Abstract
In this study, effect of chitosan films combined with orange (Citrus sinensis (L.) Osbeck) peel essential oil on the shelf life of deepwater pink shrimp (Parapenaeus longirostris Lucas 1846) was aimed. Chitosan (CH) and 2% orange peel essential oil (OPEO) combinated chitosan (CH+OPEO) were used for preparing film forming solution. Thickness and microstructure of the films, nutritional composition, sensory and melanosis evaluation, chemical, physical, and microbiological analyses were performed periodically and shelf-life was performed during the storage period of 15 days. The combination of chitosan film with OPEO was effective in prolonging the shelf life of fresh shrimps to 15 days (CH+OPEO), whereas the only chitosan-coated group had a shelf life of 10 days (CH) and the samples packaged without chitosan film had a shelf life of 7 days (control). The results of the study suggested that edible chitosan coatings together with OPEO preserved the shrimps and maintained the shelf life throughout the refrigerated storage period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shrimp is one of the most important seafood products worldwide (Oosterveer 2006). However, shrimp is highly perishable seafood (Alparslan et al. 2016) and their deterioration is mainly from biological reactions such as oxidation of lipids, protein degradation, or decomposition mediated by endogenous or microbial enzymes. These activities lead to a short shelf life of seafood products (Hosseini et al. 2015). The most important causes of shrimp spoilage are accumulation of undesirable compounds as a result of microbiological growth and biochemical reactions, and melanosis (discoloration) originated by the polymerization of phenols into insoluble black pigments, i.e., melanins (Nirmal and Benjakul 2011). Black spots (melanosis) form on prawns and other shellfish within a few hours after harvest, without refrigeration (Diouf et al. 2016). Generally, shrimps are processed with polyphenol oxidase (PPO) inhibitors such as metabisulfite and 4-hexylresorcinol, drained and then packed with ice in order to maintain the quality (Mendes 2006). Nevertheless, the use of sulfiting agents in fishery products are forbidden by regulations. Therefore, studies on alternative methods to prolong the shelf life are gaining importance.
For the past decades, research on edible films or coatings in foods is driven by food engineers due to the high demand of consumers for longer shelf life and better quality of fresh foods as well as of environmentally friendly packaging (Siracusa et al. 2008). Chitosan, the deacetylated derivative of chitin, is a natural biopolymer similar to cellulose that can be extracted from the shells of seafood (Rinaudo 2006, Koç and Özkan 2011).
In order to increase the efficiency of application of edible films on foods, natural plant extracts, especially essential oils (EOs) possessing antimicrobial and antioxidant activities, are recommended (Pranoto et al. 2005). To enhance the functional properties of edible films, EO addition is extensively preferred. Essential oils can be applied to the formulation of edible films to extend shelf life and reduce or inhibit food borne pathogens (Zivanovic et al. 2005). Essential oils (also called volatile or ethereal oils) are aromatic oily liquids characterized by a strong odor and produced by different plant materials (flowers, buds, seeds, leaves, twigs, bark, herbs, wood, fruits, and roots) as secondary metabolites (Palazzolo et al. 2013). Citrus oil is an essential oil obtained from citrus fruit. It is extracted as a byproduct of juice extraction by centrifugation, producing cold pressed oil and it is found in all citrus fruits like lemon, orange, sweat lime, and so on (Sikdar et al. 2016).
The objective of this study was to indicate the potential of chitosan and chitosan incorporated with orange peel essential oil (OPEO) as an antioxidant and antimicrobial coating in preservation of deepwater pink shrimps, besides determining the effects of melanosis inhibition.
Material and Methods
Sampling
Deepwater pink shrimps (Parapenaeus longirostris L. 1846; mean length 11.04 ± 0.78 cm and mean weight 9.03 ± 1.34 g) caught around Sığacık Gulf of Aegean Sea (Izmir) of Turkey were used as raw material. Thirty-kilogram shrimps were obtained from a vessel harvesting shrimps by trawl and were packed into an insulated polystyrene box with ice and immediately transported to the Seafood Processing Laboratory in the Fisheries Faculty at Muğla Sıtkı Koçman University, within 2 h of harvesting.
Extraction and Analysis of Essential Oil
Orange peels were obtained from a fruit juice company in Köyceğiz, Mugla. About 50 kg of orange peel were used in this study to obtain the required amount of essential oil. The essential oil was obtained by hydro distillation, using a Clevenger apparatus (Edutek Instrumentation, Haryana, India) with 150 g of dry orange peel and 1500 mL of water. The oil was obtained after 3 h of distillation at boiling temperature (100 °C) in water and stored at (4 ± 1) °C in airtight glass vials covered with aluminum foil. The gas chromatography-mass spectrophotometer (GC-MS) analysis of the obtained essential oil was conducted at the ARGEFAR-ÇEG Laboratory of Aegean University (İzmir, Turkey), using an Agilent gas chromatograph model 6890 equipped with an Agilent mass selective detector (MSD) model 5973 (Agilent Technologies, Santa Clara, CA, USA). Identification of components present in the essential oil was carried out with the Wiley 275 mass spectral library (NIST, Wiley, New York, NY, USA).
