Abstract
William Bartlett (Pyrus communis) pears were diced, packaged and subjected to a short continuous microwave treatment (4.7 min) which increased the temperature from 19.92 ± 1.36 to 101.11 ± 2.50 °C. Retorted samples were processed at 95.40 °C for 18.0 min for comparison. Recorded temperature data were used to calculate C, D and F values. Brix, pH, colour, texture, microbial counts, microstructure and volatile profiles of both treated samples were analysed immediately after process and after 1, 2, 4 and 6 weeks of storage at 4 °C. The C, D and F values of microwaved pears were significantly (p < 0.05) lower compared to those of retorted samples. Both methods of processing resulted in products with standard plate count of < 10 cfu/g during 6 weeks of storage at 4 °C. Microwave processing offered a final product with a total colour difference in the same range as the retorted samples. Polyphenol oxidase enzyme was inactivated below the detection level by both microwave processing and retorting. Microwaved samples also maintained cell wall integrity, although the intercellular intactness was slightly weakened and prevented the final product from developing water-soaked appearance. During storage, volatile profiles of microwaved samples showed significant (p < 0.05) differences, whereas the retort samples showed minimal changes. In comparison to conventional retorting, a short microwave treatment can result in a superior quality end-product with a comparable microbiological quality and a minimum shelf life of 6 weeks at 4 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fruits are an important part of a healthy diet which provide sugars, vitamins, minerals, fibres and phytochemicals (Sánchez-Moreno et al., 2012). World Health Organisation recommended 400 g of fruit per capita per day in order to prevent cardio vascular and degenerative diseases (WHO, 2003).
Pre-packaged fruits are popular amongst modern populations who request ready to eat foods due to their time poor life style. Fresh cut fruits (FCF) are prepared using sanitised washing, treatment with antimicrobial, antibrowning and texture maintaining agents, and placing in modified atmosphere packaging followed by refrigerated storage. FCF are usually prepared from fruits that do not comply with fresh market standards due to size, deformity or blemishes, but at optimal ripeness to ensure their sensorial properties. FCF require efficient distribution chain and storage at 2–4 °C to retain quality for 7–10 days (Martín-Belloso et al., 2012). Short self-life is the main limitation of FCF (Balla et al., 2012). To overcome this limitation, thermal pasteurisation has been examined to extend the shelf life of pre-packaged fruit. Conventionally, canning or the thermal sterilisation is used for obtaining a shelf life of 2 years at room temperature (Shen et al., 2012). The quality of canned fruits, however, is compromised due to the sterilisation process, e.g. soft texture, discolourisation and non-enzymatic browning (Shen et al., 2012).
Microwave heating has been used as a continuous rapid heat treatment for tempering of frozen products, pre cooking of bacon, drying of various foods and blanching of vegetables (Ahmed & Ramaswamy, 2004; Vadivambal & Jayas, 2007, 2010). Microwave pasteurisation has been applied to fruit juices, e.g. apple juice (Siguemoto et al., 2018; Siguemoto & Gut, 2016), strawberry purée (Marszałek et al., 2015, 2016), kiwifruit purée (Benlloch-Tinoco et al., 2012, 2014a, 2015) and apple purée (Picouet et al., 2009). The impact of microwave treatment on physicochemical properties safety, quality, sensorial attributes and heat distribution has been reported in these studies. This study was focused on the evaluation of the impact of continuous microwave treatment on fruit pieces that were vacuum packaged in punnets, which allowed the sealed punnets to be served in situ after microwave processing. To the best of our knowledge, there are no scientific publications on microwave pasteurised/sterilised fruit pieces. However, preparation of pasteurised fruit slices through a series of medium temperature (up to 60 °C) microwaving, hot water bathing and cold-water bathing of pre-packaged fruit slices has been patented by Perdue (2004).
Our study was focused on the comparison between microwave pasteurisation and traditional retort processing to extend the shelf life of diced pears beyond 10 days at refrigerated storage. In order to evaluate the inactivation of quality-related enzymes in the treated diced pears, the levels of residual polyphenol oxidase (PPO) and pectin methylesterase (PME) were assessed. Standard plate count (SPC) and yeast and mould count (Y&M) were assessed to ensure microbial safety of the treated diced pears. To evaluate the quality of the treated diced pears, colour, total soluble solids, pH and hardness were measured. Microstructure and volatile analyses were also evaluated as indicators of sensorial quality of treated diced pear.
Materials and Methods
Materials
William Bartlett pears (Pyrus communis, 2016 harvest) were purchased from a local Coles Supermarket (Werribee, VIC, Australia), and stored under refrigeration at 4 °C. Pears were conditioned at room temperature prior to processing. Pears recorded a penetrometer (Fruit Pressure Tester FT 327, Facchini Srl., Alfonsine, Italy) reading of 5 to 8 kg on the day of processing when tested with a 0.8-cm-diameter plunger. Ascorbic acid (SKU F00185) was purchased from The Food Ingredient Depot, Melbourne, VIC, Australia.
Preparation of Pear Punnets
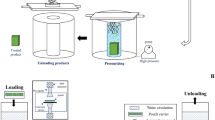
Pears were peeled, cored and diced (approximately 2 cm × 2 cm × 2 cm) using an Anliker dicer (Model GSM, Brunner-Anliker AG, Kloten, Switzerland), and submerged in 1000 ppm ascorbic acid solution to prevent enzymatic browning prior to either microwave or retort treatment. Diced pear pieces were weighed (100, 110 or 120 g) into punnets made of polypropylene (Sealed Air Corporation, Tullamarine, VIC, Australia; 90.6 mm × 61.1 mm, thickness of 0.3 mm) and vacuum sealed at 0.8 bar total pressure with polypropylene lid film (Reipack Supersealer, Tullamarine, VIC, Australia; gauge of 60 μm) using ILPRA Food Pack packaging machine (Model Basic V/G, ILPRA SPA, Vigevano, Pavia, Italy). Samples were microwaved immediately after packing.
Microwave Processing
A microwave tunnel system (Fig. 1a) with a pentagonal cross-section described in Gamage et al. (2015) was used without the hot air flow for all product treatments. A set of three magnetrons on a side of the pentagon was activated to give a burst of microwave energy, followed by an on-off-cycle to allow for internal temperature equilibration in the pear punnets. The on-off-cycle was repeated in a clockwise direction activating magnetrons mounted on each side of the pentagonal tunnel during treatment of the product. The conveyor belt used to transport the punnets was programmed to move forward at a fixed speed (0.53 cm/s) only when magnetrons are activated. Punnets of pears with three different weights 100, 110 and 120 g were subjected to microwave heating since they were expected to generate different amounts of heat. The corresponding microwave treatments were named W100, W110 and W120, respectively. The microwave treatment was performed in triplicate batches for pear punnets with the three different weights. A 4-channel signal conditioner for fibre optic temperature measurement with 4 × 6 m fibres (Model ReFlex-4, Neoptix Inc., Quebec City, Quebec, Canada) was used to record real-time temperature changes inside the pear punnets as the fibre optic probes moved through the microwave tunnel. The fibre probes were inserted into selected punnets (approximately one in every 20 punnets) to obtain a representative temperature measurement. Fibre optic sensors were inserted in the top, bottom and two middle layers of pear dices (Fig. 1b). Temperatures were recorded at 1-s intervals. The temperature data were used to calculate the equivalent time at a target temperature above 90 °C to inactivate browning enzyme (Montogomery & Petropakis, 1980).
