Abstract
The concurrent extraction of lipids, proteins, and carbohydrates can be achieved by aqueous and enzymatic extraction processes, circumventing the low extractability by mechanical pressing and the use of flammable solvents. The use of alkaline protease, preceded or not by carbohydrase pretreatments, was evaluated on the extractability of oil, protein, and carbohydrates from full-fat chickpea flour and protein functionality. Enzymatic extraction increased oil and protein extractability from 49.8 to 72.0–77.1% and 62.8 to 83.5–86.1%, respectively. Although the carbohydrase pretreatments before the addition of protease did not increase oil and protein extractability, the carbohydrate content of the extracts increased from 7.68 to 9.17−9.33 mg/mL, accompanied by the release of new oligosaccharides in the extracts, as revealed by LC–MS/MS characterization. Enzymatic extraction yielded proteins with significantly higher solubility (25.6 vs. 68.2–73.6%) and digestibility (83.8 vs. 90.79–94.67%). Treatment of the extracts with α-galactosidase completely removed the flatulence-causing oligosaccharides (stachyose and raffinose). This study highlights the effectiveness of environmentally friendly bioprocessing strategies to maximize lipid, protein, and oligosaccharide extractability from full-fat chickpea flour with concurrent improvements in protein solubility and in vitro digestibility, reduction of flatulence related oligosaccharides, and generation of a more diverse pool of oligosaccharides for subsequent prebiotic evaluation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increasing world population and the popularity of alternative protein sources, plant-based proteins are becoming the forefront of sustainable food production. Plant-based protein sources provide many benefits including decreased risk of degenerative diseases and reduced environmental impact from its production (González et al., 2011; WHO, 2003). Such benefits have promoted increased production and processing of plant-based products, which in turn requires the development of a critical understanding of the impact of key processing conditions (i.e., extraction and recovery) on the extractability and functionality of many plant-based compounds (i.e., proteins, lipids, and carbohydrates).
An area of growing interest is the processing of pulses, which are part of the legume family. Pulses are generally low in fat and high in protein and fiber (Shevkani et al., 2019). Chickpeas, a member of the pulse family, are an example of a good source of carbohydrates (~60 g/100 g), proteins (19 g/100 g), lipids (6 g/100 g), dietary fiber (~17 g/100 g), and other minor constituents (U.S. Department of Agriculture, 2019). The increasing popularity and use of chickpeas in the food industry can be explained by its nutritional value and health benefits associated with its consumption (i.e., low glycemic index, prevention of cardiovascular disease, type-2 diabetes) (Wallace et al., 2016). Used in food products worldwide, most notably for hummus production, chickpeas can be a main source of protein in vegan and vegetarian diets (Duranti & Gius, 1997). Chickpea protein isolates can also be used not only to improve the nutritional value but the physical and rheological properties of gluten-free food products (Shaabani et al., 2018).
Chickpea proteins, lipids, and carbohydrates can be extracted using numerous methods. The presence of lipids in a food matrix entails the upstream removal of lipids to release proteins and carbohydrates. Traditionally, upstream lipid removal has been accomplished either by solvent extraction or by the use of mechanical pressing, the selection of which depends on the composition of the material used (de Moura et al., 2009). Despite the environmental and safety issues associated with flammable solvent extraction and low extraction yields associated with mechanical pressing, a protein-rich by-product with varying amounts of residual oil (cake) or compromised functionality (solvent defatted flour) can be obtained (Kim et al., 2021; L’hocine et al., 2006). This sequential approach means that the cake or the defatted flour must be subjected to another processing step to extract proteins and carbohydrates, in addition to removing the remaining lipids using flammable organic solvents.
Alternatively, aqueous extraction processes (AEP) and enzyme-assisted aqueous extraction processes (EAEP) have been used to simultaneously extract lipids, proteins, and carbohydrates from a food matrix without upstream removal of lipids (Campbell & Glatz, 2009; De Moura et al., 2011b). This environmentally friendly processing strategy eliminates the negative impact of flammable and hazardous solvents conventionally used for defatting, thanks to the solubilization and transport of proteins to the exterior of the solid matrix, which creates a more porous structure that favors the washing of the oil droplets by the extraction medium (Cheng et al., 2018; Dias et al., 2020). A further improvement upon the AEP process is the enzyme-assisted extraction process (EAEP), which utilizes enzymes such as proteases and carbohydrases to maximize processing extractability. Increased oil and protein extractability in the EAEP has been attributed to enzymatic hydrolysis of the lipid body membrane, proteins, and cell walls (De Moura et al., 2008; Nadar et al., 2017).
The successful development of extraction methods for new protein sources depends on the development of fundamental knowledge of the impact of the processing conditions employed (De Moura et al., 2011a) on the extractability, composition, and functional properties of the extracted compounds. Controlled hydrolysis of chickpea protein isolates by immobilized Alcalase has been shown to produce hydrolysates with higher solubility, oil absorption, foaming capacity, and stability (Yust et al., 2010). However, limited emphasis has been given to the development of a holistic understanding of the effects of key extraction parameters (i.e., solids-to-liquid ratio, pH, temperature, incubation time, amount and type of enzyme) on the overall extractability of both lipids and proteins from full-fat chickpea flour and their impact on the functional properties of the extracted proteins.
Because extraction conditions affect yields and the functionality of the target compounds, they play a key role in the processing feasibility and potential applications of the extracted compounds. This work was undertaken to uncover the effects of different enzymatic extraction strategies on the simultaneous extraction of lipids, proteins, and carbohydrates from full-fat chickpea flour and on the functionality of the extracted proteins. Specifically, we evaluated the effectiveness of an upstream enzymatic pretreatment with carbohydrases (cellulase, hemicellulase, and xylanase) before the use of proteases, with respect to lipids and protein extractability, solubility and in vitro digestibility of the extracted proteins, and carbohydrate profiling of the extracts. Our working hypothesis was that the use of carbohydrases before the addition of proteases could hydrolyze the cell wall and potentially release new oligosaccharides while also favoring the formation of a more porous structure that could aid in protein solubilization by the aqueous medium, as well as hydrolysis of the protein bodies and oleosin membrane surrounding the lipid bodies by the protease. That could in turn improve the overall process extractability and concurrently produce more soluble and digestible protein hydrolysis products and release a more diverse pool of oligosaccharides with potential health-promoting effects. High-performance anion-exchange chromatography with pulsed amperometric detection and LC–MS/MS were used to determine the carbohydrate profile of the chickpea extracts.
Materials and Methods
Full-Fat Chickpea Flour and Enzymes Used in the Enzymatic Extraction
Commercial steamed chickpea flour of the Kabuli variety was kindly provided by Natural Products, Inc (Grinnell, Iowa, USA). Partially dehulled chickpeas (to increase the fiber content of the final product) were steamed to inactivate enzymes and achieve microbial stability before milling (as described by the manufacturer). The chickpea flour contained 7.4 ± 0.1% oil, 25.87 ± 0.07% protein, and 4.69 ± 0.09% moisture, which were determined as described in the “Proximate Analysis” section.
The following commercial enzymes were used to assist the enzymatic extraction process (EAEP):
-
(i)
FoodPro alkaline protease (also known as Protex 6L) is a bacterial alkaline endoprotease from Bacillus licheniformis (pH activity from 8.0 to 10.5, temperature from 45 to 75 ℃, and enzyme activity of 580,000–650,000 DU/g) was provided by the Genencor Division of Danisco (Rochester, NY, USA);
-
(ii)
cellulase from Trichoderma reesei, with multiple cellulolytic activities (endo- and exo-cellulase, β-glucosidase, β-glucanase, hemicellulose, pectinase, and xylanase) and enzyme activity of 200,000 CU/g at optimal pH from 4.0−6.5 and 45−70 °C was provided by Bio-Cat (Troy, VA, USA);
-
(iii)
hemicellulase from Aspergillus niger, with enzyme activity of 600,000 HCU/g and optimal activity at pH 2.0−8.0 and 25−90 °C, was provided by Bio-Cat (Troy, VA, USA); and
-
(iv)
xylanase from Trichoderma longibrachiatum, with enzyme activity of 200,000 XU/g and optimal activity at pH 3.5–6.5 and 40–70 °C, was provided by Bio-Cat (Troy, VA, USA).