Preparation of Film Forming Solution
Preparation of edible film coatings was slightly modified from Garcia et al. (1998). Chitosan-based film coatings were prepared by dissolving 1.5% w/v chitosan (Sigma Chemical Co., St. Louis, MO., USA; 450 kDa, 75–85% deacetylated) in 1.5% v/v acetic acid (Merck, Germany) while stirring on a magnetic stirrer/hot plate at 45 °C. 1.5 mL glycerol (Sigma Chemical Co., St. Louis, MO., USA) was added per gram of chitosan as a plasticizer. 0.2 mL Tween 20 (Merck, Germany) per 100-mL essential oil was added to film forming solutions (FFS) as an emulsifier. After 1 h of stirring, OPEO concentration was added to chitosan solution to reach a final concentration of 2% (v/v). According to the preliminary analysis, it was observed that the higher amounts of orange peel essential oil repressed the specific odor and color of the shrimp which directly affected the sensorial characteristics. Thus, antimicrobial and antioxidant analysis revealed that 2% OPEO concentration was the most effective that did not alter the analysis conditions. Film forming solutions were homogenized by emulsifying equipment (IKA T18-Digital Ultra Turrax, Germany) at 21000 rpm for 1 min. After cooling to room temperature, the film forming solutions (200 mL) were casted on 10 × 15-cm glass plates, and then dried for 3 days at ambient conditions (24 ± 1 °C). CH group was prepared in the same without adding OPEO. Each plate represented one piece of film.
Antimicrobial Activity of Chitosan Film Forming Solutions Combined with OPEO
For determining the optimum OPEO concentration, the antimicrobial activity of chitosan FFS combined with OPEO was tested following a well diffusion assay over five food pathogen microorganisms. These are standard reference strains that are routinely used for the evaluation of antimicrobial compounds: Bacillus subtilis ATCC 25922, Stapylococcus aureus ATCC 43300, Escherichia coli ATCC 35218, Pseodomonas aeruginosa ATCC 15442, and Candida albicans ATCC 10231 obtained from Ankara Refik Saydam Hıfzısıhha Institutes Culture Collection. Microorganisms were cultured in Nutrient Broth (NB) at appropriate temperatures. Inoculums were prepared by adjusting the turbidity of the medium to match the 0.5 McFarland Standard Dilutions. Twenty milliliters of Mueller Hinton Agar (MHA) is sterilized in separated flasks and cooled to 45–50 °C. After injecting the microorganism cultures to sterile plates (1000 μL), media was distributed and mixed homogenously. Of the solutions, 20 μL were injected to the wells of 6 mm in diameter (NCCLS 1993). Three different concentrations of chitosan + orange peel essential oil combination were evaluated for antimicrobial activity: 0.5, 1, and 2%. After the proper incubation period for each microorganism, antimicrobial activity was evaluated by measuring the zone of inhibition against the tested microorganisms. Measurements were performed in triplicate. The most effective OPEO concentration on the microbial inhibition was used to prepare the FFS for coating shrimps.
Radical Scavenging Activity of Chitosan Film Forming Solutions Combined with OPEO
Antioxidative effects of the prepared concentrations of chitosan films combined with OPEO were also determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity. Chitosan films containing 0.5, 1, and 2.0% (v/v) OPEO were prepared on foam plates. After drying, 0.1 g of film of each concentration was dissolved in distilled water (2 mL) at 45 °C and after the addition of ethanol (4 mL), were centrifuged (NUVE, NF 400R, Turkey) at 4000×g for 10 min at 20 °C (Bao et al. 2009). Pellets were discharged and the filtrate was analyzed for DPPH scavenging activity. Chitosan film without OPEO was also analyzed.
DPPH radical scavenging activity was determined according to the method of Yen and Hsieh (1995) with minor modifications. A 500-μL aliquot of ethanol extract was mixed vigorously with 5.5 mL of 0.036 mmol L−1 DPPH in ethanol and allowed to stand at room temperature in the dark for 30 min. The absorbance of the mixture at 517 nm was measured using a spectrophotometer. The control was measured in the same manner, except that ethanol was used instead of sample. DPPH radical scavenging activity was calculated according to the following equation:
where As is the absorbance of the sample, Ac is the absorbance of the control and A0 is the absorbance of the mixture of 5.5 mL of ethanol and 500 μL of sample.
Film Thickness and Microstructure of Chitosan-OPEO Films
Film thickness of CH and CH+OPEO was measured using a digital micrometer screw gauge (model MDC-25M, Mitutoyo, Kanagawa, Japan), averaging ten different locations. Microstructure analysis of the chitosan films was carried out by using scanning electron microscopy (SEM) technique in a JSM-7600F (Jeol, Japan) field emission scanning electron microscope (FESEM). Pieces were cut from the prepared films and mounted on copper stubs using double-side adhesive tape, sputter coated with gold and observed at an accelerating voltage of 15 kV.
Application of Film Coatings and Storage
Three different groups were created for analysis: non-coated control group (C), chitosan film coating without essential oil (CH), and chitosan coating with 2% orange peel essential oil (CH+OPEO). For non-coated control group, ten shrimps were put onto sterile foam dishes and vacuum packaged (Culinary, ATM Machinery 7483 BV, Haaksbergen, The Netherlands). For CH and CH+OPEO, one film was placed on foam plates and after ten shrimps were disposed, it was closed with the second film. Then, shrimps coated with film by both sides, were put into separate vacuum packages (Polyamide, >160 cm3/m2/day O2 and >8.5 g/m2/day moisture permeability, Polinas, Turkey) and vacuum packaged. Forty packages were prepared for each group and stored at (4 ± 1) °C.
Nutritional Composition Analysis
The shrimp samples were analyzed in triplicate for nutritional composition: lipid content of shrimp by the Bligh and Dyer method (Bligh and Dyer 1959), ash content by AOAC (1990, 950.46) method, moisture content by AOAC ( 2006a, 934.01) method, and total crude protein analysis by AOAC (2006b, 984.13) kjeldahl method. In the beginning and at the end of storage period, total free amino acid analysis for shrimp was determined according to in house method (Kazlıçeşme Ar-Ge Test laboratuary, İstanbul) using modified Agilent Eclipse AAA method by HPLC system (Agilent 1260 Infinity, high-performance liquid chromatography) after pre-column derivatization with OPA and FMOC.
