can be seen in the IR intensity of the product packed in T0 and T2. With the initiation of oxidation processes, the ratio of peak intensity at 2854 cm−1 to the intensity of broad peak between 3600 and 3100 cm−1 decreases. This lesser ratio indicates higher oxidation, as suggested in previous studies (Candoğan et al., 2021). Similar results were obtained in products packed in T0 and T2. A lower ratio was observed for a product packed in T0 (4.77) compared to T2 (8.00), which indicated lesser oxidation in T2. At 1650 cm−1 representing the C = C bond, the peak of the product packed in T0 is higher than the T2, indicating the formation of cis-allylic and carbonylic compounds, which also occurs during the oxidation of oil during frying (Jamwal et al., 2021). This also indicates lower oxidation in products packed in T2 than T0. A peak at 1533 cm−1 indicates RCOO- stretching bonds, which represent fatty acids/soap linkages (Lynch et al., 1996); the higher intensity of acid was observed in the product packed in T0, representing higher fatty acids in the product packed in T0, thus higher level of oxidation in T0. Moreover, the ketone group, indicated by the peak at 1015 cm−1, is sharply increased in products packed in T0. This increase in the ketone group indicates more oxidation of the product. Therefore, the oxidation of the product packed in T2 was lower when compared to T0.
The mechanism of antioxidants in the case of fried potato fingers, when packed in antioxidant packaging, can be explained as follows: the lipids can be affected by various factors such as light, oxygen, and intrinsic free radicals (Taghvaei & Jafari, 2015). Furthermore, propagation occurs due to the auto-oxidation of radicalized lipid peroxide. Finally, the oxidized lipids may combine with free radicals of lipids (Choe & Min, 2006). However, when packed in antioxidant chitosan film containing TEO, the presence of phenolic components in TEO, such as eugenol, reduces or inhibits free radicals via hydrogen atom transfer from its hydroxyl group. The phenolic compounds react with a peroxyl radical (ROO*) to create comparatively stable phenolic oxygen radicals, resulting in the elimination of free radicals. The hydrogen cation is transferred from the phenol to the radical, generating a transition state of an O–H bond with one electron (Santos-Sánchez et al., 2019). As a result, the eugenol released from the antioxidant film was able to suppress the oxidation in the oxidative stability test of fried potato fingers.
Conclusion
TEO was successfully incorporated in chitosan film. The FE-SEM analysis confirmed the incorporation as the roughness of the films increased with an increase in TEO concentration. Further, the presence of TEO was confirmed with the help of FTIR, particularly with the increase of hydroxyl groups denoting eugenol and linalool in the chitosan. Thermal analysis was performed, and it was concluded that there was no significant difference in degradation pattern for T0, T1, T2, and T3. But T4, with the highest essential oil, showed high weight loss due to the vaporization of TEO. A decrement in the percentage of moisture content, water absorption, and film water solubility was observed due to the strong networking of chitosan with the TEO. Slight improvement in the water barrier property of films was noted with increased essential oil concentration but was insignificant. In terms of mechanical property, the tensile and elongation at break increased with the increase in TEO for T1 and T2 films, indicating an increase in intermolecular interaction between chitosan and TEO. Further, the plasticization effect was induced by essential oil addition, thus enhancing the molecular mobility of the polymeric chain. As the TEO concentration increased (T3 and T4), the mechanical properties deteriorate, possibly due to structural discontinuities. Finally, the antioxidant activity was increased with the increased TEO content analyzed with the DPPH and H2O2 method. This increase in antioxidant activity would be due to the active compounds present in TEO, such as eugenol, linalool, and methyl chavicol. DPPH- and H2O2-scavenging assays confirmed excellent antioxidant properties. For the fried potato fingers when packed in T0 and T2 films, it was observed that oxidation was retarded in the T2 films, which were analyzed with the FTIR spectrum of the fried potato fingers.
Further, the study of active films can be done for sensory evaluation and will be conducted in our future research. In conclusion, chitosan with TEO can potentially retard the oxidation of lipids present in fried products, thus increasing oxidative stability. Oil-containing non-fried food such as fish and meat products can also be investigated for industrial applications.
Data Availability
All data generated or analyzed during this study are included in this manuscript.