Tailoring Enzyme Use to Maximize the Simultaneous Extraction of Lipids, Proteins, and Carbohydrates from Full-Fat Chickpea Flour
The effect of using protease (EAEP), alone or in combination with different carbohydrase pretreatments, was evaluated on the extractability of proteins, lipids, and carbohydrates from chickpea flour (Fig. 1). A non-enzymatic aqueous treatment (AEP) was used as the control.
Process flow diagram for each extraction treatment. AEP, no enzyme; EAEP 1, 0.5% protease; EAEP2, 0.25% of cellulase and 0.25% of hemicellulase (total of 0.5% of carbohydrases); EAEP3, 0.17% of cellulase, 0.17% of hemicellulase, and 0.17% of xylanase (total of 0.5% of carbohydrases). w/w, weight of enzyme/weight of flour
The AEP (control, no enzyme use) was carried out by dispersing 50 g of chickpea flour into 500 mL of water to achieve a 1:10 solids-to-liquid ratio (SLR). The slurry pH was adjusted to pH 9.0 to favor protein solubility and extractability (de Almeida et al., 2019) and kept at 50 °C under constant stirring for 60 min. For the EAEP, the potential benefits of using an upstream treatment with carbohydrases, before the alkaline protease addition, were evaluated. The following enzymatic strategies were evaluated: EAEP 1: 0.5% (w/w) of alkaline protease at pH 9.0 for 60 min; EAEP 2: 0.5% (w/w) of carbohydrases (0.25% of cellulase + 0.25% of hemicellulase) at pH 6.0 for 30 min followed by the addition of 0.5% of alkaline protease (w/w) at pH 9.0 for 60 min; and EAEP 3: 0.5% (w/w) of carbohydrases (0.17% of cellulase + 0.17% of hemicellulase + 0.17% of xylanase) at pH 6.0 for 30 min followed by the addition of 0.5% of alkaline protease (w/w) at pH 9.0 for 60 min. For the EAEP, extractions were performed at the same SLR and temperature as the AEP, and pH conditions were selected based on the enzyme manufacturer’s recommendations.
After extracting, the resulting slurry was centrifuged at 3000 × g for 30 min at 4 °C to separate the insoluble fraction (containing the unextracted compounds) from the liquid phase (containing the extracted compounds). The liquid fraction was placed in a separatory funnel and allowed to settle overnight at 4 °C to separate the oil-rich fraction (cream and free oil) from the protein- and carbohydrate-rich fraction (skim). Each extraction condition was carried out in triplicate.
Chickpea full-fat flour (starting material for the extraction) and all fractions generated by the AEP and EAEP were characterized for oil, protein, and carbohydrate contents (as described in the “Proximate Analysis” and “Carbohydrate Profile, Quantification, and α-Galactosidase Treatment of AEP and EAEP Skim Fractions” sections). Total oil extraction yield (TOE), oil distribution in the fractions (free oil yield, oil yield in the cream, skim, and insoluble), total protein extraction yield (TPE), and protein distribution in the fractions (protein yield in the cream, skim, and insoluble) were determined according to Eqs. (1) and (2), respectively (Souza et al., 2019):
* The fractions relate to free oil, cream, skim, and insoluble.
Proximate Analysis
Oil (acid hydrolysis–AOCS method 989.05), dry matter (AOCS method 925.09), and protein content (AOAC 992.23- Dumas combustion method, 6.25 of nitrogen conversion factor) were determined in the starting material and fractions generated in the extraction. Analyses were performed in duplicate for each extraction (n = 6).
Protein Degree of Hydrolysis of Skim Fractions
The protein degree of hydrolysis (DH) of AEP and EAEP skim fractions was determined by the o-phthaldialdehyde (OPA) method (Nielsen, 2001) in a spectrophotometer at 340 nm using L-serine solution as the standard. The DH was determined as the ratio of h (number of hydrolyzed bonds) and htot (total number of peptide bonds per protein equivalent–7.22 for chickpeas (Kou et al., 2013).
Low Molecular Weight Polypeptide Profile Characterization of AEP and EAEP Skim Proteins by Sodium Dodecyl Sulfate–Polyacrylamide
Sodium dodecyl sulfate–polyacrylamide (SDS-PAGE) of the skim fractions was performed using a 12% acrylamide gel and 30 µg of protein/ well. A low molecular weight (MW) range standard ladder (14.4–97.4 kDa) (Bio-Rad, Hercules, CA, USA) was used. Relative quantification and polypeptide distribution were performed using an Imager system and Image Lab software (Bio-Rad, Hercules, CA, USA).
Solubility of Skim Proteins
Approximately 15 mL of AEP and EAEP skims from each extraction replicate (n = 3) were freeze-dried on a FreeZone 4.5 Liter Benchtop Freeze Dry System (Labconco, Kansas City, MO, USA) and stored at −20 °C for subsequent solubility tests. Protein solubility of freeze-dried AEP and EAEP skim proteins was evaluated by preparing a 10 mL of a 1% (w/v) skim solution in a 30-mL beaker and adjusting the pH of the protein solution to 4.0 and 9.0 by the addition of 1 M HCl or 1 M NaOH solution. Solutions were vigorously mixed at 150 rpm for 1 h at room temperature and then centrifuged at 10,000 × g at 20 °C for 10 min. The total protein content was determined as described in item 2.3. All samples were analyzed in duplicate. Skim protein solubility was expressed as the percentage ratio of the supernatant protein content to the sample protein content.
Carbohydrate Profile, Quantification, and α-Galactosidase Treatment of AEP and EAEP Skim Fractions
The use of carbohydrases during the extraction process and as a post-extraction strategy can result in the production of chickpea extracts containing a diverse oligosaccharide profile while eliminating flatulence promoting oligosaccharides such as raffinose and stachyose, which are present in high amounts in pulses, including chickpeas. The effects of the use of different carbohydrases during the extraction and post-extraction on the content and profile of carbohydrates of the skim fractions were evaluated by different analytic techniques.
Quantification of Total Carbohydrates by Spectrophotometry
The total carbohydrate content of the skim fractions was determined using the Phenol–sulfuric method (Masuko et al., 2005). A total of 15 µL of the sample along with 15 µL of nanopure water were added to the well. The well plate was shaken at 300 rpm for 1 min then 150 µL of 98% sulfuric acid was added to each well. The microplate was then incubated at 85 °C for 15 min in an incubating thermal shaker (Thermalshake, VWR, Radnor, Pennsylvania, USA), followed by the addition of 30 µL of 5% (w/v) phenol/water solution. A calibration curve made using glucose as a standard (from 4 to 20 µg, R2 = 0.9937) was used to quantify the total carbohydrates. After vigorous mixing, the samples were measured using a microplate reader (SpectraMax iD5, Molecular Devices, San Jose, CA) at 490 nm.
Quantification of Oligosaccharides and Simple Sugars By High-Performance Anion-Exchange Chromatography
Soluble carbohydrate profiles of skims were quantified by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD, Dionex ICS-5000+, Thermo Fisher Scientific, Sunnyvale, CA, USA). Aliquots of 200 μL of samples were mixed with 400 μL of ethanol in 1.5 mL tubes, vortexed, and incubated at −30 °C for 1 h. After being centrifuged at 4 °C for 30 min (13,000 × g), the supernatant was dried under vacuum (MiVac Quattro concentrator, Genevac Ltd., Ipswitch, UK). Samples were diluted as appropriate and filtered through a 0.2-μm syringe filter into 1.5 mL vials with septa. Glucose, galactose, and fructose were separated on a CarboPac PA10 column (4 × 250 mm) with a CarboPac PA10 guard column (4 × 50 mm) at a flow rate of 1.0 mL/min. The mobile phase was maintained at an isocratic condition of 10 mM NaOH for 12 min and was increased to 100 mM NaOH in 13 min. Sucrose, raffinose, and stachyose were separated on a CarboPac PA200 column (3 × 250 mm) with a CarboPac PA200 guard column (3 × 50 mm) by isocratic elution using a mobile phase of 50 mM NaOH at a flow rate of 0.5 mL/min. Both columns were washed with 200 mM NaOH for 5 min after each run and equilibrated with the respective initial mobile phases for 10 min before the next injection. Calibration curves were built by using 1–60 μg/mL of glucose, galactose, and fructose, and 0.1–10 μg/mL of sucrose, raffinose, and stachyose. An analytical replicate was conducted for each replicate of extraction and α-galactosidase treatment (n = 3).