Energy (E) generated in the samples during microwave treatment was calculated from the temperature data using Eq. 1 (Toledo, 2007). The energy efficiency was later calculated using Eq. 2 using the energy generated during microwave treatments and the energy supplied by the magnetrons. The cook (C) value was calculated to relate heat treatment with respect to physical and chemical changes on food quality which occurred during processing (Bindu et al., 2007). C value at the thermal centre of the treated samples was obtained from the time-temperature data using Eq. 3 (Rinaldi et al., 2020). The process lethality (F) value was calculated using Eq. 4 (Awuah et al., 2007).
With
- E:
-
energy, J
- m:
-
mass samples, kg
- Cp:
-
heat capacity, J/(kg K)
- T0:
-
initial temperature, K
- T:
-
temperature, K
With
- C:
-
cook value, min
- t:
-
time, min
- T:
-
temperature at a given time, °C
- Tref:
-
reference temperature, set at 100 °C
- z:
-
temperature increase that induced a 10-fold increase of chemical reaction rate, set at 33 °C
With
- F:
-
process lethality value, min
- t:
-
time, min
- T:
-
temperature at a given time, °C
- Tref:
-
reference temperature, set at 93.3 °C (Rouweler, 2015)
- z:
-
temperature sensitivity or temperature changes that results in a 10-fold change in the D value, set at 8.9 °C (Rouweler, 2015)
The decimal reduction (D) values were calculated using Eq. 5 to compare the heating time that results in 90% reduction of the existing microbial population (Awuah et al., 2007).
With
- D:
-
decimal reduction value, min
- t1:
-
heating for time t1, min
- t2:
-
heating for time t2, min
- A:
-
survivor counts following heating for time t1
- B:
-
survivor counts following heating for time t2
Retort Processing
The pear packs for thermal processing were prepared by placing 110 g of diced pears in clear stand-up-retort pouches (Amcor Flexibles Australasia, Smithfield, NSW, Australia; 140 mm × 185 mm × 80 mm) made of 3-ply laminate (PET (12 μm)/AlOx/OPA (15 μm)/PP (70 μm)). Pouches were immediately vacuum sealed using a Webomatic packaging machine (Model E50G, Webomatic Maschinenfabrik GmbH, Bochum, NRW, Germany) up to − 0.8 bar. Samples were stored at 23 °C for further processing.
The pouches were processed in a full water immersion batch retort (Model 42300, Barriquand, Roanne, ARA, France). The pouches were placed in metal basket that held 20 pouches. The process was designed to achieve a lethality (F value) during a hold time of 18.0 min. Reference temperature was 93.3 °C and z value was 8.9 °C at pH 4.3–4.5 (Rouweler, 2015). Water at 100 °C was used as the heating medium. Triplicate batches of retorted pears were processed. The retort processing was named R110. Energy (E) absorbed by the samples during retort processing was calculated from the temperature data using Eq. 1 while the C, F and D values were obtained using Eqs. 3, 4 and 5, respectively.
Following processing, punnets/pouches were held at room temperature (23 °C) until product temperature reached equilibrium. All the samples were then moved to cold storage at 4 °C and relative humidity of 70% and held for 6 weeks.
Product Analyses
Quality of the samples were analysed immediately after processing and after 1, 2, 4 and 6 weeks of storage at 4 °C. Untreated pear samples were used as control.
Instrumental Colour, Total Solids (°Brix) and pH
The colour of the pears in opened packs in triplicate samples was measured during storage using a chroma meter (Model CR-300, Minolta Corp., Osaka City, Osaka, Japan). Readings were obtained in CIELAB scale (L*, a* and b* values). Total colour difference (ΔE*) was calculated according to Eq. 6 (Pathare et al., 2013) and was used to compare colour changes between treated and control samples. Photographs of punnets at each sampling point were also taken to track the visual colour development.
With
- ΔE*:
-
total colour difference
- ΔL*:
-
difference in lightness
- Δa*:
-
difference in green-red coordinate
- Δb*:
-
difference in blue-yellow coordinate
Total solids (°Brix) and pH of the blended pear samples were also measured in triplicate using a handheld digital refractometer (Model PAL-ALPHA, Atago Co. Ltd., Saitama City, Saitama, Japan).
Hardness
The texture of the pear samples was measured using an Instron Universal Testing Instrument (Model 5564, Instron Corp., Norwood, MA, USA) with 100 N load cell. The hardness was determined by measuring the force required for a plastic blade to cut the pear samples under the applied compression of 0.33 mm/s, starting from 22 mm above the baseline and allowing the blade to go downward for 15 mm. The hardness of 15 pear pieces from triplicate packs was measured for each treatment replication. All results were expressed as the maximum force (N) required for cutting the diced pear pieces.
Microstructural Analysis
A confocal laser scanning microscopy (CLSM, Model SP5, Leica Microsystems GmbH, Wetzlar, LDK, Germany) was used to characterise the microstructure of pears. Pear samples were thinly sliced, and each cut surface was stained with three drops of Congo Red (Merck, Darmstadt, Hesse, Germany, 0.1% in deionised water). Cover slips (Model 7201, Lab Medical Sail Brand, CNWT Co. Ltd., Haimen City, Jiangsu, China) were placed on top of the samples. The microscopic analysis was performed at room temperature with a HC PL APO 20×/0.70 IMM WATER objective lens. Congo Red was excited by Argon 488 nm laser and the emitted light was recorded at 544–663 nm. The examination depth varied from approximately 20 to 120 μm over the visualised areas of the samples.
