Abstract
Fruit juices have become an important product for the healthy food world. In the last 5 years, the sales of industrial juices treated by non-thermal preservation technologies such as high-pressure processing (HPP) have strongly increased. In the present study, the effect of the application of two stabilization treatments, mild heating (MH; 80 °C for 7 min) and high-pressure processing (HPP; 350 MPa for 5 min), on multi-fruit smoothies was compared on a wide range of quality parameters immediately after treatment and during a refrigerated storage of 21 days. From the physico-chemical and instrumental colour point of view, immediately after treatment, HPP smoothies were more similar to the fresh product than those treated by MH. During storage, the colour of MH smoothies was more stable although HPP ones showed lower browning index and viscosity more similar to the untreated product. Additionally, HPP provided smoothies with better sensory properties and higher nutritional quality than MH. In general, HPP smoothies were more similar to the untreated product. However, HPP smoothies kept a residual enzyme activity which is likely to limit the shelf life of this multi-fruit smoothie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit and vegetables are important components of a healthy diet, and their sufficient daily consumption could help prevent major diseases. The World Health Organization recommends a minimum of 400 g of fruit and vegetables per day (excluding potatoes and other starchy tubers) for the prevention of chronic diseases such as heart disease, cancer, diabetes and obesity, as well as for the prevention and alleviation of several micronutrient deficiencies, especially in less developed countries. In Europe, fruit and vegetable intake varies between countries (Lobstein et al. 2004, but according to the compiled results of European Food Safety Authority (2008), the average consumption is 386 g per day.
Consumers consider lack of convenience as an important barrier for fruit consumption. According to Briz et al. (2009)), the need of washing, peeling and cutting is a difficulty pointed out by the consumers regarding fruit consumption, and this is a reason for the growing demand of ready-to-eat or take-away fruit products. In addition, over the last years, there has been an increase in consumer demand for minimally processed foods, more similar to fresh products, without the presence of additives. However, one of the main problems in manufacturing healthy ready-to-eat products, such as those based on fresh fruit and vegetables, is their short shelf life. Therefore, extending the shelf life using mild processing technologies that minimally affect the sensory and nutritional properties of these products is a challenge for the food industry.
In recent years, fruit-based smoothies have gained in popularity across the world and are currently one of the major segments of the soft drinks market. This is primarily driven by rising health consciousness among the public and the consumption convenience and taste and naturalness offered by smoothies. From an industrial standpoint, high-pressure processing (HPP) is used by companies, mainly small- and medium-sized ones, to obtain a high-quality product with a good cost/benefit ratio (Rodrigo et al. 2010). For an acidic product (pH <4.5) such as smoothie juice, HP commercial conditions range from 300 to 600 MPa with holding time between 1 to 5 min (Sampedro et al. 2010; Andrés et al. 2016).
In general, HPP at low or moderate temperatures causes the inactivation of microbial vegetative cells and enzymes without promoting much change in the sensory and nutritional properties of the food. HPP affects the viability of microbial cells (Patterson et al. 2012) and the structure of proteins/enzymes (Lopez-Malo et al. 1998; Rastogi et al. 2007), while leaving mostly unaffected low-molecular weight food compounds, such as vitamins, pigments, flavouring agents and other compounds related to sensory, nutritional and health properties of the product (Fernández-García et al. 2001; Butz et al. 2003; Nienaber and Shellhammer 2001a, b; Oey et al. 2008; Barba et al. 2012).
