Abstract
Isoelectric precipitation and whey nanofiltration were evaluated in recovering protein from skim fractions produced by enzyme-assisted aqueous extraction processing (EAEP) of extruded full-fat soybean flakes. Countercurrent two-stage EAEP was performed at 1:6 solids-to-liquid ratio, 50 °C, pH 9.0, and 120 rpm for 1 h to extract oil and protein from soybeans. Two protein recovery strategies were applied to skim fractions produced by different extraction treatments: Treatment 1 using 0.5% protease (wt/g extruded flakes) in both extraction stages; Treatment 2 using 0.5% protease only in the 2nd extraction stage; and Treatment 3 using no enzyme in either extraction stage. Protein recovery by using isoelectric precipitation was inversely related to the extent of hydrolysis with recoveries of 27, 61, and 87% of skim proteins from Treatments 1, 2, and 3, respectively. Overall protein recoveries of 26, 54, and 57% of the original protein in the extruded full-fat flakes were achieved when combining extraction treatments and isoelectric precipitation. Nanofiltering isoelectric wheys (500-Da membrane) achieved protein retentate yields of 96.3, 94.5, and 91.8% (1.9–2.8 concentration factor) with permeate fluxes up to 1.35 kg/h m2. About 97, 98, and 99% of skim protein were recovered by isoelectric precipitation and whey nanofiltration for Treatments 1, 2, and 3, respectively. Overall protein recoveries of 93, 87, and 65% of the protein in the extruded flakes were achieved for Treatments 1, 2, and 3, respectively. Although high protein retentions were achieved, very low permeate fluxes were observed for whey nanofiltration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High nutritional quality and favorable cost of production have spurred interest in soybean protein utilization; however, the dominant use of soybean protein remains livestock feed [1]. For food use, most soybean protein forms are sold in bulk as ingredients for manufacturing fabricated food with sensory properties that consumers desire [2].
Soy proteins are mainly recovered as soy protein concentrates (SPC) or soy protein isolates (SPI). Both SPC and SPI are prepared from defatted flakes with the aim of removing the carbohydrate fraction and fiber in the case of SPI. SPCs are mainly prepared by aqueous alcohol extraction or acid leaching (pH 4.5), while SPIs are prepared by mild alkali extraction (pH 7–10) of proteins and sugar followed by isoelectric precipitation (IEP) of proteins at pH 4.5 [3]. Protein contents on a moisture-free basis are at least 65 and 90% for SPC and SPI, respectively [4].
Enzyme-assisted aqueous extraction processing (EAEP) was developed to achieve simultaneous oil and protein extraction from soybeans [5–11]. In addition to being an environmentally friendly technology, EAEP achieves high levels of fractionation of soybeans into co-products having wide applications, making EAEP an attractive alternative to replace hexane to extract oil from soybeans.
We previously demonstrated mechanical and enzymatic treatments in a countercurrent two-stage extraction process to improve oil and protein extraction yields from the typical value of ~60% to 99 and 96%, respectively, and to reduce water usage by 40%, while producing protein products with different extents of hydrolysis and functional properties [8–11]. We recently evaluated alternative modes of protease addition in countercurrent two-stage EAEP. The use of protease in both extraction stages, only in the second stage, and without enzyme in either stage yielded 96, 89, and 66% protein extraction, respectively [12]. Different extents of proteolysis were associated with changes in protein functionality [13]. Higher extents of hydrolysis favored protein solubility, rate of foaming, and foam stability but reduced emulsification properties. Essential amino acid compositions and in vitro protein digestibilities were not adversely affected by either extrusion or extraction treatments. Although significant reduction in water use was achieved by adopting countercurrent two-stage extraction [9], the skim fraction remained dilute, thus protein recovery constitutes a significant challenge in EAEP of soybeans.
