Abstract
The objective of this research was to incorporate grape seed extract (GSE) into electrospun nanofibers produced from rye flour, whey protein concentrate (WPC), and polyethylene oxide (PEO). The effects of rye flour concentration (4% and 6%) and heating methods (conventional and microwave) on the properties of solutions and nanofibers were studied. Rheology results showed that microwave-heated solutions containing 6% rye flour had higher viscosity. According to the SEM images, the developed fibers obtained from this solution had larger diameter as compared to the ones obtained from conventionally heated solutions. Microwave pretreatment resulted in beadless and homogeneous fibers. GSE addition had a positive effect on viscosity and diameter size of microwave-heated samples. The physical and thermal properties of GSE encapsulated nanofibers were determined by X-ray diffraction (XRD), water vapor permeability (WVP), differential scanning calorimeter (DSC), thermogravimetric analyzer (TGA), and Fourier transform infrared (FTIR) analyses. The GSE addition made strong interactions within polymer matrix which improved thermal stability of films. Although GSE was not so stable at high pH environment, antioxidant activities of GSE containing samples with 4% and 6% rye flour were found to be 41.62 and 42.78%, respectively. GSE loading efficiency of electrospun nanofibers was improved with increasing rye flour concentration from 54.16 to 61.15%, and loading efficiency was improved with increased rye flour content. The results showed that rye flour and GSE are good candidates for electrospinning application, and their nanofiber films can be suggested to be used in combination with other materials as multilayered packaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrospinning is a physical method that produces nanoscale materials such as fibers and particles by exposing the polymer solution into high-voltage electric field (Cerqueira et al., 2016). Since electrospun nanofibers, which have a diameter ranging from a few to several hundred nanometers (Lembach et al., 2010), exhibit high porosity, large surface area to volume ratio, and homogeneity, this novel and cost-effective technique has applications in many diverse areas, including drug delivery, tissue engineering, biosensors, and packaging (Moomand & Lim, 2015; Shao et al., 2018). Especially the active packaging, which utilizes bioactive substances to enhance the shelf life of the food product by incorporating these compounds into packaging materials, benefits from electrospinning technology (Altan et al., 2018; Cerqueira et al., 2016).
Natural bioactive phenolic compounds have several beneficial properties such as antioxidant, anti-microbial, and anti-inflammatory activities (Locilento et al., 2019). Despite the broad range of properties, they are likely to be susceptible to environmental factors like light, temperature, or oxygen, which cause degradation and limit the bioavailability of the bioactive compounds. For this reason, the encapsulation technique has been used to protect and stabilize the bioactive compounds by coating them with another substance as a physical barrier (Nedovic et al., 2011). In that respect, electrospinning method showed successful results for preserving antioxidant properties of the materials by encapsulation. While carvacrol-loaded zein nanofibers showed antioxidant activity of 65% (Altan et al., 2018), another study succeeded at obtaining 74% antioxidant activity and 62% loading efficiency (LE) for gallic acid encapsulation into lentil flour–based electrospun nanofibers (Aydogdu, Yildiz, et al., 2019b). Grape seed extract is a mixture of various polyphenols, including catechin, epicatechin, and gallic acid (Marqués et al., 2013). Being a waste and byproduct of wine and fruit juice industry makes grape seed more preferable among other phenolic and antioxidant sources due to its low cost and sustainability (Faki et al., 2019). It showed excellent cytocompatibility and antioxidant effect when used in silk fibroin nanofibers and did not change the morphology of the fibers (Lin et al., 2016). However, there is no study in literature on encapsulation of grape seed extract by electrospinning method which could be used as active packaging material as inhibiting the oxidation of the food product by its antioxidant property.
Increasing environmental and human health concerns lead scientists to search petroleum-free food packaging materials. Accordingly, usage of biopolymers such as polysaccharides and proteins became a recent trend to replace synthetic polymers with natural ones (Aman Mohammadi et al., 2018). Several studies show that biopolymers-based nanofibers are produced successfully from food-grade polysaccharides and proteins, which are renewable sources. So far, biodegradable polymers, including, but not limited to, cellulose, alginate, chitosan, starch, wheat, pullulan, whey, silk, and gelatin, have resulted in functional electrospun nanofibers (Mendes et al., 2017; Torres-Giner, 2011). As a biodegradable and low-cost option, cereals, for example, oats, rye, wheat, and rice, are found abundantly due to being staple foods in several countries (Bach Knudsen et al., 2017). Among them, rye has the highest dietary fiber content (Andersson et al., 2009) and contains different types of bioactive compounds (Jonsson et al., 2018). In addition, high starch (66–73%) and pentosan (4–7%) contents give rye flour a considerable water-binding capacity (Rosentrater & Evers, 2018). This feature of rye flour might be advantageous for the production of nanofibers since holding more water would change the viscosity of the polymer solution, which is found to be one of the critical parameters for the electrospinning process (Oguz et al., 2018). However, starch-based nanofibers often showed brittleness and poor processability (Liu et al., 2017). Some previous studies have investigated that different flour types could be good candidates to form nanofibers on their own or blended with other materials (Aydogdu, Yildiz, et al., 2019b; Tam et al., 2017; Woranuch et al., 2017). To the best of our knowledge, rye flour has never been used as nanofiber material.
On the other hand, protein-based films have shown exceptional gas permeability and several functional properties (Hammann & Schmid, 2014). Besides having outstanding emulsification, gelation, and foaming functionalities, whey proteins are successful at encapsulating the active compounds as electrospinning material (Drosou et al., 2018). However, the hydrophilic nature of protein films causes poor water vapor permeability (WVP) (Hammann & Schmid, 2014). For food packaging purposes, combining polysaccharides and proteins in the solution material might bring out their advantages and discard the drawbacks of the films. For example, it was observed that the thermal stability of the whey protein isolate nanofibers was improved when pullulan was added to the fiber solution (Drosou et al., 2018). In this regard, rye flour and whey protein could be good candidates as packaging materials with improved properties. As a biodegradable and biocompatible material, polyethylene oxide (PEO) could help form more homogeneous nanofibers along with rye flour and whey protein enhancing the electrospinnability of solutions since using PEO along with biopolymers had a decreasing effect on the repulsive forces between molecules which hindered the possibility of sufficient molecular entanglement during electrospinning (Vega-Lugo & Lim, 2012). For example, in the study of Aydogdu, Yildiz, et al. (2019b), while pure lentil resulted in nanofibers with beads, addition of PEO provided homogeneous and beadless nanofibers due to the increased molecular entanglement by PEO and amino acid in lentil flour interaction.
