Abstract
The aim of this study was to prepare electrospun gelatin (G) and gelatin-cross-linked tannic acid (GT) nanofibers loaded with orange essential oil. Four G/GT-to-orange essential oil ratios (1:0.25, 1:0.5, 1:0.74, and 1:1) were used to prepare orange oil-loaded nanofibers. The best encapsulation efficiency (69 and 52.6%) and oil content (34.45 and 26.26%) were observed at the ratio of 1:1 for both G and GT, respectively. The morphology and thermal properties of the electrospun fibers as well as the release and storage stability of loaded orange essential oil were investigated at the selected ratio. The results indicated that the fibrous structure of the orange essential oil-loaded samples was maintained. It is found that both G and GT provided suitable controlled release of orange essential oil and were successful in improving its storage stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Orange essential oil, as a natural flavoring agent, is one of the most applicable essential oils used in orange juice (to compensate the flavor lost during thermal processes) as well as in beverages, ice cream, chewing gums, candies, and cakes (Hosni et al. 2010). However, due to its volatility and sensitivity to deterioration, it may lose its function during processing and storage and cannot be applied as a long-term flavoring agent. Therefore, it is necessary and desirable to extend its resistance to environmental factors like heat, light, oxygen, and humidity and even control its release (Sutaphanit and Chitprasert 2014). In order to achieve these goals, different methods including ionic gelation (Benavides et al. 2016), spray drying (Rodea-González et al. 2012), coacervation (Sutaphanit and Chitprasert 2014), emulsion (Edris and Bergnståhl 2001), and co-crystallization (Beristain et al. 1996) have been used to encapsulate orange essential oil.
Electrospinning is a simple, cost-effective method that uses electrical field to fabricate non-woven mats of nano- to micro-meter-scale diameters. In comparison to micro-scale capsules, the fibers in nanometer scale would possess high surface-to-volume ratio contributing to mass transfer and effective delivery (Li et al. 2009). Also, due to the subcellular size of fibers, the antimicrobial properties of essential oil increase (Donsì et al. 2011). Besides, the porous structure of fibers and the absence of heating during electrospinning make it an appropriate method for encapsulation of heat-sensitive aromatic compounds (Li et al. 2009). A variety of electrospinning methods can be used for encapsulation such as blend electrospinning, co-axial electrospinning, emulsion electrospinning, and surface modification of electrospun fiber mats (Neo 2014). Recently, different bioactive compounds have been encapsulated via electrospinning method. Charernsriwilaiwat et al. (2013) loaded Garcinia mangostana extract in electrospun chitosan-based nanofibers. In another study, Li et al. (2009) studied the efficacy of electrospun zein fibers to incorporate epigallocatechin gallate. Moreover, the encapsulation of candeia essential oil in polylactic acid fiber mats was investigated by Mori et al. (2015).
Gelatin is a natural biopolymer which has been widely used for encapsulation of bioactive compounds such as basil essential oil (Sutaphanit and Chitprasert 2014; Tongnuanchan et al. 2016), bergamot and lemongrass oil (Mehraj et al. 2012), Origanum vulgare L. essential oil (Hosseini et al. 2016), and orange leaf essential oil (Alparslan et al. 2016). However, cross-linking of gelatin would be necessary due to its high solubility in aqueous mediums. Different compounds including glutaraldehyde (Ratanavaraporn et al. 2010), genipin (Panzavolta et al. 2011), dextran (Jalaja et al. 2014), transglutaminase (Chambi and Grosso 2006), and phenolic compounds such as caffeic acid (Kosaraju et al. 2010), catechin (Balange and Benjakul 2009), gallic acid (Yan et al. 2011), and tannic acid (Cao et al. 2007) have been used to cross-link gelatin and improve its functionality. Phenolics are natural compounds which abound in many plants with a relatively low cost (Hager et al. 2012). They are non-toxic, generally recognized as safe (GRAS), and benefit from antioxidant activities (Tavassoli-Kafrani et al. 2017). Among different phenolic compounds, tannic acid has sufficient hydroxyl groups to strongly bind gelatin and it can be used as a food additive with the range of 10–400 mg/L (Balange and Benjakul 2010). Moreover, in our previous paper (Tavassoli-Kafrani et al. 2017), we studied the cross-linking activity of tannic acid, gallic acid, ferulic acid, and caffeic acid toward gelatin nanofibers. As post cross-linking procedure seems to negatively affect the fiber morphology (Slemming-Adamsen et al. 2015), we first cross-linked gelatin and then electrospinning was conducted. Tannic acid showed the highest cross-linking activity (13.3%); therefore, it was used as a cross-linking agent in the present study.
