Abstract
Bio-based active food packaging containing natural antioxidants has gaining great attention these days. The food industry resulted in huge amount of waste rich in natural antioxidant and utilization of these wastes is very important from environmental viewpoint. In this study, apple peel was used to produce apple peel nanoparticles and later, chitosan (CS) and gelatin (G) based novel functional films were successfully fabricated. The prepared films were characterized for their structure, potential interaction and thermal stability. In addition, tensile strength and physical properties were also determined. Scanning electron microscopy (SEM) results revealed that higher concentration of apple peel ethanolic extract (APEE) triggered the sintering of nanoparticles within the films. The data of Fourier transform-infrared spectroscopy (FT-IR) and thermo-gravimetric analysis (TGA) revealed that the presence of apple peel related compounds in the films resulted decrease in availability of hydroxyl groups within the polymer matrix. The addition of APEE into CS/G significantly enhanced the physical properties of the film by increasing its thickness while solubility, swelling ratio, and water vapor permeability were decreased. It could be inferred that CS/G-APEE films exhibited good antioxidant properties, indicating that it could be developed as a bio-nanocomposite food packaging material for the food industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive use of petroleum-based plastic materials has caused serious environmental pollution because these materials are hardly degradable [1]. Conversely, several eco-friendly bio-based polymeric materials including proteins, polysaccharides, and lipids have received increasing worldwide attention as replacements to plastic materials [2]. Further, growing consumer concerns about health have prompted the use of natural antioxidant compounds or the use of extracts rich in antioxidants, rather than using synthetic materials for food packaging [3, 4]. Recently, different natural antioxidants have been studied to enhance the antioxidant properties of bio-based films, such as rutin, epicatechin [5], red grape extract [6], and mango kernel extract [7]. Polyphenols, including gallic acid (GA), grape seed extract, thyme extract, murta leaf extract and tea polyphenols, have been widely used to prepare the films with antioxidant activity which can protect food against oxidation [8, 9].
Apple peel contains appreciable amount of polyphenols and antioxidants, but its industrial use is limited to the extraction of pectin and fiber [10]. The main bioactive compounds present in apple peel are phenolic acids, flavonols, flavon-3-ols, anthocyanins and dihydrochalcon [11]. Procyanidins (mainly constituted by (−)-epicatechin units), (+)-catechin, (−)-epicatechin, chlorogenic acid, phloridzin and quercetin conjugates are also found in apple peel [12].
Chitosan (CS), a deacetylated derivative of chitin, is a linear cationic polysaccharide. It has been widely used in agriculture, biomedicine and food due to its non-toxic, biodegradable, biocompatible and intrinsic antimicrobial properties [13, 14]. Edible films made from CS and gelatin (G) blends are superior to films based on single components due to their compact structure, enhanced physical, mechanical and transport properties [15]. Besides, plasticizers such as glycerol are able to reduce the intermolecular forces of the polymer, increase the mobility of the polymeric chains, improve the mechanical characteristics, and affect the water barrier property of the films [16]. Furthermore, the plasticity and the water vapor permeability (WVP) of films are affected by both mechanical and mass transfer property which suggests that there is an interaction between glycerol and Tween 20 [17].
The nano-composites obtained by the addition of low percentages of nanoparticles to polymers exhibit improved mechanical, water barrier, thermal and oxidative properties. These have been successfully utilized in various fields such as biomedicine, environment engineering, purification technology, and electrical electronics [18]. The solvent displacement method constitutes a straightforward and rapid route to the fabrication of nanoparticles by reducing the effect of the solvent in which a solute is dissolved [19]. The formation of nanoparticles is very rapid and it needs only one single step to complete the procedure. When the solute solution is added to the non-solvent, a rapid desolvation of the solute leads to the formation of nano-precipitation. The solute starts precipitating as soon as the solute-containing solvent diffuses into the dispersing medium. Several works have reported the incorporation of different compounds (drugs, antioxidants) into polymer nanoparticles by solvent displacement method [20].