Microwave heating has many advantages over conventional heating, such as time and energy saving, selective heating, and process control (Sumnu, 2001). Moreover, it affects the structural and functional characteristics of both carbohydrates and proteins. It was previously shown that microwave treatment made an increase in the denaturation proportion of whey proteins in the milk samples as compared to the conventionally heated ones (Villamiel et al., 1996). Additionally, stronger and fine-stranded whey protein isolate gels were formed with microwave application (Gustaw & Mleko, 2007). Since microwave caused internal heating, amylose was effectively leached out from lotus seed starch and made complexes with green tea polyphenols (Zhao et al., 2019). Moreover, in the study where carob flour and rice starch were used as nanofiber materials, it was shown that homogeneous and bead-free electrospun nanofibers were obtained in microwave-heated sample (Uygun et al., 2020). This was explained by internal heating observed in microwave heating leading to higher internal pressure, which is most likely to have an effect on releasing amino groups and increasing solution viscosity. However, information on microwave pretreatment of flour- and protein-based nanofilms obtained via electrospinning is very limited. Also, to the best of our knowledge, there is no study on the effects of microwave pre-heating on electrospinning of nanofibers containing phenolic compounds. Thus, the aim of this study was to fabricate grape seed extract–loaded nanofibers from rye flour–whey protein biopolymers by electrospinning. In addition, the effects of microwave heating on the physical properties of the solutions and nanofibers were also examined.
Materials and Methods
Materials
Rye flour was purchased from Smart Chemical Trading Co. Inc. (Izmir, Turkey), and whey protein concentrate (WPC) (80% protein on a dry weight basis) was supplied from Proteinocean Gıda Co. Inc. (Ankara, Turkey). Polyethylene oxide having a molecular weight of 900 kDa was obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Polyethylene sorbitan monooleate (Tween 80) (density: 1.064 g/m3, viscosity: 400–620 cps at 25 °C) was provided from Merck (Darmstadt, Germany). Grape seed extract, extracted by using aqueous ethanol (70% [v/v]) and containing 75% (w/v) solid GSE, was bought from Arpas Arifoglu Trading Co. Inc. (İstanbul, Turkey).
Solution Preparation
Solutions of 2% (w/v) were obtained by dissolving PEO in distilled water at room temperature and at 400 rpm overnight using a magnetic stirrer (Daihan Scientific, Seoul, Korea). Rye flour (4% and 6% [w/v]) and whey protein (4% [w/v]) were added to PEO solutions at different concentrations and mixed with high-speed homogenizer (IKA T25 Digital Ultra Turrax, Staufen, Germany) at 10,000 rpm for 3.5 min for complete homogenization. Then, 8M NaOH solution was added to the solutions to adjust pH to 12 by using a pH meter (SG2 SevenGo pH, Mettler Toledo, USA). Heating of the solutions was conducted according to the previous work, and the process parameters were set up similarly to be able to reach adequate starch gelatinization and protein denaturation for homogeneous fiber formation (Uygun et al., 2020). Conventionally heated solutions were prepared by heating up to 80 °C in a water bath (GFL, Type 1086, Germany). Then, heating was continued on magnetically stirred hot plates at 80 °C and 750 rpm for 2 h. Others were heated by using a microwave oven (Kenwood MW-767, Hampshire, UK) at 450 W for 2.5 min to reach 80 °C. After heating was complete, solutions were cooled down to room temperature. Tween 80 at a concentration of 2% (w/v) was added to the solutions afterward as a non-ionic surfactant with the purpose of producing more homogeneous fibers by decreasing the surface tension (Tam et al., 2017; Vega-Lugo & Lim, 2012). Grape seed extract was bought as a product which contained 75% (w/v) solid GSE and added to the electrospinning solutions by arranging 20% (w/w) of GSE content in the solid fibers.
Solution Properties
Rheological Properties
The rheological properties of the solutions were measured by a controlled strain rheometer (Kinexus, Malvern, UK) equipped with cone and plate geometry (4° cone, 40 mm diameter, and 1 μm gap). The shear stress data were obtained at a controlled shear rate between 0.1 and 100 s−1 at 25 °C. Shear stress data were recorded with respect to shear rate, and measurements were conducted in duplicates.
Electrical Conductivity
Electrical conductivity of the solutions was measured at 25 ± 1 °C using conductivity meter (InoLab®Cond 7110, Wissenschaftlich-Technische Werkstatten GmbH, Wheilheim, Germany) in duplicates.
Total Phenolic Content (TPC) of Solutions
TPC of the electrospinning solutions with and without GSE addition were determined by the modified Folin-Ciocalteau method (Luca et al., 2013). Ethanol solution (70% [v/v]) was used to dilute the sample solutions; 2.5 mL of 0.2 N Folin-Ciocalteau reagent was added to 0.5 mL of the sample. After storing the vortexed mixture for 5 min in a dark place, 2 mL of 75 g/L sodium carbonate solution was added. The final mixture was kept in the dark for 2 h. By using a spectrophotometer (UV 2450, Columbia, USA), absorbance of the solutions was recorded at 760 nm. Gallic acid was used to create a calibration curve. TPC values were presented as milligrams of gallic acid equivalent (GAE) per gram of dry weight.
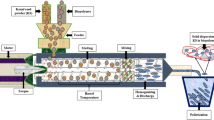
Electrospinning Process
The polymer solutions were electrospun by using Nano-Web 103 (Mersin, Turkey). Each solution was loaded into a 5 mL syringe having 11.53 mm inner diameter and a 21-gauge metallic needle. The needle was connected to the positively charged electrode after the syringe was mounted on the syringe pump horizontally. Fibers were collected onto the aluminum foil–covered metal collector, which was connected to the negatively charged electrode of the high-voltage power supply. The collector was placed 30 cm away from the needle tip. The flow rate of the solutions and the voltage were kept constant at 0.6 mL/h and 12 kV, respectively. Experiments were carried out at 25 ± 1 °C and 25–35% relative humidity.
Films were symbolized according to heat treatment, rye flour, and GSE concentration, and the nomenclature is given in Table 1. For example, M4R20 denotes film with microwave treatment, 4% (w/v) rye flour, and 20% GSE.
Characterization of Films
Morphological Analysis
Fiber morphology of the RF/WPC/PEO films was observed from the images taken by using a scanning electron microscope (SEM) (Nova NanoSEM 430, Oregon, USA). Approximately 100 fibers on the SEM images of each sample were selected randomly to determine the average diameter by using Image J analysis software (Maryland, USA).