Most of the studies published up to date were carried out in single-fruit juices such as tomato, orange, apple, watermelon, peach, kiwi and litchi juices (for a review, see Chakraborty et al. 2014), and little research has been conducted into blended fruit products. In multi-fruit smoothies containing sterilized apple juice, Keenan et al. (2010) found that mild thermal treatment had a lower effect on antioxidant activities than HPP at 450 MPa during a storage period of 30 days. More recently, the same group (Keenan et al. 2012) confirmed that HPP (450 and 600 MPa), unlike mild heat (MH), was hardly effective in inhibiting fruit enzymes, while all three treatments presented the same degradation pattern for vitamin C. As reported by Keenan et al. (2012), as smoothies contain a mixture of intracellular contents from the different fruit components, the mixture may exhibit very different biochemical behaviours to those of their individual components. Although the microbiological benefit of HPP treatment in fruit products is well understood, the degradation of nutrient and sensory attributes in a complex matrix including different fruits, such as in smoothies, requires further evaluation. The aim of the present work was to evaluate the effect of both mild heating and high-pressure industrial treatments on the physico-chemical parameters, microbiology, nutritional content and sensory properties of a multi-fruit smoothie throughout refrigerated storage to obtain a fresh-like product.
Materials and Methods
Sample Preparation
Smoothie formulation was based on commercial smoothies but selected for their sensory properties. Apples (Pyrus malus v. Golden delicious), strawberries (Fragaria ananassa v. Pájaro), bananas (Musa cavendishii v. Pequeña enana) and oranges (Citrus sinensis v. Navel-late) were purchased from a local stoking house. Smoothie composition by weight consisted of apple juice (33 %; squeezed apple juice), orange juice (33 %), strawberry (14 %), whole apple (10 %; pulp + juice) and banana (10 %). Juices were obtained using a juicer (Robot Coupé C40, Bourgogne, France) and blended to achieve the aforementioned composition. During sample preparation, room temperature was stabilized at 14 °C, and during production, fruit cuts were kept at 1–2 °C before blending. Smoothies subjected to HPP were packaged in 250-mL polyethylene terephthalate (PET) bottles (Sunbox, Madrid, Spain), while a specific HT300 pouch (Seal Air Cryovac, Milano, Italy) was used in MH samples. Pouches were sealed with a Tecnotrip vacuum sealing system EV-13L-CD (Tecnotrip, Barcelona, Spain). Both packages were selected to avoid the effect of packaging materials on the quality of the smoothie.
Thermal and High-Pressure Treatments
For MH treatment, the samples were introduced into an ILRAPLUS autoclave (Ilpra Systems, Mataró, Spain) and heated to 80 °C for 7 min including an initial ramp of 5.7 °C min−1; the total heating lasted for 27 min. The internal temperature of the smoothies during treatment was continuously recorded using a datalogger (PicoVacq, TMI Orion, France), and the accumulated lethality achieved for the pertinent pathogen, Escherichia coli O157:H7 (FDA 2001), was calculated according to the following equation:
where T corresponds to the registered temperature, T ref to the reference temperature (60 °C), z to the thermal resistance and t to the time.
The consequent log reductions of E. coli O157:H7 were calculated according to the following equation:\( \mathrm{Log}\ \mathrm{reduction} = \mathrm{lethality}/{D}_{T_{\mathrm{ref}}} \)where lethality is the value as described above and \( {D_T}_{{}_{\mathrm{ref}}} \) corresponds to the decimal reduction time (min) at T ref of the pertinent pathogen.
According to literature, the thermal inactivation parameters of E. coli O157:H7 in acid fruit juices (pH = 3.9) are D 60 = 0.8–1.7 min and z = 4.8–5.9 °C (Mazzotta 2001). Conservative values were used for the calculations (e.g. D 60 = 1.17 min and z = 5.9 °C). Only temperatures above 55 °C were used for the calculation of the accumulated lethality.
The HPP stabilization treatment consisted in the pressurization at 350 MPa for 5 min at an initial temperature of 9–10 °C in a HPP system Wave 6500 of 120 l (Hiperbaric, Burgos, Spain). This treatment was selected based on the results of Hurtado et al. 2015. The pressure ramp was 200 MPa min−1, total cycle was 7.3 min and the expected increase of temperature is 10–11 °C (Patazca et al. 2007). After treatments, samples were cooled and stored at 4 ± 1 °C in darkness. Both treatments produce the 5-log reduction in the target pathogen recommended by FDA (FDA 2001; Erkmen and Dogan 2004).