With this backdrop, this study was designed to utilize apple peel as an active ingredient for the production of CS/G-based active packaging film. Apple peel is regarded as a food industry waste and offers excellent bioactive and antioxidant properties. In the present study, apple peel ethanolic extract (APEE) was used to produce apple peel nanoparticles using solvent displacement method. To the best of our knowledge, this is the first report of using APEE containing nanoparticles with CS/G to develop bio-based films. The prepared films were characterized for structural, thermal, physicochemical, optical, mechanical and antioxidant properties.
Materials and Methods
Chemicals
Folin–Ciocalteau reagent and CS (degree of deacetylation ≥ 90.0%, molecular weight of 4 × 105 Da) were purchased from Kayon Biological Technology Co., Ltd. (Shanghai, China). GA and G were purchased from Sigma Chemical Co., Ltd. (St. Louis, MO, USA). Glycerol and Tween 20 were procured by Solarbio Science & Technology Co., Ltd. (Beijing, China). All reagents used were of analytical grade.
Preparation of Apple Peel Ethanolic Extract
Fresh apples were purchased from the local green market in Nanjing and transferred to the lab in fruit carton boxes. After washing with tap water, peeling was done by stainless steel kitchen knife. Peels were immediately placed in a freezer at − 18 °C for overnight, freeze-dried for 48 h and then grounded into a powder using kitchen-type grinder (MJ-M176P, Panasonic, Japan). The polyphenols were extracted with 80% ethanol using ultrasonic bath (KQ5200DE, Kunshan Co., Jiangsu, China) with fixed frequency of 40 kHz, 150 W power and 60% amplitude level and extract was filtrated through 0.45 μm pore size filter.
Determination of Total Polyphenols Content
The polyphenols content was determined by Folin-Ciocalteu method described by Jabbar et al. [21] using GA as a standard. Total polyphenols content was expressed as mg of GA equivalent (GAE)/mL. All experiments were performed in three replicates.
Determination of Total Flavonoids Content
The total flavonoids content present in apple peel extract was determined using aluminum chloride colorimetric assay by the method of Ramic et al. [22]. Total flavonoids were expressed as mg of catechin equivalents (CE) on dry weight of apple peel (mg CE/mL). All experiments were performed in three replicates.
Preparation of Apple Peel Nanoparticles and Solubility in Solvent–Antisolvent Systems
Apple peel nanoparticles were prepared and solubility of apple peel polyphenols in different solvent-antisolvent ratios was measured as reported previously [23]. Different volumes of APEE and distilled water were blended in order to obtain ethanol to water ratios of 1:5 to 1:20. The mixtures were shaken continuously at room temperature (25 °C) for 24 h and filtered. The solubility (S) of the apple peel polyphenols in the different ratios was calculated using the following equation:
where TPCi is the total polyphenols content in APEE and TPCf is the remaining polyphenols content in the water–ethanol solvent system after precipitation.
Particle Size Distribution Analysis of Apple Peel Nanoparticles
The mean particle size of apple peel nanoparticles (APNPs) was determined by a Zetasizer Nano ZS 3300 (Malvern Instruments Ltd., UK) on the basis of dynamic light scattering (DLS) technique. Filtered samples were placed in glass cuvette with square aperture and the scatter intensity was measured at 25 °C.
Film Preparation
Film solution was prepared by dissolving CS (2%, w/v), G (1.5% w/v), glycerol (0.5% w/v) and Tween 20 (0.15% w/v) in acetic acid aqueous solution (1.0%, v/v) with stirring (800 rpm) for 6 h at 40 °C. Different mass of APEE (5, 10, 15 and 20 g, hereafter referred as APEE5, APEE10, APEE15 and APEE20, respectively) containing nanoparticles were used to replace the corresponding acetic acid mass according to Table 1. These solutions were then degasified for 1 h to remove air bubbles. Finally, the CS/G-APEE solutions (70 mL) were casted over the petri dishes (diameter of 15 cm) for 48 h at 25 °C. The CS/G-APEE films were carefully peeled and stored in a humidity chamber at 25 °C for 48 h for further analysis.