We aimed herein to encapsulate orange essential oil in gelatin and gelatin-cross-linked tannic acid nanofibers by emulsion electrospinning. For this purpose, different ratios of gelatin/cross-linked gelatin solution to orange essential oil were firstly evaluated based on encapsulation efficiency and oil content. The best ratio was then selected for electrospinning, and the fiber morphology, thermal properties, orange essential oil stability, and release were determined.
Materials and Methods
Materials
Orange essential oil (EO) composed of mainly limonene 95.8%, terpinen 1%, and linalool 0.75% (Osare Sabz Co., Isfahan, Iran); gelatin type A, bovine skin, and bloom 180 (Behin Azma Co., Shiraz, Iran) were purchased. Tannic acid was provided by Sigma-Aldrich. All other chemicals were of analytical grade and obtained from Merck.
Preparation of Cross-linked Gelatin
Gelatin cross-linked with tannic acid (GT) was prepared by the method described by Pena et al. (2010) with some modifications. For the preparation of gelatin solution, 12.5-g gelatin powder was dissolved in 40 mL distilled water while stirring at 30 °C for 30 min. In order to complete its dissolution, the temperature was raised to 50 ± 5 °C. Separately, tannic acid solution (15% w/w gelatin) was prepared by dissolving it in 10 mL distilled water. The pH of each solution was adjusted to 11 with 1 N NaOH solution. Then, tannic acid solution was subjected to oxygen bubbling for 1 h. Cross-linking reaction was conducted by mixing two solutions and stirring for 30 min at 50 ± 5 °C. The prepared solution was freeze-dried at − 40 °C for 24 h (FD-5003-BT, Sanat Pardaz Dana Co., Iran). The resultant powder was used to prepare further solutions.
Emulsion Preparation
The emulsion preparation was performed based on the methods of Sutaphanit and Chitprasert (2014) and Gómez-Mascaraque and López-Rubio (2015). Gelatin solution (15% w/w) was prepared by dissolving gelatin powder in 40% acetic acid solution at 40 °C for 30 min. EO was added dropwise to gelatin solution at ratios of 87:13, 90:10, 93:7, and 96:4 (gelatin solution/EO, v/v) while homogenizing at 18,000 rpm for 2 min (T25 D, Ultra-Turrax, Germany) (the final ratios of gelatin powder/EO, w/w, would be 1:1, 1:0.74, 1:0.5, and 1:0.25, respectively). An ice bath was used to prevent overheating during homogenization. The emulsions were placed in a 10-mL container and then sonicated for 2 min at a power of 300 W (APU, Adeeco, Iran) with intervals of 3 s in an ice bath without agitation. The same procedure was used to prepare GT emulsion. Gelatin and GT solutions (15% w/w) in 40% acetic acid were used as blanks.
Electrospinning of Emulsions
Electrospinning procedure was done by an electrospinning system (Vita Teb Koosha, Iran). Emulsions were loaded in a 5-mL syringe connected to a metal capillary (0.6-mm inner diameter, gauge 20). The syringe pump to which the syringe was connected was used to maintain the flow rate of 0.3 mL/h. The positive electrode of a direct current was connected to the needle. The needle was aligned horizontally to the collector, and a voltage of 13 kV was applied. The distance between the tip and the collector was chosen at 15 cm. Electrospinning was conducted at room temperature (25 °C) and at a relative humidity of 33%.
Characterization of Electrospun Gelatin Nanofibers
Determination of Encapsulation Efficiency and Oil Content
The encapsulation efficiency and oil content of nanofiber mats were measured as previously reported by Sutaphanit and Chitprasert (2014). First, EO was dissolved in ethanol at a concentration of 500 ppm and further scanned over the range of 200–600 nm spectrophotometrically (T60 UV-Visible spectrophotometer, PG Instruments, USA). The maximum absorbance was observed at 219 nm. Encapsulation efficiency and oil content were determined by the following method: 3 mg each of fiber sample was mixed with 3 mL ethanol under magnetic stirring at 100 rpm for 15 min (IKA, Germany). Then, the suspensions were filtered through nylon syringe filter (0.45-μm pore size) and the absorbance was measured at 219 nm. The blanks were gelatin (G) and GT fibers without EO. Surface EO concentration was calculated based on a calibration curve (R 2 = 0.99):
The calculation of encapsulation efficiency and oil content was performed using the following equations:
Where Wi is the initial weight of EO added for encapsulation, Ws is the weight of surface EO calculated based on calibration curve, and Wt is the total weight of EO-loaded nanofibers.