α-Galactosidase Treatment of Raffinose and Stachyose in the Skim Fractions
Because of the presence of flatulence promoting oligosaccharides in the skim fractions, an α-galactosidase treatment was used to reduce the concentration of stachyose and raffinose in the skim fractions. Because the amount of stachyose and raffinose was not statistically different within the enzymatic treatments, the EAEP 1 skim was selected to demonstrate the effectiveness of the α-galactosidase treatment. 1000 µL of EAEP 1 skim was adjusted to pH 6 with 1 M HCl, and α-galactosidase (Bio-Cat, Inc., Troy, VA, USA) was added to achieve a 0.25% (w/v) concentration. The EAEP 1 skim was incubated at 40 °C for 0, 15, 30, and 60 min at 90 rpm in a water bath. Skim samples were placed in an ice bath to stop the reaction and stored at 4 °C until analyzed. The quantification of simple sugar (glucose, galactose, fructose, and sucrose) and oligosaccharide (raffinose and stachyose) was carried out by HPAEC-PAD as described in the “Quantification of Oligosaccharides and Simple Sugars By High-Performance Anion-Exchange Chromatography” section.
Mass Spectrometry Characterization of Oligosaccharides in the Extracts
The oligosaccharide profile of the skims was characterized by liquid chromatography-tandem mass spectrometry (LC–MS/MS). The reconstituted supernatant fractions obtained from ethanol precipitation (described in the “Quantification of Oligosaccharides and Simple Sugars By High-Performance Anion-Exchange Chromatography” section) were further purified by solid-phase extraction (SPE). Mixed-mode SPE cartridges, which retain compounds by both hydrophobic interaction and strong cation exchange, were used for separating oligosaccharides from peptides (Huang et al., 2022a). The reconstituted samples (150 μL, equivalent to 30 μL of skims) were premixed with 150 μL 0.2% formic acid and then loaded to Strata-X-C SPE cartridges (30 mg/1 mL, Phenomenex, Torrance, CA, USA) preconditioned with acetonitrile and 0.1% formic acid. Oligosaccharides were eluted with 3 mL 0.1% formic acid and further loaded to porous graphitic carbon SPE microplate (Glygen, Columbia, MD, USA) preconditioned with 80% acetonitrile with 0.1% trifluoroacetic acid and water. The microplate wells were washed with water for eliminating salts and flushed sequentially with 40% acetonitrile (fraction 1) and 40% acetonitrile with 0.1% trifluoroacetic acid (fraction 2) for eluting oligosaccharides. The collected oligosaccharide fractions were dried in a centrifugal evaporator. Fractions 1 and 2 were combined after dissolving the dried samples in water. For oligosaccharide characterization, the combined samples of the three replicates of extraction were pooled and injected into the LC–MS/MS (one injection for each treatment). For relative quantification, one injection was made for each extraction replicate.
LC–MS/MS analysis was performed on an Agilent 6520 Accurate-Mass Q-TOF LC–MS with a Chip Cube interface (Agilent Technologies, Santa Clara, CA, USA) equipped with an Agilent PGC-Chip II (porous graphitized carbon chip with a 40-nL enrichment column and a 75 μm × 43 mm analytical column). The capillary pump-delivered 3% acetonitrile with 0.1% formic acid (v/v/v) at a flow rate of 4 μL min−1 and loaded samples into the enrichment column. The injection volume was 2 μL for each sample. The nano pump-delivered mobile phase was composed of 3% acetonitrile with 0.1% formic acid (v/v/v) (solvent A) and 89.9% acetonitrile with 0.1% formic acid (v/v/v) (solvent B). The analytes were separated at a flow rate of 0.3 μL min−1 with 0% B from 0.0–2.5 min; 0–16% B from 2.5–20.0 min; 16–44% B from 20.0–30.0 min; 44–100% B from 30.0–35.0 min; 100% B 35.0–45.0 min. The mobile phase was switched to 100% A and equilibrated for 15 min before the next injection. The capillary voltage was set at 1850 V to maintain a stable spray. The drying gas was set at 350 °C at a flow rate of 5 L min−1. The scanning mass range was m/z 150–2500 for MS and 50–2500 for MS/MS. Collision energy for tandem MS was set by a formula of [0.02 × (m/z) − 3.5]. Data analysis was conducted in MassHunter Qualitative Analysis B.07.00 (Agilent Technologies). Oligosaccharides were identified by inspecting fragmentation patterns in tandem MS spectra. For relative quantification, peak areas of the identified oligosaccharides were integrated from merged extracted ion chromatograms, including the precursor ions and corresponding in-source fragment ions, to approach the real relative quantities (Huang et al., 2022b).
In vitro Skim Protein Digestibility
Protein digestibility of AEP and EAEP skim proteins was measured as described by Bornhorst and Singh (2013) and de Souza et al. (2020). Five milliliters of liquid skim fractions were mixed with 3.33 mL of SSF (Simulated Saliva Fluid) and vortexed. Subsequently, 6.66 mL of SGF (simulated gastric fluid) was added. Afterward, the pH was adjusted to 3.0 and the samples were placed into a water bath (37 °C, 140 rpm, 2 h). Then, 10 mL of SIF (simulated intestinal fluid) was added, and the pH was adjusted to 7.0. The samples were incubated in a water bath at 37 °C, 140 rpm, for 2 h. To stop the digestion, samples were heated in a water bath at 85 °C for 3 min. Trichloroacetic acid (TCA) was added in a 1:1 (v/v) proportion to the samples to achieve a final 12% (w/w) TCA concentration. The samples were centrifuged at 3578 × g for 30 min at 4 °C. The precipitate, protein nitrogen fraction (PN), was analyzed for the protein content. A before digestion control with sample and water, instead of simulated liquids, was performed and an enzyme blank with water, instead of the sample, was also performed. The digestibility was calculated as described by Zhong et al. (2012).
where PNbefore = protein before digestion, PNafter = protein after digestion, PNenzyme blank = enzyme blank. The PN (protein nitrogen fraction) was measured in the samples by the Dumas method using a conversion factor of 6.25 (Vario MAX cube, HE, DE) before and after the digestion.
Statistical Analysis
Extractions were performed in triplicate and the functional analyses were performed in duplicate. The results were expressed as the mean ± standard deviation (SD) of the replicates. Replicates of each measurement were analyzed by ANOVA with generalized linear models from the Statistica software (version 13.5.0.17 1984–2018, TIBCO Software Inc, Palo Alto, CA, USA). Multiple comparisons of least-square means were made by Tukey’s adjustment with the level of significance set at p < 0.05. Statistical significant differences were denoted by different letters, with the letter “a” being assigned to the highest value.
Results and Discussion
The Effects of Extraction Conditions on Oil and Protein Extraction Yields
The use of selected enzymes to assist the extraction of plant-based matrices has been successfully used as an effective strategy not only to increase the extractability of desired compounds (i.e., lipids, proteins, carbohydrates, phenolics) but to impart structural modifications in the food matrix that can lead to the production of compounds with desired functional and biological properties (i.e., higher protein digestibility and solubility, release of prebiotic oligosaccharides and antioxidants) (de Souza et al., 2020; Dias & de Moura Bell, 2022)).
The effectiveness of using alkaline protease, preceded or not by the use of selected carbohydrases, on the extractability of lipids and proteins from full-fat chickpea flour is shown in Fig. 2A and B. Enzymatic extraction significantly increased the overall extractability of lipids from full-fat chickpea flour (Fig. 2A) compared with the control (AEP, no enzyme use). When not using enzymes (AEP), 49.78 ± 2.08% of the available oil in the chickpea flour was extracted. However, oil extraction yields increased to 77.15 ± 5.87% for the EAEP 1 (using only protease), followed by 73.45 ± 1.54% for the EAEP 2 (cellulase + hemicellulase pretreatment followed by protease) and 72.02 ± 1.19% for the EAEP 3 (cellulase + hemicellulase + xylanase pretreatment followed by protease). The higher oil extraction yields observed for EAEP treatments can be primarily attributed to the modes of action and effectiveness of the protease used. Proteases can hydrolyze the oleosin membrane of the lipid bodies, releasing free oil into the aqueous medium (Campbell et al., 2011). In addition, protein removal from the matrix by solubilization or proteolysis leaves behind a more porous structure that facilitates the release of the oil. On the other hand, the AEP relies primarily on the solubilization of the proteins into the aqueous medium, without the benefit of proteolysis above described. Therefore, lipids are solely extracted through washing out of the matrix. Despite the higher EAEP oil extraction yields, compared with the AEP, oil extraction yields were not statistically different among the enzymatic treatments. Additional carbohydrase pretreatments did not significantly increase lipid extractability, therefore not justifying the additional use of enzyme, energy, and time. When looking at the oil distribution for the EAEP 1, although 77.15% of the chickpea flour oil was extracted, only 0.43% of the total extracted oil was present as free oil, while 42 and 57% of the extracted oil were present in the cream (oil-rich emulsion) and skim fractions, respectively. Comparatively, for the AEP, only 0.15% of the extracted oil was present as free oil, with 15 and 32% being present in the cream and skim, respectively. While the amount of free oil extracted by the AEP and EAEP was not statically different (0.14 vs. 0.24–0.29% yield), the use of enzyme in EAEP 1 and 2 significantly increased the oil yield in the cream (16.06 vs. 32.23–32.56% yield). Since there are no methods available to recover the diluted oil from the skim fraction, shifting more lipids into the cream fraction is of key importance to favor the overall recovery of the extracted oil, which entails the development of additional demulsification studies to breakdown the cream emulsion (De Moura & Johnson, 2009), which is beyond the scope of this work. Our results are in agreement with the literature (Dias et al., 2020; Souza et al., 2019), which demonstrates that most of the oil extracted through the AEP and EAEP is entrapped in the cream fraction.