Water Vapor Permeability
Determination of WVP of nanofiber films was made according to a modified version of ASTM E-96 standard method (Bertuzzi et al., 2007). The measurement cups having a diameter of 0.04 m were filled with 30 mL of water. The films were sealed to the cups by using screws, and any leakage was prevented by rubber joint. Then, the cups were placed and stored in the desiccator equipped with silica gels. Until steady state was reached, each cup was weighed with 2 h intervals. From the plot of the weight loss versus time, the slope was used to determine the water vapor transmission rate for each sample (WVTR; g m−2 s−1). Then, water vapor permeability was calculated by using the equation below:
where P1 is the partial pressure of water vapor at the inner surface of the film (Pa) and P2 is the partial pressure of the water vapor at the outer surface of the film (Pa). Δx is the thickness of the film (m). During the measurement, relative humidity (RH) and temperature inside the desiccator were recorded using a digital hydrometer (ThermoPro TP50, USA). RH inside the cup was assumed as 100%. Measurements were performed in duplicates.
X-Ray Diffraction
X-ray diffractometry (XRD) for the films was obtained by using Ultima IV X-ray diffractometer (Rikagu, Japan). Operation conditions were determined as voltage of 40 kV and current of 30 mA under Cu source. 2theta range for the measurements was 5–70° for all samples with a scanning rate of 2°/min.
Differential Scanning Calorimetry (DSC)
Differential scanning calorimeter (Pyris 6 DSC, PerkinElmer, USA) was used to determine thermal analysis of films. Approximately 5 mg of sample from each film was placed in an aluminum pan and then sealed. An empty pan was used as a reference. Each pan was cooled down to – 60 °C first and then heated up to 100 °C with a rate of 10 °C/min. The DSC thermograms were used to determine glass transition temperature, melting temperature, and melting enthalpy of each sample. The DSC measurements were conducted in duplicates.
Thermogravimetric Analysis (TGA)
Thermogravimetric analysis of the samples was conducted by using Pyris STA 6000 simultaneous thermal analyzer (PerkinElmer, USA). Approximately 5 mg nanofiber, rye flour, whey protein, and PEO were heated from room temperature to 500 °C with a heating rate of 10 °C/min with nitrogen. Measurements were performed in duplicates.
Fourier-Transform Infrared (FTIR) Analysis
FTIR analyses of the electrospun nanofibers, rye flour, WPC, and PEO were performed by using FTIR spectrophotometer (Pyris STA 6000, PerkinElmer, USA) which characterizes gas evolved from TGA. Data were recorded in the wavenumber range of 4000–500 cm−1 at 2 cm−1 resolution.
Total Phenolic Content of Electrospun Fibers
TPC of electrospun nanofibers were found by using the modified Folin-Ciocalteau method (Luca et al., 2013). The same procedure with the TPC determination of electrospinning solution was applied. Instead of sample solutions, 0.1 g nanofiber was dissolved in 70% (v/v) ethanol solution in the first place. Absorption of final solutions was measured by using a spectrophotometer (UV 2450, Shimadzu, USA). The measurements were done in duplicates. The LE of GSE into nanofibers was found by using the formula below:
Antioxidant Activity of Electrospun Nanofibers
The antioxidant activity of GSE-loaded nanofibers was measured with a modified version of the method in which 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was used (Luca et al., 2013). Nanofiber film sample of 0.1 g was mixed with 2 mL of 70% (v/v) ethanol solution and waited for 2 h for complete dissolution. The extract was obtained by filtering through 0.45 μm filter. The 0.1 mL of filtered sample was added to 3.9 mL of 0.6 mM DPPH solution and kept in the dark for 1 h. The absorption (Asample) was recorded at 517 nm by using a spectrophotometer (UV 2450, Shimadzu, USA). Control sample was prepared by mixing 0.1 mL of 70% (v/v) ethanol solution with 3.9 mL of 0.6 mM DPPH solution. The absorbance of the control (Acontrol) was measured at 517 nm. Methanol was used as blank. The measurements were carried out in duplicates. The antioxidant activity (%AA) of the fibers was calculated by using the formula below:
Statistical Analysis
Statistical analysis was conducted by using Minitab software (Minitab Inc., State College, USA). Analysis of variance (ANOVA) was used to observe if there were any significant differences between treatments. Tukey’s multiple comparison test was performed for the data with significant differences (p ≤ 0.05).
Results and Discussion
Physical Properties of Solutions
Rheological Properties
Electrospinning process can only be successful in production of homogeneous and beadless nanofibers if elongation of the solution is enough to be extended by the electric field (Stijnman et al., 2011). Since the process and ambient parameters were kept constant during the experiment, rheological properties such as viscosity were expected to have an important effect on bead formation and diameter of the fibers. While too low viscosity restricts the elongation and continuous fiber formation, too high viscosity makes the ejection of the polymer solution difficult. Therefore, the optimum viscosity of the solution is required to produce desired electrospun nanofibers by adjusting the polymer and solvent type and concentration (Aydogdu, Sumnu, & Sahin, 2019a). Table 2 shows the consistency index (k), flow behavior index (n), and apparent viscosity of the solutions. All the electrospinning solutions obeyed the Power Law model and showed high coefficient of determination (r2 = 0.999) values. Also, they have flow behavior index value (n) ranging between 0.776 and 0.953 which indicates shear-thinning property as being smaller than 1. Conventionally heated solutions, containing 2% (w/v) PEO, 4% (w/v) WPC, and 4% (w/v) and 6% (w/v) rye flour (C4R0 and C6R0), have lower k values and higher n values as compared to the microwave-heated solutions with the same composition (M4R0 and M6R0). Moreover, they showed lower apparent viscosity at 60 s−1. While conventionally heated samples resulted in nanofibers with beads (Fig. 1), microwave-treated ones formed homogeneous nanofiber without beads (Fig. 1). Similarly, carob flour–based electrospun nanofibers had higher viscosity and more homogeneous nanofibers when pretreated by microwave heating as compared to the conventionally heated ones (Uygun et al., 2020). It was explained by the internal pressure formed by microwave heating which accelerated the protein unfolding and amino group releasing; therefore, the solution viscosity was increased. As the composition of the solutions containing whey protein is considered, it is possible to say that microwave heating could yield more impact in terms of viscosity. When the effects of rye flour concentrations were studied, it was seen that the increasing rye flour content resulted in higher viscosity for both conventionally and microwave-heated samples. High starch content in rye flour makes the solution more viscous as starch granules are exposed to heat and swell during gelatinization process. Another factor that makes difference between viscosity values was the addition of grape seed extract into the microwave-heated solutions. Microwave-heated solutions containing 2% (w/v) PEO, 4% (w/v) WPC, and 4% (w/v) and 6% (w/v) rye flour and 20% (w/w) GSE (M4R20 and M6R20) had higher viscosity than solutions with no GSE (M4R0 and M6R0). This result could be related to the polyphenol content of the grape seed extract which could contribute to the crosslinking and entanglement of polymer chains. In another study, it was found that addition of tea polyphenols increased the solution viscosity where the pullulan and carboxymethylcellulose were used as the electrospinning solution materials (Shao et al., 2018).