Fiber Morphology
Scanning electronic microscopy (SEM) (× 130, Philips, Netherlands) with an acceleration voltage of 20 kV was used to investigate the morphological appearance of electrospun fiber mats. Prior to observation, the samples were sputter-coated with gold. The mean fiber diameters were measured with ImageJ software.
Essential Oil Release
This assay was conducted according to the method of Gómez-Mascaraque et al. (2015) with some modifications. 2.5 mg each of fiber sample was suspended in 7.5 mL ethanol, kept under room temperature (25 °C), and stirred (20 rpm). At specific time intervals, an aliquot of 1.5 mL solution was removed and analyzed spectrophotometrically at 219 nm. The same volume as that of the aliquot (1.5 mL) was replaced with fresh ethanol at each interval.
Essential Oil Stability During Storage
The storage stability of nanofibers was investigated based on the method reported by Li et al. (2013) at 33% relative humidity and 25 °C. The relative humidity was achieved by placing saturated MgCl2 into the desiccator. Nanofibers (2 mg each) and free EO (as control sample) were kept in open micro-tubes and placed in the desiccator. For free EO, every 2 h for the first 12 h and after that, every 24 h, samples were dissolved in ethanol and the absorbance was recorded at 219 nm. For encapsulated EO, the max absorbance of EO in the water/ethanol (1:1) solution was measured at 211 nm. In order to investigate the storage stability of encapsulated EO, the time intervals were every 4 days, samples were dissolved in 3 mL ethanol/water (1:1), and the absorbance was read at 211 nm. The EO concentration was calculated based on the calibration curve:
Results were expressed as the percent of remaining EO in comparison to the amount of EO calculated at 0 time.
Thermal Properties
Differential scanning calorimeter (DSC) (DSC 302, BUHR, Germany) was used to analyze the thermal properties of electrospun nanofibers. The temperature ranged between 10 and 250 °C, and the heating rate was 10 °C/min.
Statistical Analysis
The results were analyzed using a completely randomized design with one-way ANOVA and least significant difference (LSD) test at a probability level of 0.05 using the SAS software. All the experiments were performed at least in duplicate. Means were calculated for each experimental series, and the data were expressed as means ± standard deviations (SD) (n = 3).
Results and Discussion
Determination of Encapsulation Efficiency and Oil Content
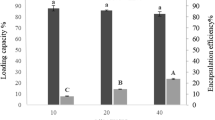
The influence of different G/GT-to-EO ratios on the encapsulation efficiency and oil content was investigated (Table 1). The encapsulation efficiency of nanofibers ranged from 35.0 to 69.0 and from 16.9 to 60.7% for G and GT nanofibers, respectively. Gelatin nanofibers showed higher encapsulation efficiency than GT samples. Moreover, in G nanofibers loaded with EO (GE), the encapsulation efficiency increased as the amount of EO enhanced and the highest encapsulation efficiency was observed for 1:1 ratio. Similarly, in GT nanofibers loaded with EO (GTE), an increase in encapsulation efficiency was observed by adding more EO while the ratio of 1:0.74 had the highest encapsulation efficiency and a decline in encapsulation efficiency was observed for 1:1 ratio. It seems that at 1:1 ratio, the amount of gelatin-tannic, against gelatin, was not sufficient to saturate the surface of oil droplets during emulsion formation and thus GT was unable to completely entrap oil in the nanofibers. The lowest capability of GT to entangle EO in all ratios might be due to its higher viscosity which could negatively affect the rate at which GT is adsorbed to the surface of oil droplets during homogenization (Surh et al. 2006).
As expected, oil content of nanofibers increased with an increment in the oil ratio to the wall material and the highest value was obtained at the highest concentration of essential oil (1:1 ratio) for both GE (34.4%) and GTE (26.2%) samples (Table 1). With respect to oil content and encapsulation efficiency, the ratio of 1:1 was selected for further analyses.