Characterization of CS/G-APEE Films
Analysis of SEM
Scanning electron microscopy (SEM, SU8010, Hitachi, Japan) at 10 kV was applied to study the film surface morphology. Pieces of films (10 × 10 mm) were cut, dried and mounted on aluminum stubs using a double-sided carbon tape and sputter-coated with gold.
Analysis of FT-IR
Fourier Transform-Infrared (FT-IR) spectrometry (Nicolet IR200, Thermo, USA) was applied to study the preliminary structures of the prepared films with or without APEE through KBr module. FT-IR spectra were recorded in the frequency range of 4000 to 400 cm−1.
Thermogravimetric Analysis
Thermogravimetric analysis (TGA) was performed using a TG 209F3 (NETZSCH, Germany) over a temperature range of 30–600 °C at the rate of 10 °C/min under a dynamic nitrogen atmosphere. The samples (2.5 mg) were put in aluminum pans, and an empty pan was used as a reference.
Analysis of Thickness
Film thickness was measured using a digital micrometer. Film density was determined from the film weight and volume. The film volume was calculated according to the area and thickness of the film.
Determination of Solubility and Swelling Index
The films were cut into 2 × 2 cm pieces for the determination of solubility and swelling index. The pieces were dried at 105 °C to constant weight to afford initial dry mass (M1). Then, they were placed in 100 mL beakers with 50 mL distilled water covered with plastic wraps and stored at 25 °C for 24 h. Next, the films were dried superficially with filter papers and dried at 105 °C to constant weight to afford final dry mass (M2). The solubility was calculated by using the following equation:
The films were put into 50 mL beakers with 30 mL distilled water for 24 h at 25 °C after weighing the films (M1). The wet films were then dried superficially with filter papers, followed by weighing the wet films (M2). The swelling index (SI) was calculated by using the following equation:
Determinations of Water Vapor Permeability
Water vapor permeability (WVP) tests of the films were carried out gravimetrically [24]. The film (5 × 5 cm) was sealed on top of the glass cups with a diameter of 4 cm containing anhydrous calcium chloride (0% RH, assay cup) or nothing (control cup). Then, the cups were placed in a desiccator maintained at 75% RH with a saturated solution of sodium chloride. Changes in the weight of the cups were periodically recorded every 1 h during the first 10 h and finally after 24 h. The slope of the weight change vs. time was calculated by linear regression. WVP was expressed by the following equation (g/m s Pa):
where L is the average film thickness (m), A is the transfer area (m2) and ∆P the partial water vapor pressure difference.
Analysis of Film Transmittance
The transmittance of the films was determined using a UV-1200 spectrophotometer (Mapada, Shanghai, China). The film was cut into 1 × 4 cm strips and the transmittance at 600 nm was recorded. All experiments were conducted in triplicate and the average values were reported.
Analysis of Mechanical Property
Tensile strength (TS) and elongation at break (EB) of the films were measured as described in the literature [25]. The experiment was performed at room temperature using a texture analyzer (TA. XT Plus, Stable Micro Systems Ltd., Surrey, UK). Strips (20 mm wide and 150 mm long) were cut from each film and mounted on the grips. The initial grip separation and the detector speed were set at 100 mm and 50 mm/min, respectively. In accordance with the values from the stress–strain curves, values of TS and EB were calculated based on ASTM D 882-10 standard method.
Assay of Antioxidant Activity
Antioxidant activity of the films was evaluated following the method of Bozic et al. [26]. Pieces of the film (5 mg) and 1.5 mL of the working solution were mixed. The working solution was prepared by diluting the stable radicals of ABTS with phosphate buffer saline (PBS, 0.2 M, pH 7.4), produced by oxidation of ABTS (7.00 mM) with potassium persulfate (K2S2O8, 4.95 mM) for 12 h in the dark at room temperature. The absorbance of the mixture was measured at 734 nm.
where A0 is the absorbance of the initial ABTS+, A1 is the absorbance of the sample, and A2 is the absorbance of a standard prepared as A1, whereas replacing ABTS with PBS.
