Abstract
The impact of continuous microwave heating (MV) at 90 and 120 °C for 10 s and at 80 and 90 °C for 7 s, as well as conventional thermal processing (CTP) at 90 °C for 15 min, on the quality of strawberry purée was studied. Product quality was determined, covering total polyphenols, anthocyanins, phenolic acids, flavonols and vitamin C content, as well as colour parameters, enzymatic activity, and finally, the sensory and microbiological quality. CTP caused higher degradation of polyphenols (ca. 7 %), anthocyanins (ca. 20 %) and vitamin C (ca. 48 %) compared to MV at 120 °C. Changes of the colour of MV-preserved purée were insignificant (dE <2), whereas CTP caused significant changes (dE >3). Only MV at 120 °C and CTP effectively decreased the level of total microbial count (<1 log cfu/g), but considering enzymes activity, only CTP was effective, inactivating ca. 98 and 100 % of polyphenol oxidase (PPO) and peroxidase (POD), respectively. The most effective inactivation of PPO (82 %) and POD (88 %) after MV was noted at 120 °C, but the best compromise between colour and nutrient preservation and enzyme inactivation was achieved at the MV at 90 °C. Considering the obtained results, it can be concluded that the microwave heating better preserved the quality of strawberry puree than conventional heat pasteurization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heat processing is the method most commonly used in the fruit processing industry to ensure the safety and storage stability of products such as juices, nectars, purées, jams, etc. The heat treatment of fruit products is aimed not only at destroying microorganisms but also inactivating the main oxidative tissue enzymes, i.e. polyphenoloxidase (PPO) and peroxidase (POD), which can lead to undesirable changes during storage. However, traditional pasteurization can contribute to the degradation of many nutritionally important but temperature-sensitive components in fruits, such as vitamin C, anthocyanins and polyphenols (Verbeyst et al. 2013, Verbeyst et al. 2010). High temperatures and the long duration of the process can also cause changes to the product’s sensory quality, affecting the taste, flavour, texture and colour (Benlloch-Tinoco et al. 2013, Fazaeli et al. 2013, Verbeyst et al. 2013).
Taking into account, the growing consumer demand for high quality food which is microbiologically safe but which has well-preserved sensory and nutritional qualities of the fresh product, attempts are being made to minimize heat treatment during processing. This can be achieved by, e.g. shortening the heating time in flow heating systems compared to the batch (immersion) pasteurization process or by using innovative processing techniques, utilizing methods other than conventional steam or hot water heating to destroy microorganisms and tissue enzymes. One of these new methods is the application of microwave energy. At present microwave heating is used in various processes in food technology, such as tempering, vacuum drying, freeze drying, dehydration, cooking, blanching, baking, roasting, pasteurization, sterilization and extraction (Orsat et al. 2005). A new way of using microwave energy is to control postharvest brown rot in stone fruit (Sisquella et al. 2013, Sisquella et al. 2014).
Conventional flow pasteurization is usually accomplished by passing the product through a metal heat exchanger, either of the shell and tube or plate type. The typical processing parameters for fruit products are 80–90 °C for 10–60 s. Heat transferred to a fluid from a metal surface results in a thin layer of low velocity fluid adjacent to the metal surface. This layer is hotter than the bulk of the product, and it is where the thermal degradation of sensitive compounds can take place, as well as scorching. To minimize this degradation, the flow rates are kept high to provide a turbulent flow, and the temperature differential is kept low by using hot water or low pressure steam as the heating medium. Some high density products, i.e. fruits purée and sauces, cannot be pumped by traditional heat exchangers and need to be pasteurized using batch (discontinuous) process. Facing these challenges, companies, as well as scientists, are looking for alternative methods for preservation of thermally sensitive food products. One of such innovative methods is continuous flow microwave heating. This emerging technology can serve as an alternative to the conventional continuous heating of some fluid products. The radiant energy heats the product directly, without heating the tube walls, so it is possible to preserve higher density of fruit products, such as purée and products with fruit particles (De Ancos et al. 1999). Rapid start-up and shutdown are possible, and less heat is lost to the environment (Nikdel and MacKellar 1992). The most important advantages of continuous microwave heating are the fast rise in temperature, controllable heat distribution and the possibility of cooling the product in the flow. The equipment is much smaller compared to the traditional heat exchanger, as well as it does not need steam installation (Benlloch-Tinoco et al. 2013, Piasek et al. 2010).
This technique is therefore being considered in the search for processes of the microbiological stabilization of fruit products, which cause minimum damage to the products’ organoleptic characteristics and nutritional value (Juan et al. 2002). Several studies have been successfully carried out on the microwave pasteurization of fruit products, i.e. apple ciders, purée and juices (Gentry and Roberts, 2005, Tajachakavit et al. 1998, Picouet et al. 2009); kiwi fruit purée (De Ancos et al. 1999, Benlloch-Tinoco et al. 2013); pomegranate and black mulberry juices (Fazaeli et al. 2013); citrus juices (Nikdel and MacKellar, 1992, Nikdel et al. 1993) and papaya and strawberry purée (De Ancos et al. 1999), showing that due to the reduced time of exposure to energy, they preserve their natural organoleptic characteristics, and this type of treatment also lowers the risk of losing essential thermolabile nutrients.
Although microwave could potentially replace conventional heat processes for some specific applications, there were still problems that were inherent in this technology, such as non-uniform product temperature distribution. Patented by Listkin (2010), unique construction of resonance chamber can solve this problem. Furthermore, several of the studies mentioned above have proven the advantages of using microwave pasteurization on different fruit juices, the impact of continuous flow microwave heating, with rapid cooling in the heat exchanger and filling up at aseptic conditions, on the quality of high-density strawberry purée has not yet been explored.
A large body of research indicates that consuming foods that are high in phenolics and flavonoids contributes to a person’s health and may reduce the risk of heart disease and cancer (Gerard and Roberts 2004, Boero 2011, Törrönen et al. 1997). Strawberries are one of the most valuable sources of phenolic antioxidants, especially ellagic acid (Da Silva Pinto et al. 2008, Häkkinen and Törrönen 2000). This fruit represents the main source of ellagic acid derivatives, corresponding to more than 50 % of all phenolic compounds. Flavonols (kaempferol, quercetin) and phenolic acids (p-hydroxybenzoic and ellagic acid) have been proposed to have beneficial effects on health as antioxidants and anticarcinogens (Da Silva Pinto et al. 2008). There is a particular interest in the determination of the total ellagic acid content in fruits because of possible chemopreventive effects. Ellagic acid is a polyphenol found in nuts and berries, such as strawberry, raspberry and blackberry. This compound can exist as free form, glycoside or linked as ellagitannins esterified with glucose, although the free form of this compound is rarely found (Häkkinen and Törrönen 2000).