Fiber Morphology
All samples showed smooth cylindrical fibrous structure with no phase separation in the structure which could confirm the successful incorporation of orange essential oil within fibers (Fig. 1). The mean fiber diameter of GT sample (145 nm) was bigger than that of G nanofibers (83.5 nm) which might be due to the large molecular size of tannic acid cross-linked with gelatin (Table 2). Jalaja and James (2015) also reported higher mean fiber diameter of gelatin-cross-linked oxidized sucrose. The addition of EO increased the mean fiber diameter which may be because of the effect of EO in lowering the electrical conductivity of G and GT solutions leading to an increase in mean fiber diameter (Mori et al. 2015; Wen et al. 2016). These results were in agreement with the findings of Charernsriwilaiwat et al. (2013).
Thermal Properties
The DSC results can be a good indicator of encapsulation efficiency of an active substance within a carrier polymer (Feyzioglu and Tornuk 2016). When the active compound is effectively incorporated inside the polymer carrier, we cannot observe the characteristic peaks of the active substance. However, separate peaks can be seen if the co-precipitation is not sufficient (Mezzomoa et al. 2012). As shown in Fig. 2, the similarity of samples with and without EO seemed to demonstrate the successful encapsulation of EO within gelatin and gelatin-cross-linked tannic acid nanofibers. The DSC thermograms of all samples showed an endothermic peak in the range of 48 to 52 °C corresponding to denaturation and helix-to-coil transition of G and GT (Table 2). The denaturation temperature increased in nanofibers containing EO which might be because of the effect of EO on enhancing the interaction of water with G and GT chains hence increasing their water-holding capacity (Feyzioglu and Tornuk 2016). According to Table 2, the enthalpy of denaturation in EO-loaded gelatin nanofiber increased while it reduced in GT containing EO. It can be argued that on the one hand, EO acts as a plasticizer and increases the mobility of gelatin molecular chains (Wen et al. 2016) which leads to the reduction of denaturation enthalpy. On the other hand, it can also interact with polypeptide chains and increase denaturation enthalpy (Feyzioglu and Tornuk 2016; Tongnuanchan et al. 2016). This interaction may be related to the interaction of gelatin hydrophobic chain with the alkyl chain and benzene ring of essential oil whereby orange essential oil is a guest molecule located inside gelatin or GT chains (Paula et al. 2011). After preparation of G and GT solutions, the polypeptide chains can partially reconstruct their initial order. When EO is added to the GT solution, it acts as a plasticizer preventing the reconstruction of its initial order; therefore, the denaturation enthalpy would reduce. However, due to better emulsifying properties, the G sample can interact more with EO which leads to an increase in denaturation enthalpy.
In the study conducted by Feyzioglu et al. (2016), the effect of encapsulation of summer savory essential oil was investigated on the thermal properties of chitosan nanoparticles. They found that the addition of essential oil to chitosan nanoparticles increased the thermal stability of nanoparticles. They argued that this increase might be due to the interaction between chitosan and essential oil. Furthermore, Tongnuanchan et al. (2016) incorporated basil essential oil, palm oil, or a mixture of them to the gelatin film. They reported a slight increase in denaturation temperature and reduction of the enthalpies of all samples containing oils. They explained this reduction in terms of the barrier effect of basil essential oil and palm oil on the reconstruction of gelatin chains during the casting and drying of gelatin films.
Essential Oil Release
The release profile of EO from G and GT nanofiber mats during 72 h was investigated. As shown in Fig. 3, for both samples, the release process can be characterized as a tri-phasic procedure, i.e., an initial burst release followed by subsequent reduction and finally a gradual release.
The preliminary burst release was indicated at the first 4 h of the experiment. This phenomenon seems to be because of the surface oil release. Obviously, during electrospinning, some content of EO would not be entrapped and placed on the surface of the formed nanofibers. Therefore, by placing the GE or GTE nanofibers in the release medium, the surface EO will be released quickly. After 8 h, the released EO started to increase gradually and reached to almost a steady concentration in both samples. There was no significant difference between G and GT in EO release during 72 h.