Sensory Analysis and Melanosis Evaluation
Sensory evaluation of the samples was conducted by six trained persons (24–36 years old) throughout the storage period. Panelists gave scores for sensory characteristics, such as appearance, color, odor, texture, and general acceptability using a 5-point descriptive scale (5 = excellent, 4 = good, 3 = moderate, 2 = borderline-clearly not fresh, 1 = unfit spoiled) (Zeng et al. 2005). One package of test group (including ten shrimps) was presented to each panelist. Melanosis was evaluated according to melanosis scale published by Otwell and Marshall (1986). Shrimps from each treatment were evaluated for degree of melanosis by an experienced seven-member panel.
Color Measurement
The color of shrimp samples was measured by a lab color meter (Pen Color Art 1L model, Artoksi MSM, Istanbul, Turkey) in accordance with the recommendations of the International Commission on Illumination (CIE 1976). The measured L*, a*, and b* color parameters indicated lightness/brightness, redness/greenness, and yellowness/blueness, respectively. The color meter was calibrated with a white standard and the color measurement was repeated three times on different parts of the surface.
Chemical Analysis of Shrimp Samples
A residual amount of SO2 in fresh shrimp meat was determined according to AOAC (2000) method. The pH values were recorded by a digital pH meter (InoLab, WTW, Weilheim, Germany) after homogenization of each 10 g of sample in 100 mL of distilled water (Manthey et al. 1988). Determination of total volatile base nitrogen (TVB-N) was carried out as described by Antonacopoulos (1973). Homogenized shrimp samples were steam-distilled and the TVB-N value (in mg of nitrogen per 100 g of meat) was determined according to the consumption of 0.1 M HCl. TMA-N value within homogenized shrimp meat was determined according to Schormüller (1968) and the results were expressed as TMA-N value in mg of per 100 g of shrimp meat. Thiobarbituric acid (TBA) reactive substances were determined according to Tarladgis et al. (1960) to evaluate the oxidation stability during chilled storage and the results were expressed as TBA value in milligram of malonaldehyde per kilogram of shrimp meat. Peroxide value (PV), expressed in meq active oxygen/kilogram lipids, was determined according to AOCS (1994).
Microbiological Analysis of Shrimp Samples
The following groups of microflora were monitored: total viable count (TVC), psychotropic bacteria count (PBC), and total coliform bacteria (TCB). A sample of 10 g was removed aseptically from the filet using a scalpel and forceps, transferred to a stomacher bag containing 90 mL of sterile peptone water (PW) solution (0.1%), and homogenized at room temperature. For each sample, further serial decimal dilutions were prepared in PW solution (0.1%). The appropriate dilutions were subsequently used for enumeration and differentiation of microorganisms. Total viable counts were determined using plate count agar (PCA, Code: 1.05463, Merck, Darmstadt, Germany) after incubation for 2 days at 37 °C, and psychotropic bacteria counts were determined after incubation at 7 °C for 10 days with the same medium (FDA/BAM 2009). The sample was diluted in serial 10-fold steps used as inoculum for the three-tube MPN procedure for total coliforms as specified in FDA/BAM (2002). Suspension, 1 mL from each diluted tube, was transferred into lauryl sulfate tryptose (LST) broth and incubated at 35 °C for 24–48 h. Inoculum, one loopful from tubes with gas formation within 48 h at 35 °C, was transferred to brilliant green lactose (BGL) broth in the Durham tube, incubated at 35 °C for 24–48 h. The tubes with turbidity and gas formation indicated the presence of coliforms. Their numbers per 1 g of sample were calculated from the MPN table.
Statistical Analysis
Experiments were performed in triplicate (n = 3) for three independent samples and a completely randomized design (CRD) was used. Data were presented as mean values ± standard deviations and a probability value of P < 0.05 was considered significant. Analysis of variance (ANOVA) was performed and the mean comparisons were done by Duncan’s multiple range tests. Statistical analysis was performed using the Statistical Package for Social Sciences v.21 Software Package (SPSS for Windows, SPSS Inc., Chicago, IL, USA).
Results and Discussion
Active Ingredients of Orange Peel Essential Oil
Active ingredient content of OPEO was identifed by GC-MS and totally 16 components were detected (Table 1) and the major component was found to be D-limonene with 82.24%. The citrus essential oils are mixture of volatile compounds and mainly consisted of monoterpene hydrocarbon. The volatile components of citrus oil were mainly composed of limonene, the most abundant compound in citrus oil (Sawamura 2011). Djenane (2015) reported that the studied oils are made up mainly of limonene (77.37%) for orange essential oil (EO). In another report, a total of 18–22 compounds were identified in the C. sinensis peel essential oils and limonene (80.9%) was the main constituent in the oils from fresh (Kamal et al. 2011). The findings of our study are similar to other studies (Sawamura 2011; Kamal et al. 2011; Djenane 2015) despite minor differences. Climate, geographic conditions, variety of the plant, the drying period, the extraction method used, and so on should be considered among these differences that may have a direct impact on the content of essential oils.
Antimicrobial Activity of Chitosan Film Forming Solutions Containing OPEO
Antimicrobial activity of chitosan FFSs containing different concentrations (0.5, 1, and 2%) of OPEO or not containing OPEO were studied on the five microorganisms (Table 2). CH and CH + 0.5% OPEO found to have no antimicrobial activity on B. subtilis. CH + 1% OPEO inhibited C. albicans growth (P < 0.05) and the antimicrobial effect did not change depending on the concentration increase. Chitosan FFS containing 2% OPEO concentration presented the highest inhibitory activity on selected microorganisms (P < 0.05). Hammer et al. (1999) reported that orange essential oil inhibited C. albicans at 1.0% (v/v), S. aureus at 2.0% (v/v), and E.coli and P. aeruginosa at >2.0%. Djenane (2015) measured the inhibition zone of orange (Citrus sinensis L.) peel essential oil against S. aureus CECT 4459 as 11.66 ± 1.50 mm. As a result of antimicrobial analysis combined simultaneously with antioxidant activity, 2% OPEO concentration was evaluated to be the effective concentration for chitosan film coating of the shrimps.