The use of ultrafiltration (UF) alone, as well as in combination with electroacidification, has been promoted and occasionally used commercially for recovering unhydrolyzed protein from aqueous extracts of defatted soy flour [14, 15]. Protein recoveries from single-stage aqueous extraction processing (AEP) and EAEP of extruded and unextruded full-fat soy flakes using IEP [16, 17], UF and ion-exchange chromatography [17] have been evaluated. Protein recovery by employing IEP (30%) was limited due to the greater solubility of hydrolyzed proteins [17]. Extrusion reduced overall soy protein yields in the curd compared to unextruded soybean white flakes from 62 to 38% in the absence of protease [16]. With protease, overall curd yields were similar, 47 and 51% for flakes and extruded flakes, respectively [16]. Curd protein purity, however, was highest for extruded flakes at 87 and 92% for extraction with and without protease, respectively, compared to curd from unextruded soy flake skim, which was 83 and 79% protein for AEP with and without protease, respectively [16]. The dominant impurity in the curd was oil, demonstrating the high oil-binding capacity for which soy protein is known and indicating the importance of removing oil from the skim prior to IEP to achieve high protein purity.
For UF of skim from EAEP of extruded flakes, Campbell and Glatz [17] achieved optimal protein purity using a 3-kDa membrane, with 0.74 protein retention and 70% purity of retentate protein. Ion-exchange chromatography enabled separating protein from the skim without oil contamination and recovering proteins with molecular weights between 12 and 30 kDa, but with low yields (15–20%). To date, the best one-step protein recovery method from EAEP of extruded flakes is UF, which achieves 60–63% overall yields, although with lower purity than when using IEP (30% protein recovery).
Because the extent of hydrolysis during extraction so strongly influenced protein recovery, the present study was undertaken to test the benefit of alternative protease addition strategies in countercurrent two-stage EAEP. Our hypothesis was that combining IEP with UF while controlling the extent of proteolysis would offer advantages over two-stage dead-end UF strategy for the same skims, which we recently reported on [12]. The objectives of the present study were to: (1) evaluate IEP as a means to recover the protein present in skim fractions from different extraction treatments; (2) evaluate upstream IEP of proteins followed by nanofiltration (NF) of the whey; and (3) identify the optimal process combination for protein recovery when incorporating both upstream extraction and downstream recovery methods.
Materials and Methods
Full-Fat Soybean Flakes
Full-fat soybean flakes were prepared from variety 92M91-N201 soybeans (Pioneer, a DuPont Business, Johnston, IA, USA) harvested in 2007. The soybeans were cracked into 4–6 pieces by using a corrugated roller mill (model 10X12SGL, Ferrell-Ross, Oklahoma City, OK, USA) and the hulls were removed from the meats (cotyledons) by aspirating with a multi-aspirator (Kice, Wichita, KS, USA). The meats were conditioned at 60 °C by using a triple-deck seed conditioner (French Oil Mill Machinery Co., Piqua, OH, USA) and flaked to approximately 0.25 mm thickness by using a smooth-surface roller mill (Roskamp Mfg, Inc., Waterloo, IA, USA).
Soybean Processing
Extruding Soybean Flakes
The moisture content of the soybean flakes was increased to 15% by spraying water onto the flakes while mixing in a Gilson mixer (model 59016A, St. Joseph, MO, USA). The moistened full-fat soybean flakes were extruded by using a twin-screw extruder (ZSE 27 mm diameter twin-screw extruder; American Leistritz Extruders, Somerville, NJ, USA). High-shear geometry screws were used in co-rotational orientation at 90 rpm screw speed. The extruder barrel (1,080 mm length) was composed of 10 heating blocks that were set for the temperature profile 30–70–100–100–100–100–100–100–100–100 °C. The extruder was manually fed to achieve an output rate of 10.5 kg/h of extruded flakes. Based on our previous results [10], the flakes were not collected in water. The collets (extruded pellets) were cooled to room temperature, placed in polyethylene bags, and stored in a cold room at 4 °C until extracted. The extruded flakes contained 20.7±1.5% oil (as is), 35.7 ± 0.5% protein (as is), and 11.3 ± 1.0% moisture.
Enzyme Treatment
Protex 6L, obtained from the Genencor Division of Danisco (Rochester, NY, USA), was used. Protex 6L is a bacterial alkaline endoprotease derived from a strain of Bacillus licheniformis and has highest activity at pH 7.0–10.0 and 30–70 °C. Protex 6L has minimum activity of 580,000 DU/g. The 0.5% enzyme dosage for the extraction was based on the weight of extruded flakes and was selected based on our previous work [8].