EO Storage Stability
The storage stability of EO was significantly (p < 0.05) improved by encapsulation within G and GT nanofibers. As can be seen in Fig. 4, free EO was completely vaporized during first 96 h while it remained for almost 20 days (480 h) of storage within G and GT nanofibers, which clearly pertains to the barrier effect of the wall material. The retention of EO in G nanofibers was higher than that of the GT sample. This might be due to the higher surface swelling of GT at 33% relative humidity. Aewsiri et al. (2009) reported that gelatin cross-linking with tannic acid decreased its surface hydrophobicity which was due to the presence of hydroxyl and carboxyl groups of tannic acid. Gelatin-tannic absorbs more water causing surface swelling, thereby creating some pores which could lead to easy EO volatilization (Li et al. 2013). Besides, the bigger fiber mean diameter in the GT sample could induce the loss of EO from nanofibers (Jalaja and James 2015). The results were in agreement with the report of Turasan et al. (2015). Their results showed an improved stability of rosemary essential oil encapsulated in whey protein concentrate and maltodextrin. In another study, Li et al. (2013) encapsulated orange essential oil in spray-dried chitosan micro-capsules. The storage stability of encapsulated orange essential oil increased from 8 to 80 days at 25 °C.
Conclusion
In the present study, the potential of gelatin and gelatin-cross-linked tannic acid nanofibers as wall materials for nanoencapsulation of orange essential oil via electrospinning was investigated. The best ratio of G or GT to EO was achieved at 87:13 with respect to the encapsulation efficiency and oil content of the fibers. The cross-linking of gelatin using tannic acid decreased its ability for encapsulation of EO. The morphology of nanofibers containing EO showed no major difference compared to electrospun G and GT, although a slight increase in the mean fiber diameter was observed. It is proved that both gelatin and gelatin-cross-linked tannic acid provided suitable controlled release of orange essential oil and strongly increased its storage stability. The storage stability of EO in gelatin nenofibers was higher than that of the gelatin-tannic type. The potential applications of these fibers are in different food or pharmaceutical products where the maximum protection is needed. Gelatin could be used as a promising delivery system for value-added enriched or fortified food products.
References
Alparslan, Y., Hasanhocaoglu Yapıcı, H., Metin, C., Baygar, T., Günlü, A., & Baygar, T. (2016). Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT - Food Science and Technology, 72, 457–466. https://doi.org/10.1016/j.lwt.2016.04.066.
Balange, A. K., & Benjakul, S. (2009). Effect of oxidised phenolic compounds on the gel property of mackerel (Rastrelliger kanagurta) surimi. LWT - Food Science and Technology, 42(6), 1059–1064. https://doi.org/10.1016/j.lwt.2009.01.013.
Balange, A. K., & Benjakul, S. (2010). Cross-linking activity of oxidised tannic acid towards mackerel muscle proteins as affected by protein types and setting temperatures. Food Chemistry, 120(1), 268–277. https://doi.org/10.1016/j.foodchem.2009.10.019.
Benavides, S., Cortés, P., Parada, J., & Franco, W. (2016). Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chemistry, 204, 77–83. https://doi.org/10.1016/j.foodchem.2016.02.104.
Beristain, C. e. I., Vazquez, A., Garcia, H. S., & Vernon-Carter, E. J. (1996). Encapsulation of orange peel oil by co-crystallization. Lebensmittel- Wissenschaft und Technologie, 29(7), 645–647. https://doi.org/10.1006/fstl.1996.0098.
Cao, N., Fu, Y., & He, J. (2007). Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocolloids, 21(4), 575–584. https://doi.org/10.1016/j.foodhyd.2006.07.001.
Chambi, H., & Grosso, C. (2006). Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Research International, 39(4), 458–466. https://doi.org/10.1016/j.foodres.2005.09.009.
Charernsriwilaiwat, N., Rojanarata, T., Ngawhirunpat, T., Sukma, M., & Opanasopit, P. (2013). Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. International Journal of Pharmaceutics, 452(1-2), 333–343. https://doi.org/10.1016/j.ijpharm.2013.05.012.
Donsì, F., Annunziata, M., Sessa, M., & Ferrari, G. (2011). Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Science and Technology, 44(9), 1908–1914. https://doi.org/10.1016/j.lwt.2011.03.003.
Edris, A., & Bergnståhl, B. (2001). Encapsulation of orange oil in a spray dried double emulsion. Nahrung, 45(2), 133–137. https://doi.org/10.1002/1521-3803(20010401)45:2<133::AID-FOOD133>3.0.CO;2-C.