Strawberries also contain substantial amounts of vitamin C. All these nutrients are thermolabile compounds and are easily lost during thermal processing as it was shown in our previous study (Marszałek et al. 2015a, b). Also the main anthocyanin compound in strawberries, pelargonidin-3-glucoside, is very unstable during heating; it undergoes considerable degradation, deteriorating the product’s colour. Strawberries also contain oxidizing enzymes, polyphenoloxidase and peroxidase, which, if not completely inactivated, make the products susceptible to undesirable changes during storage (Häkkinen et al. 2000, Igual et al. 2010, Marszałek et al. 2015a, b). All the above-mentioned qualities make strawberries a suitable model material for studying the impact of different processing methods, i.e. high pressure processing (Marszałek et al. 2011, Marszałek et al. 2015a, b); supercritical carbon dioxide (Marszałek et al. 2015a, b) or microwave (Marszałek and Mitek 2012) on the quality of strawberry products.
The goal of this study was to determine the quality of strawberry purée preserved using the unique continuous microwave heating in comparison with the quality of the product subjected to conventional thermal processing. The key quality factors of purée were examined: total polyphenols, anthocyanins, phenolic acids, flavonols and vitamin C content, as well as colour parameters, enzymatic activity, and finally, the sensory and microbiological quality.
Materials and Methods
Production of Strawberry Purée
Strawberries cv. Senga Sengana, were harvested at the end of June 2010 and in July 2011, and pre-treated (washing, destalking, freezing) at the company “Ulmer” Sp.j. (Stare Zadybie, Poland). After freezing in a fluidization tunnel (UniDex, Poland), the strawberries were sorted (Niagara Sortex, Bühler, Switzerland) according to colour and size (45–55 mm), and stored at –24 °C until further processing. After defrosting (12 h at 4 °C), they were mashed up in a laboratory food processor (CL-30, Robot Coupe, France). The resultant semi–liquid strawberry pulp was homogenized (Mz-50, Fryma, Switzerland, Ø ≤ 0.5 mm) and deaerated at 0.06 MPa (LVE, Fryma, Switzerland). The temperature of the purée did not exceed 0 °C throughout the whole processing procedure. This fresh strawberry purée was used as a control sample (CS1—strawberries from 2010 and CS2—strawberries from 2011).
Preservation of Strawberry Purée
Microwave Treatment
Samples of strawberry purée were preserved using the innovative EnbioJet® continuous semi-industrial microwave heating system patented by Listkin in 2010 (2.45 GHz, 63 A, maximum power 20 kW, maximum efficiency 600 L/h, Enbio Technology Co., Kosakowo, Poland). EnbioJet® (Fig. 1) is capable of heating a sample up to 140 °C in a very short time 2–12 s (depending on the flow). The product was pumped into the microwave chamber (ca. 0.4 m width and 0.9 m length) through a Teflon tube (0.02 m diameter and 1.9 m length). In order to examine a wide range of device operation range, as well as based on preliminary experiment (Marszałek et al. 2012), the strawberry purée from 2010 was subjected to microwave heating (MV1) at a temperature of 90 ± 1 °C at atmospheric pressure or at 120 ± 1 °C and 0.35 MPa and holding time of 10 s (flow rate 2.0 L/min), whereas the purée from 2011 was subjected to microwave heating (MV2) at a temperature of 80 ± 1 or 90 ± 1 °C at atmospheric pressure and holding time of 7 s (flow rate 3.5 L/min). The come-up time during all processes was 2–5 s. After microwave heating, the purée was cooled in a continuous heat exchanger at up to 30 ± 1 °C. The inlet and outlet temperatures were monitored continuously using thermocouples inserted centrally in the tubes and attached to the data logger. All the parameters (temperature, pressure and time) were processed and reported using EnbioJet® DTL software. The average residence time of the test liquid in the microwave heat exchanger was obtained by dividing the total volume of the test sample inside the oven by the steady state volumetric flow rate of the liquid through the system. To prevent any contamination, the outlet of the sampling port was placed in an aseptic laminar chamber and samples were poured into 100 mL sterile jars.
Conventional Thermal Processing
The purée prepared using CS1 was pasteurized in 100 mL glass jars (55 mm diameter and 75 mm height) in a stainless steel, gas-heated bath pasteurizer (volume ca. 60 L). Process parameters of 90 °C for 15 min assured the purée’s microbial stability (Marszałek et al. 2011, Marszałek et al. 2014). The come-up time during pasteurization was 44 min. Data regarding the time and temperature were recorded inside the jars in the coldest spot, using a monitoring-measuring device (9004, Ellab, Denmark) designed for measuring the temperature inside a package and the temperature of the heating medium during the course of the process.
Pasteurization Units Calculation
The pasteurization units of microwave heating (MV) and conventional thermal (CTP) processes were calculated using the following equation: \( \mathrm{P}\mathrm{U}={\displaystyle {\int}_0^t10\left(\frac{{}^T(t){-}^T\mathrm{r}\mathrm{e}\mathrm{f}}{z}\right)}\mathrm{d}\mathrm{t} \), where t is temperature time (s), T (t) is product temperature at each treatment time T ref 80 °C (Heinz et al. 2003) and z is temperature sensitivity (13.62 °C) for Listeria monocytogenes (Benlloch-Tinoco et al. 2014).
Chemical Reagents
The following standards were used in the study: p-hydroxybenzoic acid (p-HBA), ellagic acid (EA), quercetin (Q), kaempferol (K), pelargonidin-3-glucoside (Pg-3-glc) and cyanidin-3-glucoside (Cy-3-glc) of HPLC purity (Extrasynthese, France); L-ascorbic acid (AA) of HPLC purity (Supelco, USA); DL-dithiothreitol (DTT) (>99 %) and polyvinylpyrrolidone (PVPP) (∼110 μm) (Fluka, USA); catechol (>99 %), gallic acid (GA) (>98 %), hydrogen peroxide (30 %), Triton X-100, and Trolox (>97 %) (Sigma-Aldrich, USA). The remaining reagents were purchased in POCh (Warsaw, Poland). Demineralized water used for the analyses was purified using Direct-Q 3 apparatus (Millipore, USA).