Sampling
The different parameters studied were measured on untreated products (day 0), after MH and HPP treatments (day 0AT) and throughout refrigerated storage at 4 ± 1 °C (days 7, 14 and 21). Microbiological, physico-chemical and nutritional analyses and instrumental colour measurements were taken on three independent samples (three different 250-mL bottles) per day of sampling. Sensory analysis was carried out using two independent samples (two different 250-mL bottles) per day of sampling. Two independent experiments/replicates (three in the case of microbiological studies) were performed on different days.
Microbiological Analyses
Microbiological analysis was performed to enumerate spoilage (aerobic mesophilic bacteria (AMB), psychrotrophic bacteria (PSY) and yeasts and moulds (YM)), hygiene (coliforms) and safety (E. coli, Salmonella spp. and Listeria monocytogenes). For each sample, an aliquot of 10 mL was diluted (1/10, w/w) with sterile saline peptone water, which contained 1 g L−1 Bacto Peptone (Difco Laboratories, Detroit, MI, USA) and 8.5 g L−1 NaCl (Merck, Darmstadt, Germany). Further decimal dilutions were made using the same diluent. Undiluted fruit smoothie was also sampled. AMB and PSY were determined on plate count agar after incubation at 30 °C for 72 h and 4 °C for 10 days, respectively. YM were counted on yeast extract glucose chloramphenicol agar (YGC, Merck) after incubation at 25 °C for 5 days. E. coli and total coliform counts (log10 [cfu mL−1]) were determined in a chromogenic E. coli/coliforms medium (ChromID Coli, bioMérieux, Marcy-l'Étoile, France) after incubation at 37 °C for 24–48 h. All determinations were performed in duplicate using the pour plate method. Counts were expressed in log10 [cfu mL−1]. The detection limit was 1.0 cfu mL−1 (0 log10 [cfu mL−1]). Additionally, at the end of storage (21 days), the presence of Salmonella spp. and L. monocytogenes was investigated in 25 mL of sample (Stollewerk et al. 2012).
Physico-Chemical Analyses
The degree of clarification in samples (bottle or pouch) kept in vertical position was determined in the upper juice portion by measuring transmittance (%) at 660 nm using pure water as a blank (100 % transmittance) in a UV2 Series UV/Vis spectrophotometer (UNICAM, Cambridge, UK). Total soluble solids (TSS), CIELAB colour, pH and total titratable acidity were determined in shaken samples. TSS was determined using an ATC-1e hand refractometer (Atago, Minato-ku, Tokyo, Japan) and expressed as grams per 100 g or degrees Brix (±0.01 accuracy and 0–32 range; AOAC 932.14). CIELAB colour was measured with a CR-200/08 Chroma Meter II (Minolta Ltd., Milton Keynes, UK) with D65 illuminant, 2° observer angle and 50-mm aperture size and calibrated with a standard white reflector plate. Readings were obtained applying the standard CIE L*a*b* (1976) colour system, where L* is a lightness value, a* indicates hue on a green (−) to red (+) axis and b* indicates hue on blue (−) to yellow (+) axis. Subsequently, samples were centrifuged at 15,430×g for 10 min in a 5804 Eppendorf centrifuge (Hamburg, Germany) to determine viscosity of the supernatant and total insoluble solids as described by Ros et al. (2004). Absolute viscosity was measured at 40 °C using a No. 100 Ostwald Cannon-Fenske viscometer tube (Sigma-Aldrich, Madrid, Spain). Total insoluble solids (TIS) were calculated as the relative weight difference between the shaken sample and the resulting supernatant after centrifugation. Finally, browning index (absorbance units) was determined according the method of Ting and Rouseff (1986). A solution of sample in methanol at 1:1 (v/v) was kept in ice for 15 min, and then, the solution was centrifuged at 15,430×g for 10 min in a 5804 Eppendorf centrifuge. The absorbance of the supernatant was measured at 420 nm in a UV2 Series UV/Vis spectrophotometer. Triplicate measurements were made for each sample. The pH was measured using a Crison pH 25 coupled to a Crison 5053 puncture electrode, (Crison Instruments S.A., Barcelona, Spain).