Assay for DPPH radical scavenging activity was performed in a similar way by mixing 5 mg of film and methanolic DPPH solution (0.2 mM, 1.5 mL) in the dark at 30 °C. After 30 min, the absorbance of the mixture at 517 nm was recorded.
where A0 is the absorbance of the initial DPPH, A1 is the absorbance of sample and A2 is the absorbance of the sample under identical conditions as A1 with methanol instead of DPPH solution.
Statistical Analysis
Data were analyzed statistically by SPSS 20.0. Analysis of variance (ANOVA) was applied to assess the difference between factors and levels. To identify the significance of differences among mean values, Tukey’s multiple range tests were executed. Differences were considered significant when p < 0.05.
Results and Discussion
Particle Size and Solubility of APEE
The total phenolic content of APEE was 2.14 mg of gallic acid equivalents (GAE) per milliliter, whereas total flavonoid content of APEE was 1.67 mg of catechin equivalents per milliliter. Size distributions of the APNPs obtained with different ethanol to water ratios are shown in Fig. 1. APNPs obtained with ethanol to water ratio 1:20 showed narrower size distribution (diameters 0.1–0.5 μm) than the particles obtained with 1:5 ratio (diameters 0.1–2.0 μm). Moreover, the solubility of the apple peel polyphenols decreased from 90 to 46%, when the concentration of APEE was changed from 5 to 20% w/w, respectively. The precipitation process consists of several stages such as generation of super-saturation, nucleation, and subsequent growth of nuclei. Generation of super-saturation is a prerequisite for the nucleation to occur. In this case, the use of APEE at 20% w/w led to a higher driving force for precipitation. The trend is in line with a recent finding reported by Lopez-Cardoba et al. [23].
Characterization of CS/G-APEE Film
SEM
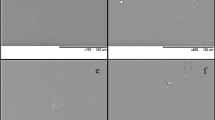
Permeability of films can be affected by the structure, morphology, and homogeneity of the matrix [27]. Figure 2 shows SEM images of the films surface with and without APEE. SEM images indicate that the surface of the control film was quite smooth, uniform, ordered and in homogeneous structure without bubbles or porous (Fig. 2). This phenomenon is in agreement with that reported by Jridi et al. [28]. It is evident from the SEM analysis that the presence of APNPs in the nano-composite films affected the structure of the samples and caused discontinuities in the matrix polymer (Fig. 2b–e). As expected, the rapid desolvation of some non-water-soluble extract components (e.g. phenolic acids), when the ethanolic extract was blended with water, led to nano-precipitation. Gonçalves et al. [29] stressed that hydrophobic nature of some antioxidants, related to hydrocarbon chain, led to the precipitation of these hydrophobic components. These incompatibilities caused morphological changes in the films (e.g. rougher surface). Similar observations are reported by Pastor et al. [30] and Chang-Bravo et al. [3] that investigated biopolymer films carrying ethanolic extracts of propolis. The samples containing the lowest concentration of apple peel ethanolic extract CS/G-APEE5 showed a good dispersion of the nanoparticles within the film matrix; while, in the CS/G-APEE20 samples some agglomerates of nanoparticles were observed (Fig. 2e). In fact, a low concentration of extract resulted in a more homogeneous particle size distribution and consequently, the nanoparticles were better incorporated within the films. Similar observations were reported by Teodoro et al. [31] who worked on the fabrication of cassava starch films containing acetylated starch nanoparticles as reinforcement.