Radical Scavenging Activity of Chitosan Film Forming Solutions Combined with OPEO
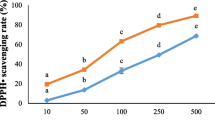
Antioxidant activity of chitosan films containing different concentrations (0.5, 1, and 2%) of OPEO or not containing OPEO was performed (Fig. 1). According to the free radical scavenging activity, the results of our study showed that chitosan film with 2% OPEO was determined to have higher antioxidant activity than other concentration and statistically was found to be significant (P < 0.05). Choi et al. (2000) found that the radical scavenging activity of 34 kinds of citrus essential oils on DPPH ranged from 17.7 to 64%. Tongnuanchan et al. (2012) studied the antioxidant activities of films from fish skin gelatin incorporated with bergamot, kaffir lime, lemon, and lime essential oil and figured out that film incorporated with bergamot essential oil showed the highest free radical scavenging activity (P < 0.05), followed by the film added with lemon essential oil.
Microstructure of Chitosan-OPEO Films
Thickness of the films was measured as 16.5 ± 1.41 and 17.25 ± 2.04 μm for CH and CH + 2% OPEO, respectively. Similarly, Altiok et al. (2010) also concluded that essential oil addition did not affect the film thickness. SEM images of the surface and sectional profile of chitosan films with and without OPEO provided remarkable data about the morphology (Fig.2a, b). The surface of the chitosan film not containing OPEO (control) was found to be compact but have aggregates on it. López-Mata et al. (2013) also underlined the nonporous surface and irregular shaped folds of chitosan films viewed by SEM. There were not much grains and porous formations. Chitosan film with 2% OPEO was flat, homogeneous with uniformly distributed holes and porous morphology. When compared with the chitosan film, OPEO combination resulted in visible oil droplet formations. Similarly, Sugumar et al. (2015) and Hafsa et al. (2016) also reported that essential oil addition caused a heterogeneous structure in which oil droplets were entrapped in the continuous polysaccharide network. They emphasized that the size of the oil droplets in the polymer matrix were larger as the essential oil concentration increased.
Nutritional Composition of Shrimp
Initial nutritional composition of the shrimps was found to be 18.51% protein, 2.15% lipid, 74.22% moisture, and 2.45% ash. According to Rosa and Nunes (2004), similar results were obtained for initial moisture, protein, lipid, and ash content of Parapenaeus longirostris as 74.6, 20.8, 0.2, and 1.9, respectively. Similarly, Dayal et al. (2013) reported that the average protein, lipid, and moisture content of fresh shrimp was 19.4, 1.15, and 76.3/100 g, respectively.
The amino acid content of the groups as initial (raw material) and at the end of the 15-day storage (C, CH, and CH+OPEO) are presented in Table 3. Ten essential and ten non-essential amino acids were detected for all groups. Arginine (an essential amino acid/EAA) and glutamine, alanine, proline, glutamic acid (non-essential amino acids/NEAA) were the most abundant amino acids in all groups including the fresh shrimps (P < 0.05). The ratio of EAA to NEAA was 0.58 for fresh shrimps. At the end of the 15-day storage, this ratio was found to be 0.52, 0.74, and 0.85 for C, CH, and CH+OPEO groups, respectively. The ratio of EAA to NEAA was higher for the chitosan groups (CH and CH+OPEO) than for the control group. Total amino acid content of the CH+OPEO group was higher than the fresh shrimps. Yanar and Celik (2006) reported the ratio of EAA to NEAA as 0.60 for Penaeus semisulcatus and 0.59 for Metapenaeus monoceros while Rosa and Nunes (2004) found this ratio as 0.93 for P. longirostris.
Sensorial Analysis Results
The initial score of overall evaluation was found to be 4.93 points. On the 10th day of storage, group C was under the borderline limit of acceptability (1 point) with 0.72 points. Similarly, group CH was also under the limit with 0.88 points on the 10th day. Group CH+OPEO did not reach the limit value throughout the storage period. There were significant differences between the group C and others (P < 0.05). The difference between the groups CH and CH+OPEO was not significant until the 10th day (P > 0.05) while the difference became significant after 10-day storage (P < 0.05). In a study of Wang et al. (2007), it was reported that shrimps coated with chitosan could maintain better sensory characteristics than the uncoated group and prolonged shelf life 2 or 3 days more.
Melanosis and Color Measurement
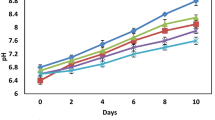
Melanosis changes together with the L* values of the shrimps throughout the storage period are given in Fig. 3. The control group reached a value of 9.93 on the 12th day of storage, while CH and CH+OPEO reached the values of 8.30 and 7.65 at the end of the storage period (15 days), respectively. There was a significant increase for melanosis in all groups throughout the storage (P < 0.05). When comparing with the control group, chitosan coating was found to be effective on melanosis until the 12th day of the storage. The shrimps got dark and had highly melanosis. So it was concluded that chitosan coating without essential oil does not affect the melanosis formation of shrimps. On the other hand, chitosan coatings incorporated with OPEO had an observable impact on the melanosis. There are many studies about preventing the melanosis of shrimps and it was concluded that natural plant extracts and coating materials could retard melanosis (Gokoglu and Yerlikaya 2008; Nirmal and Benjakul 2009; Huang et al. 2012).