Electrical Conductivity
Electrical conductivity is another important parameter since electrostatic charges are required in the electrospinning solution so that fiber from the syringe would be transferred through the electric field and collected on the plate. Conductivity value of the solution should be high enough to overcome the force of the electric field created by the applied voltage in the electrospinning machine. Once it is overcome, the solution forms a sufficient elongation and fabricates uniform nanofiber. Therefore, solutions with zero electrical conductivity cannot be used in electrospinning (Angammana & Jayaram, 2011). On the other hand, too high conductivity, which had a limit of about 1 S/m (Fernández de la Mora, 2007), would result in unstable jet formation that cannot reach up to collector plate (Seethu et al., 2020). Also, adding more NaOH solution with the purpose of increasing conductivity would lower the viscosity of solution, which would result in beaded and nonuniform fibers. Electrical conductivity values of solutions were displayed in Table 2. It can be observed that conductivity values of solutions containing 6% (w/v) rye flour were lower than those with 4% (w/v). This could be related to increase in polymer interaction in the solutions with more biopolymer content. Similarly, lower conductivity results were obtained when sugar concentration (Luo et al., 2012) and pullulan content (Drosou et al., 2018) were increased in electrospinning solutions. The reason for lower electrical conductivity of microwave-treated solutions might be linked to intermolecular interactions that increased with more unfolded proteins coming from WPC since microwave heating could promote the release of free amino groups by internal heating principle (Uygun et al., 2020). Moreover, GSE incorporation to the solutions reduced conductivity values. It was previously studied that polyphenol addition could decrease electrical conductivity, which was associated with increase in molecular entanglement in polymer solution (Shao et al., 2018). As being another decisive factor on fiber morphology, electrical conductivity showed a negative correlation with fiber diameter. Larger diameter values were obtained when solution had higher viscosity and lower conductivity combination for electrospinning process (Table 2). Also, microwave heating had a contribution to the increase in fiber diameter by resulting in solutions with higher viscosity and decreased conductivity as compared to conventionally heated samples.
Characterization of Electrospun Nanofibers
Fiber Morphology
The fiber formation and film properties were directly affected by solution properties, ambient conditions, and process parameters (Liu et al., 2017). In this study, the impact of solution properties was investigated by keeping the ambient conditions and process parameters constant. The morphology of the electrospun nanofibers was examined to evaluate the effects of heating method, rye flour concentration, and antioxidant material addition, which also play significant role on the rheological properties and the electrical conductivity of solutions. Figure 1 displays the SEM images and the size distribution of the nanofibers. Conventionally heated solutions yielded a few bead formations on the fibers. Table 2 shows that they also had smaller fiber diameter comparing to microwave-heated ones. As explained previously, microwave heating could promote the unfolding of proteins, which increases the solution viscosity and decreases electrical conductivity. High electrical conductivity of conventionally heated solutions might be exposed to higher attractive forces on the way to the collector, which forms faster travel of polymer jet. Thus, there is not enough time for solvent to evaporate, and beads are formed due to sticky nanofibers (Aydogdu, Yildiz, et al., 2019b). Similarly, electrospun fibers from flaxseed mucilage having larger diameter were obtained when electrical conductivity of solutions was lower (Hadad & Goli, 2019). Table 2 shows that average fiber diameter of the nanofibers varied between 295 and 338 nm. Solutions with higher viscosity and lower electrical conductivity yielded fibers with larger diameter. When rye flour concentration and GSE addition were considered, it was shown that both factors had a positive effect on the solution viscosity and their nanofibers had larger diameters. The positive correlation between viscosity and fiber diameter was studied in different researches before. Diameter of electrospun zein fibers was shown to increase as consistency index of solution increased due to enhanced molecular entanglement in the solution (Moomand & Lim, 2015). Likewise, the increasing viscosity of amaranth–pullulan solutions with increasing amaranth content resulted in electrospun nanofibers with larger diameter (Blanco-Padilla et al., 2015). Another study, in which lentil flour and hydroxypropyl methylcellulose were used for electrospinning, indicated that fiber diameter was higher when viscosity of the solution was increased by higher lentil flour concentration (Tam et al., 2017).
Water Vapor Permeability
Since films separate two environments from each other and control the moisture transfer between them, water vapor permeability is a significant characteristic. Particularly, the film with less WVP is more preferable as a packaging material (Chinma et al., 2012). Table 3 shows the WVP values of the electrospun nanofibers, and they were ranged between 1.09 × 10−10 and 1.94 × 10−10 g m−1 s−1 Pa−1. The effect of antioxidant incorporation on permeability could be observed through the comparison of WVP values of GSE-loaded and not GSE-loaded samples as keeping the polymer content constant (Table 3). The statistical analysis showed that barrier property of the films was significantly different when GSE was introduced to the samples. WVP of M4R0 film increased from 1.22 × 10−10 to 1.94 × 10−10 g m−1 s−1 Pa−1 when GSE was added into it. Similarly, WVP of M6R0 film was lower than WVP of M6R20 film. The reason behind that was explained in the study where GSE-loaded chitosan films had higher WVP values than of chitosan films without GSE by hydrophilic nature of GSE (Rubilar et al., 2013). The presence of hydrophilic GSE might let water molecules form hydrogen bonds more which causes a rise in WVP. Moreover, GSE might reduce crystallinity of the films; hence, less ordered fibers would probably result in higher permeability. Similarly, it was reported that GSE caused a rise in WVP which could be again related to the hydrophilicity of GSE (Sogut & Seydim, 2018).
X-Ray Diffraction
According to the XRD result shown in Fig. 2, all four samples showed similar diffraction pattern with three main peaks. Thus, it can be interpreted that the composite nanofiber samples are semi-crystalline materials both having amorphous and crystalline structures. All samples showed peaks at 2θ = 19° and 2θ = 23°. Those peaks could be attributed to the crystallinity coming from PEO. In a previous study, pure PEO showed peaks at 19.2° and 23.3° in XRD analysis (Xu et al., 2012). In between those peaks, another diffraction peak appeared at 2θ = 21.59°, 2θ = 20.8°, 2θ = 22.04°, and 2θ =21.41° for M6R0, M6R20, M4R0, and M4R20, respectively. Therefore, it can be stated that incorporation of GSE reduced the crystallinity of rye flour/WPC/PEO nanofibers since GSE might have an enhancing effect on the interaction of water molecules with polymer chains due to its hydrophilic character (Tavassoli-Kafrani et al., 2018). Similar result was found in the study of Lin et al. (2016) where silk fibroin and PEO were used as electrospun nanofiber material, and the introduction of GSE decreased the crystallinity of the nanofibers (Lin et al., 2016).