FT-IR
FT-IR spectroscopy was performed to investigate the intermolecular interaction between CS/G and APEE that was related to the physical and mechanical characters of films, and the spectra are shown in Fig. 3a. The CS/G and CS/G-APEE films showed characteristic bands at approximately around 3416 cm−1 (O–H stretching), 2926 cm−1 (C–H stretching from alkyl groups). Besides, the peaks at 1644, 1599, 1485 and 1182 cm−1 corresponded to C=O, N–H bending vibration, C–N stretching and C–O–C band stretching, respectively [32]. The FT-IR peaks of CS/G-based nano-composite films were comparatively similar to those of CS/G film as control. After addition of APNPs into CS/G film, no additional peaks and no significant wavelength shift were observed, indicating that no covalent bonds between APEE and CS/G were detected [33]. As it could be seen, shifting to higher or lower wavenumbers of some of the peaks has occurred with an increase of APEE concentration. Moreover, the film CS/G-APEE20 showed a greater reduction in the OH amount compared with those for control CS/G film. This could be attributed to the existence of APNPs, which intermingled with the glycerol-polymer matrix, and subsequently changed the OH availability within the polymer network [34].
TGA
Thermal decomposition pattern and thermal stability of the prepared films were investigated by TGA. TGA curves of CS/G-APEE composite films incorporated with APEE at different concentrations are shown in Fig. 3b. The thermal degradation of the CS/G films without extract followed the pattern described previously [32]. Figure 3b shows two mass loss stages for all samples. The first stage of mass loss observed at 80–120 °C was related to the volatilization of physical and chemical bounded water and residual acetic acid from films [35]. The second weight loss at 220 to 300 °C was due to thermal degradations of all films. The loss rate was similar in each weight loss stage. The second phase was linked to the purging of glycerol, depolymerization and pyrolytic decomposition of the biopolymers [36]. For the films containing APEE, the degradation temperatures showed a trend towards lower values, as compared to those of CS/G control film. The thermal stability of macromolecules is directly related to the internal crystalline structure, which means that higher crystallinity will result in higher thermal stability, because more energy (heat) is required to break the higher crystalline structure [32]. This fact also supported the results of SEM.
Physical Properties of CS/G-APEE Film
Thickness
An increased in film thickness was observed from 0.092 ± 0.0017 mm for the control CS/G film to 0.129 ± 0.0026 mm for the prepared film with 20% (w/w) APEE (Table 2). The thickness of the active nano-composite films was strongly dependent on the amount of APEE added. The increase in the extract concentration led to an increase in the film thickness. Similar trends were presented for gelatin film containing different essential oils [37].
Solubility
Water solubility and swelling index are two important characters of a film, affecting its water resistance property. The solubility of CS/G film in distilled water was 28.50%, which is in accordance with the values reported by Rui et al. [38]. It is clear from the data that as the concentration of APEE in the film matrix increased the water solubility decreased. This could be related to the formation of strong hydrogen bonds between CS/G matrix and the nanoparticles. These hydrogen bonds were unable to break by water molecules, leading to decrease solubility of CS/G-APEE20 (Table 2). Polyphenols might establish the interactions with CS/G molecules through the potential hydrogen bonding, which might limit the interactions between hydrophilic groups of the CS/G and water molecules due to the competitive binding effect. Similar results have been reported in the literature [39].
Swelling Index
The swelling index (SI) of the films were evaluated after immersion of film samples in water for 24 and the SI values of prepared films are presented in Table 2. SI of control CS/G film prepared at room temperature was 35.79% which is in agreement with that of earlier reports [40]. The intrinsic swelling property of CS/G films is related to the presence of hydrophilic groups such as carboxylic groups in their structures, which can easily interact with water [41]. In the present study, SI values of the films in water were reduced (p < 0.05) when the films were enriched with APEE. The reason for a decreasing trend of SI in the CS/G-APEE films could be a double-natured one: (i) the existence of intermolecular interactions between polymer and incorporated components which are taking up CS’s functional groups and thus preventing the establishment of chitosan-water hydrogen bonding, and (ii) a hydrophobic nature of the incorporated nanoparticles. The strong intermolecular interactions among polyphenols, CS, G, glycerol and Tween 20 weakened the intermolecular distances and then formed a more compact network. Similar trend has also been reported in the literature for the preparation of CS/genipin/poly (N-vinyl-2-pyrrolidone) films [42].