Polyphenol Oxidase Extraction and Assay
Polyphenol oxidase (PPO) activity was determined in the microwaved pear samples stored for 0, 1 and 2 weeks at 4 °C. Since the three consecutive PPO analysis was below detection level (bdl), no PPO analysis was performed on microwaved pear samples stored for 4 and 6 weeks. Retorted samples were tested before and after processing (0 days), and after 6 weeks of storage at 4 °C. The PPO enzyme was extracted from pears using the method described by Gaulliard and Richard-Forget (1997) and Carbonara and Mattera (2001). Samples were freeze-dried (Dynavac Model FD-5, Dynapumps, Melbourne, VIC, Australia) at − 40°C for approximately 48 h while McIlvaine citric acid phosphate buffer at pH 6.5 was prepared from citric acid anhydrous (Sigma-Aldrich, St. Louis, MO, USA) and disodium hydrogen phosphate (Sigma-Aldrich, St. Louis, MO, USA). Freeze-dried pear samples (1.5 g) were homogenised with 12.5 mL of McIlvaine citric acid phosphate buffer, pH 6.5, containing 0.05 M sodium dodecyl sulphate (Sigma-Aldrich, St. Louis, MO, USA), using a homogeniser (Model Ultra Turrax T-25, IKA Labortechnik, Staufen, GI, Germany) at 9500 rpm for 2 min. All subsequent steps were also performed at 4 °C. The suspension was centrifuged (Model J6-MI, Beckman Coulter, Brea, CA, USA) at 3000×g for 15 min followed by 23,700×g for another 15 min. The supernatant was filtered (Whatman Grade 4 Qualitative Filter Paper, Whatman plc, Maidstone, Kent, UK) and used as the enzyme extract to determine PPO activity. The PPO activity was determined in a reaction mixture (1.5 mL) containing 0.5 mL of enzyme extract, 0.9 mL of 0.1 M citric acid phosphate buffer (pH 6.5) and 0.1 mL of 0.4 mM chlorogenic acid solution (14 mg chlorogenic acid, Sigma-Aldrich, St. Louis, MO, USA, in 100 mL of citric acid phosphate buffer, pH 6.5) in a quartz cell. The absorbance of the mixture was measured at 400 nm for 3 min at 5-s intervals at 37 °C using a spectrophotometer (Model UV-1700 Pharma spec, Shimadzu Corp., Kyoto City, Kyoto, Japan). One unit of PPO activity was defined as a change in absorbance of 0.001 OD/min (Benlloch-Tinoco et al., 2013). The reaction rate was estimated from the initial linear portion of the plotted curve.
The relative PPO activity of the samples was calculated using Eq. 7.
With
- At:
-
PPO activity of treated pear sample
- A0:
-
PPO activity of untreated pear sample
Pectin Methylesterase Extraction and Assay
Pectin methylesterase (PME) was extracted from the pear samples stored for 0, 1, 2, 4 and 6 weeks at 4 °C following the method proposed by Hagerman and Austin (1986) with some modifications. Puréed pear samples containing 2.5 g of total solids were mixed with deionised water to obtain 42.5 g of mixture. The mixture was then homogenised with 3.75 g of sodium chloride (Sigma-Aldrich, St. Louis, MO, USA) using a homogeniser (Model Ultra Turrax T-25, IKA Labortechnik, Staufen, GI, Germany) at high speed (13,000 rpm) for 15 s. Final concentration of the NaCl in the homogenate was 8.8% w/v. The homogenate was stirred for 15 min and then centrifuged (Model Sorvall RC 5C Plus, Thermo Fisher Scientific, Waltham, MA, USA) at 13,000 rpm for 25 min at 4 °C. The supernatant was assayed for PME activity by a titration of the free carboxylic groups produced from the pectin with 0.01 N NaOH (Sigma-Aldrich, St. Louis, MO, USA) solution using an automatic pH stat-titrator (Radiometer 854, titration Workstation, Lyon, ARA, France) at pH 7.5 and 30 °C (Duvetter et al., 2005). A 20 mL aliquot of a solution containing 0.15 M NaCl and 0.5% w/v apple pectin (Sigma-Aldrich, St. Louis, MO, USA, 70–75% esterification) was equilibrated to 30 °C and pH adjusted to 7.5. Following the addition of a 0.6–1.0 mL PME extract (depending on the remaining activity of the extract), the pH was quickly readjusted to 7.5 which was then maintained up to 20 min by the titration with 0.01 N NaOH solution. The volume of base (VNaOH) added was recorded as a function of time. All samples were measured in triplicate and the slope (S = VNaOH /t) of the initial linear part of the titration curve was determined covering a period of 5–10 min. The slope is directly proportional to the activity of PME per mL of the sample (activity). PME activity is expressed as microequivalents of ester hydrolysed per minute per milliliter of sample at pH 7.5 and 30 °C. The units were multiplied by 103 for easy interpretation as shown in Equation 8 (Basak & Ramaswamy, 1996).
With
- S:
-
slope of titration, mL/min
- NNaOH:
-
concentration of base, μmol/mL
- V:
-
volume of sample, mL
- VNaOH:
-
volume of base, mL
- t:
-
time of reaction, min
Microbiological Analysis
Fresh diced pear pieces and processed samples were stored for 0, 1, 2, 4 and 6 weeks at 4 °C. The fresh diced pear pieces were used to assess standard plate count (SPC) and yeast and mould count (Y&M). SPC was examined by diluting the puréed samples in 0.1% w/v sterile peptone water (Oxoid™, Thermo Fisher Scientific, Waltham, MA, USA) and enumerating the viable cells in Plate Count Agar (Oxoid™, Thermo Fisher Scientific, Waltham, MA, USA), following the Australian Standard procedure (AS 5013.13–2004). For Y&M assessment, the diluted samples were spread on Dichloran Rose-Bengal Chloramphenicol (Oxoid™, Thermo Fisher Scientific, Waltham, MA, USA), following the Australian Standard procedure (AS 5013.29–2009). The cultured agar plated was incubated at 30 °C for 72 h in the case of SPC and at 25 °C for 5 days in the case of Y&M.
Headspace Volatile Analysis
Volatile compounds of the unprocessed and microwaved pear samples stored for 0, 2 and 6 weeks at 4 °C were analysed using headspace solid phase micro extraction (SPME). Volatile compounds of the retorted samples stored for 0 and 6 weeks at 4 °C were also analysed. For each sample, 0.5 g of the purée was analysed for volatile profiles in duplicate.
Headspace analysis was performed using an Agilent Technologies gas chromatograph-mass spectrometer (GCMS) system (GC Model 6890 N, MS Model 5975 series B, Agilent Technologies Inc., Palo Alto, CA, USA) equipped with a Combi PAL robotic auto sampler (CTC Analytics AG, Zwingen, Basel-Landschaft, Switzerland). The pear volatiles were sampled on to SPME fibre (Model CAR/PDMS StableFlex fibre, phase thickness 85 μm, length 1 cm; Supelco, Bellefonte, PA, USA) for 30 min at 45 °C. SPME-sampled volatiles were thermally desorbed in the GC inlet (Programmable Temperature Vaporizing inlet, 260 °C, set in splitless mode, splitless time 1.0 min; Agilent Technologies Inc., Palo Alto, CA, USA) and chromatographed on a VF-WAXms column (30 m × 0.32 mm × 1.0 μm; Agilent Technologies Inc., Palo Alto, CA, USA) using a temperature gradient of 35–225 °C at 10 °C/min with initial and final hold times of 3 and 10 min, respectively. Eluted compounds were detected by the MS detector. MS conditions were as follows: capillary direct interface temperature, 200 °C; ionization energy, 70 eV; mass range, 30–300 amu; and scan rate, 5 scans/s. GCMS system was interfaced with the computer using Chemstation Workstation software (Agilent Technologies Inc., Palo Alto, CA, USA). Compounds were identified by comparison of collected mass spectra with spectra in the NIST11 database (National Institute of Standards Technology mass spectral search program, Gaithersburg, MD, USA), linear retention indices (determined using a set of saturated alkanes C7–C22), and spectra of an authentic standard. Quantitative analysis was performed using internal standard methodology.