Countercurrent Two-Stage EAEP
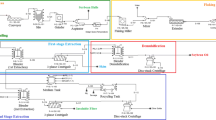
The extruded flakes were subjected to countercurrent two-stage extraction and the liquid fraction (skim + cream + free oil) obtained in the second extraction stage of one trial was recycled to the first extraction stage of the next trial (incoming fresh flakes) on the following day (Fig. 1). On the first day of extraction, the first extraction-stage EAEP was performed with 1 kg of extruded flakes using 1:6 solids-to-liquid ratio. The slurry pH was adjusted to 9.0 before adding 0.5% Protex 6L (wt/extruded flakes) and stirred for 1 h at 120 rpm and 50 °C by using an electric motor (Opti Chem, Chemglass, Vineland, NJ, USA) connected to a controller with digital display. The reaction was carried out in a 20-L jacketed glass reactor. The slurry obtained in the first extraction stage was centrifuged at 3000×g to remove the insoluble fraction. After removing the insoluble fraction, the liquid phase (skim, cream, and free oil) was placed in a separatory funnel (5-L jacketed reactor) and allowed to settle overnight at 4 °C. The liquid phase was then separated into three fractions (skim, cream, and free oil). The insoluble fraction obtained in the first extraction stage (1st insolubles) was then subjected to a second extraction stage. Prior to the second extraction stage, the 1st insoluble fraction was dispersed in water to obtain 1:6 solids-to-liquid ratio and the same extraction conditions were used in the second extraction stage as in the first extraction stage. The slurry obtained in the second extraction stage was centrifuged to separate the insoluble and liquid fractions.
The liquid phase was recycled to the first extraction stage on the next day in two different ways: 1) without any heat treatment (active enzyme in both stages; Treatment 1); or 2) recycled liquid phase was heated for 10 min at 85 °C to inactivate the enzyme prior to the first-stage of countercurrent extraction (Treatment 2). Treatment 3 used the same recycle method as Treatment 1, but enzyme was not used in either extraction stage. Extractions were carried out in this manner for 4 days, with steady-state being achieved after the second day [10]. The skim fractions obtained from the third extraction trial (steady-state extraction) were used as starting material for IEP and whey NF.
Isoelectric Precipitation of Skim Proteins
About 100 g of skim fractions were adjusted to pH 4.5 with 2 N HCL under constant stirring (~440 rpm) by using a magnetic stirrer (R0 10 power, IKA-Werker, Wilmingto, NC, USA). After adjusting the pH, the skim fractions were stirred for 1 h at ambient temperature, and then centrifuged at 15,008 × g for 30 min at 25 °C to separate whey (supernatant fraction) and protein curds. IEP was performed in duplicate with each skim fraction.
Dead-End NF of Whey Fractions
Laboratory-scale dead-end NF was performed by using Amicon stirred cells with 200-mL capacities (model 8020; Billerica, MA, USA) and effective membrane area of 41.8 cm2. About 50 g of the whey fraction generated after isoelectric precipitating each skim fraction was nanofiltered with a 500-Da cellulose acetate (YC05) membrane at 4.81 bar transmembrane pressure to achieve 1.87–2.80 concentration factor (CF = volume of feed/volume of retentate). The membranes were manufactured by Millipore (Billerica, MA, USA) and each experiment was conducted in duplicate with a new membrane. The permeates were analyzed for protein content.
Protein Retention
The protein retention coefficient (R) was calculated according to Eq. 1 [18]:
where C R is the protein concentration in the retentate; C 0 is the initial feed concentration of protein; CF is the concentration factor (initial volume/final volume) and R is the average retention coefficient of protein. Permeate samples were collected at intervals during NF for protein determination. Retentate protein content was determined by mass balance.
Membrane Fouling and Resistance
Global flux resistance was determined according to the Darcy equation:
where J is membrane flux, ΔP is transmembrane pressure, μ 0 is the permeate viscosity, and ΣR, the global resistance, is the sum of all resistances (i.e. membrane, fouling, cake formation, polarization, etc.). Clean membrane resistance was determined by measuring clean water flux before NF of the sample. In order to determine the critical transmembrane pressure, sample filtration was performed at negligible concentration factor (CF max = 1.1, after sampling was completed), by recycling the permeate to the filtration cell at transmembrane pressures ranging from 2.7 to 4.8 bar. Irreversibly fouled membrane resistance was determined by measuring clean water flux after NF to CF = 2. Based on the higher protein solubility at pH 4.5, because the proteins were hydrolyzed into small peptides, whey from IEP of skim proteins from Treatment 1 (enzyme in both extraction stages) was used to determine the fouling contribution on permeate flux reduction. Small peptides, such as those from Treatment 1, clog membrane pores much quicker than larger peptides from other treatments [18].