WVP
The main function of preservative film is to retard the deterioration of food products from the surrounding atmosphere. Film is considered better if the moisture transfer between surrounding environment and food is as low as possible. WVP is one of the most essential characters of the bio-composite films for food packaging as it has direct contact with food products hence has great influence on its shelf life. The higher WVP of the composite edible films is a problem for food industry. Thus, nano-science can be applied to overcome this problem and prevent water migration by the formation of tightly linked three dimensional networks. For control CS/G film, the WVP was 10.95 ± 0.21 × 10−11 g/m s Pa, which is similar to those reported in literature [38]. The inclusion of APEE into CS/G film matrix significantly decreased the WVP as compared to CS/G control film (Table 2). The cross-linking produced by CS/G, APEE, glycerol and Tween 20 might result in decreasing the interaction of polymer matrix and causing less water molecule interactions in films, and water vapor molecule would take long time to permeate from the film due to increase in the film thickness. The inclusion of nanoparticles and its interaction with matrix restricted the mobility of protein molecules which has resulted in decrease value of WVP for fabricated films [43]. Martelli et al. [44] reported a reduction in water vapor permeation rate in the film prepared by banana puree incorporated with CS nanoparticles. Similar results on the water vapor behavior of glycerol plasticised-starch/CSNPs composites have been reported by Chang et al. [45].
Transparency
Film transparency has great impact on the appearance of food and hence directly related to the film functionality [46]. Transparency values of CS/G film and CS/G-APEE films are presented in Table 2. However, the CS/G-APEE active films showed lower transparency than the control films, indicating that the addition of APEE into film matrix caused a decrease in film transparency. Consequently, the opaque appearance of the CS/G-APEE composite films reflected the UV light, thereby hindered light transmission through the films. Therefore, CS/G-APEE films would have excellent barrier properties against UV light. These findings are in line with previous reports [23].
Mechanical Property of the Films
Mechanical properties are important in edible films, because adequate mechanical strength ensures the integrity of the film and its freedom from minor defects such as pinholes [23]. The presented values of control films in this study were in the range of those reported previously [15]. CS/G-based films showed good mechanical properties as compared to only CS-based film [47]. The results of the present study showed that APEE significantly affected the mechanical property of the CS/G films (Fig. 4a). It has been reported that the mechanical properties of a biodegradable film are usually related to the film network microstructure and the intermolecular force [48]. The lower mechanical properties in the films incorporated with APEE films could be due to the interactions of phenolic compounds with CS/G molecules by hydrogen bonds and/or hydrophobic interactions [49]. These changes enhanced the inter-chain distances in the film network and subsequently decreased TS and EB of resulting films [50].
Mechanical properties of CS/G-based films with different concentrations of APEE. Different letters indicate significant differences (p < 0.05). Values are expressed as mean ± standard deviation. a Scavenging activities of the CS/G-based APEE films on DPPH and ABTS radicals (b). Values are mean (n = 3) ± SD, and a–e represent significant differences (p < 0.05) in the same band
Antioxidant Activity
The main attribute of an active food packaging film is its antioxidant potential. Figure 4b showed the scavenging activities against DPPH and ABTS radicals of the control and CS/G-APEE films. The control films showed a low DPPH and ABTS scavenging activities, which is similar to previous study [51]. DPPH and ABTS radicals increased (p < 0.05) with the addition of APEE. However, incorporating the APEE significantly improved the antioxidant activity of the CS/G-based films and the antioxidant activity was enhanced with increased concentration of APEE. The possible explanation of the increased antioxidant activity is the presence of phenolic groups in APEE. The total phenolic contents increased with increased concentration of APEE in CS/G-APEE films. Similar results have also been reported previously [52].