Principal Component and Statistical Analysis
Analysis of variance (ANOVA) and principal component analysis (PCA) were conducted using XLSTAT version 2017 software (Addinsoft, New York, NY, USA), an add on for Microsoft Excel.
Results and Discussion
Energy, C Value, F Value and D Value
The amount of energy generated in each punnet during microwave processing experiment was calculated using Eq. 1, with 3.73 kJ/(kg K) as the heat capacity (Cp) of William Bartlett pear (Singh & Sarkar, 2005). This Cp value was for temperature range of 4–32 °C and was assumed to remain constant throughout the microwave and retort processing. Equation 1 was applied on temperature data recorded during the pre-heating and the heating sections of either microwave or retort treatment. The representative time-temperature profiles of the microwave and retort treatments are in Fig. 2a and b. Based on these time-temperature profiles, the C and F values were calculated.
C values of all microwave treatments (1.89–2.35 min) were significantly (p < 0.05) lower than that of retort processing (14.54 min, Fig. 3d), indicating a rapid heating. This resulted less impact on quality attributes of the microwaved samples compared to that of the retorted samples. Rinaldi et al. (2020) reported that processing protocols with lower C values led to less depreciation of product quality. Products that have undergone processing protocols with C values in the region of 100–200 min are usually considered to have an impaired quality (Awuah et al., 2007).
Temperature maximum (Tmax) (a), energy per punnet (b), energy efficiency (c), C values (d), F value (e) and D value (f) of diced pear samples during the pre-heating and the heating sections of microwave (W100, W110 and W120) and retort (R110) treatments. Different superscript letters (abcd) within each block indicate values are significantly different at the level of p < 0.05. The efficiency of retort processing is not available (na*). No statistical analysis was applied to energy efficiency
In our study, retort processing showed the highest F value (33.52 min, Fig. 3e) and thus it was significantly (p < 0.05) different from those of all microwave processing (5.20–11.90 min). All F values for microwave processing were either within or slightly above the recommended range for maintaining quality of pear (1.3–10 min) as reported by Rouweler, (2015).
Similarly, all three microwave processing protocols displayed no significant (p > 0.05) difference in D values (5.32–6. min), which were significantly (p < 0.05) less than that of the retort processing (20.33 min, Fig. 3f).
The maximum temperature and the generated energy per punnet were significantly (p < 0.05) different when the mass of pears in punnets were varied, i.e. 100, 110 and 120 g (Fig. 3b). According to Eq. 1, the increase of energy could happen due to an increase in either mass or temperature difference, or combination of both when the heat capacity remains constant. Nonetheless, Tmax of the three microwave treatments (Fig. 3a) exceeded 90 °C. It is reported that deactivation of browning enzyme in William Bartlett pear occurred at 90 °C (Montogomery & Petropakis, 1980). The electromagnetic energy emitted by the magnetrons was 2920 kJ and was used to process 50 punnets per each run. Thus, the energy efficiency of all three microwave treatments ranged from 52.12 to 59.83% (Fig. 3c).
Instrumental Colour
The colour of raw diced pears was measured before each processing experiment and their values were used as the reference in calculating the ΔL*, Δa* and Δb* in Eq. 6. Lightness (L*) of all samples decreased immediately after either microwave or retort processing This reduction in lightness was also observed by naked eyes (Fig. 4b–i). Since the Tmax of all microwaved samples exceeded the deactivation temperature of browning enzyme for William Bartlett pear (90 °C) (Montogomery & Petropakis, 1980), the decrease of lightness of pear samples upon microwave processing was very likely due to either non-enzymatic browning or cell wall disruption that led cell contents leak into intercellular voids, or a combination of both.
Diced pear samples after 2 min being submerged in 1000 ppm ascorbic acid without further treatment (a), with either subsequent microwave processings W100 (b, f), W110 (c, g) and W120 (d, h), or subsequent retort processing R110 (e, i), with those stored for 0 day are placed in the left hand side (b–e) while those stored for 2 weeks at 4 °C are placed in the right hand side (f–i)
Reduced lightness of pear purée due to heat treatment was previously reported by Ibarz et al. (1999). The lightness of 11 °Brix industrial pear purée decreased faster when the heating temperature increased from 80 to 98 °C and followed a first-order kinetic. In addition, more steam may have been produced at intracellular spaces at a higher temperature and this may have contributed to cell wall disruption and penetration of cellular fluid from cells into intercellular voids, resulting in reduction of lightness. In contrast, increased of lightness was reported in microwave treated kiwifruit (Benlloch-Tinoco et al., 2012) and strawberry (Marszałek et al., 2015) purées, which was most likely due to degradation of pigments.
Nonetheless, all pear samples did not show any significant (p > 0.05) changes in total colour difference (ΔE*) during storage up to 6 weeks at 4 °C (Fig. 5a). This implied that PPO enzymes deactivation was nearly complete. However, small browning spots were observed in some pear samples after 2 weeks at 4 °C following microwave processing at W120 (Fig. 4h).
Total Solids (°Brix) and pH
Total solids (°Brix) of all samples (Table 1) did not show any significant (p > 0.05) differences between treatments or storage intervals. Similar results were reported in a previous study conducted on microwaved kiwifruit purée by Benlloch-Tinoco et al. (2012). Microwave processing did not alter the physical structure of pear samples dramatically; they remained as solids with small amounts of exudates (data not shown). Therefore, their °Brix did not show significant changes after processing.
Similarly, pear samples also did not show a significant (p > 0.05) change in pH following microwave processing and storage (Table 1). Previously, Benlloch-Tinoco et al. (2015) also showed that there were no significant pH changes in kiwifruit purée following microwave treatment. In our study and in Benlloch-Tinoco et al.’s (2015) study on kiwifruit purée, microwave treatments were applied for a relatively short time duration (283 s (in heating section) and 340 s, respectively). Our pear samples reached the maximum temperature of ~ 103 °C while their kiwifruit samples reached about ~ 85 °C (Benlloch-Tinoco et al., 2014b). However, in both pear and kiwi fruit samples, the heat treatments may have contributed to the inactivation of enzymes that could lead to pH changes.
Polyphenol Oxidase
All three microwave treatments deactivated polyphenol oxidase (PPO) enzyme to less than the minimum detectable residual activity (less than 2%) and it was assumed to be 0% (Table 2). Storage at 4 °C for 2 weeks did not change these values significantly. Retort processing also decreased PPO enzyme activity in pear samples; however, it left higher level of residual PPO enzyme (15.43%) than microwave processing (Table 2). This may be due to the differences in time temperature profiles (Fig. 2). The durations of retort and microwave processing were 18.0 and 4.7 min, respectively. However, the Tmax of retort processing was 96.10 °C while the Tmax of microwave processing ranged between 99.75 and 103.15 °C (Fig. 3a). This result indicated that microwave treatment has a greater impact on PPO enzymes deactivation in pears compared to retort treatment. The superior performance of microwave treatment on PPO enzyme deactivation in kiwifruit purée compared to conventional heat treatment (circulating thermostatic water bath) was reported by Benlloch-Tinoco et al. (2015).