Differential Scanning Calorimetry Analysis
The calorimetrically detectable transitions of nanofibers were obtained by DSC. Those including the glass transition temperature (Tg), the melting temperature (Tm), and enthalpy change during melting (ΔHm) are shown in Table 3. The presence of both Tm and Tg for all samples indicated that nanofibers had semi-crystalline structure since Tg represented a transition from glassy state to rubbery state. The melting temperature values of nanofibers varying around 55 °C were found to be not significantly different from each other and lower than Tm of PEO which was 71.5 °C according to previous studies (Uygun et al., 2020). Such a decrease has been reported for electrospun nanofibers in which carob flour (Uygun et al., 2020), chitosan (Kuntzler et al., 2018), and soy protein isolate (Xu et al., 2012) were used as polymers along with PEO. The reason behind the Tm depression of PEO might be explained by disruption of crystalline structure of PEO as it was forming strong interactions with the polymers used, which were rye flour and whey protein in this study. The glass transition temperature of nanofibers was higher with the incorporation of GSE since there was a reduction in chain mobility with formation of intermolecular bonds (Wen et al., 2016). Also, as it was argued previously, hydrophilic nature of GSE might have a decreasing impact on the crystalline structure of the films. Melting enthalpy values decreased with increasing rye flour content. Although there was no significant difference between them, the decrease could be explained by disrupted crystalline structure of polymers with increased interaction (Aydogdu et al., 2018)
Thermogravimetric Analysis
TGA thermograms in Fig. 3 show the weight change profile of the samples as a function of temperature. The TGA of all nanofibers have a minor initial weight loss between 30 and 100 °C because of vaporization of free water and two-stage degradation. The first degradation occurred between 200 and 300 °C which could be associated with polysaccharide degradation coming from rye flour content and whey protein decomposition. While the onset temperature of degradation (Tonset) of rye flour was found near 275–280 °C, Tonset of WPC was around 270 °C. Similarly, in the study of pullulan–whey protein electrospun nanofibers, the first thermal degradation between 250 and 350 °C was attributed to the polysaccharide degradation (Drosou et al., 2018). The second degradation of nanofibers has Tonset around 400 °C which may represent the degradation of PEO since pure PEO showed one single-stage degradation near 400 °C. Similar result was obtained in the study of PEO–lentil flour electrospun nanofibers; the second Tonset was reported as 400 °C (Aydogdu, Yildiz, et al., 2019b). In the first-stage degradation, a slight decrease in the weight loss for nanofibers containing GSE could be observed. As explained in the DSC results, it can be argued that GSE had an enhancing effect on intermolecular interactions, and thermal stability was improved. As previously reported, TGA curve of GSE has a large peak in a range of 120–290 °C, followed by two peaks between 450 and 505 °C which were not displayed for that temperature range on the TGA of nanofibers. The absence of second thermal degradation peaks after 400 °C could be associated with encapsulation of GSE due to its dispersion/dissolution before encapsulation (Locilento et al., 2019).
FTIR Analysis
FTIR measurement is a useful analysis to obtain information on functional groups in the samples and their interaction between the constituents of each nanofiber by examining their characteristic peaks displayed on spectra of each sample. The FTIR spectra of electrospun nanofibers, PEO, rye flour, and whey protein concentration were shown in Fig. 4. Nanofibers had peaks located around 1100 cm−1, which was related to the stretching vibrations that occurred at the ether bond found on the backbone of the PEO chain (Vega-Lugo & Lim, 2012). The characteristic peak at near 2800 cm−1 originating from stretching of methylene group (CH2) was also observed at PEO (Sullivan et al., 2014). These are indications of the presence of PEO in nanofibers after the electrospinning process. The peaks located at 840–960 cm–1 band in both nanofibers and rye flour were attributed to vibrations coming from C−O−C of α-1,4 glycosidic linkages (Kizil et al., 2002). Similar results were observed in which lentil flour (Aydogdu, Yildiz, et al., 2019b) and carob flour (Uygun et al., 2020) were used along with PEO in fabrication of electrospun nanofibers. Spectra for the nanofibers showed characteristic peak around at 1630 cm−1 which was associated to the Amide I region and was found in proteins. This peak was also seen at spectrum of WPC due to N−H scissoring. Similarly, in the studies where whey protein isolate and PEO were used for electrospinning process, peak at 1650 cm−1 was observed (Colín-Orozco et al., 2015; Sullivan et al., 2014). Differing from nanofibers without GSE addition, GSE-loaded nanofibers had broad new bands around 1350 and 3500 cm−1. These are the characteristic absorption peaks of GSE positioning at 1000–1300 cm−1 and 3250–3300 cm−1 (Lin et al., 2016). Since GSE has a great amount of phenolic compounds including catechin, epicatechin, and gallic acid, the bands are typically observed at 3300 and 1283 cm−1 due to stretching of different −OH groups and ester C−O stretching, respectively (Locilento et al., 2019). The shifting of the bands could be associated with indication of an interaction between GSE and polymer. These results demonstrate the successful incorporation of GSE into rye flour/WPC/PEO electrospun nanofibers.
Total Phenolic Content and Antioxidant Activity of Fibers
Total phenolic content of the electrospinning solutions with and without GSE was shown in Table 4. Before GSE addition, solutions with 4% (w/v) and 6% (w/v) rye flour had TPC values of 2.98 and 3.19 mg GAE/g dry matter, respectively. It showed that there were phenolic compounds in the solution materials apart from GSE. Considering that TPC values increased with increasing rye flour content, it could be argued that source of the phenolic compounds was mostly rye flour. Previous studies showed that rye flour grains had TPC content varying between 2.61 and 3.37 mg GAE/g dry matter, and t-ferulic acid was found to be the most abundant among other phenolic compounds in rye grains (Kulichová et al., 2019). Since ferulic acid had thermal stability up to 245 °C (Fiddler et al., 1967) and had a resistance to high pH (Friedman & Jürgens, 2000), heat treatment and alkaline conditions of this study were not destructive for rye flour–sourced TPC in the solutions which did not contain GSE. Also, microwave treatment might have an increasing effect on TPC because such a food process could release phenolics bound in cell walls (Acosta-Estrada et al., 2014). GSE contains high amount of phenolic compounds including mainly catechin, epicatechin, and gallic acid (Monagas et al., 2003). TPC of GSE-added solutions increased to 10.61 and 13.98 mg GAE/g dry matter for solutions with 4% (w/v) and 6% (w/v) rye flour, respectively. Despite the high phenolic content of GSE, TPC of the solutions were not elevated considerably. Since pH of the solutions was adjusted to 12 during preparation, the degradation might be related to high pH of the solutions which was destructive to gallic acid (Aydogdu, Yildiz, et al., 2019b). However, epigallocatechin and epicatechin were found to be relatively stable to alkaline pH, which could be an explanation to the rise in TPC when GSE was added. Table 4 shows the loading efficiency and antioxidant activity of electrospun nanofibers. The loading efficiency of the nanofiber containing 6% (w/v) rye flour was higher than of the one containing 4% (w/v) rye flour. Similarly, it was found in the study of carvacrol encapsulation in starch and poly-ε-caprolactone by electrospinning that encapsulation efficiency was increased by increasing polymer concentration (Tampau et al., 2017). GSE has significant antioxidant capacity due to its oligomeric proanthocyanidin content which prevents oxidization by providing electrons to the free radical (Huh et al., 2004). As displayed in Table 4, both of the nanofiber samples showed antioxidant activity around 40%. This showed that antioxidant property of the samples was preserved in electrospun nanofibers. Similarly, in another study, GSE encapsulation to silk fibroin was performed by electrospinning which resulted in films with remarkable antioxidant capacity (Lin et al., 2016).