Conclusion
Apple peel, a food processing waste, is a rich source of antioxidant compounds. In the present study, APNPs were prepared by solvent displacement method and incorporated into CS/G to fabricate the CS/G-based packaging films. The amount of extract added to the formulations significantly affected the precipitation. With the addition of APEE into CS/G-based film, the thickness increased while swelling degree, water solubility and vapor permeability were decreased, suggesting that water barrier property of the film was enhanced. FT-IR spectra of CS/G-APEE films suggested that the interactions between APEE and CS/G were likely to be non-covalent. Higher extract concentration provoked the formation of agglomerates of nanoparticles within the films. Tensile properties of the films were also influenced by the addition of APEE. Overall, these results suggest that the CS/G-based APEE films containing APNPs could find useful applications in food packaging.
References
Liu J, Liu S, Chen Y, Zhang L, Kan J, Jin C (2017) Food Hydrocoll 71:176–186
Koshy RR, Mary SK, Thomas S, Pothan LA (2015) Food Hydrocoll 50:174–192
Chang-Bravo L, Lopez-Cordoba A, Martino M (2014) React Funct Polym 85:11–19
Colín-Chavez C, Vicente-Ramírez EB, Soto-Valdez H, Peralta E, Auras R (2014) Food Bioprocess Technol 7:3504–3515
Friesen K, Chang C, Nickerson M (2015) Food Chem 2015(172):18–23
Ciannamea EM, Stefani PM, Ruseckaite RA (2016) LWT Food Sci Technol 74:353–362
Maryam Adilah ZA, Jamilah B, Nur Hanani ZA (2018) Food Hydrocoll 74:207–218
Moradi M, Tajik H, Razavi Rohani SM, Oromiehie AR, Malekinejad H, Aliakbarlu J (2012) LWT Food Sci Technol 46:477–484
Talon E, Trifkovic KT, Nedovic VA, Bugarski BM, Vargas M, Chiralt A (2017) Carbohydr Polym 157:1153–1161
Shalini R, Gupta DK (2010) J Food Sci Technol 47:365–371
Boyer J, Liu RH (2004) Nutr J 3:1–15
Karaman S, Tutem E, Baskanb KS, Apak R (2013) J Sci Food Agric 93:867–875
Hamed I, Ozogul F, Regenstein JM (2016) Trends Food Sci Technol 48:40–50
Usman A, Zia KM, Zuber M, Tabasum S, Rehman S, Zia F (2016) Int J Biol Macromol 86:630–645
Mohammadi R, Mohammadifar MA, Rouhi M, Kariminejad M, Mortazavian AM, Sadeghi E, Hasanvand S (2018) Int J Biol Macromol 107:406–412
Muller CMO, Yamashita F, Laurindo JB (2008) Carbohydr Polym 72:82–87
Rodrıguez M, Oses J, Ziani K, Mate JI (2006) Food Res Int 39:840–846
Yun YH, Youn HG, Shin JY, Yoon SD (2017) Int J Biol Macromol 104:1150–1157
Joye IJ, McClements DJ (2013) Trends Food Sci Technol 34:109–123
Kakran M, Sahoo NG, Tan IL, Li L (2012) J Nano Res 14:1–11
Jabbar S, Abid M, Wu T, Hashim MM, Saeeduddin M, Hu B, Lei S, Zeng XX (2015) J Food Process Preserv 39:1878–1888
Ramic M, Vidovic S, Zekovic Z, Vladic J, Cvejin A, Pavlic B (2015) Ultrason Sonochem 23:360–368
Lopez-Cordoba A, Medina-Jaramillo C, Pineros-Hernandez D, Goyanes S (2017) Food Hydrocoll 71:26–34
Hassannia-Kolaee M, Khodaiyan F, Shahabi-Ghahfarrokhi I (2016) J Food Sci Technol 53:1294–1302