Analyses
Total Content of Polyphenols
Strawberry purée (10 g) was extracted for 5 min in an ultrasound bath (40 KHz, 100 W, 25 °C, ITR, Poland) using 30 g of an extraction mixture: methanol/water/hydrochloric acid (80:19.9:0.1; v/v/v) in centrifuge flasks. Afterwards, the sample was centrifuged in a laboratory centrifuge (MPW-350R, MPW Med. Instruments, Poland) at 3.670×g and 4 °C for 5 min. Extraction was repeated five times, supernatants were combined, and the methanol was evaporated under vacuum (B-481, Büchi, Switzerland). The residue was transferred quantitatively to a volumetric flask and filled up to 50 mL with 0.1 % (v/v) o-phosphoric acid.
The total content of polyphenols was determined spectrophotometrically using the Folin-Ciocalteu method modified by Gao et al. (2000), and was expressed in milligrammes of gallic acid equivalent per 100 g of the purée’s fresh weight (mg GAE/100 g FW).
HPLC Analysis of Phenolic Acids and Flavonols
The following free phenolic compounds: p-hydroxybenzoic acid (p-HBA), ellagic acid (EA), quercetin (Q) and kaempferol (K) were determined using the modified method of Odriozola-Serrano et al. (2008). Most of phenolic acids and flavonols are chemical bonded. To determine the total contents of phenolic compounds, acidic hydrolysis was conducted using the method used by Da Silva Pinto et al. (2007). Forty millilitres of a methanol/6 M hydrochloric acid/water mixture (56:25:19; v/v/v) was added to 1 mL of the extract (described in TCP section). The sample was heated at 120 °C for 90 min, then cooled and neutralized with 6 M NaCl to pH ca. 3.5.
The analysis of phenolic acids and flavonols in the HPLC system equipped with a DAD SPD-10Avp detector, thermostat CTD-10AsVp and DEGASEXTM DG-4400 degasser (Shimadzu, Japan) was carried out on a reversed phase Luna C18 column (250 × 4.6 mm, 5 μm, Phenomenex, USA) at a temperature of 30 °C, flow rate of 1 mL/min and detection at 260 nm (phenolic acids) and 360 nm (flavonols). The water/formic acid mixture (99:1, v/v) and acetonitrile were used as eluents (eluent A and B, respectively) in the following gradient programme: 10 % B (10 min), from 10 to 45 % B (15 min), from 45 to 70 % B (5 min), from 70 to 10 % B (3 min) and 10 % B (4 min). Selected phenolic compounds were quantified using p-HBA, EA, Q and K calibration curves. The contents of all compounds were expressed in mg/100 g FW. Peak identities were as described by Odriozola-Serrano et al. (2008) (Table 1).
HPLC Analysis of Anthocyanins
The HPLC determination of the anthocyanin content was carried out using the method described by Goiffon et al. (1999), with modifications consisting of changing the isocratic elution into gradient elution, and shortening the analysis time to 22 min. Ten millilitres of the extract (described in TCP section) were absorbed and purified on Sep-Pak C18 minicolumns (Waters, USA). Anthocyanins were eluted with 5 mL of a methanol/water/hydrochloric acid mixture (75:24.9:0.1; v/v/v), and then filtered on PTFE filters with a pore size of 0.45 μm (Waters, USA). Analyses were carried out in a gradient system using the equipment described in the previous section at a temperature of 25 °C, flow rate of 1 mL/min and detection at 520 nm. A water/formic acid mixture (89:11, v/v) and acetonitrile were used as eluents (A and B, respectively) in the following programme: 9 % B (15 min), from 9 to 20 % B (1 min), from 20 to 30 % B (1 min), from 30 to 9 % B (2 min) and 9 % B (4 min). Monomers of the anthocyanins were identified by comparing their retention times with those of the standards and with data from the literature. Pelargonidin-3-glucoside (Pg-3-glc), followed by cyanidin-3-O-glucoside (Cy-3-glc) contents were calculated based on their standard calibration curves. Pelargonidin-3-O-rutinoside (Pg-3-rut) was expressed as Pg-3-glc. Total content of anthocyanins (TCA) was calculated as the sum of Pg-3-glc, Cy-3-glc and Pg-3-rut. All results were expressed in mg/100 g FW.
HPLC Analysis of AA and DHAA
The total vitamin C content, expressed as L-ascorbic acid (AA) and L-dehydroascorbic acid (DHAA), was determined as described by Odriozola-Serrano et al. (2007). Approximately 1 g of purée was transferred to a volumetric flask and filled to 10 mL with 0.01 % of m-phosphoric acid. The extract was then filtered through PTFE 0.45 μm filters. In order to assay the DHAA, it was reduced to AA with 0.1 % DL-dithiothreitol (DTT) using 1 mL of the following mixture: extract/DTT (50:50; v/v), and leaving it in the dark for 1 h. The DHAA content was calculated using the formula: DHAA = (AA + DHAA) − AA.
The HPLC analysis (2695, Waters, USA) was carried out using a reversed phase SunFire C18 column (250 × 4.6 mm, 5 μm, Waters, USA) and a photodiode detector (DAD) (2996, Waters, USA). The analysis was carried out using the isocratic method using 0.01 % m-phosphoric acid at a flow rate of 1 mL/min and column temperature of 25 °C. AA was quantified using external calibration curves. The contents of AA and DHAA were expressed in mg AA/100 g FW. Peak identities were as described by Odriozola-Serrano et al. (2007).
Determination of PPO and POD Activities
The activity of selected tissue enzymes was determined as described by Terefe et al. (2010). The extraction mixture comprised 0.2 M phosphate buffer (pH = 6.5) containing 4 % (w/v) polyvinylpyrrolidone (PVPP), 1 % (v/v) Triton X-100 and 1 M NaCl. The strawberry purée and the mixture (4.5:4.5 g, w/w) were treated with ultrasound (40 KHz, 100 W, 25 °C, ITR, Poland) for 3 min and centrifuged (MPW-350R, MPW Med. Instruments, Poland) at 17,700×g for 30 min at 4 °C. The supernatant, after filtration through blotting filter paper, was used to determine PPO and POD activity.
For the PPO activity assay, 100 μL of the supernatant was introduced into 3 mL of 0.05 M phosphate buffer (pH 6.5) containing 0.07 M catechol, and the absorbance was measured spectrophotometrically (UV-1650PC, Shimadzu, Japan) at λ = 420 nm and 25 °C for 10 min. A blank sample was prepared in the same way, by substituting the supernatant with a phosphate buffer. The PPO activity was expressed as a change in the absorbance/min/g of FW of the analyzed sample.