References
Abdollahi, M., Rezaei, M., & Farzi, G. (2012). Improvement of active chitosan film properties with rosemary essential oil for food packaging. International Journal of Food Science and Technology, 47(4), 847–853. https://doi.org/10.1111/j.1365-2621.2011.02917.x
Alparslan, Y., & Baygar, T. (2017). Effect of chitosan film coating combined with orange peel essential oil on the shelf life of deepwater pink shrimp. Food and Bioprocess Technology, 10(5), 842–853. https://doi.org/10.1007/s11947-017-1862-y
Altiok, D., Altiok, E., & Tihminlioglu, F. (2010). Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. Journal of Materials Science: Materials in Medicine, 21(7), 2227–2236. https://doi.org/10.1007/s10856-010-4065-x
ASTM. (2017). Standard test methods for water vapor transmission of materials. https://doi.org/10.1520/E0096-00E01
Atarés, L., & Chiralt, A. (2016). Essential oils as additives in biodegradable films and coatings for active food packaging. Trends in Food Science and Technology, 48, 51–62. https://doi.org/10.1016/j.tifs.2015.12.001
Babakhani, A., Farvin, K. H. S., & Jacobsen, C. (2016). Antioxidative effect of seaweed extracts in chilled storage of minced Atlantic mackerel (Scomber scombrus): Effect on lipid and protein oxidation. Food and Bioprocess Technology, 9(2), 352–364. https://doi.org/10.1007/s11947-015-1630-9
Bai, T., Li, J., Murtaza, A., Iqbal, A., Zhu, L., Zhang, J., et al. (2022). Scavenging of ROS after eugenol treatment as mechanism of slowing down membrane lipid metabolism to maintain the surface color of fresh-cut yam. Food and Bioprocess Technology, 1821–1835. https://doi.org/10.1007/s11947-022-02833-0
Bakkali, F., Averbeck, S., Averbeck, D., & Idaomar, M. (2008). Biological effects of essential oils - A review. Food and Chemical Toxicology, 46(2), 446–475. https://doi.org/10.1016/j.fct.2007.09.106
Bof, M. J., Jiménez, A., Locaso, D. E., García, M. A., & Chiralt, A. (2016). Grapefruit seed extract and lemon essential oil as active agents in corn starch–chitosan blend films. Food and Bioprocess Technology, 9(12), 2033–2045. https://doi.org/10.1007/s11947-016-1789-8
Cai, L., & Wang, Y. (2021). Physicochemical and antioxidant properties based on fish sarcoplasmic protein/chitosan composite films containing ginger essential oil nanoemulsion. Food and Bioprocess Technology, 14(1), 151–163. https://doi.org/10.1007/s11947-020-02564-0
Candoğan, K., Altuntas, E. G., & İğci, N. (2021). Authentication and quality assessment of meat products by Fourier-transform infrared (FTIR) spectroscopy. Food Engineering Reviews, 13(1), 66–91. https://doi.org/10.1007/s12393-020-09251-y
Choe, E., & Min, D. B. (2006). Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety, 5(4), 169–186. https://doi.org/10.1111/j.1541-4337.2006.00009.x
da Silva, W. M. F., Kringel, D. H., de Souza, E. J. D., da Rosa Zavareze, E., & Dias, A. R. G. (2022). Basil essential oil: Methods of extraction, chemical composition, biological activities, and food applications. Food and Bioprocess Technology, 15(1), 1–27. https://doi.org/10.1007/s11947-021-02690-3
Dadex. (2016). The impact of light and oxidation, photo-oxidation, packaging on fats and oils. Retrieved July 15, 2022, from http://dadex.ca/article/the-impact-of-light-and-oxidation-photo-oxidation-packaging-on-fats-and-oil
de Souza, K. C., Correa, L. G., da Silva, T. B. V., Moreira, T. F. M., de Oliveira, A., Sakanaka, L. S., et al. (2020). Soy protein isolate films incorporated with Pinhão (Araucaria angustifolia (Bertol.) Kuntze) extract for potential use as edible oil active packaging. Food and Bioprocess Technology, 13(6), 998–1008. https://doi.org/10.1007/s11947-020-02454-5
Demarco, F., Paula, A., Alexandre, R., Ivane, A., & Tonial, B. (2022). Effects of natural antioxidants on the lipid oxidation, physicochemical and sensory characteristics, and shelf life of sliced salami. Food and Bioprocess Technology, (0123456789). https://doi.org/10.1007/s11947-022-02877-2
Durazzo, A., Kiefer, J., Lucarini, M., Camilli, E., Marconi, S., Gabrielli, P., et al. (2018). Qualitative analysis of traditional Italian dishes: FTIR approach. Sustainability (Switzerland), 10(11). https://doi.org/10.3390/su10114112