Analyses
Whey and Curd Fractions After IEP of Skim Proteins
Oil, protein, and dry matter (solids) contents were determined on the skims, whey, and curd fractions obtained after IEP. Oil content was determined by using the acid hydrolysis Mojonnier method (AOCS method 922.06), protein content by using the Dumas method and the conversion factor of 6.25 (vario MAXCN Elementar Analysensysteme Gmbh, Hanau, Germany), and total solids by weight after drying the samples in a vacuum oven at 110 °C for 3 h (AACC Method 44–40). Analyses were performed in duplicate. Yields of oil, protein, and solids were expressed as percentages of each component in each fraction relative to the initial amounts in the skim fractions and in the extruded flakes. Whey fractions were used as starting material for dead-end NF.
Size-Exclusion Chromatography (SEC) of Whey Proteins from IEP of Skim Fractions
Low-MW polypeptides were characterized by using high-performance liquid chromatography (HPLC) equipped with a 300 × 7.8 mm Biobasic SEC 300 size-exclusion column (BioRad Laboratories, Ltd, Hercules, CA, USA). Samples were prepared in mobile phase buffer (2 M guanidine HCl) at about 1 mg/mL protein concentration and then filtered through a 0.45-μm regenerated cellulose membrane (Millipore Corporation, Billerica, MA, USA). Injection size was 10 μL at 1 mL/min mobile phase flow rate. Absorbance was measured at 215 nm. MW markers (Sigma, St. Louis, MO, USA) were aprotinin from bovine lung (6511 Da), insulin chain B (3595 Da), angiotensin II human acetate (1060 Da), and leucine enkephalin acetate hydrate (555 Da).
SDS PAGE of Wheys and Curds from IEP of Skim Fractions
High-MW profiles were determined by SDS PAGE after diluting samples to 1.5 mg/mL protein concentration with 10 mM phosphate buffer (pH 7.5), mixing 1 part sample with two parts sample buffer (62.5 mM Tris–HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, 5% 2-mercaptoethanol), heating in boiling water for 5 min, and loading onto 4–15% gradient polyacrylamide gel (BioRad Laboratories, Ltd. Hercules, CA, USA) using 15-μL aliquots for loading 7.5 μg of protein.
Statistical Analyses
The experiment was a completely randomized design. The data were analyzed by Analysis of Variance (ANOVA) by using mixed models from the SAS system (version 8.2, SAS Institute, Inc., Cary, NC, USA). Means were compared using F-protected contrasts and the level of significance was set at P < 0.05.
Results
Effects of IEP of Skim Proteins on Oil, Protein, and Solids Distributions in the Precipitate (Protein Curd) and Supernatant Fractions (Whey)
The more extensive the proteolysis treatment, the lower was the protein recovery by IEP (Fig. 2). Means of protein yields within the fractions (whey and curd) were statistically different at P < 0.05. Reducing enzyme use (Treatment 1 vs. Treatment 3) increased IEP of protein from 27.0 to 87.0%. Increased protein solubility at the isoelectric point is a direct result of hydrolysis [16, 17]. Similar to protein recovery in the curd, oil yield in the protein curd also increased (P < 0.05) when reducing enzyme use (44.0–74.0% for Treatments 1 and 3, respectively). The amount of oil in the curd was related to the amount of protein recovered in the curd. No difference (P < 0.05) in oil yield in the protein curd was observed when comparing means of Treatments 2 (enzyme in the second extraction stage only) and 3 (no enzyme used). Soy proteins can bind large quantities of fat, up to 150 mL oil/mg protein for SPI [19]. It was likely that emulsified oil was entrapped in the protein curd. Oil and protein also form complexes during extrusion [6]. While there was a significant increase (P < 0.05) in the fraction of protein precipitated between skims of Treatments 2 (enzyme in the second extraction stage only) and 3 (no enzyme), there was not a significant increase in the fraction of oil in the curd. We are currently investigating the nature of the oil-protein interactions in the skim. Yields of solids in the curd and whey followed the same trend as was observed for oil and protein.