For the POD activity assay, 1.5 mL of 0.05 M phosphate buffer (pH = 6.5) was added to the mixture containing 200 μL of the supernatant, 200 μL of 0.05 M phosphate buffer containing 1 % p-phenylenediamine (w/v) and 200 μL of 1.5 % (v/v) hydrogen peroxide. Mixture absorbance was measured at λ = 485 nm, 25 °C for 10 min. The POD activity was expressed as a change in the absorbance/min/g of FW of the analyzed sample.
Changes in Colour Parameters
The colour of the strawberry purée was determined using a CM-3600d colorimeter (Konica Minolta, Japan), in glass cuvettes with an optical path of 10 mm. The measurement was made on the CIEL*a*b* system, using illuminant D65. The values of the L*, a* and b* parameters enabled calculating the absolute difference of the samples’ colour after preservation compared to the control sample using two coefficients: ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2 and L* × a*/b*, where L* (lightness/darkness), a* (red/green) and b* (yellow/blue).
Microbiological Analyses
Yeasts and moulds were analyzed according to the ISO 21527-1:2008 standard. Purée samples were diluted using sterile normal saline (0.85 % sodium chloride), spread plated on sterile nutrient agar with dichloran rose bengal chlortetracycline (DRBC) and incubated at 25 °C for 5 to 7 days.
The total microbial count (TMC) was determined according to the EN ISO 4833:2003 standard. Samples diluted with sterile normal saline were plated count agar (PCA); plates were incubated at 30 °C for 72 h. All the experiments were conducted in duplicates, and mean values of 10 log cfu/g sample have been reported.
Sensory Analysis
The sensory analysis of strawberry products was conducted as described in ISO 4121:1998. A six-point scale was used to evaluate colour, appearance, consistency, aroma and taste, and an overall quality assessment was conducted using a nine-point hedonic scale; assessments were made by a trained sensory panel of eight persons (age 27–55 years). All samples were evaluated independently in a test room complying with ISO 8589:2007 requirements.
Statistical Analysis
All analyses were conducted using Statistica 10 StatSoft® software. The significance of the differences was computed based on an analysis of the variance with Tukey’s test (p value < 0.05). The Pearson coefficient of determination was used to establish the dependence of the measured values.
Three independent samples subjected to simultaneous treatment under the same conditions were analyzed in duplicate.
Results and Discussion
Pasteurization Unit
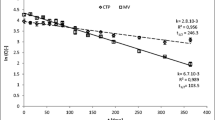
The temperature was monitored during both CTP and MV processes (Fig. 2). Based on this information, it was possible to calculate PU for comparison of both processes of different nature. Despite shorter time of heating, the highest level of PU was recorded for MV1/120 process (171.84 min) just before CTP (162.73 min). This was mainly due to much higher process temperature (MV at 120 °C) than during CTP (at 90 °C) regarding to T ref. During other MV processes, there were recorded much lower PU at 1.04, 0.77 and 0.15, respectively, for MV1 at 90 °C; and MV2 at 90 and 80 °C. This result shows that MV could be much more sensitive for product compared to CTP (batch pasteurization).
Temperature data during different processes. (CTP, white diamond suit) conventional thermal processing at 90 °C, 15 min. (MV1/90, white square) microwave heating at 90 °C, 10 s. (MV1/120, white up-pointing triangle) microwave heating at 120 °C, 10 s. (MV2/80, white circle) microwave heating at 80 °C, 7 s. (MV2/90, plus sign) microwave heating at 90 °C, 7 s. All microwave processes refer to the bottom timeline
Matsui et al. (2008a, b), in the study on the kinetic inactivation of specific enzymes during microwave and conventional heating, showed that z parameter depended on heating method (with similar parameters of both methods). Apart from this weak side of the above method, it can be used to show the differences in the heating dose between CTP and MV.
Content of Phenolic Compounds
TCP in fresh strawberry purée was 221 and 282 mg GAE/100 g FW for CS1 and CS2 samples, respectively (Table1). The decrease in TCP after CTP was 13.8 %, whereas microwave treatment at all temperatures and time used caused only a 3.6–6.6 % loss of these compounds.
Most of phenolic compounds in strawberries exist in bounded form; therefore, two phenolic acids: p-hydroxybenzoic acid (p-HBA) and ellagic acid (EA), and two flavonols: quercetin (Q) and kaempferol (K), known as the major phenolics of strawberries, were quantified, in both free and bounded form. Free phenolic acids and flavonols were determined after extraction from purée, whereas the total content of this compounds were determined after acidic hydrolysis. Fresh purées contained only small amounts of free EA, Q and K, whereas free p-HBA was not detected. The sum of free compounds constituted only 1.6 and 2.8 % of TCP in the CS1 and CS2 samples, respectively, which is consistent with the data reported by Cao et al. (2011). The analysis of the total content of these phenolic acids and flavonols, carried out after hydrolysis, showed that they had a 20 % contribution in TCP, in both fresh purée samples. The most abundant phenolic acid was EA, constituting 9 and 15 % of the TCP, respectively, for CS2 and CS1. The quercetin concentration found in two fresh purée samples differed significantly and was 4.6 times higher in CS2 than in CS1. The other flavonol, kaempferol, was present in much smaller amounts, similar in both samples, ca. 3 mg/100 g FW.
The contents of free ellagic acid, quercetin and the total contents of both phenolic acids and quercetin significantly increased after CTP, as was previously observed (Cao et al. 2011, Odriozola-Serrano et al. 2008). This phenomenon could be connected with spontaneous hydrolysis of bounded form to free compounds, as well as more intensive extraction of free form from tissue to intercellular juice during long-time heating. Although the PU for CTP and MV1 at 120 °C was similar the total time of heating was much longer during CTP, therefore, higher increase of phenolic compound after CTP is justified. The content of phenolics for free K (after MV2 at 80 °C) in purées MV1 and MV2 treated with microwaves at all time and temperature conditions used also showed a significant increase from 15 % for free Q to 42 % for total p-HBA compared to the control samples. Increasing the process temperature did not significantly affect TCP and individual compounds with the exception of the total content of kaempferol in the MV1 sample. The effect of conventional and microwave heating on the increase in the contents of the assayed polyphenol components is usually linked with the better extraction of these potentially antioxidative compounds from fruit tissue (Gerard and Roberts 2004).