Ferreira A, Nunes C, Castro A, Ferreira P, Coimbra MA (2014) Carbohydr Polym 113:490–499
Bozic M, Gorgieva S, Kokol V (2012) Carbohydr Polym 87:2388–2398
Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F (2015) Food Hydrocoll 44:172–182
Jridi M, Hajji S, Ayed HB, Lassoued I, Mbarek A, Kammoun M, Souissi N, Nasri M (2014) Int J Biol Macromol 67:373–379
Gonçalves CMB, Tome LC, Garcia H, Brandao L, Mendes AM, Marrucho IM (2013) J Food Eng 116:562–571
Pastor C, Sanchez-Gonzalez L, Chafer M, Chiralt A, Gonzalez-Martínez C (2010) Carbohydr Polym 82:1174–1183
Teodoro AP, Mali S, Romero N, de Carvalho GM (2015) Carbohydr Polym 126:9–16
Qiao C, Ma X, Zhang J, Yao J (2017) Food Chem 235:45–50
Lee DS, Woo JY, Ahn CB, Je JY (2014) Food Chem 148:97–104
Pineros-Hernandez D, Medina-Jaramillo C, Lopez-Cordoba A, Goyanes S (2017) Food Hydrocoll 63:488–495
Jahed E, Khaledabad MA, Almasi H, Hasanzadeh R (2017) Carbohydr Polym 164:325–338
Fan JM, Ma W, Liu GQ, Yin SW, Tang CH, Yang XQ (2014) Food Hydrocoll 36:60–69
Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F (2016) Food Chem 194:1266–1274
Rui L, Minhao X, Bing H, Li Z, Danyang Y, Zeng XX (2017) Carbohydr Polym 173:473–481
Voon HC, Bhat R, Easa AM, Liong MT, Karim AA (2012) Food Bioprocess Technol 5:1766–1774
Mayachiew P, Devahastin S (2010) Food Chem 118:594–601
Kakaei S, Shahbazi Y (2016) LWT Food Sci Technol 72:432–438
Aldana AA, González A, Strumia MC, Martinelli M (2012) Mater Chem Phys 134:317–324
Vanin FM, Hirano MH, Carvalho RA, Moraes ICF, Bittante AMQB, Sobral PJA (2014) Food Res Int 63:16–24
Martelli MR, Barros TT, De Moura MR, Mattoso LHC, Assis OBG (2013) J Food Sci 78:98–104
Chang PR, Jian R, Yu J, Ma X (2010) Food Chem 120:736–740
Bilbao-Sainz C, Bras J, Williams T, Senechal T, Orts W (2011) Carbohydr Polym 86:1549–1557
Riaz A, Lei S, Akhtar HMS, Wan P, Chen D, Jabbar S, Abid M, Hashim MM, Zeng XX (2018) Int J Biol Macromol 114:547–555
Elsabee MZ, Abdou ES (2013) Mater Sci Eng C 33:1819–1841
Hoque MS, Benjakul S, Prodpran T (2011) Food Hydrocoll 25:1085–1097
Chen H, Hu X, Chen E, Wu S, McClements DJ, Liu S, Li B, Li Y (2016) Food Hydrocoll 61:662–671
Cheng YS, Wang BJ, Weng YM (2015) LWT Food Sci Technol 63:115–121
Xuejiao W, Yumei X, Hanjing G, Lin C, Jiali W, Shuang Z, Yan G, Zhixi L, Xianchao F (2018) Carbohydr Polym 179:207–220
Acknowledgements
This work was partially supported by a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the first author would like to express his thanks to the Ministry of Education of China for financial assistance through the Chinese Government Scholarship Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Riaz, A., Lagnika, C., Abdin, M. et al. Preparation and Characterization of Chitosan/Gelatin-Based Active Food Packaging Films Containing Apple Peel Nanoparticles. J Polym Environ 28, 411–420 (2020). https://doi.org/10.1007/s10924-019-01619-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01619-4