Mechanical Properties of Films
The main goal of this study was to use rye flour as an electrospinning material for the first time and incorporate grape seed extract to those electrospun films successfully. The produced nanofiber films showed weak mechanical properties which were not adequate to be measured by a texture analyzer. In this regard, these electrospun nanofiber films with an antioxidant capacity can be used in combination with another packaging material, and multilayered packaging material is formed. As shown by many other studies in the literature, nanofibers are not used alone to package the food material since their mechanical properties are weak. For example, fibers can be collected on the inner surface of another packaging material. As a successful example, gallic acid–loaded hydroxypropyl methylcellulose/PEO nanofibers, which was fixed to the inner surface of PA/PE film and wrapped on walnuts, can be given (Aydogdu, Sumnu, & Sahin, 2019a).
Conclusions
In this research, grape seed extract as an antioxidant compound was encapsulated into rye flour and whey protein–based electrospun nanofibers successfully. Microwave heating pretreatment to solutions was found to be more effective than conventional heating in terms of obtaining homogeneous and beadless nanofibers with shorter processing time. The effect of GSE incorporation into rye flour and whey protein–based electrospun nanofibers material was confirmed by chemical and thermal analyses. The increase in rye flour content resulted in larger fiber diameter. On the other hand, higher rye flour content had a positive impact on loading efficiency of grape seed extract. The addition of GSE provided an increase in fiber diameter due to improved molecular entanglement and intermolecular interactions which yielded in nanofibers with improved thermal stability. Thus, grape seed extract–incorporated nanofibers produced in this study from rye flour and whey protein can be suggested as a promising material for biodegradable film with high antioxidant activity and enhanced thermal stability. In particular, using these nanofiber films with antioxidant capacity in combination with another packaging material to form a multilayered packaging can be suggested due to having weak mechanical properties. Further studies are required to examine the other physical properties of nanofibers, including the release kinetics and stability of antioxidants to evaluate their potential use in active packaging and many other applications.
Data Availability
Not applicable
References
Acosta-Estrada, B. A., Gutiérrez-Uribe, J. A., & Serna-Saldívar, S. O. (2014). Bound phenolics in foods, a review. Food Chemistry, 152, 46–55. https://doi.org/10.1016/j.foodchem.2013.11.093.
Altan, A., Aytac, Z., & Uyar, T. (2018). Carvacrol loaded electrospun fibrous films from zein and poly(lactic acid) for active food packaging. Food Hydrocolloids, 81, 48–59. https://doi.org/10.1016/j.foodhyd.2018.02.028.
Aman Mohammadi, M., Rostami, M. R., Raeisi, M., & Tabibi Azar, M. (2018). Production of electrospun nanofibers from food proteins and polysaccharides and their applications in food and drug sciences. Jorjani Biomedicine Journal, 6(4), 62–77. https://doi.org/10.29252/jorjanibiomedj.6.4.62.
Andersson, R., Fransson, G., Tietjen, M., & Åman, P. (2009). Content and molecular-weight distribution of dietary fiber components in whole-grain rye flour and bread. Journal of Agricultural and Food Chemistry, 57(5), 2004–2008. https://doi.org/10.1021/jf801280f.
Angammana, C. J., & Jayaram, S. H. (2011). Analysis of the effects of solution conductivity on electrospinning process and fiber morphology. IEEE Transactions on Industry Applications, 47(3), 1109–1117. https://doi.org/10.1109/TIA.2011.2127431.
Aydogdu, A., Sumnu, G., & Sahin, S. (2018). A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohydrate Polymers., 181, 234–246. https://doi.org/10.1016/j.carbpol.2017.10.071.
Aydogdu, A., Sumnu, G., & Sahin, S. (2019a). Fabrication of gallic acid loaded hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydrate Polymers, 208(July 2018), 241–250. https://doi.org/10.1016/j.carbpol.2018.12.065.
Aydogdu, A., Yildiz, E., Aydogdu, Y., Sumnu, G., Sahin, S., & Ayhan, Z. (2019b). Enhancing oxidative stability of walnuts by using gallic acid loaded lentil flour based electrospun nanofibers as active packaging material. Food Hydrocolloids, 95(April), 245–255. https://doi.org/10.1016/j.foodhyd.2019.04.020.
Bach Knudsen, K. E., Nørskov, N. P., Bolvig, A. K., Hedemann, M. S., & Lærke, H. N. (2017). Dietary fibers and associated phytochemicals in cereals. Molecular Nutrition and Food Research, 61(7), 1–15. https://doi.org/10.1002/mnfr.201600518.
Bertuzzi, M. A., Castro Vidaurre, E. F., Armada, M., & Gottifredi, J. C. (2007). Water vapor permeability of edible starch based films. Journal of Food Engineering, 80(3), 972–978. https://doi.org/10.1016/j.jfoodeng.2006.07.016.
Blanco-Padilla, A., López-Rubio, A., Loarca-Piña, G., Gómez-Mascaraque, L. G., & Mendoza, S. (2015). Characterization, release and antioxidant activity of curcumin-loaded amaranth-pullulan electrospun fibers. LWT - Food Science and Technology, 63(2), 1137–1144. https://doi.org/10.1016/j.lwt.2015.03.081.
Cerqueira, M. A., Fabra, M. J., Castro-mayorga, J. L., Bourbon, A. I., Pastrana, L. M., Vicente, A. A., & Lagaron, J. M. (2016). Use of electrospinning to develop antimicrobial biodegradable multilayer systems: Encapsulation of cinnamaldehyde and their physicochemical characterization. Food and Bioprocess Technology, 9(11), 1874–1884. https://doi.org/10.1007/s11947-016-1772-4.