SEC of Polypeptides and SDS PAGE of Whey and Curd Proteins from IEP of Skim
Figures 3 and 4 show SDS PAGE and SEC profiles, respectively, for whey and curd proteins recovered from EAEP skim fractions. When using no enzyme (Treatment 3), whey proteins were primarily intact proteins (i.e., albumins) with MWs of <37 kDa. The proteins in the curd fraction are primarily the intact α, α′, and β subunits of β-conglycinin and the intact acid (As) and basic (Bs) subunits of glycinin. When enzyme was used in the second extraction stage only (Treatment 2), the intensities of the whey proteins, MWs ranging from 37 to 25 kDa, decreased showing evidence of hydrolysis, and no apparent difference was noted for the curd proteins. When using enzyme in both extraction stages (Treatment 1), the whey proteins were hydrolyzed to <20 kDa and all curd proteins except the basic subunits of glycinin were also hydrolyzed to <15 kDa. Proteolysis resistance of the basic subunit of glycinin has been reported by Kapchie et al. [21]. Apparently the hydrolyzed whey proteins were < 10 kDa because there were no intense bands at the bottom of the gel.
SDS PAGE profiles of whey and curd proteins at pH 4.5. Major subunits of soy storage proteins glycinin and β-conglycinin are indicated [20]
SEC profiles of whey from IEP of skim protein from Treatments 1, 2 and 3. Treatment 1, without any heat treatment (active enzyme in both stages; Treatment 1); Treatment 2, heated for 10 min at 85 °C to inactivate the enzyme prior to the first stage of countercurrent extraction; and Treatment 3, without enzymes in either extraction stages
When using only IEP as means to recover protein in each skim fraction, it resulted in 27, 61, and 87% protein recoveries for skims of Treatment 1 (enzyme in both extraction stages), Treatment 2 (enzyme in the second extraction stage only), and Treatment 3 (no enzyme), respectively. This is also in agreement with Campbell and Glatz [17] who reported 30% protein recovery when using EAEP skim and IEP to recover proteins from single-stage aqueous extraction of soy extrudate using P6L, which represents 26% overall protein recovery and providing similar proteolysis as Treatment 1. We recently reported protein extraction yields of 96, 89, and 66% when using Treatments 1, 2, and 3, respectively [12]. Combining extraction yields and protein recovery yields from IEP of the skims, results in overall recoveries of 26, 54, and 57% of the protein in the extruded soybean flakes. To capture protein remaining in the whey, NF was evaluated.
Nanofiltration of Wheys from IEP of Skim Fractions (500-Da membrane)
Analysis of flux as a function of transmembrane pressure (TMP) for the 500 Da NF of whey from IEP of skim from Treatment 1 (enzyme used in both extraction stages) at CF < 1.1 (Fig. 5) showed that 4.8 bar was below the critical TMP, although osmotic pressure of the bulk retentate solution for this case was high. Extrapolation of the flux versus TMP curve crossed the x-axis at 2.4 bar suggesting an osmotic pressure of 2.4 bar. This would explain the severe flux decline seen at CF = 2. Doubling the retentate concentration (and, hence at least doubling the osmotic pressure) would reduce the effective TMP (ΔP-osmotic pressure) to near zero. It should be noted that the pressure used in the present study (4.8 bar), which was limited by the experimental apparatus, was somewhat lower than what is commonly encountered in NF, which could entail low permeate fluxes. After concentrating the sample to CF = 2, the fouled membrane resistance was on average 7.4 × 1013 m−1, being slightly greater than the clean membrane resistance, which was about 6.54 × 1013 m−1. Membrane resistance plus fouling was about 20% of global resistance, which using an osmotic pressure of 2.4 bar, ranged from 3.3 × 1014 to 3.7 × 1014 m−1.