Although the lipid content in AEP and EAEP skim fractions is low (~0.3 and 0.4%, respectively), the high volume of skim produced accounts for a significant portion of the oil in the chickpea flour (up to 47.5% yield). On a dry basis, the oil contents of AEP and EAEP skims are 7.77 and 7.91%, respectively. When comparing the oil distribution with previous studies for other food matrices, lower oil yields have been reported for skim fractions produced by enzymatic hydrolysis (Protex 6L) of extruded soybean flakes (14% yield, De Moura et al., 2008) and almond cake (14% yield, and Souza et al., 2019). This could be attributed to differences in the composition (i.e., lipids, protein) of the starting materials and processing conditions used (i.e., milling, flaking, extruding, type of enzyme). As an example, the initial oil content in the chickpea flour is very low (7%) compared to that of soybeans (21%) and almonds cake (16.25%). Because there are no methods available to recover oil from the skim fraction, and lipids can reduce the skim protein solubility (de Almeida et al., 2019), it is important to identify processing conditions leading to reduced oil in the skim fraction, which should, in turn, increase the lipid content in the cream fraction for subsequent recovery as free oil.
Overall, the addition of a carbohydrase pretreatment in EAEP 2 and 3 did not significantly increase oil extractability compared with the use of protease alone (EAEP 1), nor altered the distribution of the extracted oil among the fractions. However, the use of protease in all enzymatic treatments significantly increased oil extractability and oil yield in the cream when compared with the AEP.
Figure 2B shows the significant increase in protein extractability when enzymes were used to assist the extraction (EAEP) compared with the control (AEP). Enzymatic extraction significantly increased protein extractability from 62.81 ± 1.68% (AEP) to 83.49–86.13% (EAEP). However, extraction yields within the enzymatic strategies evaluated were very similar (83.49 ± 0.19% for EAEP 1, 84.04 ± 0.49% for EAEP 2, and 86.13 ± 1.51% for EAEP 3). The small increase in protein extractability observed for EAEP 3, compared with EAEP 1, could be attributed to the carbohydrase pretreatment applied before the addition of the protease, indicating the breakdown of the cell walls by the carbohydrases and the additional extraction time (30 min) helped with the additional release of proteins from the chickpea flour. Nonetheless, considering the additional use of 0.5% of enzyme and additional reaction time (30 min) when performing the carbohydrase pretreatment, the modest increment in protein extractability observed compared with the use of the protease alone (83.5 vs. 84.0–86.0%) does not justify the inclusion of the additional pretreatment. As expected, the higher protein extractability observed for EAEP treatments led to the production of skim fractions with higher yields (77.49–82.62%) compared with the AEP (62.14%). From the 83.5–86% protein extracted, 77–83% and 61% of the extracted protein was present in the EAEP and AEP skims, respectively. The higher extractability of the EAEP was reflected by the higher protein content of the EAEP skims (2.34–2.36%) compared with the one from the AEP skim (1.83%).
Importantly, the distribution of extracted proteins was influenced by the different modes of action of the enzymes used in the EAEP treatments. While the AEP produced a skim fraction with the lowest protein yield (62.14%), the use of cellulase + hemicellulase + xylanase before the addition of the protease (EAEP 3) led to higher protein yield in the skim (82.62%) for subsequent recovery, compared with the use of cellulase + hemicellulase before the use of protease (EAEP 2) (77.49%) or protease alone (EAEP 1) (77.61%). A similar trend was observed for the cream fraction, where EAEP 1 and 2 led to the production of a cream fraction with a higher protein yield (5.88 and 6.56%, respectively) compared with the AEP (0.67%).
To the best of our knowledge, there are no reports describing the effectiveness of aqueous and enzymatic extraction processes to simultaneously extract lipids and proteins from full-fat chickpea flour, which hinders the comparison of our data with the literature. Our findings are consistent with the ones presented for AEP and EAEP of other food matrices. De Moura et al. (2008) reported protein extraction yields of 85% when using Protex 6L to assist the extraction of extruded soybean flakes and Souza et al. (2019) reported an increase in protein extractability from 69.6% (AEP) to 75% when Protex 6L was used to assist the extraction from the almond cake.
The Effects of Extraction Conditions on the Degree of Hydrolysis and Low MW Polypeptide Profile of AEP and EAEP Skim Proteins
During proteolysis, the breakdown of peptide bonds results in an increased concentration of primary amines, corresponding to an increase in the degree of hydrolysis (DH). Because the DH often has a significant impact on the functional properties of the extracted proteins (Ghribi et al., 2015a), understanding the effects of extraction conditions on the DH and protein functionality becomes necessary to further identify possible industrial applications for the extracted proteins.
Enzymatic extraction significantly increased the DH from 10.0% (AEP) to 23.3, 25.0, and 25.5% for the EAEP 2, EAEP 1, and EAEP 3, respectively (Fig. 3). No significant difference was observed for the DH amongst the enzymatic treatments, in agreement with the use of the same amount of protease in all EAEP treatments. Our results are in agreement with the literature where the use of enzymes to assist the extraction leads to a higher DH (Ghribi et al., 2015b).
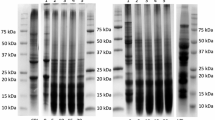
The protein profile of chickpea skim proteins is shown in Fig. 3. AEP skim proteins (unhydrolyzed proteins) presented a band at ~66 kDa that could be attributed to convicilin, a protein with molecular weight between 68 and 70 kDa (Tzitzikas et al., 2006), corresponding to 18.1% of the protein in the lane. Another intense band can be seen at 45−47 kDa, which might correspond to the vicilin protein, which has three different polypeptide subunits with molecular weights of 53, 47, and 43 kDa (Romero et al., 1975). The major bands observed at ~40 and 20 kDa can be attributed to the acidic (\(\alpha\)) subunit of legumins and the basic (\(\beta\)) subunit of legumins, respectively (Boulter & Croy, 1997). Moreover, the bands at 37 kDa and 27 kDa could indicate the presence of lectins (Sathe, 2002). The legumin alpha-subunit and the lectins correspond to 12.3% of the protein in the lane. Our results agree with the ones reported by Chang et al. (2012), which reported globulin protein 11S legumins and 7S vicilins as the major protein fractions and 2S albumin as the minor protein fraction in chickpea flour.
The use of enzymes to assist the extraction (EAEP 1–3) promoted the complete hydrolysis of proteins with MW > 21 kDa, indicating total hydrolysis of convicilin, vicilin, and legumin α-subunit, and partial hydrolysis of the legumin β-subunit, in congruence with the significantly higher degree of hydrolysis of those samples. Moreover, a significant increase in the relative abundance of protein hydrolysis products and peptides with MW < 14 kDa can be observed for most EAEP samples. Ghribi et al. (2015a) showed a significant decrease in the ~45–66 kDa and ~34–45 kDa molecular weight bands due to increasing enzymatic hydrolysis of chickpea proteins.
The Effects of Extraction Conditions on Protein Solubility
Solubility is an important functional property of proteins because of its impact on food applications. Soluble proteins can be integrated into food products whose pH can vary widely, while insoluble proteins may be limited in their application and therefore its desirability. Chickpea protein peptides are needed to be functional, and specifically soluble, to enhance their applications in the food industry. Boye et al. (2010) reported that unhydrolyzed extracted chickpea proteins had higher water and oil absorption capacities, and emulsifying capabilities than other pulse proteins while having similar solubility and gelation capabilities. As the result of proteolysis, smaller peptides are released which can be significantly more soluble than larger protein bodies (Carbonaro et al., 1997).