At the beginning of storage, lightness (L*), redness (a*), and yellowness (b*) values of fresh shrimps were determined as 53.56, 0.60, and 6.98, respectively. When comparing with the other groups, the L* value of the control group decreased significantly throughout the storage (P < 0.05). It was detected that chitosan coatings had a positive effect on the color of shrimps according to control. Bingöl et al. (2015) reported that lightness (L*) value directly associated with melanosis decreased continuously on the storage period. The a* value in the sample-coated chitosan containing essential oil was determined to increase during storage (P < 0.05). The b* value of the control group was statistically different than in the other samples (both with and without essential oil coated) (P < 0.05). Chitosan-coated shrimps were found to be more red and blue during storage period and redness is good for consumer acceptability. However, yellowness (b*) value, which is associated with deterioratio, was increased in relation to the microbial growth. Gokoglu and Yerlikaya (2008) investigated the effects of grape seed extracts on inhibition of melanosis in shrimp and concluded that the results of L* value were correlated with sensory melanosis scores in all treatments. Sathivel (2005) has reported that chitosan coating have no impact on L*, a*, and b* values.
Results of Chemical Analysis
Residual sulfur dioxide (SO2) of the fresh shrimps was detected as 0.25 mg/kg. This amount corresponds to 0.37 mg/kg as sodium metabisulfite residue. For not cooked crustaceans, these limits range from 150 to 300 mg kg−1 (Iammarino et al. 2014). Measured residual SO2 level of our samples may be originated from the contaminated equipments used during transportation and they were found to be below the limit values.
The results of pH, TVB-N, and TMA-N measurements of the C, CH, and CH+OPEO groups are shown in Fig. 4. It was reported that for shrimp, a pH of 7.7 or less indicates prime quality, 7.70–7.95 shows poor but acceptable quality, and 7.95 or greater represents unacceptable quality (Çolakoğlu et al. 2006). In this study, the pH value of fresh shrimp was 6.44. Group C and CH exceeded the acceptable upper limit of 7.95 on days 7 and 12 of the storage, respectively. Group CH+OPEO remained acceptable throughout the storage period. The increase pH in the control group was statistically significant than in the other coated groups (P < 0.05). In a study of garlic oil incorporation to chitosan edible coatings, Aşik and Candoğan (2014) concluded that chitosan edible coating application onto the surface of the shrimp meat resulted in the decrease of pH values of the samples, as compared with the C group (P < 0.05), likely because of the low pH of the chitosan coating solution, resulting from acetic acid incorporation into the formulation.
The upper limit value of 30 mg/100 g TVB-N for spoilage has been used for marine products (Harpaz et al. 2003). In this study, the initial value of TVB-N content in fresh shrimps was 17.32 mg/100 g of shrimp. The TVB-N values of the control and coated samples increased significantly throughout the storage (P < 0.05). Control (37.73 mg/100 g), CH (34.49 mg/100 g), and CH+OPEO (35.64 mg/100 g) exceeded the acceptable limit value on days 7, 10, and 15 of the storage, respectively. During the storage period, the total volatile base content of all groups increased; however, this increase was slightly moderate in the shrimp treated with OPEO. Aşik and Candoğan (2014) who measured the initial TVB-N value (day 0) of shrimp meat as 20.72 mg/100 g reported that chitosan coating application significantly reduced TVB-N value in shrimps as compared with the control, whereas no noticeable effect of garlic oil incorporated into chitosan was observed.
The initial value of TMA-N content in shrimps was 1.15 mg/100 g of meat. Varlik et al. (2000) reported the initial TMA-N value for fresh P. longirostris stored at 4 °C as 1.75 mg/100 g of meat. Varlik et al. (1993) reported that the consumable limit of TMA-N value should remain between 1 and 8 mg/100 g and seafood that contains >8 mg/100 g TMA-N value should be considered as spoiled. The TMA-N values of the control and coated samples increased significantly throughout the storage (P < 0.05). Control, CH, and CH+OPEO exceeded the acceptable limit value of TMA-N on days 7, 10, and 15 days of the storage, respectively. There were statistically significant differences between control and the others groups in terms of TMA-N values (P < 0.05).
Thiobarbituric acid (TBA) and peroxide values (PV) of the samples during 15-day storage are presented in Fig. 5. Depending on the storage time, the increase of TBA values for the control group was found to be statistically significant different than the other groups (P < 0.05). CH and OPEO on TBA has been more effective than the control group and TBA value of this group remained at very low levels (P < 0.05).
A PV scale for freshness has been suggested at 0–2 mmol of O2/kg for very good, 2–5 for good, 5–8 for acceptable, and 8–10 mmol of O2/kg for spoiled ( Ludorff and Meyer 1973). Okpala et al. (2014) monitored the quality changes in PV of Pacific white shrimp during iced storage and reported that the initial PV value was 1.56 ± 0.27 meq active oxygen/kg lipids. In this study, the initial PV value of the shrimps was 0.79 meq active oxygen/kg lipids. The PV values of the control and coated samples increased significantly (P < 0.05) with storage time; however, significant differences (P < 0.05) were observed in the PV for the control group. The results of the present study indicate that orange peel is effective in retarding the production of PV in shrimp stored by refrigeration (4 ± 1 °C).