Chinma, C. E., Ariahu, C. C., & Abu, J. O. (2012). Development and characterization of cassava starch and soy protein concentrate based edible films. International Journal of Food Science and Technology, 47(2), 383–389. https://doi.org/10.1111/j.1365-2621.2011.02851.x.
Colín-Orozco, J., Zapata-Torres, M., Rodríguez-Gattorno, G., & Pedroza-Islas, R. (2015). Properties of poly (ethylene oxide)/ whey protein isolate nanofibers prepared by electrospinning. Food Biophysics, 10(2), 134–144. https://doi.org/10.1007/s11483-014-9372-1.
Drosou, C., Krokida, M., & Biliaderis, C. G. (2018). Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocolloids, 77, 726–735. https://doi.org/10.1016/j.foodhyd.2017.11.014.
Faki, R., Gursoy, O., & Yilmaz, Y. (2019). Effect of electrospinning process on total antioxidant activity of electrospun nanofibers containing grape seed extract. Open Chemistry, 17(1), 912–918. https://doi.org/10.1515/chem-2019-0098.
Fernández de la Mora, J. (2007). The fluid dynamics of Taylor Cones. Annual Review of Fluid Mechanics, 39(1), 217–243. https://doi.org/10.1146/annurev.fluid.39.050905.110159.
Fiddler, W., Parker, W. E., Wasserman, A. E., & Doerr, R. C. (1967). Thermal decomposition of ferulic acid. Journal of Agricultural and Food Chemistry, 15(5), 757–761. https://doi.org/10.1021/jf60153a003.
Friedman, M., & Jürgens, H. S. (2000). Effect of pH on the stability of plant phenolic compounds. Journal of Agricultural and Food Chemistry, 48(6), 2101–2110. https://doi.org/10.1021/jf990489j.
Gustaw, W., & Mleko, S. (2007). Gelation of whey proteins by microwave heating. Milchwissenschaft, 62(4), 439–442.
Hadad, S., & Goli, S. A. H. (2019). Improving oxidative stability of flaxseed oil by encapsulation in electrospun flaxseed mucilage nanofiber. Food and Bioprocess Technology, 829–838. https://doi.org/10.1007/s11947-019-02259-1
Hammann, F., & Schmid, M. (2014). Determination and quantification of molecular interactions in protein films: A review. Materials, 7(12), 7975–7996. https://doi.org/10.3390/ma7127975.
Huh, Y. S., Hong, T. H., & Hong, W. H. (2004). Effective extraction of oligomeric proanthocyanidin (OPC) from wild grape seeds. Biotechnology and Bioprocess Engineering, 9(6), 471–475. https://doi.org/10.1007/BF02933488.
Jonsson, K., Andersson, R., Bach Knudsen, K. E., Hallmans, G., Hanhineva, K., Katina, K., Kolehmainen, M., Kyrø, C., Langton, M., Nordlund, E., Lærke, H. N., Olsen, A., Poutanen, K., Tjønneland, A., & Landberg, R. (2018). Rye and health—Where do we stand and where do we go? Trends in Food Science and Technology, 79(July), 78–87. https://doi.org/10.1016/j.tifs.2018.06.018.
Kizil, R., Irudayaraj, J., & Seetharaman, K. (2002). Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. Journal of Agricultural and Food Chemistry, 50(14), 3912–3918. https://doi.org/10.1021/jf011652p.
Kulichová, K., Sokol, J., Nemeček, P., Maliarová, M., Maliar, T., Havrlentová, M., & Kraic, J. (2019). Phenolic compounds and biological activities of rye (Secale cereale L.) grains. Open Chemistry, 17(1), 988–999. https://doi.org/10.1515/chem-2019-0103.
Kuntzler, S. G., Costa, J. A. V., & de Morais, M. G. (2018). Development of electrospun nanofibers containing chitosan/PEO blend and phenolic compounds with antibacterial activity. International Journal of Biological Macromolecules, 117, 800–806. https://doi.org/10.1016/j.ijbiomac.2018.05.224.
Lembach, A. N., Tan, H., Roisman, I. V, Gambaryan-roisman, T., Zhang, Y., Tropea, C., & Yarin, A. L. (2010). Drop impact, spreading , splashing , and penetration into electrospun nanofiber mats. 26(9), 9516–9523. https://doi.org/10.1021/la100031d
Lin, S., Chen, M., Jiang, H., Fan, L., Sun, B., Yu, F., Yang, X., Lou, X., He, C., & Wang, H. (2016). Green electrospun grape seed extract-loaded silk fibroin nanofibrous mats with excellent cytocompatibility and antioxidant effect. Colloids and Surfaces B: Biointerfaces, 139, 156–163. https://doi.org/10.1016/j.colsurfb.2015.12.001.
Liu, G., Gu, Z., Hong, Y., Cheng, L., & Li, C. (2017). Electrospun starch nanofibers: Recent advances, challenges, and strategies for potential pharmaceutical applications. Journal of Controlled Release, 252, 95–107. https://doi.org/10.1016/j.jconrel.2017.03.016.
Locilento, D. A., Mercante, L. A., Andre, R. S., Mattoso, L. H. C., Luna, G. L. F., Brassolatti, P., & Anibal, F. de F., & Correa, D. S. (2019). Biocompatible and biodegradable electrospun nanofibrous membranes loaded with grape seed extract for wound dressing application. Journal of Nanomaterials, 2019, 1–12. https://doi.org/10.1155/2019/2472964.
Luca, A., Cilek, B., Hasirci, V., Sahin, S., & Sumnu, G. (2013). Effect of degritting of phenolic extract from sour cherry pomace on encapsulation efficiency-production of nano-suspension. Food and Bioprocess Technology, 6(9), 2494–2502. https://doi.org/10.1007/s11947-012-0880-z.
Luo, C. J., Loh, S., Stride, E., & Edirisinghe, M. (2012). Electrospraying and electrospinning of chocolate suspensions. Food and Bioprocess Technology, 5(6), 2285–2300. https://doi.org/10.1007/s11947-011-0534-6.
Marqués, J. L., Porta, G. D., Reverchon, E., Renuncio, J. A. R., & Mainar, A. M. (2013). Supercritical antisolvent extraction of antioxidants from grape seeds after vinification. Journal of Supercritical Fluids, 82, 238–243. https://doi.org/10.1016/j.supflu.2013.07.005.
Mendes, A. C., Stephansen, K., & Chronakis, I. S. (2017). Electrospinning of food proteins and polysaccharides. Food Hydrocolloids, 68, 53–68. https://doi.org/10.1016/j.foodhyd.2016.10.022.
Monagas, M., Gómez-Cordovés, C., Bartolomé, B., Laureano, O., & Ricardo Da Silva, J. M. (2003). Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. Journal of Agricultural and Food Chemistry, 51(22), 6475–6481. https://doi.org/10.1021/jf030325+.