The NF of wheys from IEP of skim proteins is shown in Fig. 6. Since higher amounts of protein were present in wheys after IEP of skims with greater protein hydrolysis [4.8, 2.4, and 0.6%, Treatment 1 (enzyme in both extraction stages), Treatment 2 (enzyme in the second stage only) and Treatment 3 (no enzyme), respectively], lower permeate fluxes were obtained for whey after IEP of skims from Treatments 1 and 2 compared with whey after isoelectric precipitation of skim from Treatment 3. Permeate flux of whey after IEP for Treatments 1, 2, and 3 decreased from 2.73 to 0.22, 5.04 to 0.65, and 8.69 to 1.35 kg/h m2 at CFs of 1.91, 2.05, and 2.80, respectively. Protein yields (from whey) in the retentates were approximately 96.3, 94.5, and 91.8% for Treatments 1, 2, and 3, respectively. Mean protein retentions during ultrafiltration for the three wheys were not statistically different at P < 0.05 (0.94, 0.92, and 0.92, respectively). Although membrane resistance and irreversible fouling corresponded to about 20% of the global resistance, reduced permeate flux is mainly a consequence of higher polarization concentration, which could result from the development of a gel layer over the membrane surface thereby reducing permeate fluxes to greater extents for wheys containing higher protein contents.
About 97, 98, and 99% of the initial skim protein were recovered when combining protein recovery in the curd and in the retentate for Treatment 1 (enzyme in both extraction stages), Treatment 2 (enzyme in second extraction stage only), and Treatment 3 (no enzyme), respectively. Overall protein recoveries (combining curd and retentate) of 93, 87, and 65% of the protein initially present in the extruded flakes were achieved when coupling IEP and NF of wheys from Treatments 1, 2, and 3, respectively. We recently reported overall protein recoveries of 87.6, 85.9, and 65.5% from flaked and extruded soybeans when combining extraction yields from Treatments 1, 2, and 3, respectively, and two-stage dead-end membrane filtration (both UF and NF) of their skim fractions [12]. Permeate fluxes ranged from 1.13 to 0.26 kg/h m2 (CF = 1.96), 3.64 to 0.53 kg/h m2 (CF = 2.13), and 5.59 to 0.56 kg/h m2 (CF = 2.96) for a two-stage membrane filtration of skims from Treatments 1, 2, and 3, respectively [12]. Removing unhydrolyzed proteins by IEP followed by whey NF achieved greater overall protein recovery for skim from Treatment 1 and similar recoveries for skims from Treatments 2 and 3 when compared to using two-stage membrane filtration of skims from the same treatments. Although wheys from IEP contained higher protein contents than skim permeates from first-stage (UF) membrane filtration [12] (4.8, 2.4, and 0.6% vs. 3.6, 1.8, and 0.25% for Treatments 1, 2, and 3, respectively), the removal of unhydrolyzed proteins may have favored whey permeate fluxes that ranged from 2.73 to 0.22 kg/h m2 (CF = 1.91), 5.04 to 0.65 kg/h m2 (CF = 2.05), and 8.69 to 1.35 kg/h m2 (CF = 2.80) for Treatments 1, 2, and 3, respectively. Although using IEP and whey NF achieved slightly greater overall protein recovery and higher permeate fluxes, at similar CFs, than using two-stage dead-end membrane filtration, functionalities of recovered proteins by IEP and membrane filtration may be different.
Conclusions
Yields of protein recovery by IEP were inversely related to protein hydrolysis. Protein recoveries by IEP increased from 27.0 to 87.0% with decreased use of enzyme during extraction. Most proteins in the whey fraction were <37 KDa MW. Low protein precipitation was associated with greater hydrolysis, therefore higher solubility. IEP recovered about 26, 54, and 57% of soybean protein when using Treatment 1 (enzyme in both extraction stages), Treatment 2 (enzyme in the second extraction stage only), and Treatment 3 (no enzyme), respectively. SDS-PAGE showed that protein subunits in the curd fractions were <25 KDa for IEP of skim from Treatment 1 and about 75–10 KDa for IEP of skims from Treatments 2 and 3. NF (500 Da membrane) of whey after IEP of skim achieved 96.3, 94.5, and 91.8% protein retentions and 0.22 (CF = 1.9), 0.81 (CF = 2.0), and 1.35 (CF = 2.8) kg/h m2 permeate fluxes, respectively. About 93, 87, and 65% of the protein from extruded flakes was recovered when coupling IEP and NF of wheys from Treatments 1, 2, and 3, respectively. Although Treatment 1 had higher overall protein recovery than Treatment 2, possible differences in protein functionalities may enable different applications and justify the lower protein recovery of Treatment 2.