Results of Microbiological Analysis
Microbial flora changes of shrimps after the treatment with chitosan coating containing OPEO are shown in Fig. 6. For fresh water and marine species, the microbiological limit recommended by the ICMSF (1986) for total viable count at 30 °C is 7 log/g or log/cm2. This value is also accepted as limit for psychrotrophic bacteria count for researchers (Sallam 2007). The initial TVC of the shrimps was 3.7 log CFU/g (Fig. 6). There was not a significant increase for control group until 7 days of storage (P > 0.05); however, the upper limit of 7 log CFU/g was exceeded after 7 days. The increases of TVC count for CH and CH+OPEO groups were significant until 12 days of storage (P < 0.05). TVC load of the CH group exceeded the upper limit after 12 days of storage. CH+OPEO groups remained under the limit TVC count for 15-day storage so it was concluded that chitosan combined with OPEO is effective for TVC growth. There are many reports about the control of microbial growth by chitosan coatings or films most of which concluded that chitosan films are effective on TVB count (Fan et al. 2009; Ojagh et al. 2010; Alak et al. 2010)
Psychrotrophic bacteria (PB) are the major group of microorganisms responsible for spoilage of aerobically stored seafood at chilled temperatures (Sallam 2007). The initial PB of the shrimps was 2.5 log CFU/g (Fig. 6). There was a significant increase for C, CH, and CH+OPEO groups (P < 0.05). The upper limit of 7 log CFU/g was exceeded after 10 and 12 days for C and CH groups, respectively. PB value of the CH+OPEO group remained under the limit, as there was non-significant increase throughout the storage period (P > 0.05). It was concluded that the orange peel essential oil combination inhibited the total PB count when comparing with the non-coated or only chitosan-coated groups. Huang et al. (2012) investigated the effects of 1.0 and 1.5% O-carboxymethyl chitosan (CMC) and 1.0 and 1.5% chitosan (CH) coatings on the quality changes of whiteleg shrimp (Litopenaeus vannamei) during refrigerated storage. They concluded that CMC and CH application retarded the PB growth throughout the storage period.
The consumable limit value of total coliform bacteria (TCB) count for seafood is reported as 160–210 MNP/g (Jay et al. 2005) while ICMSF (1986) and Ababouch et al. (2005) stated the limit for fresh and frozen fish as <100 MNP/g. The initial TCB count was detected as 1.5 MNP/g for fresh shrimp. There was a rapid increase for control group after 7 days of storage (P < 0.05) and reached 9 MNP/g after 12 days. Small increases were observed for TCB count of CH group when comparing with the control group (P < 0.05) while there was a steady increase for the CH+OPEO group. TCB count of all groups was under the limit value throughout the storage period. It can be concluded that OPEO inhibited the TCB growth of shrimps under refrigerated conditions.
Conclusions
The results of the present study pointed out that the combination of chitosan coating and orange peel essential oil was effective in extending the shelf life of fresh shrimps to 15 days. Chitosan combined with orange peel essential oil had a positive effect on the sensorial and microbiological parameters of the shrimps. Microbiological and sensorial analysis revealed out that control group samples had 7-day shelf life whereas only chitosan-coated group had a shelf life of 10 days (CH). Therefore, the addition of orange peel essential oil to chitosan coating solution inhibited the lipid oxidation and microbial growth, so extended the shelf-life of shrimps for nearly 8 days more when compared to the non-coated group. Besides, the composite chitosan coating was a transparent layer from a sensorial point of view and prevented black spot development. This coating could therefore be useful for preserving shrimp quality during cold storage with a shelf life extension in shrimps of about 8 days. In conclusion, chitosan film enriched with orange peel essential oil provides an effective coating that prolongs the shelf life and maintains the quality attributes of the shrimps. Moreover, the use of different coating material combined orange peel essential oils will also contribute on preventing melanosis and prolonging the shelf life of shrimp.
References
Ababouch, L., Gandini, G., & Ryder, J. (2005). Causes of detentions and rejections in international fish trade. FAO Fisheries Technical Paper, No:473, Rome, FAO, pp.110.
Alak, G., Hisar, S. A., Hisar, O., Kaban, G., & Kaya, M. (2010). Microbiological and chemical properties of bonito fish (Sarda sarda) fillets packaged with chitosan film, modified atmosphere and vacuum. Journal of the Faculty of Veterinary Medicine, 16, 73–80.
Alparslan, Y., Yapıcı, H. H., Metin, C., Baygar, T., Günlü, A., & Baygar, T. (2016). Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT-Food Science and Technology, 72, 457–466.
Altiok, D., Altiok, E., & Tihminlioglu, F. (2010). Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. Journal of Materials Science: Materials in Medicine, 21(7), 2227–2236.
Antonacopoulos, N. (1973). Bestimmung des Flüchtigen Basenstickstoofs. In W. Ludorf & V. Meyer (Eds.), Fische und Fischerzeugnisse (pp. 224–225). Berlin: Aulage Verlag Paul Parey.
AOAC (1990). Official methods of analysis (13th ed.). Washington: D.C., USA: Association of Official Analytical Chemists. Official method 950.46.
AOAC (2000). Sulfites in foods and beverages, ion exclusion chromatographic method. AOAC Official Methods of Analysis. Official Method 990.31, Sec. 47.3.46.
AOAC (2006a). Official methods of analysis, ( [Revised] (18th ed.). Washington: DC., USA: Association of Official Analytical Chemists. Official method 934.01.
AOAC (2006b). Official methods of analysis, ( [Revised] (18th ed.). Washington: DC., USA: Association of Official Analytical Chemists. Official method 984.13.
AOCS. (1994). Official methods Cd 8-53 and recommended practices of the American Oil Chemists Society. Champaign, IL: American Oil Chemists Society.
Aşik, E., & Candoğan, K. (2014). Effects of chitosan coatings incorporated with garlic oil on quality characteristics of shrimp. Journal of Food Quality, 37(4), 237–246.
Bao, S., Xu, S., & Wang, Z. (2009). Antioxidant activity and properties of gelatin films incorporated with tea polyphenol-loaded chitosan nanoparticles. Journal of the Science of Food and Agriculture, 89(15), 2692–2700.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Bingöl, E. B., Bostan, K., Uran, H., Alakavuk, D. Ü., & Sivri, N. (2015). Effects of chitosan treatment on the quality parameters of shrimp (Parapenaeus longirostris) during chilled storage. Turkish Journal of Fisheries and Aquatic Sicences, 15(4), 821–831.