Because enzymatic hydrolysis can significantly affect protein functionality, we evaluated the impact of the AEP and EAEP on the solubility of extracted proteins at pH 4.0 (which is close to the isoelectric point of chickpea proteins (4.3, (Sánchez-Vioque et al., 1999) and pH 9.0 (Fig. 4A, B).
At pH 4, where chickpea protein solubility is unfavored by the proximity to its isoelectric point (pI), enzymatic extraction significantly improved protein solubility (25.6% AEP vs. 68.2–73.6% EAEP) (Fig. 4A). However, no statistically significant differences were observed amongst the enzymatic treatments. These results demonstrate that the use of enzymes during the extraction can indeed generate smaller and more soluble peptides, in agreement with previous studies reporting the beneficial effects of proteolysis on the solubility of almond proteins (Almeida et al., 2019; Souza et al., 2019). Increased solubility of EAEP skim proteins at pH 4.0, compared with AEP skim proteins at the same pH, agrees with the higher DH of EAEP skim proteins. However, at pH 9.0, AEP and EAEP skim proteins exhibited similar high solubility, with values ranging from 85 to 88% (Fig. 4B). Higher solubility of AEP and EAEP skim proteins at pH 9.0 is attributed to a higher negative net charge of the proteins, which enhances electrostatic repulsion between protein molecules thus favoring its solubility. Conversely, at the isoelectric point, the net-zero charge of the proteins enhances the attractive forces within the protein molecules, which in turn reduces their solubility in the aqueous medium (Zayas, 1997). It is not surprising that chickpea protein solubility at acidic pH, which is near the isoelectric point, is lower than that at alkaline pH. In that view, all enzymatic treatments significantly increased protein solubility at acidic pH. Such increase in solubility has been attributed to an enhanced net charge of the hydrolysates, which can heighten molecular electrostatic repulsion, thus favoring the unfolding of proteins and increasing protein-water interactions (Ghribi et al., 2015b). Increased protein solubility at acidic pH is of particular importance as it can open up potential uses of the hydrolysates in specific industrial food formulations involving acidic pH (e.g., protein-rich beverages, protein supplements).
It is important to highlight the potential impact of enzymatic extraction on the functional properties (e.g., foaming, gelling, and emulsification properties) of the extracted protein. For instance, enzymatic extraction of almond cake proteins resulted in hydrolyzates with reduced foaming and emulsification properties, suggesting that extensive hydrolysis (DH > 10%) can reduce some functional properties (Souza et al., 2020). Similar results were reported for chickpea protein hydrolysates, with higher DH resulting in hydrolyzates with reduced emulsification properties (Ghribi et al., 2015b). However, moderate hydrolysis during the enzymatic extraction of almond flour (DH ~7) resulted in the production of hydrolyzates with higher emulsifying properties and foaming capacity at pH values close to the protein isoelectric point (Dias & de Moura Bell, 2022). Therefore, a holistic evaluation of the impact of extraction conditions on the protein structure and functionality, which depends on the matrix characteristics and upstream unit operations employed, is necessary to identify potential applications of the extracted protein and re-evaluate the selection of the extraction conditions.
The Effects of Extraction Conditions on Carbohydrate Content, Profile, and a-Galactosidase Treatment of AEP and EAEP Skim Fractions
Quantification of Oligosaccharides and Free Monosaccharides Sugars by Spectrophotometry and High-Performance Anion-Exchange Chromatography
In addition to being a source of raffinose, stachyose, and verbascose, chickpeas are a source of dietary fiber (18–22 g per 100 g of flour), from which 10–18 g is comprised of insoluble fiber and 4–8 g is comprised of soluble flour (Tosh & Yada, 2010). The use of carbohydrase pretreatments, before proteolysis, was evaluated as a strategy to improve the bio-functionality of the protein extracts through the release of potentially prebiotic oligosaccharides into the skim fractions via the breakdown of the cell wall polysaccharides. The effects of the extraction methods used on the total carbohydrate content of the skim fractions and oligosaccharides which are known to exist in chickpeas and for which high-purity standards exist were evaluated by two assays (Table 1).
Indeed, the enzymatic treatments significantly increased the total carbohydrate content of the skim fractions from 7.68 mg/mL (AEP) to 8.37−9.33 mg/mL (EAEP1–3) (Table 1). The higher carbohydrate content of EAEP 2 and 3 skim fractions can be attributed to the use of cellulase and hemicellulase in the EAEP 2 and cellulase, hemicellulase, and xylanase in EAEP 3, which likely promoted the breakdown of the cell wall cellulose and hemicellulose (Reese et al., 1950) into smaller carbohydrate structures. While the EAEP increased the overall extractability of chickpea carbohydrates, the amount of sucrose (4.28–4.70 mg/mL) and major oligosaccharides raffinose (0.83–0.97 mg/mL) and stachyose (2.53–2.80 mg/mL) in the skim fractions was not statistically different within the extraction processes evaluated (AEP vs. EAEP 1–3). This is not surprising because the enzymes used in the EAEP 1–3, including alkaline protease, cellulase, hemicellulase, and xylanase, do not target glycosidic linkages in sucrose, stachyose, and raffinose to cause their degradation. Sucrose, stachyose, and raffinose were also not expected to be generated, under the action of the carbohydrases, since they are not part of cell wall polysaccharides’ structures. Besides, possibly due to the small size of sucrose, stachyose, and raffinose, their extractability was already high in the AEP and did not further increase when the alkaline protease and carbohydrases were used (EAEP 1–3). Because the increment in the total carbohydrate content in the EAEP skims was not associated with the release of free monosaccharides (all in trace concentration) nor with an increase in sucrose and major oligosaccharides such as raffinose or stachyose, LC–MS/MS was used to evaluate the potential release of oligosaccharide by the enzymatic treatments and characterize the composition of the newly generated oligosaccharides.
α-Galactosidase Treatment of Raffinose and Stachyose in the Skim Fractions
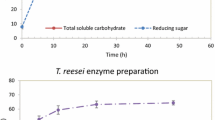
Chickpeas are rich in raffinose, stachyose, and verbascose, whose simplicity of monosaccharide composition (galactose, glucose, and fructose) render them easily fermentable by a variety of intestinal bacteria in a non-selective way that results in the production of undesirable gases that can cause abdominal bloating and discomfort (Sánchez-Mata et al., 1998). To reduce the concentration of flatulence-causing oligosaccharides stachyose and raffinose in the skims, an α-galactosidase was applied to hydrolyze the glycosidic bonds within raffinose and stachyose (Fig. 5).
From Fig. 5, we can observe that the α-galactosidase treatment completely hydrolyzed raffinose and stachyose in just 15 min, which was corroborated by the concurrent increase in the concentration of galactose released from the cleavage of the α-glycosidic bonds. Although sucrose concentration should increase with the α-galactosidase treatment, the observed decrease in sucrose reflects the hydrolysis of the glycosidic bonds between glucose and fructose by the α-galactosidase (De Moura et al., 2008), indicating that the enzyme preparation also possesses invertase activity. This can be observed by the simultaneous reduction in the sucrose concentration and increase in the glucose and fructose concentration. Our results are in agreement with the ones reported by De Moura Bell et al. (2013), who reported the complete reduction of stachyose in the protein extracts generated from AEP/EAEP of soybeans by the α-galactosidase treatment. Our results demonstrate that the α-galactosidase treatment can certainly be an effective and fast treatment to reduce the presence of flatulence-causing oligosaccharides in the chickpea extracts and could therefore be introduced during the extraction process if adequate pH values are selected to favor the activity of the enzymes used.