References
Witte NH (1995) Soybean meal processing and utilization. In: Erickson DR (ed) Practical handbook of soybean processing and utilization. AOCS Press, USA, pp 93–116
Lusas EW, Rhee KC (1995) Soybean protein processing and utilization. In: Erickson DR (ed) Practical handbook of soybean processing and utilization. AOCS Press, USA, pp 117–160
Hettiarachchy N, Kalapathy U (1997) Soybean protein products. In: Liu K (ed) Soybeans: chemistry, technology and utilization. Aspen Publishers, USA, pp 379–411
Endres JG (2001) Soy protein products: characteristics, nutritional aspects, and utilization. AOCS Press, USA, pp 4–9
Rosenthal A, Pyle DL, Niranjan K (1998) Simultaneous aqueous extraction of oil and protein from soybean: mechanisms for process design. Trans Inst Chem Eng Part C 76:224–230
Lamsal BP, Murphy PA, Johnson LA (2006) Flaking and extrusion as mechanical treatments for enzyme-assisted aqueous extraction of oil from soybeans. J Am Oil Chem Soc 83:973–979
Freitas SP, Hartman L, Couri S, Jablonka FH, Carvalho CWP (1997) The combined application of extrusion and enzymatic technology for extraction of soybean oil. Fett/Lipid 99:333–337
de Moura JMLN, Campbell K, Mahfuz A, Jung S, Glatz CE, Johnson LA (2008) Enzyme-assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification. J Am Oil Chem Soc 85:985–995
de Moura JMLN, Johnson LA (2009) Two-stage countercurrent enzyme-assisted aqueous extraction processing of oil and protein from soybeans. J Am Oil Chem Soc 86:283–289
de Moura JMLN, de Almeida NM, Johnson LA (2009) Scale-up of enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc 86:809–815
de Moura JMLN, de Almeida NM, Jung S, Johnson LA (2010) Flaking as a pretreatment for enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc 87:1507–1515
de Moura JMLN, Campbell K, Almeida NM de, Glatz CE, Johnson LA (2011) Protein extraction and membrane recovery in enzyme-assisted aqueous extraction processing of soybeans. J Am Oil Chem Soc. doi:10.1007/s11746-010-1737-0
de Almeida, NM, de Moura JMLN, and Johnson LA (2010) Functional properties of protein produced by two-stage countercurrent enzyme-assisted aqueous extraction. In: Abstracts, 101st American Oil Chemists’ Society Annual Meeting and Expo, Phoenix, AZ, 16–19 May
Kumar NS, Yea MK, Cheryan M (2003) Soy protein concentrates by ultrafiltration. J Food Sci 68:2278–2282
Mondor M, Ippersiel D, Lamarche F, Boye JI (2004) Production of soy protein concentrates using a combination of electroacidification and ultrafiltration. J Agric Food Chem 52:6991–6996
Jung S, Mahfuz AA (2009) Low temperature dry extrusion and high-pressure processing prior to enzyme-assisted aqueous extraction of full fat soybean flakes. Food Chem 114(3):947–954
Campbell KA, Glatz CE (2009) Protein recovery from enzyme-assisted aqueous extraction of soybean. Biotechnol Prog, Published on-line, Nov. 25
Cheryan M (1998) Ultrafiltration and microfiltration handbook. Technomic Publishing, USA
Nielsen NC (1985) In: Altschul AA, Wilcke HL (eds) New protein foods, vol. 5: Seed Storage Proteins. Academic Press, New York, NY
Wu S, Murphy PA, Johnson LA, Reuber MA, Fratzke AR (2000) Simplified process for soybean glycinin and β-conglycinin fractionation. J Agric Food Chem 48:2702–2708
Kapchie VN, Towa LT, Hauck C, Murphy PA (2010) Recycling of aqueous supernatants in soybean oleosome isolation. J Am Oil Chem Soc 87:223–231
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
de Moura, J.M.L.N., Campbell, K., de Almeida, N.M. et al. Protein Recovery in Aqueous Extraction Processing of Soybeans Using Isoelectric Precipitation and Nanofiltration. J Am Oil Chem Soc 88, 1447–1454 (2011). https://doi.org/10.1007/s11746-011-1803-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-011-1803-2