de Filho, J. G., & O., de Deus, I. P. B., Valadares, A. C. F., Fernandes, C. C., Estevam, E. B. B., & Egea, M. B. (2020). Chitosan film with citrus limonia essential oil: Physical and morphological properties and antibacterial activity. Colloids and Interfaces, 4(2), 1–10. https://doi.org/10.3390/colloids4020018
Gan, I., & Chow, W. S. (2018). Antimicrobial poly(lactic acid)/cellulose bionanocomposite for food packaging application: A review. Food Packaging and Shelf Life, 17(February), 150–161. https://doi.org/10.1016/j.fpsl.2018.06.012
Hafsa, J., Smach, M., & ali, Ben Khedher, M. R., Charfeddine, B., Limem, K., Majdoub, H., & Rouatbi, S. (2016). Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT - Food Science and Technology, 68, 356–364. https://doi.org/10.1016/j.lwt.2015.12.050
Hemalatha, T., UmaMaheswari, T., Senthil, R., Krithiga, G., & Anbukkarasi, K. (2017). Efficacy of chitosan films with basil essential oil: Perspectives in food packaging. Journal of Food Measurement and Characterization, 11(4), 2160–2170. https://doi.org/10.1007/s11694-017-9601-7
Hromiš, N. M., Lazić, V. L., Markov, S. L., Vaštag, ŽG., Popović, S. Z., Šuput, D. Z., et al. (2015). Optimization of chitosan biofilm properties by addition of caraway essential oil and beeswax. Journal of Food Engineering, 158, 86–93. https://doi.org/10.1016/j.jfoodeng.2015.01.001
Jamwal, R., Amit, K., & S., Balan, B., Kelly, S., Cannavan, A., & Singh, D. K. (2021). Rapid and non-destructive approach for the detection of fried mustard oil adulteration in pure mustard oil via ATR-FTIR spectroscopy-chemometrics. Spectrochimica Acta - Part a: Molecular and Biomolecular Spectroscopy, 244, 118822. https://doi.org/10.1016/j.saa.2020.118822
Jordan. (2022). Plastic antioxidant market - Growth, trends, COVID-19 impact, and forecasts (2022 - 2027)- Product image plastic antioxidant market - Growth, trends, COVID-19 impact, and forecasts (2022 - 2027). Research and Markets. Retrieved February 6, 2022, from https://www.researchandmarkets.com/reports/5119456/plastic-antioxidant-market-growth-trends?utm_source=GNOM&utm_medium=PressRelease&utm_code=88s4jl&utm_campaign=1449550+-+Worldwide+Plastic+Antioxidant+Industry+to+2025+-+Asia-Pacific+Set+to+Dominate+the+Ma
Kalaycıoğlu, Z., Torlak, E., Akın-Evingür, G., Özen, İ, & Erim, F. B. (2017). Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. International Journal of Biological Macromolecules, 101, 882–888. https://doi.org/10.1016/j.ijbiomac.2017.03.174
Kalpana, S., Priyadarshini, S. R., Maria Leena, M., Moses, J. A., & Anandharamakrishnan, C. (2019). Intelligent packaging: Trends and applications in food systems. Trends in Food Science and Technology, 93(October 2018), 145–157. https://doi.org/10.1016/j.tifs.2019.09.008
Kaya, M., Khadem, S., Cakmak, Y. S., Mujtaba, M., Ilk, S., Akyuz, L., et al. (2018). Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Advances, 8(8), 3941–3950. https://doi.org/10.1039/c7ra12070b
Kumar, H., Pandey, A., Negi, Y. S., & Kadam, A. A. (2022). Green materials and their application in food packaging. In Encyclopedia of Green Materials (pp. 1–10). Singapore: Springer Nature Singapore. https://doi.org/10.1007/978-981-16-4921-9_85-1
Li, Q., Liu, J., Cao, L., Zhang, L., Bredie, W. L. P., Otte, J., & Lametsch, R. (2022). Effects of γ-glutamylated hydrolysates from porcine hemoglobin and meat on kokumi enhancement and oxidative stability of emulsion-type sausages. Food and Bioprocess Technology, 1851–1865. https://doi.org/10.1007/s11947-022-02851-y
Lord, M. S., Cheng, B., McCarthy, S. J., Jung, M. S., & Whitelock, J. M. (2011). The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials, 32(28), 6655–6662. https://doi.org/10.1016/j.biomaterials.2011.05.062
Ludwicka, K., Kaczmarek, M., & Białkowska, A. (2020). Bacterial nanocellulose—a biobased polymer for active and intelligent food packaging applications: Recent advances and developments. Polymers, 12(10), 1–23. https://doi.org/10.3390/polym12102209
Lynch, M. L., Pan, Y., & Laughlin, R. G. (1996). Spectroscopic and thermal characterization of 1 : 2 sodium soap / fatty acid Acid - Soap Crystals, 357–361.