Content of Anthocyanins
The characteristics of anthocyanins presented in Table 2 indicate that the prevailing compound in fresh purée was pelargonidin-3-O-glucoside (Pg-3-glc), followed by cyanidin-3-O-glucoside (Cy-3-glc) and pelargonidin-3-O-rutinoside (Pg-3-rut). The relative content of anthocyanin monomers in CS1 and CS2 was on average ca. 8:88:4 (±1) % (w/w) for Cy-3-glc/Pg-3-glc/Pg-3-rut, respectively. These results were similar to those reported by Odriozola-Serrano et al. (2008) and Cao et al. (2011), who found the same main monomeric anthocyanins in strawberries with similar relative contribution. The total content of anthocyanins (TCA) was 92.60 and 96.99 mg/100 g, respectively, for CS1 and CS2.
The effect of processing on the content of all investigated anthocyanins depended on the type of process used, as well as PU calculated based on temperature-time history. The greatest losses in these pigments, ca. 43 %, were observed after traditional pasteurization. Microwave heating was less destructive; the highest observed TCA losses were 23.3 % for MV1 at 120 °C. There was no correlation between anthocyanins degradation and calculated PU, but it can be explained by the different nature of the examined processes (higher temperature and much shorter time in MV). In most cases (except for Cy-3-glc of CS1), the decrease in the content of individual compounds, as well as TCA, did not intensify when a higher temperature was applied. Lack of changes may be due to small differences in PU (except for MV1 at 120 °C). On the other hand, a longer heating time caused more intensive degradation of the anthocyanin pigments. The decrease in the TCA was considerably higher (ca. 8 %) in MV1 at 90 °C compared to MV2 at the same temperature, which correlates with the level of PU for this samples. The results of other studies also show that anthocyanin degradation was more pronounced when using the conventional thermal processing compared to microwave heating (Piasek et al. 2010, Marszałek and Mitek 2012, Fazaeli et al. 2013). These differences may be caused by the shorter heating time and the uniform temperature distribution used in microwave equipment.
The relative content of anthocyanin monomers in CTP and microwave-treated samples was similar to the untreated purée ca. 8:88:4 (±1) % (w/w) for Cy-3-glc/Pg-3-glc/Pg-3-rut, respectively. Pg-3-rut appeared to be slightly more susceptible than other anthocyanins to conventional heating and microwave processing for a longer (10 s) time (MV1), especially at 120 °C. This might indicate that the Pg-3-rut is sensitive to high doses of heat in a short time. In milder conditions, during 7 s heating at 80 and 90 °C (MV2), the most unstable pigment was Pg-3-glc. Most authors have demonstrated, however, that Pg-3-glc is the most sensitive anthocyanin in strawberries (Verbeyst et al. 2010, Verbeyst et al. 2012). The thermal stability of anthocyanins depends on the equilibrium of flavylium cations, pseudo-bases and chalcones. Increasing the heating temperatures disturbs this equilibrium and induces the formation of colourless chalcones which, during long-term heating, are transformed into coloured (brownish) polymers (Özkan 2002, Fazaeli et al. 2013). These transformations are very important for determining the shelf life of these products.
Contents of AA and DHAA
The vitamin C content, expressed as the sum of AA and DHAA, in control samples was 53.38 and 55.37 mg/100 g FW in CS1 and CS2, respectively (Table 2). Ascorbic acid constituted ca. 70 % of the total vitamin C content in both samples of fresh purée. Similar results, from 32.4 to 84.7 mg of vitamin C in six strawberry cultivars, were reported by Hakala et al. (2003).
Thermal pasteurization significantly degraded vitamin C, reducing its content by 62 %. The level of vitamin C also decreased significantly in microwave-preserved purée, but the losses only reached from 4 % (MV1 at 90 °C) to 22 % (MV2 at 90 °C). The AA to DHAA ratio in purée did not change significantly after CTP and after MV conducted for 7 s (MV2 samples). However, in purée subjected to microwave treatment for 10 s at both temperatures (MV1 samples), an intensive oxidation of L-ascorbic acid to dehydroascorbic acid was noted. Although degradation of total vitamin C content was higher in MV2 samples (12–22 %), the equilibrium between AA and DHAA were strongly disrupted in MV1 samples. DHAA constituted 50 % of the total vitamin C content in these samples, compared to 20–26 % in MV2 samples. Considering that DHAA is less stable and more rapidly degraded than AA, the faster oxidation of AA during microwave heating for a longer exposure time may seem undesirable. In CTP sample, the equilibrium noted between AA and DHAA was similar to CS1. This may be due to a long heating time and much faster degradation of DHAA compared to the samples heated in microwave for a shorter time. A statistical analysis of the results showed that the destruction of vitamin C was influenced by the method of heating (CTP or MV), as well as the duration and temperature of the microwave treatment, and this may be due to differences in PU delivered to the product during heating. Some authors found that the degradation of vitamin C in orange juice was the highest during microwave heating compared to ohmic, infrared and conventional heating (Virkam et al. 2005), when others did not observe differences in vitamin C content of apple purée treated with microwaves and untreated samples (Picouet et al. 2009). They found that the AA content decreased, changing the proportion of DHAA from 39 to 57 %. The different and sometimes contradictory results concerning the stability of vitamin C during processing could be due to the different conditions used in each survey. Even a slight change of time, temperature, oxygen access, or the presence of metal or other reactive ions could significantly influence the degradation rate of AA.
Activity of PPO and POD
Peroxidase activity in both control samples was 1.59 ± 0.05 OD/min/g FW and was three times greater than the activity of polyphenoloxidase, which was 0.43 ± 0.02 OD/min/g FW (Fig. 2). Similar activities of these enzymes in different strawberry cultivars, ranging from 0.27 to 2.31 OD/min/g FW for POD and from 0.04 to 2.24 OD/min/g FW for PPO, were reported by other authors (Cano et al. 1997, Terefe et al. 2010, Terefe et al. 2013).
Only thermal pasteurization led to the effective inactivation of both analyzed enzymes. A substantial, ca. 80 %, decrease in PPO activity was observed in the strawberry purée preserved with microwaves at 90 and 120 °C, regardless of the heating time. The lower temperature 80 °C, in connection with higher flow rate resulting in a shorter (7 s) time of heating, was significantly less effective in inhibiting PPO, retaining ca. 38 % of its initial activity.