Pectin Methylesterase
In this current study, control (raw) samples prior to microwave processing showed similar value of residual pectin methylesterase (PME) enzyme, indicating similar maturity of pears (Nagel & Patterson, 1967). Meanwhile, the pears subjected to retort processing were less mature which was shown by lower value of residual PME enzyme (Table 2). Nonetheless, the values from both control samples were not significantly (p > 0.05) different. The residual PME enzyme levels after microwave or retort processing were also not significantly (p > 0.05) different. Therefore, this current study did not provide conclusive results to indicate the comparative advantage of microwave processing over retort processing for PME enzyme deactivation in diced pears.
The difficulty to deactivate PME enzyme of pear sample was possibly due to the presence of the enzyme in situ both in active and dormant forms. According to Balogh et al. (2004), purified PME enzyme from carrot was more prone to thermal degradation compared to PME in carrot juice while PME in carrot pieces was the most thermostable one.
PME is required for demethoxylation of pectin, which is a polysaccharide that builds plant cell walls. The strength of the cell wall increases through aggregation with calcium during demethoxilation. The resulting demethoxilated pectin, however, becomes a substrate for polygalacturonase and pectate lyase; both are enzymes which catalyse pectin depolymerisation. The later reaction reduces cell wall firmness (Jolie et al., 2010).
During ripening and maturation, the amount of PME enzyme in pear fruit increases until a maximum value is reached (Nagel & Patterson, 1967). As PME enzyme remains stable, the amount of polygalacturonase enzyme increases, which results in softening of pears during ripening. The amount of both enzymes drops when the pear becomes soft (Ahmed & Labavitch, 1980). Therefore, to avoid further softening of the final product, PME should be completely deactivated.
Texture and Microstructure
There was a significant reduction in hardness of the samples after microwave processing (59.7–71.3%) or retorting (88.5%) (Fig. 5b). The hardness of microwaved samples immediately after processing was higher than that of retorted samples, although were not significantly (p > 0.05) different. There was no significant (p > 0.05) interaction effect between microwave treatments and storage intervals. Martínez-Hernández et al. (2013) reported a 13.2% decrease of texture in kailan-hybrid broccoli stem following a sous vide microwave treatment at 900 W for 2.5 min. Sous vide refers to cooking products under vacuum packaged condition. Besides the applied high temperature, conventional boiling induced an osmosis-based cell fluid leaching and thus the boiled products experienced great loss of firmness.
Even after 2 weeks of storage at 4 °C, the CLSM image of microwaved sample (Fig. 6b) showed intact cell walls, although the intracellular adhesion amongst cells was no longer retained and the intracellular voids were filled with juice. This possibly resulted in a reduction in hardness compared to that of the control sample. The hardness of microwaved samples continued to decrease slightly when the storage was extended to 6 weeks (Fig. 5b), most likely due to the presence of residual PME enzyme (Table 2).
Confocal images of diced pear samples that undergone 2 min submerging in 1000 ppm ascorbic acid without further treatment (a), with microwave processing W110 followed by 2 weeks storage at 4 °C (b), and retort processing R110 followed by 2 weeks storage at 4 °C (c). The length of the scale bar is 250 μm of 1024 × 1024 μm images
Retort processing resulted in a more obvious cell wall rupture and damage (Fig. 6c) and thus the hardness of pears decreased, lower than those of microwaved samples (Fig. 5b). Even though there was residual PME enzyme activity (Table 2), the hardness of retorted samples remained unchanged during 6 weeks of storage at 4 °C (Fig. 5b).
Microbiology
Approximately 2 log reduction of SPC was obtained after pear samples were subjected to microwave processing (Table 3). The final SPC of retorted and microwaved samples was also < 10. The SPC of both microwaved and retorted samples remained < 10 during 6 weeks of storage at 4 °C. This result was supported by relatively constant pH of processed pear samples during storage (Table 1), indicating very low to no aerobic microbiological growth.
The average initial counts were less than 3.0 log cfu/g, indicating its origin from fresh and good quality fruits (Tournas et al., 2006). Both initial Y&M in the control samples may have been resulted from the surface microflora of pear fruits. Yeast growth, however, is more likely to take place in foods with high acidity (low pH), high sugar or high salt contents, foods preserved with weak acids and frozen foods. Fruit products, including fruit salads, are prone to fermentation by yeasts (Fleet, 2011). Mould also could survive in acidic foods and high sugar foods and the resultant spoiled foods are often accompanied by off-odour and mycelial mats growing on their surface (Lima Tribst et al., 2009).
Y&M decreased to < 100 following either microwave or retort processing and remained the same during storage at 4 °C for 6 weeks (Table 3). The low number throughout cold storage implies that the remaining yeast and mould in the samples could not grow at refrigeration temperature. Samples during cold storage (Fig. 4f–i) did not show either visual signs of spoilage or alcoholic odours.
PCA of Physicochemical Properties
PCA was applied to physicochemical (°Brix, pH, ΔE* and hardness) properties of both microwaved and retorted samples that were stored for 0, 1, 2, 4 and 6 weeks at 4 °C. PCA of volatile compounds was performed separately. Predicted values for some missing data were obtained using a linear interpolation.
The four parameters were not correlated based on Pearson analysis (r < 0.7). The top two principle components (PC1 and PC2) scored F1 = 44.93% and F2 = 30.61%, and thus the total variance of both PCs exceeded 70%. The eigenvalue of each PC was more than 1. In PC1, the corresponding loadings were positive for ΔE* (0.89), pH (0.81) and °Brix (0.56) and negative for hardness (− 0.20). In PC2, the loading factors were high and positive for °Brix and hardness (0.63 and 0.89, respectively) and low and negative for pH (− 0.01) and ΔE* (− 0.19).
There are four clusters in the PCA biplot (Fig. 7). Cluster A consisted of samples No. 1, 5 and 9, which were undergone microwave processing W100 and stored at 4 °C for 0, 1 and 2 weeks, respectively. This cluster was characterised with higher °Brix and pH values compared to those of other samples even though the difference was not significant (p > 0.05, Table 1). Samples in this cluster also displayed moderate hardness in comparison to other processed samples.
Microwaved samples W100 after storage at 4 °C for 4 and 6 weeks (samples No. 13 and 17, respectively) experienced decrease in hardness, and therefore they belonged to cluster B together with all retorted samples. In comparison to others, cluster B is distinctive due to its lower hardness and greater ΔE*.
Cluster D consisted of diced pears that were subjected to microwave processing with either W110 or W120 followed by storage at 4 °C for 0, 1 and 2 weeks. Samples in cluster D exhibited moderate hardness and ΔE*. When the storage was continued to 4 and 6 weeks, microwaved samples W110 and W120 displayed further decrease in hardness and thus they formed cluster C. Samples in this cluster also showed decrease in pH during storage, although it was not significant (p > 0.05).