Characterization Of Oligosaccharides in the Skim Fractions with LC–MS/MS
A total of 60 oligosaccharides were identified in the AEP and EAEP (1−3) skim fractions by inspecting the fragmentation patterns tandem MS spectra (Figs. 6 and 7). With the masses of the precursor ions and fragment ions, the monosaccharide compositions of the oligosaccharides were determined. Among the 60 oligosaccharides, 46 contained only hexoses with a degree of polymerization range of 3 to 16 (Fig. 6A–E). Stachyose, raffinose, and verbascose peaks, which were identified by comparing the retention times with the authentic standards, were the first three tallest peaks among the 46 hexose oligosaccharides (Fig. 6A). As some oligosaccharides could originate from the enzyme formulations used in the EAEP treatments, the presence of oligosaccharides in the four enzymes used during the extraction was also examined. The results showed that no oligosaccharides were found in the alkaline protease, whereas 31 hexose oligosaccharides found in the skim fractions were also present in at least one of the three carbohydrases used in EAEP 2 and 3 (Fig. 6C–E). It could therefore be confirmed that the remaining 12 oligosaccharides composed of 3–5 hexose residues and present in similar abundances in the AEP and the three EAEP skim fractions (Fig. 6B–D) are endogenous oligosaccharides in chickpeas.
Ciceritol is a digalactosyl-pinitol present in the skim fractions in a high abundance (with peak areas close to stachyose; Fig. 6F) firstly identified in chickpea (Quemener & Brillouet, 1983). Besides ciceritol, seven oligosaccharides with relatively lower abundances possess similar structures to ciceritol (Fig. 6F). Among them, four are ciceritol isomers containing two hexose residues and one methyl-inositol (Hex2 + methyl-inositol); two are composed of three hexose residues and one methyl-inositol; one is composed of four hexose residues and one methyl-inositol. In addition to ciceritol, digalactosyl-pinitol B and tri-galactosyl-pinitol A were identified in chickpeas in previous studies (Ruiz-Aceituno et al., 2013, 2017). Although it was not possible to fully elucidate the exact structures (i.e., types of hexoses, methyl-inositols, and glycosidic linkages) of the other hexosyl-methyl-inositol derivatives, this work represents the first report of their existence in chickpeas, to the best of our knowledge.
Of interest, six oligosaccharides were exclusively found in the skim fractions generated by EAEP 2 and 3 (Fig. 7). To ensure that the oligosaccharides aforementioned were generated from polysaccharide depolymerization under the action of the carbohydrase enzymes used during the extraction, we analyzed all the enzyme preparations used in the extraction. No oligosaccharides were identified in the enzyme preparations, confirming the de-novo origin of the said structures originated from the process. The monosaccharide composition of the six oligosaccharides, with signal intensities from high to low, were as follows: Hex4Pent3, Hex3dHex3Pent3HexA1, Hex2Pent2, Pent7HexA1, Hex5Pent3, and Hex2dHex3Pent3Hex1. Because these oligosaccharides all contain multiple pentose units and other non-hexose monosaccharide units, they were not derived from the depolymerization of cellulose, which only consists of linear chains of β-1,4-linked glucan. Based on the monosaccharide compositions, it is likely that the oligosaccharides were generated from hemicellulose (e.g., xyloglucans) and pectin (e.g., from its component rhamnogalacturonan) partial hydrolysis (Tosh & Yada, 2010; Wood et al., 2014; Yoo et al., 2012). Due to the lack of digestive enzymes in the human gastrointestinal tract able to break down glycosidic linkages of plant cell wall polysaccharides, the newly generated oligosaccharides with diverse monosaccharide compositions in the skims of EAEP 2 and 3 could be novel prebiotics.
It was initially expected that more oligosaccharides could be generated through hydrolyzing polysaccharides by the carbohydrases used in EAEP 2 and 3, but in reality, only six oligosaccharides were found in the two skim samples in relatively low abundances. One plausible explanation for this result might be related to the polysaccharides’ tangled structure and steric hindrance, which would in turn reduce enzyme accessibility. According to Brummer et al. (2015), chickpea soluble fiber polysaccharides have a number average molecular weight (Mn) of 419 kDa and a weight average molecular weight (Mw) of 2,103 kDa. The massive size of the soluble polysaccharides may create steric hindrance issues for the endo-cleaving enzymes decreasing their accessibility and performance. Additionally, it is worth considering that some of the products generated by the enzymatic depolymerization of the polysaccharides might be larger than the size of oligosaccharides (3–20 monosaccharide units) and therefore not measured by LC–MS/MS. The reduction in molecular weight or the increase in solubility of cell wall polysaccharides could lead to a higher total carbohydrate content in the skims of EAEP 2 and 3 than EAEP 1, as the total carbohydrate quantification reported in Table 1. Moreover, the insoluble polysaccharides, which are more abundant than the soluble ones in chickpeas (Tosh & Yada, 2010), might have even larger molecular sizes which are not possible to measure with the current analytical tools. Thus, a more intense enzymatic treatment might be needed to further hydrolyze those larger molecules into oligosaccharides. To further increase the concentration and diversity of novel oligosaccharides from chickpea polysaccharides, additional enzyme screening and process optimization would be needed.
The Effects of Enzymatic Extraction on In vitro Digestibility of Skim Proteins
Enzymatic extraction can significantly alter the in vitro digestibility of the extracted protein. In addition to being soluble, increased digestibility of chickpea proteins is another important functional property as it can promote nutritional benefits through higher intestinal absorption. The larger proteins observed in the AEP skim (“The Effects of Extraction Conditions on the Degree of Hydrolysis and Low MW Polypeptide Profile of AEP and EAEP Skim Proteins” section) can hinder digestibility, while the hydrolysis of these larger structures could improve its overall breakdown during human digestion (Sánchez-Vioque et al., 1999).
The in vitro digestibility of skim proteins from the non-enzymatic and the three enzymatic treatments are shown in Fig. 8. The digestibility of all EAEP skim proteins was significantly higher than that of the AEP skim (unhydrolyzed), highlighting the effectiveness of the use of enzyme during the extraction to enhance protein digestibility. Enzymatic hydrolysis significantly increased protein digestibility from 83.81% ± 1.86 (AEP), to 94.67% ± 8.70, 94.67% ± 6.47, and 90.79% ± 7.21 for the EAEP 1, 2, and 3, respectively, with no significant differences within the enzymatic treatments. As expected, the addition of carbohydrases did not significantly alter the in vitro digestibility of the EAEP skim proteins. Increased protein digestibility of EAEP skim proteins can be attributed to the breakdown of large proteins into smaller sizes by the protease (He et al., 2015; de Souza et al., 2020), which corroborates with the DH and molecular weight results (“The Effects of Extraction Conditions on Protein Solubility” section). Clemente et al. (1998) reported that the digestibility of raw chickpeas increased from 71.8 ± 1.0 to 83.5 ± 0.1% after cooking, similar to the findings of Attia et al. (1994). Cooking chickpeas lead to protein denaturation and unfurling of the protein bodies, which improves the access to proteolysis by the saliva, gastric, and intestinal fluids. The digestibility of the AEP skim is similar to that of cooked chickpeas found by Clemente et al. (1998), which could be attributed to the steaming of the chickpeas before milling. Goertzen et al. (2020) reported digestibility values of 73.71 and 82.22% for untreated chickpea flour and isolate, respectively. However, no improvements in protein digestibility were observed when pepsin, trypsin, or papain were used to hydrolyze the chickpea protein isolate. It is worth mentioning that in their study, enzymes were used to hydrolyze the chickpea protein isolate while in our work, and enzymes were used to extract proteins from the chickpea flour, which lead to a significant increase in protein digestibility (from 83.8 to 94.6%). Our results highlight that the use of selected enzymes to assist the extraction of full-fat chickpea flour is an effective strategy not only to improve protein extractability but to significantly enhance protein in vitro digestibility and solubility.
Conclusions
The extraction methods proposed in this work improved the overall protein extractability from full-fat chickpea flour and significantly enhanced the nutritional quality and functionality of the extracted proteins, without the need of performing upstream lipid removal by solvent extraction or mechanical pressing. The use of alkaline protease in the extraction increased oil (49.8 to 77.2%) and protein extractability (62.8 to 84.0%) from chickpea flour while releasing smaller and more soluble proteins. Proteolysis resulted in increased protein solubility at acidic pH (73%), where chickpea protein solubility is unfavored. Importantly, it did increase in vitro protein digestibility to 94.6%, which can therefore enhance the nutritional value of the extracted protein. While the use of carbohydrase pretreatments did not increase oil and protein extractability, it did release new oligosaccharides as revealed by LC–MS/MS, warranting future investigation of the potential prebiotic properties of the novel oligosaccharides. The α-galactosidase post-extraction treatment eliminated the presence of flatulence-causing oligosaccharides in the extracts. These results demonstrate that enzymatic modifications can be exploited to provide the food industry with plant-based proteins that are highly functional, applicable, and produced by an environmentally friendly process. The impact of proteolysis on the sensory properties of food products containing chickpea hydrolyzates would merit further investigation.