Choi, H. S., Song, H. S., Ukeda, H., & Sawamura, M. (2000). Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-diphenyl-2-picrylhydrazyl. Journal of Agricultural and Food Chemistry, 48(9), 4156–4161.
CIE (1976). 18th Session, London, U.K., Sept. 1975. CIE Publication 36, Paris, France.
Çolakoğlu, F. A., Ormancı, H. B., & Altın, A. (2006). Determination of the shelf life of fresh shrimp (Parapaneus longirostris) treated with Frische-star. Ege Journal of Fisheries and Aquatic Sciences, 23(1/3), 383–386.
Dayal, J. S., Ponniah, A. G., Khan, H. I., Babu, E. M., Ambasankar, K., & Vasagam, K. K. (2013). Shrimps—a nutritional perspective. Current Science, 104(11), 1487–1491.
Djenane, D. (2015). Chemical profile, antibacterial and antioxidant activity of algerian citrus essential oils and their application in Sardina pilchardus. Foods, 4(2), 208–228.
Diouf, A., Fall, J., Ayessou, N., Ndour, M. M., Diouf, N., & Seydi, M. (2016). Contribution to the revision of the senegalese law on the use of sulfites for the treatment of crustaceans: the case of shrimp. Journal of Biology and Life Science, 7(1), 122–137.
Fan, W., Sun, J., Chen, Y., Qiu, J., Zhang, Y., & Chi, Y. (2009). Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chemistry, 115(1), 66–70.
FDA/BAM. (2002). Food and drug analyses/bacteriological analytical manual, chapter 4: total coliform bacteria count. Silver Spring, MD, USA: U.S. Food and Drug Administration.
FDA/BAM. (2009). Food and drug analyses/bacteriological analytical manual, chapter 3: aerobic plate count. Silver Spring, MD, USA: U.S. Food and Drug Administration.
Garcia, M. A., Martino, M. N., & Zaritzky, N. E. (1998). Plasticized starch-based coatings to ımprove strawberry (Fragaria ananassa) quality and stability. Journal of Agricultural and Food Chemistry, 46, 3758–3767.
Gokoglu, N., & Yerlikaya, P. (2008). Inhibition effects of grape seed extracts on melanosis formation in shrimp (Parapenaeus longirostris). International Journal of Food Science & Technology, 43(6), 1004–1008.
Hafsa, J., Ali Smach, M., Khedher, M. R. B., Charfeddine, B., Limem, K., Majdoub, H., & Rouatbi, S. (2016). Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT-Food Science and Technology, 68, 356–364.
Hammer, K. A., Carson, C. F., & Riley, T. V. (1999). Antimicrobial activity of essential oils and other plant extracts. Journal of Applied Microbiology, 86(6), 985–990.
Harpaz, S., Glatman, L., Drabkin, V., & Gelman, A. (2003). Effects of herbal essential oils used to extend the shelf life of freshwater-reared Asian sea bass fish (Lates calcarifer). Journal of Food Protection, 66(3), 410–417.
Hosseini, S. F., Rezaei, M., Zandi, M., & Farahmand Ghavi, F. (2015). Effect of fish gelatin coating enriched with oregano essential oil on the quality of refrigerated rainbow trout fillet. Journal of Aquatic Food Product Technology, 25(6), 835–842.
Huang, J., Chen, Q., Qiu, M., & Li, S. (2012). Chitosan-based edible coatings for quality preservation of postharvest whiteleg shrimp (Litopenaeus vannamei). Journal of Food Science, 77(4), C491–C496.
Iammarino, M., Di Taranto, A., & Ientile, A. R. (2014). Monitoring of sulphites levels in shrimps samples collected in Puglia (Italy) by ion-exchange chromatography with conductivity detection. Food Additives & Contaminants: Part B, 7(2), 84–89.
ICMSF (1986). International Commission on Microbiological Specifications for foods. In: Microorganisms in foods. Sampling for microbiological analysis: principles and scientific applications, Vol. 2. Toronto, Canada. ICMSF (eds). University of Toronto Press.
Jay, J. M., Loessner, M. J., & Golden, D. A. (2005). Modern food microbiology (p. 56). New York: Springer Science & Business Media.
Kamal, G. M., Anwar, F., Hussain, A. I., Sarri, N., & Ashraf, M. Y. (2011). Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. International Food Research Journal, 18(4), 1275–1282.
Koç, B. E., & Özkan, M. (2011). Utilization of chitosan in food industry. Food, 36(3), 161–168.
López-Mata, M. A., Ruiz-Cruz, S., Silva-Beltrán, N. P., Ornelas-Paz, J. D. J., Zamudio-Flores, P. B., & Burruel-Ibarra, S. E. (2013). Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules, 18(11), 13735–13753.
Ludorff, W., & Meyer, V. (1973). Fische und fischerzeugnisse (Vol. 6). Paul Parey.
Manthey, M., Karnop, G., & Rehbein, H. (1988). Quality changes of European catfish from warm-water aquaculture during storage ice. International Journal of Food Science and Technology, 23, 1–9.
Mendes, R. (2006). Guidebook on melanosis inhibitors and processing technology of crustaceans. INIAP/IPIMAR: Project QLK1-CT-2002-71517 (CRUSTAMEL New approaches to the crustaceans prevention of melanosis and quality indices), p.41.
NCCLS (1993). Performance standards for antimicrobial disc suspectibility tests. Approved Standard NCCLS Publication M2- A5, Villanova, PA, USA.
Nirmal, N. P., & Benjakul, S. (2009). Melanosis and quality changes of Pacific white shrimp (Litopenaeus vannamei) treated with catechin during iced storage. Journal of Agricultural and Food Chemistry, 57(9), 3578–3586.