Volatile Compounds
Thirty-six compounds were identified through SPME analysis. These compounds with the sample codes were assessed using PCA to outline the differences in volatile profiles among samples. Whenever the absolute values of their correlations were at least 0.7, one compound from each pair was taken for subsequent analysis. Principal components (PCs) were extracted until the cumulative contribution accounted for more than 70% of the total variance. The abscissa and ordinate in Fig. 8 revealed that the contributions of the first and the second principal components (PC1 and PC2) were F1 = 48.31% and F2 = 25.70%, respectively. The eigenvalue of either PC1 or PC2 exceeded 1 and both accounted for 74.01% of the total variance. In PC1, the corresponding loadings were positive for butyl acetate, 1-butanol and furfural, for which the factor loadings were 0.69, 0.84 and 0.87, respectively. In the same PC, the loadings for d-limonene and furfural were − 0.47 and − 0.53, respectively. In PC2, all corresponding loading was positive, i.e. for butyl acetate (0.11), hexanal (0.64), 1-butanol (0.49), d-limonene (0.75) and furfural (0.24).
The biplot (Fig. 8) showed the similarity of raw samples No. 1, 2 and 3, while raw sample No. 4 showed a much lower 1-butanol concentration. According to Jennings (1961), 1-butanol as well as 1-hexyl alcohol were two major alcohols present in ripe pears. Thus, the lower content of 1-butanol in sample No. 4 was possibly because this sample was less ripened than the other raw samples, which was also supported by its greenish colour (data not shown). Similarly, the concentration of d-limonene in William Bartlett pear also depends on the fruit maturity. Those which were ripened during storage showed a higher amount of d-limonene (Zlatić et al., 2016).
The amount of 1-butanol, furfural and butyl acetate of pear samples increased in microwave processed samples and further during storage (Fig. 9). This could be traced in the biplot (Fig. 8), for example raw sample No. 1 was subjected to microwave treatment W100 and the resultant microwaved sample was coded as No. 5. Sample No. 5 showed increase of 1-butanol, furfural and butyl acetate. The amount of these volatile compounds further increased during storage for 2 and 6 weeks, as displayed by samples No. 9 and 13, respectively. Microwave treatments, W110 and W120, and the subsequent storage also altered the volatile profile of pear samples similar to microwave treatment W100. Retort processing R110, however, did not change the volatile profile dramatically as seen in the biplot (Fig. 7). Raw sample No. 4 was not far from retorted sample No. 8. Storage did not change the volatile profile of retorted samples. All retorted samples were close to each other in cluster R (Fig. 8).
Concentrations (milligrams per kilograms) of butyl acetate (a), hexanal (b), 1-butanol (c), d-limonene (d) and furfural (e) from raw, microwaved (W100, W110 and W120), and retorted (R110) samples after 0, 2 and 6 weeks of storage at 4 °C. Different superscript letters (abc) within each graph indicate values are significantly different at the level of p < 0.05. Prediction values (*) are given for retorted (R110) samples after 2 weeks of storage
The increased concentration of furfural derivatives due to processing and storage was anticipated, although the significant (p < 0.05) parameter was treatment only (Fig. 9e). According to Hernandez et al. (1997), furfural derivatives were associated with Maillard reaction products and their concentration in fruit juices increased with either increasing temperature or time. This was also seen in the samples in this study, regardless of the type processing.
Butyl acetate, along with hexyl acetate, are the two dominant compounds, which occupied 33% and 39%, of the total volatiles of William Bartlett pears (Suwanagul & Richardson, 1998), respectively. They are generally associated with fruity aroma. Their release increased during softening of fresh William Bartlett pears and further increased during storage at 0 °C for 7–14 days (Makkumrai et al., 2014). 1-Hexanol and 1-butanol were the major alcohols and 1-butanol was also associated with fruity aroma of William Bartlett pears (Makkumrai et al., 2014). These only made up 1% of total volatiles since they were the corresponding precursor of butyl and hexyl acetates (Suwanagul & Richardson, 1998). Since the concentration of 1-butanol is very low, while the odour threshold is much higher than that of butyl acetate, the contribution of 1-butanol to the overall aroma perception of Wiiliam Bartlett pears is minor (Makkumrai et al., 2014).
Increase of both butyl acetate and 1-butanol concentrations was only experienced in microwaved samples (Fig. 9a and c, respectively). The content of both compounds was significantly (p < 0.05) affected by treatment. The butyl acetate content was also significantly (p < 0.05) affected by the storage time. Concentrations of these two compounds decreased upon retort processing and thus separated the retorted sample No. 8 from microwaved samples (Fig. 8). This difference suggested that microwave and retort processing delivered heat in different mechanisms at cellular levels, which further led to different volatile formations.
Hexanal is responsible for green and sour odour in pears. Hexanal is abundant in Granny smith apple, and thus hexanal is believed to be responsible for apple aroma in pears (Makkumrai et al., 2014). Hexanal is also present in some pear varieties; it was reported by Makkumrai et al. (2014) to appear in William Bartlett pears, though Suwanagul and Richardson (1998) did not mention any aldehydes in the headspace volatile profile of William Bartlett pears. The concentration of hexanal in the pear samples investigated in this study decreased dramatically to 5–25% of its initial concentration soon after processing (Fig. 9b), regardless of the applied treatments, and further decreased during storage. Su and Wiley (2006) observed that apple juice which was pasteurised in water bath at 85 °C for 10 min experienced a non-significant (p > 0.05) decrease of hexanal concentration. Meanwhile, Aguilar-Rosas et al. (2007) reported a 70% decrease of hexanal after apple juice was subjected to high temperature short time (HTST) pasteurisation at 90 °C for 30 s. Both studies suggested a thermal degradation as a reason for the decrease of hexanal in apple juice upon processing. Thermal degradation may be the cause of hexanal reduction in the microwaved and retorted samples in this study, since both treatments led to a temperature above 90 °C.
Thermal degradation is also a possible cause of d-limonene reduction in the pear samples in this study (Fig. 9d). The d-limonene compound is a cyclic terpene with sweet orange/citrus smell, which is present in William Bartlett pear at a much lower concentration compared to esters and dienoates (Zlatić et al., 2016). The degradation of d-limonene in orange juice was temperature dependent (Boff et al., 2003; Min et al., 2003). Therefore, d-limonene in orange juice that was subjected to non-thermal processing such as pulsed electric field (40 kV/cm for 97 ms) which led to temperature exposure at 58 °C for 5 s (Min et al., 2003) or high pressure processing at 600 MPa and 25 °C for 346 s (Boff et al., 2003) retained 87% of d-limonene in comparison to that of freshly squeezed orange juice. Meanwhile, conventionally pasteurised (held at 90 °C for 30–90 s) orange juice only retained 60–70% of d-limonene (Min et al., 2003).
In the present study, the concentration of d-limonene content of retorted samples decreased to a lesser extent when compared to the microwave treated samples. Further significant (p < 0.05) reduction of d-limonene content was observed during storage of microwave treated samples.