Data Availability
Data can be made available upon reasonable request.
References
Attia, R. S., Shehata, A. M. E. T., Aman, M. E., & Hamza, M. A. (1994). Effect of cooking and decortication on the physical properties, the chemical composition and the nutritive value of chickpea (Cicer arietinum L.). Food Chemistry, 50(2), 125–131. https://doi.org/10.1016/0308-8146(94)90108-2
Bornhorst, G. M., & Singh, R. P. (2013). Kinetics of in vitro bread bolus digestion with varying oral and gastric digestion parameters. Food Biophysics, 8(1), 50–59. https://doi.org/10.1007/s11483-013-9283-6
Boulter, D., & Croy, R. R. D. (1997). The Structure and biosynthesis of legume seed storage proteins: A biological solution to the storage of nitrogen in seeds. Advances in Botanical Research, 27, 1–92. https://doi.org/10.1016/S0065-2296(08)60280-3
Boye, J. I., Aksay, S., Roufik, S., Ribéreau, S., Mondor, M., Farnworth, E., & Rajamohamed, S. H. (2010). Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International, 43(2), 537–546. https://doi.org/10.1016/j.foodres.2009.07.021
Brummer, Y., Kaviani, M., & Tosh, S. M. (2015). Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Research International, 67, 117–125. https://doi.org/10.1016/j.foodres.2014.11.009
Campbell, K. A., & Glatz, C. E. (2009). Mechanisms of aqueous extraction of soybean oil. Journal of Agricultural and Food Chemistry, 57(22), 10904–10912. https://doi.org/10.1021/jf902298a
Campbell, K. A., Glatz, C. E., Johnson, L. A., Jung, S., De Moura, J. M. N., Kapchie, V., & Murphy, P. (2011). Advances in aqueous extraction processing of soybeans. JAOCS, Journal of the American Oil Chemists’ Society, 88(4), 449–465. https://doi.org/10.1007/s11746-010-1724-5
Carbonaro, M., Cappelloni, M., Nicoli, S., Lucarini, M., & Carnovale, E. (1997). Solubility-digestibility relationship of legume proteins. Journal of Agricultural and Food Chemistry, 45(9), 3387–3394.
Chang, Y. W., Alli, I., Molina, A. T., Konishi, Y., & Boye, J. I. (2012). Isolation and characterization of chickpea (Cicer arietinum L.) seed protein fractions. Food and Bioprocess Technology, 5(2), 618–625. https://doi.org/10.1007/s11947-009-0303-y
Cheng, M. H., Sekhon, J. J. K., Rosentrater, K. A., Wang, T., Jung, S., & Johnson, L. A. (2018). Environmental impact assessment of soybean oil production: Extruding-expelling process, hexane extraction and aqueous extraction. Food and Bioproducts Processing, 108, 58–68. https://doi.org/10.1016/j.fbp.2018.01.001
Clemente, A., Sánchez-Vioque, R., Vioque, J., Bautista, J., & Millán, F. (1998). Effect of cooking on protein quality of chickpea (Cicer arietinum) seeds. Food Chemistry, 62(1), 1–6. https://doi.org/10.1016/S0308-8146(97)00180-5
de Almeida, N. M., Dias, F. F. G., Rodrigues, M. I., & de Moura Bell, J. M. L. N. (2019). Effects of processing conditions on the simultaneous extraction and distribution of oil and protein from almond flour. Processes, 7(11), 844. https://doi.org/10.3390/PR7110844
De Moura Bell, J., Maurer, D., Yao, L., Wang, T., Jung, S., & Johnson, L. A. (2013). Characteristics of oil and skim in enzyme-assisted aqueous extraction of soybeans. JAOCS, Journal of the American Oil Chemists’ Society, 90(7), 1079–1088. https://doi.org/10.1007/s11746-013-2248-6
De Moura, J., & Johnson, L. A. (2009). Two-stage countercurrent enzyme-assisted aqueous extraction processing of oil and protein from soybeans. JAOCS, Journal of the American Oil Chemists’ Society, 86(3), 283–289. https://doi.org/10.1007/s11746-008-1341-8
de Moura, J., Nobrega, M. L., de Almeida, N. M., & Johnson, L. A. (2009). Scale-up of enzyme-assisted aqueous extraction processing of soybeans. Journal of the American Oil Chemists’ Society, 86(8), 809–815. https://doi.org/10.1007/s11746-009-1406-3
De Moura, J. M. L. N., Campbell, K., De Almeida, N. M., Glatz, C. E., & Johnson, L. A. (2011a). Protein recovery in aqueous extraction processing of soybeans using isoelectric precipitation and nanofiltration. JAOCS, Journal of the American Oil Chemists’ Society, 88(9), 1447–1454. https://doi.org/10.1007/s11746-011-1803-2
De Moura, J. M. L. N., Campbell, K., Mahfuz, A., Jung, S., Glatz, C. E., & Johnson, L. (2008). Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification. JAOCS, Journal of the American Oil Chemists’ Society, 85(10), 985–995. https://doi.org/10.1007/s11746-008-1282-2
De Moura, J. M. L. N., Maurer, D., Jung, S., & Johnson, L. A. (2011b). Pilot-plant proof-of-concept for integrated, countercurrent, two-stage, enzyme-assisted aqueous extraction of soybeans. JAOCS, Journal of the American Oil Chemists’ Society, 88(10), 1649–1658. https://doi.org/10.1007/s11746-011-1831-y
de Souza, T. S. P., Dias, F. F. G., Oliveira, J. P. S., de Moura Bell, J. M. L. N., & Koblitz, M. G. B. (2020). Biological properties of almond proteins produced by aqueous and enzyme-assisted aqueous extraction processes from almond cake. Scientific Reports, 10(1), 1–12. https://doi.org/10.1038/s41598-020-67682-3
Dias, F. F. G., Almeida, N. M., Souza, T. S. P., Taha, A. Y., & Bell, J. M. L. N. M. (2020). Characterization and demulsification of the oil-rich emulsion from the aqueous extraction process of almond flour. Processes, 8(10), 1228. https://doi.org/10.3390/pr8101228
Dias, F. F. G., & de Moura Bell, J. M. L. N. (2022). Understanding the impact of enzyme-assisted aqueous extraction on the structural, physicochemical, and functional properties of protein extracts from full-fat almond flour. Food Hydrocolloids, 127, 107534. https://doi.org/10.1016/J.FOODHYD.2022.107534
Duranti, M., & Gius, C. (1997). Legume seeds: Protein content and nutritional value. Field Crops Research, 53(1–3), 31–45. https://doi.org/10.1016/S0378-4290(97)00021-X
Ghribi, A. M., Maklouf Gafsi, I., Sila, A., Blecker, C., Danthine, S., Attia, H., et al. (2015a). Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chemistry, 187, 322–330. https://doi.org/10.1016/j.foodchem.2015.04.109
Ghribi, A. M., Sila, A., Przybylski, R., Nedjar-Arroume, N., Makhlouf, I., Blecker, C., et al. (2015b). Purification and identification of novel antioxidant peptides from enzymatic hydrolysate of chickpea (Cicer arietinum L.) protein concentrate. Journal of Functional Foods, 12, 516–525. https://doi.org/10.1016/j.jff.2014.12.011
Goertzen, A. D., House, J. D., Nickerson, M. T., & Tanaka, T. (2020). The impact of enzymatic hydrolysis using three enzymes on the nutritional properties of a chickpea protein isolate. Cereal Chemistry, 98(2), 275–284. https://doi.org/10.1002/cche.10361
González, A. D., Frostell, B., & Carlsson-Kanyama, A. (2011). Protein efficiency per unit energy and per unit greenhouse gas emissions: Potential contribution of diet choices to climate change mitigation. Food Policy, 36(5), 562–570. https://doi.org/10.1016/j.foodpol.2011.07.003
He, S., Simpson, B. K., Ngadi, M. O., & Ma, Y. (2015). In vitro studies of the digestibility of lectin from black turtle bean (Phaseolus vulgaris). Food Chemistry, 173, 397–404. https://doi.org/10.1016/j.foodchem.2014.10.045
Huang, Y., Robinson, R. C., Dias, F. F. G., Bell, J. M. L. N. D. M., & Barile, D. (2022a). Solid-Phase extraction approaches for improving oligosaccharide and small peptide identification with liquid chromatography-high-resolution mass spectrometry: A Case study on proteolyzed almond extract. Foods, 11(3), 340. https://doi.org/10.3390/FOODS11030340
Huang, Y. P., Robinson, R. C., & Barile, D. (2022b). Food glycomics: Dealing with unexpected degradation of oligosaccharides during sample preparation and analysis. Journal of Food and Drug Analysis, 30(1), 62–76. https://doi.org/10.38212/2224-6614.3393
Kim, T. K., Yong, H. I., Kim, Y. B., Jung, S., Kim, H. W., & Choi, Y. S. (2021). Effects of organic solvent on functional properties of defatted proteins extracted from Protaetia brevitarsis larvae. Food Chemistry, 336, 127679. https://doi.org/10.1016/j.foodchem.2020.127679
Kou, X., Gao, J., Zhang, Z., Wang, H., & Wang, X. (2013). Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT - Food Science and Technology, 50(2), 591–598. https://doi.org/10.1016/j.lwt.2012.08.002
L’hocine, L., Boye, J. I., & Arcand, Y. (2006). Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. Journal of Food Science, 71(3), C137–C145. https://doi.org/10.1111/j.1365-2621.2006.tb15609.x
Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimura, S. I., & Lee, Y. C. (2005). Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Analytical Biochemistry, 339(1), 69–72. https://doi.org/10.1016/j.ab.2004.12.001
Nadar, S. S., Pawar, R. G., & Rathod, V. K. (2017). Recent advances in enzyme extraction strategies: A comprehensive review. International Journal of Biological Macromolecules, 101, 931–957. https://doi.org/10.1016/J.IJBIOMAC.2017.03.055
Nielsen, P. M. (2001). Improved method for determining protein hydrolysis. Journal of Food Science, 66(5), 642–646.