Nirmal, N. P., & Benjakul, S. (2011). Retardation of quality changes of Pacific white shrimp by green tea extract treatment and modified atmosphere packaging during refrigerated storage. International Journal of Food Microbiology, 149(3), 247–253.
Ojagh, S. M., Rezaei, M., Razavi, S. H., & Hosseini, S. M. H. (2010). Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chemistry, 120(1), 193–198.
Okpala, C. O. R., Choo, W. S., & Dykes, G. A. (2014). Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) freshly harvested and stored on ice. LWT-Food Science and Technology, 55(1), 110–116.
Oosterveer, P. (2006). Globalization and sustainable consumption of shrimp: consumers and governance in the global space of flows. International Journal of Consumer Studies, 30(5), 465–476.
Otwell, W.S., & Marshall, M. (1986). Studies on the use of surfices to control shrimp melanosis (blackspot). Florida sea grant college technical paper No. 46, Florida, pp. 1–18.
Palazzolo, E., Laudicina, V. A., & Germanà, M. A. (2013). Current and potential use of citrus essential oils. Current Organic Chemistry, 17(24), 3042–3049.
Pranoto, Y., Rakshit, S. K., & Salokhe, V. M. (2005). Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT-Food Science and Technology, 38(8), 859–865.
Rinaudo, M. (2006). Chitin and chitosan: properties and applications. Progressin Polymer Science, 31, 603–632.
Rosa, R., & Nunes, M. L. (2004). Nutritional quality of red shrimp, Aristeus antennatus (Risso), pink shrimp, Parapenaeus longirostris (Lucas), and Norway lobster, Nephrops norvegicus (Linnaeus). Journal of the Science of Food and Agriculture, 84(1), 89–94.
Sallam, K. I. (2007). Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control, 18(5), 566–575.
Sathivel, S. (2005). Chitosan and protein coatings affect yield, moisture loss, and lipid oxidation of pink salmon (Oncorhynchus gorbuscha) fillets during frozen storage. Journal of Food Science, 70(8), e455–e459.
Sawamura, M. (Ed.). (2011). Citrus essential oils: flavor and fragrance. Hoboken: John Wiley & Sons, Inc..
Schormüller, J. (1968). Handbuch der Lebensmittel Chemie. Band III/2 Teil. Tierische Lebensmittel Eier, Fleisch, Buttermilch, Springer Verlag, Berlin, Heidelberg, New York: 1482–1537.
Sikdar, D. C., Menon, R., Duseja, K., Kumar, P., & Swami, P. (2016). Extraction of citrus oil from orange (Citrus sinensis) peels by steam distillation and its characterizations. International Journal of Technical Research and Applications, 4(3), 341–346.
Siracusa, V., Rocculi, P., Romani, S., & Dalla Rosa, M. (2008). Biodegradable polymers for food packaging: a review. Trends in Food Science & Technology, 19(12), 634–643.
Sugumar, S., Mukherjee, A., & Chandrasekaran, N. (2015). Eucalyptus oil nanoemulsion-impregnated chitosan film: antibacterial effects against a clinical pathogen, Staphylococcus aureus, in vitro. International Journal of Nanomedicine, 10(Suppl 1), 67–75.
Tarladgis, B.G., Watts, B.M,. Younathan, M.T., & Dugan, T.L. (1960). A distillation method for quantitative determination of malonaldehyde in rancid foods. Journal of the American Oil Chemists’ Society, 37, 44–48.
Tongnuanchan, P., Benjakul, S., & Prodpran, T. (2012). Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chemistry, 134(3), 1571–1579.
Varlik, C., Baygar, T., Özden, Ö., Erkan, N., & Mol, S. (2000). Sensory evaluation and determination of some physical and chemical characteristics of shrimp during gold storage. Turkish Journal of Veterinary and Animal Sciences, 24(3), 181–186.
Varlik, C., Gökoğlu, N., & Gün, H. (1993). The effect of temperature on the penetration of vinegar/salt. Food, 18(4), 223–228.
Wang, X. J., Zhang, K. S., & Ren, Y. X. (2007). Study on preservation of shrimp by coating with chitosan. Food Science, 7, 131.
Yanar, Y., & Celik, M. (2006). Seasonal amino acid profiles and mineral contents of green tiger shrimp (Penaeus semisulcatus De Haan, 1844) and speckled shrimp (Metapenaeus monoceros Fabricus, 1789) from the Eastern Mediterranean. Food Chemistry, 94(1), 33–36.
Yen, G. C., & Hsieh, P. P. (1995). Antioxidative activity and scavenging effects on active oxygen of xylose-lysine maillard reaction products. Journal of the Science of Food and Agriculture, 67(3), 415–420.
Zeng, Q. Z., Thorarinsdottir, K. A., & Olafsdottir, G. (2005). Quality changes of shrimp (Pandalus borealis) stored under different cooling conditions. Journal of Food Science, 70(7), 459–466.
Zivanovic, S., Chi, S., & Draughon, A. F. (2005). Antimicrobial activity of chitosan films enriched with essential oils. Journal of Food Science, 70(1), M45–M51.
Acknowledgements
This study is a part of Ph.D. thesis and supported by the Scientific Research Projects Unit of Mugla Sitki Kocman University (Project Number: 12/97). The authors would like to thank Hatice Hasanhocaoğlu Yapıcı, Cansu Metin, and Tuba Baygar for their contribution and Daniela Giannetto for English editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alparslan, Y., Baygar, T. Effect of Chitosan Film Coating Combined with Orange Peel Essential Oil on the Shelf Life of Deepwater Pink Shrimp. Food Bioprocess Technol 10, 842–853 (2017). https://doi.org/10.1007/s11947-017-1862-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1862-y