Conclusion
This study has demonstrated the potential of both continuous microwave treatment (4.7 min) and retort (18.0 min) processing for preparation of ready to eat (RTE) pear snacks with 6 weeks shelf life at 4 °C as indicated by the microbiology and quality parameters. All three microwave treatments and the retort processing resulted in a 2-log reduction of SPC with final SPC below 10. The physicochemical properties of microwave treated pear samples were superior to those of retorted pear samples. Microwave processing was effective in reducing the residual PPO enzyme level to below detection level (less than 2%) and, thus, prevented enzymatic browning. The texture of microwave treated samples was harder than that of the retorted samples. Microstructure investigation revealed the presence of a more intact cellular structure which may have led to higher hardness values of microwave treated pear samples. The volatile profiles of microwave treated samples were characterised with increased furfural, 1-butanol and butyl acetate, and thus made them different from those of retorted samples. Short microwave processing treatments (4.7 min) could be used to extend the shelf life of diced pears for a minimum of 6 weeks at 4 °C which is a fourfold increase in shelf life compared to fresh cut products that have a shelf life of 7 to 10 day at 4 °C. Further investigation is required to observe sensorial acceptance of this microwave processed pear snack.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aguilar-Rosas, S. F., Ballinas-Casarrubias, M. L., Nevarez-Moorillon, G. V., Martin-Belloso, O., & Ortega-Rivas, E. (2007). Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. Journal of Food Engineering, 83(1), 41–46.
Ahmed, E. A., & Labavitch, J. M. (1980). Cell wall metabolism in ripening fruit. Plant Physiology, 65(5), 1014–1016.
Ahmed, J., & Ramaswamy, H. S. (2004). Microwave pasteurization and sterilization of foods. Food Science and Technology-New York-Marcel Dekker, 167, 691.
Australian StandardTM AS 5013.1 (2004). Food Microbiology, Method 1: Examination for specific organisms – Standard plate count.
Australian StandardTM AS 5013.29 (2009). Food Microbiology, Method 29: Examination for specific organisms – Colony count of yeasts and moulds.
Awuah, G. B., Ramaswamy, H. S., & Economides, A. (2007). Thermal processing and quality: Principles and overview. Chemical Engineering and Processing, 46(6), 584–602.
Balla, C., Farkas, J., & Dalmadi, I. (2012). Developments in minimal processing of fruits. In N. K. Sinha, J. S. Sidhu, J. Barta, J. S. Wu, & M. Pilar Cano (Eds.), Handbook of Fruits and fruit processing (2nd ed., pp. 153–173). Wiley.
Balogh, T., Smout, C., Nguyen, B. L., Van Loey, A. M., & Hendrickx, M. E. (2004). Thermal and high-pressure inactivation kinetics of carrot pectinmethylesterase: From model system to real foods. Innovative Food Science & Emerging Technologies, 5(4), 429–436.
Basak, K., & Ramaswamy, H. S. (1996). Ultra high pressure treatment of orange juice: a kinetic study on inactivation of pectin methyl esterase. Food Research International, 29(7), 601–607.
Benlloch-Tinoco, M., Varela, P., Salvador, A., & Martínez-Navarrete, N. (2012). Effects of microwave heating on sensory characteristics of kiwifruit puree. Food and Bioprocess Technology, 5(8), 3021–3031.
Benlloch-Tinoco, M., Igual, M., Rodrigo, D., & Martínez-Navarrete, N. (2013). Comparison of microwaves and conventional thermal treatment on enzymes activity and antioxidant capacity of kiwifruit puree. Innovative Food Science & Emerging Technologies, 19, 166–172.
Benlloch-Tinoco, M., Igual, M., Salvador, A., Rodrigo, D., & Martínez-Navarrete, N. (2014a). Quality and acceptability of microwave and conventionally pasteurised kiwifruit puree. Food and Bioprocess Technology, 7(11), 3282–3292.
Benlloch-Tinoco, M., Martínez-Navarrete, N., & Rodrigo, D. (2014b). Impact of temperature on lethality of kiwifruit puree pasteurization by thermal and microwave processing. Food Control, 35(1), 22–25.
Benlloch-Tinoco, M., Igual, M., Rodrigo, D., & Martínez-Navarrete, N. (2015). Superiority of microwaves over conventional heating to preserve shelf-life and quality of kiwifruit puree. Food Control, 50, 620–629.
Bindu, J., Ravishankar, C. N., & Gopal, T. K. S. (2007). Shelf life evaluation of a ready-to-eat black clam (Villorita cyprinoides) product in indigenous retort puches. Journal of Food Engineering, 78(3), 995–1000.
Boff, J. M., Truong, T. T., Min, D. B., & Shellhammer, T. H. (2003). Effect of thermal processing and carbon dioxide-assisted high-pressure processing on pectinmethylesterase and chemical changes in orange juice. Journal of Food Science, 68(4), 1179–1184.
Carbonara, M., & Mattera, M. (2001). Polyphenoloxidase activity and polyphenol levels in organically and conventionally grown peach (Prunus persica L., cv. Regina bianca) and pear (Pyrus communis L., cv. Williams). Food Chemistry, 72(4), 419–424.
Duvetter, T., Fraeye, I., Van Hoang, T., Van Buggenhout, S., Verlient, I., Smout, C., et al. (2005). Effect of pectinmethylesterase infusion methods and processing techniques on strawberry firmness. Journal of Food Science, 70(6), S383–S388.
Fleet, G. H. (2011). Yeast spoilage of foods and beverages. In C. P. Kurtzman, J. W. Fell, & T. Boekhout (Eds.), The Yeasts: A Taxonomic Study (Vol. 3, 5th ed.). Elsevier Science.
Gamage, T. V., Sanguansri, P., Swiergon, P., Eelkema, M., Wyatt, P., & Leach, P. (2015). Continuous combined microwave and hot air treatment of apples for fruit fly (Bactrocera tryoni and B. jarvisi) disinfestation. Innovative Food Science & Emerging Technologies, 29, 261–270.
Gaulliard, F., & Richard-Forget, F. (1997). Polyphenoloxidases from Williams pear (Pyrus communis L, cv Williams): Activation, purification, and some properties. Journal of the Science of Food and Agriculture, 74(1), 49–56.
Hagerman, A. E., & Austin, P. J. (1986). Continuous spectrophotometric assay for plant pectin methyl esterase. Journal of Agricultural and Food Chemistry, 34(3), 440–444.
Hernandez, T., Ausín, N., & Bartolomé, B. (1997). Variations in the phenolic composition of fruit juices with different treatments. Zeitschrift für Lebensmitteluntersuchung und -Forschung A, 204(2), 151–155.
Ibarz, A., Pagán, J., & Garza, S. (1999). Kinetic models for colour changes in pear puree during heating at relatively high temperatures. Journal of Food Engineering, 39(4), 415–422.
Jennings, W. G. (1961). Volatile esters of Bartlett pears. Journal of Food Science, 26(6), 564–568.
Jolie, R. P., Duvetter, T., Van Loey, A. M., & Hendrickx, M. E. (2010). Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydrate Research, 345(18), 2583–2595.
Lima Tribst, A. A., Ana, d. S. S., & Rodriguez de Massaguer, P. (2009). Review: Microbiological quality and safety of fruit juice—past, present and future perpectives. Critical Reviews in Microbiology, 35(4), 310–339.