Moomand, K., & Lim, L. (2015). Properties of encapsulated fish oil in electrospun zein fibres under simulated in vitro conditions. 431–444. https://doi.org/10.1007/s11947-014-1414-7
Nedovic, V., Kalusevic, A., Manojlovic, V., Levic, S., & Bugarski, B. (2011). An overview of encapsulation technologies for food applications. Procedia Food Science, 1, 1806–1815. https://doi.org/10.1016/j.profoo.2011.09.265.
Oguz, S., Tam, N., Aydogdu, A., Sumnu, G., & Sahin, S. (2018). Development of novel pea flour-based nanofibres by electrospinning method. International Journal of Food Science and Technology, 53(5), 1269–1277. https://doi.org/10.1111/ijfs.13707.
Rosentrater, K. A., & Evers, A. D. (2018). Bread-baking technology. In Kent’s Technology of Cereals. https://doi.org/10.1016/b978-0-08-100529-3.00008-6
Rubilar, J. F., Cruz, R. M. S., Silva, H. D., Vicente, A. A., Khmelinskii, I., & Vieira, M. C. (2013). Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. Journal of Food Engineering, 115(4), 466–474. https://doi.org/10.1016/j.jfoodeng.2012.07.009.
Seethu, B. G., Pushpadass, H. A., Emerald, F. M. E., Nath, B. S., Naik, N. L., & Subramanian, K. S. (2020). Electrohydrodynamic encapsulation of resveratrol using food-grade nanofibres: Process optimization, characterization and fortification. Food and Bioprocess Technology, 13(2), 341–354. https://doi.org/10.1007/s11947-019-02399-4.
Shao, P., Niu, B., Chen, H., & Sun, P. (2018). Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. International Journal of Biological Macromolecules, 107(Pt B), 1908–1914. https://doi.org/10.1016/j.ijbiomac.2017.10.054.
Sogut, E., & Seydim, A. C. (2018). Development of chitosan and polycaprolactone based active bilayer films enhanced with nanocellulose and grape seed extract. Carbohydrate Polymers, 195(April), 180–188. https://doi.org/10.1016/j.carbpol.2018.04.071.
Stijnman, A. C., Bodnar, I., & Hans Tromp, R. (2011). Electrospinning of food-grade polysaccharides. Food Hydrocolloids, 25(5), 1393–1398. https://doi.org/10.1016/j.foodhyd.2011.01.005.
Sullivan, S. T., Tang, C., Kennedy, A., Talwar, S., & Khan, S. A. (2014). Electrospinning and heat treatment of whey protein nanofibers. Food Hydrocolloids, 35, 36–50. https://doi.org/10.1016/j.foodhyd.2013.07.023.
Sumnu, G. (2001). A review on microwave baking of foods. International Journal of Food Science and Technology, 36(2), 117–127. https://doi.org/10.1046/j.1365-2621.2001.00479.x.
Tam, N., Oguz, S., Aydogdu, A., Sumnu, G., & Sahin, S. (2017). Influence of solution properties and pH on the fabrication of electrospun lentil flour/HPMC blend nanofibers. Food Research International, 102(July), 616–624. https://doi.org/10.1016/j.foodres.2017.09.049.
Tampau, A., González-Martinez, C., & Chiralt, A. (2017). Carvacrol encapsulation in starch or PCL based matrices by electrospinning. Journal of Food Engineering, 214, 245–256. https://doi.org/10.1016/j.jfoodeng.2017.07.005.
Tavassoli-Kafrani, E., Goli, S. A. H., & Fathi, M. (2018). Encapsulation of orange essential oil using cross-linked electrospun gelatin nanofibers. Food and Bioprocess Technology, 11(2), 427–434. https://doi.org/10.1007/s11947-017-2026-9.
Torres-Giner, S. (2011). Electrospun nanofibers for food packaging applications. Multifunctional and Nanoreinforced Polymers for Food Packaging, 108–125. https://doi.org/10.1533/9780857092786.1.108
Uygun, E., Yildiz, E., Sumnu, G., & Sahin, S. (2020). Microwave pretreatment for the improvement of physicochemical properties of carob flour and rice starch–based electrospun nanofilms. Food and Bioprocess Technology, 13(5), 838–850. https://doi.org/10.1007/s11947-020-02440-x.
Vega-Lugo, A. C., & Lim, L. T. (2012). Effects of poly(ethylene oxide) and pH on the electrospinning of whey protein isolate. Journal of Polymer Science, Part B: Polymer Physics, 50(16), 1188–1197. https://doi.org/10.1002/polb.23106.
Villamiel, M., Corzo, N., Martínez-Castro, I., & Olano, A. (1996). Chemical changes during microwave treatment of milk. Food Chemistry, 56(4), 385–388. https://doi.org/10.1016/0308-8146(95)00196-4.
Wen, P., Zhu, D. H., Feng, K., Liu, F. J., Lou, W. Y., Li, N., Zong, M. H., & Wu, H. (2016). Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chemistry, 196, 996–1004. https://doi.org/10.1016/j.foodchem.2015.10.043.
Woranuch, S., Pangon, A., Puagsuntia, K., Subjalearndee, N., & Intasanta, V. (2017). Rice flour-based nanostructures via a water-based system: Transformation from powder to electrospun nanofibers under hydrogen-bonding induced viscosity, crystallinity and improved mechanical property. RSC Advances, 7(32), 19960–19966. https://doi.org/10.1039/c7ra01485f.
Xu, X., Jiang, L., Zhou, Z., Wu, X., & Wang, Y. (2012). Preparation and properties of electrospun soy protein isolate/polyethylene oxide nanofiber membranes. ACS Applied Materials and Interfaces, 4(8), 4331–4337. https://doi.org/10.1021/am300991e.
Zhao, B., Sun, S., Lin, H., Chen, L., Qin, S., Wu, W., Zheng, B., & Guo, Z. (2019). Physicochemical properties and digestion of the lotus seed starch-green tea polyphenol complex under ultrasound-microwave synergistic interaction. Ultrasonics Sonochemistry, 52(July 2018), 50–61. https://doi.org/10.1016/j.ultsonch.2018.11.001.
Code Availability
Not applicable
Author information
Authors and Affiliations
Contributions
Gizem Aslaner: Conceptualization, Methodology, Investigation, Writing. Gulum Sumnu: Conceptualization, Supervision, Reviewing and Editing. Serpil Sahin: Conceptualization, Supervision, Reviewing and Editing
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslaner, G., Sumnu, G. & Sahin, S. Encapsulation of Grape Seed Extract in Rye Flour and Whey Protein–Based Electrospun Nanofibers. Food Bioprocess Technol 14, 1118–1131 (2021). https://doi.org/10.1007/s11947-021-02627-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02627-w