Feyzioglu, G. C., & Tornuk, F. (2016). Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT - Food Science and Technology, 70, 104–110. https://doi.org/10.1016/j.lwt.2016.02.037.
Gómez-Mascaraque, L. G., Lagarón, J. M., & López-Rubio, A. (2015). Electrosprayed gelatin submicroparticles as edible carriers for the encapsulation of polyphenols of interest in functional foods. Food Hydrocolloids, 49, 42–52. https://doi.org/10.1016/j.foodhyd.2015.03.006.
Gómez-Mascaraque, L. G., & López-Rubio, A. (2015). Protein-based emulsion electrosprayed micro- and submicroparticles for the encapsulation and stabilization of thermosensitive hydrophobic bioactives. Journal of Colloid and Interface Science. doi:http:// dx.doi.org/https://doi.org/10.1016/j.jcis.2015.11.061.
Hager, A.-S., Vallons, K. J. R., & Arendt, E. K. (2012). Influence of gallic acid and tannic acid on the mechanical and barrier properties of wheat gluten films. Journal of Agricultural and Food Chemistry, 60(24), 6157–6163. https://doi.org/10.1021/jf300983m.
Hosni, K., Chrif, R., Abid, I., Medfei, W., Kallel, M., Brahim, N. B., et al. (2010). Composition of peel essential oils from four selected Tunisian citrus species: evidence for the genotypic influence. Food Chemistry, 123(4), 1098–1104. https://doi.org/10.1016/j.foodchem.2010.05.068.
Hosseini, S. F., Rezaei, M., Zandi, M., & Farahmandghavi, F. (2016). Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chemistry, 194, 1266–1274. https://doi.org/10.1016/j.foodchem.2015.09.004.
Jalaja, K., & James, N. R. (2015). Electrospun gelatin nanofibers: a facile cross-linking approach using oxidized sucrose. International Journal of Biological Macromolecules, 73, 270–278. https://doi.org/10.1016/j.ijbiomac.2014.11.018.
Jalaja, K., Kumar, P. R., Dey, T., Kundu, S. C., & James, N. R. (2014). Modified dextran cross-linked electrospun gelatin nanofibres for biomedical applications. Carbohydrate Polymers, 114, 467–475. https://doi.org/10.1016/j.carbpol.2014.08.023.
Kosaraju, S. L., Puvanenthiran, A., & Lillford, P. (2010). Naturally crosslinked gelatin gels with modified material properties. Food Research International, 43(10), 2385–2389. https://doi.org/10.1016/j.foodres.2010.09.008.
Li, Y., Ai, L., Yokoyama, W., Shoemaker, C. F., Wei, D., Ma, J., & Zhong, F. (2013). Properties of chitosan-microencapsulated orange oil prepared by spray-drying and its stability to detergents. Journal of Agricultural and Food Chemistry, 61(13), 3311–3319. https://doi.org/10.1021/jf305074q.
Li, Y., Lim, L. T., & Kakuda, Y. (2009). Electrospun zein fibers as carriers to stabilize (-)-epigallocatechin gallate. Journal of Food Science, 74(3), C233–C240. https://doi.org/10.1111/j.1750-3841.2009.01093.x.
Mehraj, A., Benjakul, S., Prodpran, T., & Agustini, T. W. (2012). Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocolloids, 28, 189–199.
Mezzomoa, N., de Paz, E., Maraschinc, M., Martín, A., Cocero, M. J., & Ferreira, S. R. S. (2012). Supercritical anti-solvent precipitation of carotenoid fraction from pink shrimp residue: effect of operational conditions on encapsulation efficiency. Journal of Supercritical Fluids, 66, 342–349. https://doi.org/10.1016/j.supflu.2011.08.006.
Mori, C. L. S. d. O., dos Passos, N. A., Oliveira, J. E., Altoé, T. F., Mori, F. A., Mattoso, L. H. C., Scolforo, J. R., & Tonoli, G. H. D. (2015). Nanostructured polylactic acid/candeia essential oil mats obtained by electrospinning. Journal of Nanomaterials, 2015, 1–9. https://doi.org/10.1155/2015/439253.
Neo, Y. P. (2014). Electrospinning as a novel encapsulation method. Auckland: The University of Auckland.