Quemener, B., & Brillouet, J. M. (1983). Ciceritol, a pinitol digalactoside form seeds of chickpea, lentil and white lupin. Phytochemistry, 22(8), 1745–1751. https://doi.org/10.1016/S0031-9422(00)80263-0
Reese, E. T., Siu, R. G., & Levinson, H. S. (1950). The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. Journal of Bacteriology, 59(4), 485–497. https://doi.org/10.1128/jb.59.4.485-497.1950
Romero, J., Sun, S. -M. M., Mcleester, R. C., Bliss, F. A., & Hall, T. C. (1975). Heritable variation in a polypeptide subunit of the major storage protein of the bean, Phaseolus vulgaris L. Plant Physiology, 56(6), 776–779.
Ruiz-Aceituno, L., Carrero-Carralero, C., Ruiz-Matute, A. I., Ramos, L., Sanz, M. L., & Martínez-Castro, I. (2017). Characterization of cyclitol glycosides by gas chromatography coupled to mass spectrometry. Journal of Chromatography A, 1484, 58–64. https://doi.org/10.1016/j.chroma.2017.01.001
Ruiz-Aceituno, L., Rodríguez-Sánchez, S., Ruiz-Matute, A. I., Ramos, L., Soria, A. C., & Sanz, M. L. (2013). Optimisation of a biotechnological procedure for selective fractionation of bioactive inositols in edible legume extracts. Journal of the Science of Food and Agriculture, 93(11), 2797–2803. https://doi.org/10.1002/jsfa.6103
Sánchez-Mata, M. C., Peñuela-Teruel, M. J., Cámara-Hurtado, M., Díez-Marqués, C., & Torija-Isasa, M. E. (1998). Determination of mono-, di-, and oligosaccharides in legumes by high-performance liquid chromatography using an amino-bonded silica column. Journal of Agricultural and Food Chemistry, 46(9), 3648–3652. https://doi.org/10.1021/jf980127w
Sánchez-Vioque, R., Clemente, A., Vioque, J., Bautista, J., & Millán, F. (1999). Protein isolates from chickpea (Cicer arietinum L.): Chemical composition, functional properties and protein characterization. Food Chemistry, 64(2), 237–243. https://doi.org/10.1016/S0308-8146(98)00133-2
Sathe, S. K. (2002). Dry bean protein functionality. Critical Reviews in Biotechnology, 22(2), 175–223. https://doi.org/10.1080/07388550290789487
Shaabani, S., Yarmand, M. S., Kiani, H., & Emam-Djomeh, Z. (2018). The effect of chickpea protein isolate in combination with transglutaminase and xanthan on the physical and rheological characteristics of gluten free muffins and batter based on millet flour. LWT - Food Science and Technology, 90, 362–372. https://doi.org/10.1016/j.lwt.2017.12.023
Shevkani, K., Singh, N., Chen, Y., Kaur, A., & Yu, L. (2019). Pulse proteins: Secondary structure, functionality and applications. Journal of Food Science and Technology, 56(6), 2787–2798. https://doi.org/10.1007/s13197-019-03723-8
Souza, T. S. P., Dias, F. F. G., Koblitz, M. G. B., & Bell, J. M. L. N. M. (2019). Aqueous and Enzymatic extraction of oil and protein from almond cake: A comparative study. Processes, 7(7), 472. https://doi.org/10.3390/pr7070472
Tosh, S. M., & Yada, S. (2010). Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Research International. https://doi.org/10.1016/j.foodres.2009.09.005
Tzitzikas, E. N., Vincken, J. P., De Groot, J., Gruppen, H., & Visser, R. G. F. (2006). Genetic variation in pea seed globulin composition. Journal of Agricultural and Food Chemistry, 54(2), 425–433. https://doi.org/10.1021/jf0519008
U.S. Department of Agriculture, A. R. S. (2019). FoodData Central. FoodData Central.
Wallace, T. C., Murray, R., & Zelman, K. M. (2016). The nutritional value and health benefits of chickpeas and hummus. Nutrients, 8(12), 766. https://doi.org/10.3390/nu8120766
WHO. (2003). Diet, nutrition and the prevention of chronic diseases (Vol. 916). World Health Organization Technical report series.
Wood, J. A., Knights, E. J., Campbell, G. M., & Choct, M. (2014). Differences between easy- and difficult-to-mill chickpea (Cicer arietinum L.) genotypes. Part I: Broad chemical composition. Journal of the Science of Food and Agriculture, 94(7), 1437–1445. https://doi.org/10.1002/jsfa.6437
Yoo, H. D., Kim, D., Paek, S. H., & Oh, S. E. (2012). Plant cell wall polysaccharides as potential resources for the development of novel prebiotics. Biomolecules and Therapeutics, 20(4), 371. https://doi.org/10.4062/biomolther.2012.20.4.371
Yust, M., Pedroche, J., del Carmen Millán-Linares, M., Alcaide-Hidalgo, J. M., & Millán, F. (2010). Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chemistry, 122(4), 1212–1217. https://doi.org/10.1016/j.foodchem.2010.03.121
Zayas, J. F. (1997). Chapter 1: Solubility of proteins. Functionality of Proteins in Foods (pp. 6–75). Berlin, Heidelberg: Springer. https://doi.org/10.1007/978-3-642-59116-7
Zhong, C., Wang, R., Zhou, Z., Jia, S. R., Tan, Z. L., & Han, P. P. (2012). Functional properties of protein isolates from caragana korshinskii Kom. Extracted by three different methods. Journal of Agricultural and Food Chemistry, 60(41), 10337–10342. https://doi.org/10.1021/jf303442u
Funding
This work was supported by the USDA Agriculture Research Service, Pulse Crop Health Initiative (PCHI 2019), Agreement number 58–3060-9–046; and USDA National Institute of Food and Agriculture, Hatch/Multi-State project [1023517]. Graphical abstract was generated using https://BioRender.com (#9DCDF04A-0002).
Author information
Authors and Affiliations
Contributions
Kazunori Machida: methodology, data curation, formal analysis, writing–review & editing. Fernanda Furlan Goncalves Dias: methodology, data curation, writing–review & editing. Yu-Ping Huang: methodology, formal analysis, data curation; writing–review & editing. Daniela Barile: conceptualization, data curation, supervision, funding, writing–review & editing. Juliana Maria Leite Nobrega de Moura Bell: conceptualization, data curation, supervision, funding, writing–review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Machida, K., Huang, YP., Furlan Gonçalves Dias, F. et al. Leveraging Bioprocessing Strategies to Achieve the Simultaneous Extraction of Full-Fat Chickpea Flour Macronutrients and Enhance Protein and Carbohydrate Functionality. Food Bioprocess Technol 15, 1760–1777 (2022). https://doi.org/10.1007/s11947-022-02847-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02847-8