The study conducted by Terefe et al. (2010) on strawberry purée from two cultivars confirmed that PPO in this fruit was highly resistant (only 28 % of inactivation) to thermal inactivation at a temperature as high as 100 °C during 30 min processing time, while POD displayed very high thermosensitivity with complete inactivation in less than 5 min at 70 °C. PPO in kiwi fruit and strawberry purées in experiments conducted by De Ancos et al. (1999) exhibited a similar susceptibility to microwave treatment, after heating with 475 W for 60 s, 70 % of its activity was retained.
POD turned out to be less resistant to microwave heating than PPO. A higher degree of inactivation (ca. 88 %) was achieved when longer, 10 s, heating time was applied. Shortening the heating time to 7 s resulted in slightly but significantly lower inactivation, amounting to only 78 % in MV2 heated at 80 °C.
Matsui et al. (2008a, b) reported that microwave inactivation of PPO and POD of green coconut water was more efficient compared with conventional processes. They also proved that natural tissue enzymes are more resistant to inactivation compared to the model experiment.
De Ancos et al. (1999) reported that the stability of the POD depends on the species of fruit, i.e. POD in strawberries was more resistant to microwave inactivation compared to the POD of papaya and kiwi fruit; the microwave heating of strawberry purée (850 W, 60 s) caused only 8 % of this enzyme’s inactivation. On the other hand, Benlloch-Tinoco et al. (2013) showed that the inactivation of the POD and PPO in kiwi fruit purée processed in desired intervals of microwave power (300–900 W) and time (100–300 s) ranged from 43 to 88 %.
Changes in Colour
The visually perceived colour of strawberry purée can be presented as a combination of red and yellow hunter a* and b* values associated with L* value, representing lightness. To monitor total changes in a food product’s colour, two coefficients are often used: L* × a* / b* and ΔE (Rodrigo et al. 2007). As shown in Fig. 3, the L* × a* / b* coefficient in both control samples was ca. 77 and increased significantly (by 4.5 units) after thermal pasteurization. A significant increase in the L* × a* / b* coefficient was also observed in all samples after microwave treatment. The highest temperature (120 °C) caused an increase in the L* value and a decrease in the a* and b* parameters (data not show), which could suggest the thermal degradation of anthocyanins. In turn, at the lowest temperature (80 °C), all colour parameters increased, which probably resulted from the electromagnetic field extraction of anthocyanin pigments from the tissue to intercellular juice (Zhang et al. 2013, Gerard and Roberts 2004). The L* × a* / b* coefficient shows only direction of colour changes based on proportion between L*, a* and b* parameters, whereas ΔE coefficient shows differences between control sample and sample after process, therefore, there were no correlation between this coefficients.
Activity of enzymes in control sample and in pasteurized and microwave-preserved strawberry puree. Data: mean ± SD (n = 3), FW fresh weight, CS1 control sample from strawberries harvested in 2010, CS2 control sample from strawberries harvested in 2011; CTP conventional thermal processing at 90 °C and 15-min sample from 2010; MV1 microwave heating—strawberry from 2010, time of heating 10 s; MV2 microwave heating—strawberry from 2011, time of heating 7 s. The percentage of residual activities ((A / A 0) × 100) are presented, where A 0 represents the activity of the enzymes in the fresh strawberries (A 0 of PPO = 0.43 OD/min/g FW; A 0 of POD = 1.59 OD/min/g FW). Mean values denoted with the same letter are not statistically significantly different, p ≤ 0.05
The highest value of ΔE, corresponding to the greatest changes in colour, was noted in thermal pasteurized purée (ΔE = 3.0). The high changes in colour in thermally pasteurized strawberry pulp were reported by Cao et al. (2011) (ΔE = 10.18). Substantial changes (ΔE = 5.67) in strawberry purée after pasteurization were also noticed by Patras et al. (2009). Significantly lesser colour changes were observed in the samples preserved using microwave energy. The highest changes were observed in MV1 samples at 120 °C (ΔE = 1.41), and the lowest in the MV2 samples at 80 °C (ΔE = 0.91) and 90 °C (ΔE = 0.65). Such low ΔE values indicate well-preserved colour of all the microwaved samples, since it has been shown that if ΔE is lower than 1.5, the changes in colour are unnoticeable by inexperienced observers (Barba et al. 2013). De Ancos et al. (1999) also reported that microwave heating caused only slight colour changes in strawberry purée. The minor changes in the colour parameters could be related to the modern flow microwave heating system connected with the continuous cooling system used in this study (Fig. 4).
Colour changes in pasteurized and HEPP-preserved strawberry puree. Data: mean ± SD (n = 3); FW fresh weight; CS1 control sample from strawberries harvested in 2010; CS2 control sample from strawberries harvested in 2011; CTP conventional thermal processing at 90 °C and 15 min—sample from 2010; MV1 microwave heating—strawberry from 2010, time of heating 10 s; MV2 microwave heating—strawberry from 2011, time of heating 7 s. Mean values denoted with the same letter are not statistically significantly different, p ≤ 0.05
The L* × a* / b* coefficient was negatively correlated (R = −0.77, p ≤ 0.05) with TAC and the main anthocyanin, Pg-3-glc content in the analyzed samples. A strong (R = 0.998, p ≤ 0.01) positive correlation has been found between ΔE values and a decrease in the Pg-3-glc content; a weaker, but also significant (R = 0.84, p ≤ 0.1) correlation of ΔE and TCA was observed. It could be concluded that both employed coefficients are a useful tool for fast and simple estimation of colour and anthocyanin changes during strawberry processing, confirming the findings of previous studies (Rodrigo et al. 2007, Terefe et al. 2009).
Microbiological Quality
Microwave and traditional thermal pasteurization were effective on yeasts and moulds, totally eliminating these potentially spoiling microorganisms (Table 3). On the other hand, only CTP and microwave treatment at 120 °C caused a reduction in the total microbial count of up to <1 log cfu/g; at lower temperatures, a significant (over two logarithmic cycles) but incomplete reduction in the TMC was achieved. The lethality of microwave heating at continuous flow against typical microorganism that could contaminate fruit products was verified by some authors. The continuous flow microwave heating (2000 W, 71 °C, 6 s) of apple cider inoculated with E. coli 25922 resulted in a 5-log10 reduction in these bacteria (Gentry and Roberts 2005). Also, Lactobacillus plantarum inoculated in orange juice were readily killed using this technique either at 70 or 90 °C with very short residence time (Nikdel and MacKellar 1992). Tajachakavit et al. (1998) reported that the destruction of Sacharomyces cereviesiae and Lactobacillus plantarum occurred much faster under microwave heating than conventional thermal heating, suggesting some contributory enhanced effects to be associated with microwave heating.