Makkumrai, W., Anthon, G. E., Sivertsen, H., Ebeler, S. E., Negre-Zakharov, F., Barrett, D. M., & Mitcham, E. J. (2014). Effect of ethylene and temperature conditioning on sensory attributes and chemical composition on 'Bartlett' pears. Postharvest Biology and Technology, 97, 44–61.
Marszałek, K., Mitek, M., & Skąpska, S. (2015). Effect of continuous flow microwave and conventional heating on the bioactive compounds, colour, enzymes activity, microbial and sensory quality of strawberry purée. Food and Bioprocess Technology, 8(9), 1864–1876.
Marszałek, K., Woźniak, Ł., Skąpska, S., & Mitek, M. (2016). A comparative study of the quality of strawberry purée preserved by continuous microwave heating and conventional thermal pasteurization during long-term cold storage. Food and Bioprocess Technology, 9(7), 1100–1112.
Martín-Belloso, O., Soliva-Fortuny, R., & Oms-Oliue, G. (2012). Fresh-cut fruits. In N. K. Sinha, J. S. Sidhu, & J. Barta (Eds.), Handbook of Fruits and Fruit Processing (2nd ed., pp. 245–262). Wiley-Blackwell.
Martínez-Hernández, G. B., Artés-Hernández, F., Colares-Souza, F., Gómez, P. A., García-Gómez, P., & Artés, F. (2013). Innovative cooking techniques for improving the overall quality of a kailan-hybrid broccoli. Food and Bioprocess Technology, 6(8), 2135–2149.
Min, S., Jin, Z. T., Yeom, H., & Zhang, Q. H. (2003). Commercial-scale pulsed electric field processing of orange juice. Journal of Food Science, 68(4), 1265–1271.
Montogomery, M., & Petropakis, H. (1980). Inactivation of Bartlett pear polyphenol oxidase with heat in the presence of ascorbic acid. Journal of Food Science, 45(4), 1090–1091.
Nagel, C. W., & Patterson, M. E. (1967). Pectic enzymes and development of the pear (Pyrus communis). Journal of Food Science, 32(3), 294–297.
Pathare, P. B., Opara, U. L., & Al-Said, F. A.-J. (2013). Colour measurement and analysis in fresh and processed foods: a review. Food and Bioprocess Technology, 6(1), 36–60.
Perdue, R. R. (2004). Packaged pasteurized fresh fruits and a method for production. USPTO 20060034980.
Picouet, P. A., Landl, A., Abadias, M., Castellari, M., & Viñas, I. (2009). Minimal processing of a Granny Smith apple purée by microwave heating. Innovative Food Science & Emerging Technologies, 10(4), 545–550.
Rinaldi, M., Litttardi, P., Paciulli, M., Ganino, T., Cocconi, E., Barbanti, D., Rodolfi, M., Aldini, A., & Chavaro, E. (2020). Impact of ohimic heating and high pressure processing on qualitative attributes of ohmic treated peach cubes in syrup. Food, 9(8), 1093.
Rouweler, J. (2015). Heat Process Values F (2nd Ed.) for several Commercial pasteurization and sterilization processes: Overview, uses, and restrictions. Retrieved from https://www.researchgate.net/publication/278033357_Heat_Process_Values_F_2_nd_Ed_for_several_Commercial_Pasteurization_and_Sterilization_Processes_Overview_Uses_and_Restrictions?channel=doi&linkId=557ad7aa08aee4bf82d59719&showFulltext=true. Accessed 17 Feb 2021.
Sánchez-Moreno, C., De Pascual-Teresa, S., De Ancos, B., & Pilar Cano, M. (2012). Nutritional quality of fruits. In N. K. Sinha, J. S. Sidhu, J. Barta, J. S. B. Wu, & M. P. Cano (Eds.), Handbook of Fruits and Fruit Processing (2nd ed., pp. 73–84). Wiley.
Shen, S.-C., Wu, M.-C., & Wu, J. S. (2012). Conventional Thermal Processing and Preservation. In N. K. Sinha, J. S. Sidhu, J. Barta, J. S. B. Wu, & M. P. Cano (Eds.), Handbook of Fruits and Fruit Processing (2nd ed., pp. 121–131). Wiley.
Siguemoto, E. S., & Gut, J. A. W. (2016). Dielectric properties of cloudy apple juices relevant to microwave pasteurization. Food and Bioprocess Technology, 9(8), 1345–1357.
Siguemoto, E. S., Pereira, L. J., & Gut, J. A. W. (2018). Inactivation kinetics of pectin methylesterase, polyphenol oxidase, and peroxidase in cloudy apple juice under microwave and conventional heating to evaluate non-thermal microwave effects. Food and Bioprocess Technology, 11(7), 1359–1369.
Singh, R., & Sarkar, A. (2005). Thermal Properties of Frozen Foods. In M. Rao, S. Rizvi, & A. Datta (Eds.), Engineering Properties of Foods (3rd ed., pp. 175–201). CRC Press.
Su, S. K., & Wiley, R. C. (2006). Changes in apple juice flavor compounds during processing. Journal of Food Science, 63(4), 688–691.
Suwanagul, A., & Richardson, D. G. (1998). Identification of headspace volatile compounds from different pear (Pyrus communis L.) varieties. Acta Horticulturae, 475, 605–624.
Toledo, R. R. (2007). Fundamentals of Food Process Engineering (3rd ed.pp. 125–152). Springer Science+Business Media, LLC.
Tournas, V. H., Heeres, J., & Burgess, L. (2006). Moulds and yeasts in fruit salads and fruit juices. Food Microbiology, 23(7), 684–688.
Vadivambal, R., & Jayas, D. S. (2007). Changes in quality of microwave-treated agricultural products—a review. Food and Bioprocess Technology, 98(1), 1–16.
Vadivambal, R., & Jayas, D. S. (2010). Non-uniform temperature distribution during microwave heating of food materials—a review. Food and Bioprocess Technology, 3(2), 161–171.
WHO. (2003). Diet, nutrition and the prevention of chronic diseases Report of a Joint WHO/FAO Expert Consultation. Geneva.
Zlatić, E., Zadnik, V., Fellman, J., Demšar, L., Hribar, J., & Željko, Č. (2016). Comparative analysis of aroma compounds in ‘Bartlett’ pear in relation to harvest date, storage conditions, and shelf-life. Postharvest Biology and Technology, 117, 71–80.
Acknowledgements
The author A.F. Devi would like to express her gratitude to the Endeavour Research Fellowship providing financial support during her research.
Author information
Authors and Affiliations
Contributions
AFD conducted the experiments, analysed the results, drafted and revised the manuscript. XNA conducted the experiments. RW designed and conducted the experiments. PS and PS provided technical assistance for microwave and retort equipment and collected data. TS collected and analysed data. SN collected data. TVG concepted, designed and conducted experiments, and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devi, A.F., Au, X.N., Weerakkody, R. et al. Microwave Pasteurised Pear Snack: Quality and Microbiological Stability. Food Bioprocess Technol 14, 1615–1630 (2021). https://doi.org/10.1007/s11947-021-02642-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02642-x