Mahdavi, V., Hosseini, S. E., & Sharifan, A. (2018). Effect of edible chitosan film enriched with anise (Pimpinella anisum L.) essential oil on shelf life and quality of the chicken burger. Food Science and Nutrition, 6(2), 269–279. https://doi.org/10.1002/fsn3.544
Moradi, M., Tajik, H., Razavi Rohani, S. M., Oromiehie, A. R., Malekinejad, H., Aliakbarlu, J., & Hadian, M. (2012). Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT - Food Science and Technology, 46(2), 477–484. https://doi.org/10.1016/j.lwt.2011.11.020
Peng, Y., Wu, Y., & Li, Y. (2013). Development of tea extracts and chitosan composite films for active packaging materials. International Journal of Biological Macromolecules, 59, 282–289. https://doi.org/10.1016/j.ijbiomac.2013.04.019
Pereda, M., Amica, G., & Marcovich, N. E. (2012). Development and characterization of edible chitosan/olive oil emulsion films. Carbohydrate Polymers, 87(2), 1318–1325. https://doi.org/10.1016/j.carbpol.2011.09.019
Priyadarshi, R., Sauraj, K., & B., Deeba, F., Kulshreshtha, A., & Negi, Y. S. (2018). Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocolloids, 85(March), 158–166. https://doi.org/10.1016/j.foodhyd.2018.07.003
Queiroz, M. F., Melo, K. R. T., Sabry, D. A., Sassaki, G. L., & Rocha, H. A. O. (2015). Does the use of chitosan contribute to oxalate kidney stone formation? Marine Drugs, 13(1), 141–158. https://doi.org/10.3390/md13010141
Ramakanth, D., Akhila, K., Gaikwad, K. K., & Maji, P. K. (2022). UV-activated oxygen scavenging system based on natural rubber latex from Hevea brasiliensis for active packaging applications. Industrial Crops and Products, 178(February), 114658. https://doi.org/10.1016/j.indcrop.2022.114658
Rodrigues, F. (2007). Composition of the leaf, flower and fruit volatile oils of Pittosporum tobira (Thunb.) WT Aiton grown in three locations in Portugal. Flavour and …, 2009(April), 311–316. https://doi.org/10.1002/ffj
Rodríguez-Núñez, J. R., Madera-Santana, T. J., Sánchez-Machado, D. I., López-Cervantes, J., & Soto Valdez, H. (2014). Chitosan/hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. Journal of Polymers and the Environment, 22(1), 41–51. https://doi.org/10.1007/S10924-013-0621-Z/TABLES/5
Santos-Sánchez, N. F., Salas-Coronado, R., Villanueva-Cañongo, C., & Hernández-Carlos, B. (2019). Antioxidant compounds and their antioxidant mechanism. In E. Shalaby (Ed.), Antioxidants. Rijeka: IntechOpen. https://doi.org/10.5772/intechopen.85270
Sharma, K., Babaei, A., Oberoi, K., Aayush, K., Sharma, R., & Sharma, S. (2022a). Essential oil nanoemulsion edible coating in food industry: A review. Food and Bioprocess Technology, (0123456789). https://doi.org/10.1007/s11947-022-02811-6
Sharma, P., Ahuja, A., Dilsad Izrayeel, A. M., Samyn, P., & Rastogi, V. K. (2022b). Physicochemical and thermal characterization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) films incorporating thyme essential oil for active packaging of white bread. Food Control, 133(November 2021). https://doi.org/10.1016/j.foodcont.2021.108688
Singh, A. K., Ramakanth, D., Kumar, A., Lee, Y. S., & Gaikwad, K. K. (2021). Active packaging technologies for clean label food products: A review. Journal of Food Measurement and Characterization, 15(5), 4314–4324. https://doi.org/10.1007/s11694-021-01024-3
Siripatrawan, U., & Harte, B. R. (2010). Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocolloids, 24(8), 770–775. https://doi.org/10.1016/j.foodhyd.2010.04.003
Souza, V. G. L., Fernando, A. L., Pires, J. R. A., Rodrigues, P. F., Lopes, A. A. S., & Fernandes, F. M. B. (2017). Physical properties of chitosan films incorporated with natural antioxidants. Industrial Crops and Products, 107(February), 565–572. https://doi.org/10.1016/j.indcrop.2017.04.056
Souza, V. G. L., Rodrigues, C., Ferreira, L., Pires, J. R. A., Duarte, M. P., Coelhoso, I., & Fernando, A. L. (2019). In vitro bioactivity of novel chitosan bionanocomposites incorporated with different essential oils. Industrial Crops and Products, 140(July), 111563. https://doi.org/10.1016/j.indcrop.2019.111563