Sensory Quality
The results of sensory analysis presented in Table 4 showed some minor but significant changes in most of the assessed attributes after preservation. The scores were lowered by no more than one point at the most. The colour was affected after the CTP and MV1 process, whereas shorter (7 s) microwave treatment MV2 did not visibly affect the colour of the samples. This was confirmed by the results of instrumental analysis. If ΔE values do not exceed 1.5, the changes in colour are unnoticeable by inexperienced observers (Barba et al. 2013). Panellists invited to this analysis were trained in accredited laboratory; therefore, they could notice more subtle differences between control sample (CS1) and microwave-heated sample (MV1 at 120 °C). Taste and aroma were slightly changed in all the preserved samples. The consistency of the purée remained unchanged despite the process conditions. The overall quality of the MV1 samples was assessed a little, although significantly lower than the MV2 sample, but still higher than the sample after CTP.
To the authors’ best knowledge, there are no publications concerning the sensory quality of strawberry purée after microwave treatment.
Conclusions
This study is an attempt at a comprehensive evaluation of the effects of continuous microwave heating on the quality of strawberry purée. The survey included the most important quality indicators, i.e. vitamin C and phenolic compounds, enzyme activity, colour parameters, sensory attributes and microbial load.
Continuous microwave treatment (2.45 GHz, 63 A, 20 kW) at 80–120 °C for 7 and 10 s turned out to be much less destructive for phenolic acids, flavonols, anthocyanins and vitamin C, compared to traditional thermal batch pasteurization. The changes in colour caused by this processing method were barely visible, the overall sensory quality changed only slightly, while the microbial load was significantly decreased and yeasts and moulds were below the detection limit. All these changes increased, to a different extent, with an increase in the duration and temperature used in the process.
None of parameters used in the experiment allowed complete inactivation of tissue enzymes. The residual activity of the PPO and POD implies that when storage stability is taken into account, a temperature of at least 90 °C and 10 s heating time should be applied. Further studies are recommended to evaluate the quality of strawberry purée preserved using microwaves during storage and the determination of its shelf-life.
References
Barba, F. J., Esteve, M. J., & Frigola, A. (2013). Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Research International, 50, 545–549.
Benlloch-Tinoco, M., Igual, M., Rodrigo, D., & Martinez-Navarrete, N. (2013). Comparison of microwaves and conventional thermal treatment on enzymes activity and antioxidant capacity of kiwifruit puree. Innovative Food Science and Emerging Technologies, 19, 166–172.
Benlloch-Tinoco, M., Martinez-Navarrete, N., & Rodrigo, D. (2014). Impact of temperature on lethality of kiwifruit puree pasteurized by thermal and microwave processing. Food Control, 35, 22–25.
Boero, R. (2011). Food quality as a public good: cooperation dynamics and economic development in a rural community. Mind & Society, 10, 203–215.
Cao, X., Zhang, Y., Zhang, F., Wang, Y., Yi, J., & Liao, X. (2011). Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. Journal of the Science of Food and Agriculture, 91, 877–885.
Cano, M. P., Hernandez, A., & De Ancos, B. (1997). High pressure and temperature effects on enzyme inactivation in strawberry and orange products. Journal of Food Science, 62(1), 85–88.
Da Silva Pinto, M., Lajolo, F. M., & Genovese, M. I. (2008). Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria x ananassa Duch.). Food Chemistry, 107, 1629–1635.
De Ancos, B., Cano, M. P., Hernandez, A., & Monreal, M. (1999). Effects of microwave heating on pigment composition and colour of fruit purees. Journal of the Science of Food and Agriculture, 79, 663–670.
Fazaeli, M., Yousefi, S., & Emam-Djomeh, Z. (2013). Investigation on the effects of microwave and conventional heating methods on the phytochemicals of pomegranate (Punica granatum L.) and black mulberry juices. Food Research International, 50, 568–573.
Gao, X., Ohlander, M., Jeppson, N., Bjork, L., & Trajkovski, V. (2000). Changes in antioxidant effect and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. Food Chemistry, 48, 1845–1890.
Gentry, T. S., & Roberts, J. S. (2005). Design and evaluation of continuous flow microwave pasteurization system for apple cider. LWT, 38, 227–238.
Gerard, K. A., & Roberts, J. S. (2004). Microwave heating of apple mash to improve juice yield and quality. LWT, 37, 551–557.
Goiffon, J. P., Mouly, P. P., & Gaydou, E. M. (1999). Anthocyanic pigment determination in red fruit juices, concentrated juices and syrups using liquid chromatography. Analytica Chimica Acta, 382, 39–50.
Hakala, M., Lapveteläinen, A., Huopalahti, R., Kallio, H., & Tahovonen, R. (2003). Effects of varieties and cultivation conditions on the composition of strawberries. Journal of Food Composition and Analysis, 16, 67–80.
Häkkinen, S. H., Karenlampi, S. O., Mykkanen, H. M., Heinonen, I. M., & Torronen, A. R. (2000). Ellagic acid content in berries: influence of domestic processing and storage. European Food Research and Technology, 212, 75–80.
Häkkinen, S. H., & Törrönen, A. R. (2000). Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: influence of cultivar, cultivation site and technique. Food Research International, 33, 517–524.
Heinz, V., Toepfl, S., & Knorr, D. (2003). Impact of temperature on lethality and energy efficiency of apple juice pasteurization by pulsed electric fields treatment. Innovative Food Science and Emerging Technologies, 4, 167–175.
Igual, M., Garcίa-Martίnez, E., Camacho, M. M., & Martίnez-Navarrete, N. (2010). Effect of thermal treatment and storage on the stability of organic acids and functional value of grapefruit juice. Food Chemistry, 118, 291–299.
Listkin, M. (2010). PATENT No WO/2010/027285 Al., Resonance chamber, especially for an apparatus for pasteurization of fluid liquid products, International Application No. PCT/PL2008/000096. http://patentimages.storage.googleapis.com/pdfs/63bcb6c38ddf2c098bf2/EP2334341B1.pdf.
Matsui, K. N., Gut, J. A. W., Oliveira, P. V., & Tadini, C. C. (2008). Inactivation kinetics of polyphenol oxidase and peroxidase in green coconut water by microwave processing. Journal of Food Engineering, 88, 169–176.
Marszałek, K., Mitek, M., & Skąpska, S. (2015a). The effect of thermal pasteurization and high pressure processing at cold and mild temperatures on the chemical compositions, microbial and enzyme activity in strawberry purée. Innovative Food Science and Emerging Technologies, 27, 48–56.