Panzavolta, S., Gioffre, M., Focarete, M. L., Gualandi, C., Foroni, L., & Bigi, A. (2011). Electrospun gelatin nanofibers: optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomaterialia, 7(4), 1702–1709. https://doi.org/10.1016/j.actbio.2010.11.021.
Paula, H. C. B., Sombra, F. M., Cavalcante, R.d. F., Abreu, F. O. M. S., & de Paula, R. C. M. (2011). Preparation and characterization of chitosan/cashew gum beads loaded with Lippia sidoides essential oil. Materials Science and Engineering: C, 31(2), 173–178. https://doi.org/10.1016/j.msec.2010.08.013.
Pena, C., de la Caba, K., Eceiza, A., Ruseckaite, R., & Mondragon, I. (2010). Enhancing water repellence and mechanical properties of gelatin films by tannin addition. Bioresource Technology, 101, 6836–6842.
Ratanavaraporn, J., Rangkupan, R., Jeeratawatchai, H., Kanokpanont, S., & Damrongsakkul, S. (2010). Influences of physical and chemical crosslinking techniques on electrospun type A and B gelatin fiber mats. International Journal of Biological Macromolecules, 47(4), 431–438. https://doi.org/10.1016/j.ijbiomac.2010.06.008.
Rodea-González, D. A., Cruz-Olivares, J., Román-Guerrero, A., Rodríguez-Huezo, M. E., Vernon-Carter, E. J., & Pérez-Alonso, C. (2012). Spray-dried encapsulation of chia essential oil (Salvia hispanica L.) in whey protein concentrate-polysaccharide matrices. Journal of Food Engineering, 111(1), 102–109. https://doi.org/10.1016/j.jfoodeng.2012.01.020.
Slemming-Adamsen, P., Song, J., Dong, M., Besenbacher, F., & Chen, M. (2015). In situ cross-linked PNIPAM/gelatin nanofibers for thermo-responsive drug release. Macromolecular Materials and Engineering, 12, 1226–1231.
Surh, J., Decker, E. A., & McClements, D. J. (2006). Properties and stability of oil-in-water emulsions stabilized by fish gelatin. Food Hydrocolloids, 20(5), 596–606. https://doi.org/10.1016/j.foodhyd.2005.06.002.
Sutaphanit, P., & Chitprasert, P. (2014). Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chemistry, 150, 313–320. https://doi.org/10.1016/j.foodchem.2013.10.159.
Tavassoli-Kafrani, E., Goli, S. A. H., & Fathi, M. (2017). Fabrication and characterization of electrospun gelatin nanofibers crosslinked with oxidized phenolic compounds. International Journal of Biological Macromolecules, 103, 1062–1068. https://doi.org/10.1016/j.ijbiomac.2017.05.152.
Tongnuanchan, P., Benjakul, S., Prodpran, T., Pisuchpen, S., & Osako, K. (2016). Mechanical, thermal and heat sealing properties of fish skin gelatin film containing palm oil and basil essential oil with different surfactants. Food Hydrocolloids, 56, 93–107. https://doi.org/10.1016/j.foodhyd.2015.12.005.
Turasan, H., Sahin, S., & Sumnu, G. (2015). Encapsulation of rosemary essential oil. LWT - Food Science and Technology, 64(1), 112–119. https://doi.org/10.1016/j.lwt.2015.05.036.
Wen, P., Zhu, D.-H., Feng, K., Liu, F.-J., Lou, W.-Y., Li, N., Zong, M. H., & Wu, H. (2016). Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/ b-cyclodextrin inclusion complex for antimicrobial packaging. Food Chemistry, 196, 996–1004. https://doi.org/10.1016/j.foodchem.2015.10.043.
Yan, M., Li, B., Zhao, X., & Yi, J. (2011). Physicochemical properties of gelatin gels from walleye pollock (Theragra chalcogramma) skin cross-linked by gallic acid and rutin. Food Hydrocolloid, 25(5), 907–914. https://doi.org/10.1016/j.foodhyd.2010.08.019.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tavassoli-Kafrani, E., Goli, S.A.H. & Fathi, M. Encapsulation of Orange Essential Oil Using Cross-linked Electrospun Gelatin Nanofibers. Food Bioprocess Technol 11, 427–434 (2018). https://doi.org/10.1007/s11947-017-2026-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-2026-9