Sun, L., Sun, J., Chen, L., Niu, P., Yang, X., & Guo, Y. (2017). Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydrate Polymers, 163, 81–91. https://doi.org/10.1016/j.carbpol.2017.01.016
Sun, W., Zhou, F., Sun, D. W., & Zhao, M. (2013). Effect of oxidation on the emulsifying properties of myofibrillar proteins. Food and Bioprocess Technology, 6(7), 1703–1712. https://doi.org/10.1007/s11947-012-0823-8
Susilowati, E., Kartini, I., Santosa, S. J., & Triyono. (2016). Effect of glycerol on mechanical and physical properties of silver-chitosan nanocomposite films. IOP Conference Series: Materials Science and Engineering, 107(1). https://doi.org/10.1088/1757-899X/107/1/012041
Sutaphanit, P., & Chitprasert, P. (2014). Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chemistry, 150, 313–320. https://doi.org/10.1016/j.foodchem.2013.10.159
Taghvaei, M., & Jafari, S. M. (2015). Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. Journal of Food Science and Technology, 52(3), 1272–1282. https://doi.org/10.1007/s13197-013-1080-1
Tanwar, R., Gupta, V., Kumar, P., Kumar, A., Singh, S., & Gaikwad, K. K. (2021). Development and characterization of PVA-starch incorporated with coconut shell extract and sepiolite clay as an antioxidant film for active food packaging applications. International Journal of Biological Macromolecules, 185(May), 451–461. https://doi.org/10.1016/j.ijbiomac.2021.06.179
Tsiraki, M. I., & Savvaidis, I. N. (2013). Effect of packaging and basil essential oil on the quality characteristics of whey cheese “Anthotyros.” Food and Bioprocess Technology, 6(1), 124–132. https://doi.org/10.1007/s11947-011-0676-6
Wang, Q., Tian, F., Feng, Z., Fan, X., Pan, Z., & Zhou, J. (2015). Antioxidant activity and physicochemical properties of chitosan films incorporated with Lycium barbarum fruit extract for active food packaging. International Journal of Food Science and Technology, 50(2), 458–464. https://doi.org/10.1111/ijfs.12623
Wang, Y., Xia, Y., Zhang, P., Ye, L., Wu, L., & He, S. (2017). Physical characterization and pork packaging application of chitosan films incorporated with combined essential oils of cinnamon and ginger. Food and Bioprocess Technology, 10(3), 503–511. https://doi.org/10.1007/s11947-016-1833-8
Xiao, Z., Xu, Z., & Zhu, G. (2017). Production and characterization of nanocapsules encapsulated linalool by ionic gelation method using chitosan as wall material. Food Science and Technology, 37(4), 613–619. https://doi.org/10.1590/1678-457x.27616
Yen, M. T., Yang, J. H., & Mau, J. L. (2008). Antioxidant properties of chitosan from crab shells. Carbohydrate Polymers, 74(4), 840–844. https://doi.org/10.1016/j.carbpol.2008.05.003
Zanela, J., Casagrande, M., Radaelli, J. C., Dias, A. P., Wagner Júnior, A., Malfatti, C. R. M., & Yamashita, F. (2021). Active biodegradable packaging for foods containing Baccharis dracunculifolia leaf as natural antioxidant. Food and Bioprocess Technology, 14(7), 1301–1310. https://doi.org/10.1007/s11947-021-02641-y
Funding
Author Himanshu Kumar would like to thank the Ministry of Education (ME), Government of India, for providing resources and financial support to carry out this research work during his PhD research.
Author information
Authors and Affiliations
Contributions
Himanshu Kumar, investigation, formal analysis, visualization, data curation, and writing. Arihant Ahuja, formal analysis, manuscript editing, review, and revision of manuscript. Ashish A. Kadam, resources, manuscript editing and review, and revision of manuscript. Vibhore Kumar Rastogi, formal analysis, revision of manuscript, and review. Yuvraj Singh Negi, conceptualization, investigation, methodology, resources, data curation, manuscript editing and review, and supervision.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, H., Ahuja, A., Kadam, A.A. et al. Antioxidant Film Based on Chitosan and Tulsi Essential Oil for Food Packaging. Food Bioprocess Technol 16, 342–355 (2023). https://doi.org/10.1007/s11947-022-02938-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02938-6