Marszałek, K., Woźniak, Ł., & Skąpska, S. (2015). Effect of supercritical carbon dioxide on the selected quality parameters of preserved strawberry juice. Żywność –Nauka Technologia Jakość, 2, (99), doi: 10.15193/zntj/2015/99/026.
Marszałek, K., Mitek, M., & Skąpska, S. (2011). Application of high hydrostatic pressure (HHP) to stabilize strawberry juices and nectars. Żywność –Nauka Technologia Jakość, 1(74), 113–123.
Marszałek, K., & Mitek, M. (2012). Effects of flow microwave preservation on changes of anthocyanins, vitamin C and colour of strawberry purée. Zeszyty Problemowe Postepów Nauk Rolniczych, 566, 135–142.
Nikdel, S., Chen, C., Parish, M., MacKellar, D., & Friedrich, L. (1993). Pasteurization of citrus juice with microwaves energy in continuous-flow unit. Journal of Agricultural and Food Chemistry, 41, 2116–2119.
Nikdel, S., & MacKellar, D. G. (1992). A microwave system for continuous pasteurization of orange juice. Proceedings of the Florida State Horticultural Society, 105, 108–110.
Odriozola-Serrano, I., Harnandes-Jover, T., & Martin-Belloso, O. (2007). Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chemistry, 105, 1151–1158.
Odriozola-Serrano, I., Soliva-Fortuny, R., & Martin-Belloso, O. (2008). Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. European Food Research and Technology, 228, 239–248.
Orsat, V., Raghavan, V., & Meda, V. (2005). Microwave technology for food processing: an overview. In H. Schubert & M. Regier (Eds.), The microwave processing of foods (pp. 105–118). Washington: Taylor and Francis, CRC Press.
Özkan, M. (2002). Degradation of anthocyanins in sour cherry and pomegranate juices by hydrogen peroxide in the presence of added ascorbic acid. Food Chemistry, 78, 499–504.
Patras, A., Brunton, N. P., Pieve, S., & Butler, F. (2009). Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purees. Innovative Food Science and Emerging Technologies, 10, 308–313.
Piasek, A., Kusznierewicz, B., Grzybowska, I., Malinowska-Pańczyk, E., Piekarska, A., Azqueta, A., Collins, A. R., Namieślnik, J., & Bartoszek, A. (2010). The influence of sterilization with EnbioJet® microwave flow pasteurizer on composition and bioactivity of aronia and blue-berried honeysuckle juices. Journal of Food Composition and Analysis, 24(6), 880–888.
Picouet, P. A., Landl, A., Abadias, M., Castellari, M., & Vinas, I. (2009). Minimal processing of Granny Smith apple purée by microwave heating. Innovative Food Science & Emerging Technologies, 10, 545–550.
Rodrigo, D., Loey, A., & Hendrickx, M. (2007). Combined thermal and high pressure colour degradation of tomato puree and strawberry juice. Journal of Food Engineering, 79, 553–560.
Sisquella, M., Vinas, I., Teixidó, N., Picouet, P., & Usual, J. (2013). Continuous microwave treatment to control postharvest brown rot in stone fruit. Postharvest Biology and Technology, 86, 1–7.
Sisquella, M., Picouet, P., Vinas, I., Teixidó, N., Segarra, J., & Usual, J. (2014). Improvement of microwave treatment with immersion of fruit in water to control brown rot in stone fruit. Innovative Food Science and Emerging Technologies. doi:10.1016/j.ifset.2014.06.010.
Tajachakavit, S., Ramaswamy, H. S., & Fustier, P. (1998). Enhanced destruction of spoilage microorganisms in apple juice during continuous flow microwave heating. Food Research International, 31(10), 713–722.
Terefe, N. S., Kleintschek, T., Gamage, T., Fanning, K. J., Netzel, G., Versteeg, C., & Netzel, M. (2013). Comparative effects of thermal and high pressure processing on phenolic phytochemicals in different strawberry cultivars. Innovative Food Science & Emerging Technologies, 19, 57–65.
Terefe, N. S., Matthies, K., Simons, L., & Versteeg, C. (2009). Combined high pressure-mild temperature processing for optimal retention of physical and nutrition quality of strawberries (Fragaria x ananassa). Innovative Food Science and Emerging Technology, 10, 297–307.
Terefe, N. S., Yang, Y. H., Knoerzer, K., Buckow, R., & Versteeg, C. (2010). High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innovative Food Science and Emerging Technology, 11, 52–60.
Törrönen, R., Hakkinen, S., Karenlampi, S., & Mykkanen, H. (1997). Flavonoids and phenolic acid in selected berries. Cancer Letters, 114, 191–192.
Verbeyst, L., Oey, I., Plancken, V. I., Hendrickx, M., & Loey, A. (2010). Kinetic study on the thermal and pressure degradation of anthocyanins in strawberries. Food Chemistry, 123, 269–274.
Verbeyst, L., Bogaerts, R., Plancken, I., Hendrickx, M., & Loey, A. (2013). Modeling of vitamin C degradation during thermal and high-pressure treatments of red fruit. Food and Bioprocess Technology, 6(4), 1015–1023.
Verbeyst, L., Hendrickx, M., & Loey, A. (2012). Characterisation and screening of the process stability of bioactive compounds in red fruit paste and red fruit juice. European Food Research and Technology, 234, 593–605.
Virkam, V. B., Ramesh, M. N., & Prapulla, S. G. (2005). Thermal degradation of nutrients in orange juice heated by electromagnetic and conventional methods. Journal of Food Engineering, 69, 31–40.
Zhang, G., Hu, M., He, L., Fu, P., & Zhou, J. (2013). Optimization of microwave-assisted enzymatic extraction of polyphenols from waste peanut shell and evaluation of its antioxidant and antibacterial activities in vitro. Food and Bioproducts Processing, 91, 158–168.
Acknowledgments
This project was financed by the National Research Center (grant no. NN 312 252540) and co-financed from the funds of the European Union within the European Social Fund (contract no. 78/ES/ZS-II/W2151.1/11). The authors would like to thank the Enbio Technology Co. for access to the EnbioJet® continuous flow microwave heating system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marszałek, K., Mitek, M. & Skąpska, S. Effect of Continuous Flow Microwave and Conventional Heating on the Bioactive Compounds, Colour, Enzymes Activity, Microbial and Sensory Quality of Strawberry Purée. Food Bioprocess Technol 8, 1864–1876 (2015). https://doi.org/10.1007/s11947-015-1543-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1543-7