Determination of the Enzymatic Activities and Antioxidant Status
Enzymatic activity of peroxidase (POD) was first extracted in triplicate from the smoothie by mixing 10 mL sample with 10 mL 0.2 M sodium phosphate buffer pH 6.5. The mixture was centrifuged for 10 min at 15,430×g. The POD activity was spectrophotometrically measured by adding 1.1 mL 0.2 M sodium phosphate buffer pH 6.5, 0.5 mL enzyme extract, 1 mL o-phenylenediamine solution (10 g L−1 in 0.2 M sodium phosphate buffer pH 6.5) as substrate (proton donor) and 0.5 mL hydrogen peroxide solution (15 g L−1 in 0.2 M sodium phosphate buffer pH 6.5) as oxidant to a 1-cm path cuvette. The formation of the coloured oxidation product (2,3-diaminophenazine) was measured as the change in absorbance at 485 nm and 25 °C for 20 min (Vervoort et al. 2011).
Enzymatic activity of polyphenol oxidase (PPO) was assessed according to the procedure of Wang et al. (2014) with slight modifications. Samples (3 g) were homogenized in 6 mL 0.2 M sodium phosphate buffer, pH 7.0, containing 10 g L−1 insoluble polyvinyl pyrrolidone (PVP) and 5 g L−1 Triton X-100. Homogenates were centrifuged at 12,000×g for 10 min, and PPO activity was determined by measuring the rate of linear increase in absorbance at 420 nm and 25 °C. Reaction material contained 2 mL of 7 mM 4-t-butyl catechol solution, 1 mL of distilled water and 0.2 mL of the extract supernatant, containing the active enzyme. The reference cuvette contained only substrate solution and distilled water. PPO activity was defined as the change in absorbance under conditions of the assay (Δ absorbance min−1 mol).
The final results were expressed as relative activities of POD and PPO expressed as percentage, which were calculated as the ratio between the treated (HPP or MH) and the untreated smoothies.
The enzymatic activity of pectin methyl esterase (PME) was determined according to the method of Li et al. (2015) with slight modifications. PME activity was measured by monitoring the release of free carboxylic groups of galacturonic acid during pectin methyl ester hydrolysis and was assayed reacting 5 mL of the sample with 50 mL of a 1 % (w/v) citrus pectin solution containing 0.2 M NaCl. During pectin hydrolysis, the pH was maintained constantly by addition of 0.01 N NaOH using a GLP21 pH meter (Crison, Barcelona, Spain). The results of PME activity (expressed as PME units g−1) were calculated by using the following formula:
The antioxidant status of smoothies was quantitatively assessed by using the 2.2-diphenyl-1-picrylhydrazyl (DPPH) free radical (radical-scavenging activity) and the ferric ion reducing antioxidant power (FRAP) methods (González-Hidalgo et al. 2012). DPPH is based on electron-transfer, which produces a violet solution in ethanol. The DPPH is reduced in the presence of antioxidants and the ethanol solution turns yellow. A polynomial regression equation (Nuñez-Mancilla et al. 2013) between the total antioxidant activity (TAA = [1-(Abs sample/Abs blank)] × 100) and the concentration of sample was used for quantification. The results (IC50) were expressed as the sample concentration required for capturing half of the DPPH free radicals. The FRAP assay (μmol equivalents Fe2+ 100 mL−1; ±3 μmol 100 mL−1) was used to measure the antioxidant ability against all the reagent oxidative species. The antioxidant ability was determined from the blue compound formed as a result of the reaction between the sample solution and the FRAP reagent (acetate buffer, 2,4,6-tripyridyl-s-triazine and ferrous chloride) at 37 °C. The absorbance of this compound was measured at 593 nm after 2 min.
Determination of the Nutritional Quality
Major nutrients (vitamin C, phenols, flavonoids and sugars) were analysed in the multi-fruit smoothies. The total vitamin C content (mg 100 mL−1; ±0.5 mg 100 mL−1 accuracy) was calculated as the sum of ascorbic acid (AA) and dehydroascorbic acid (DHAA) according to the method of Gil et al. (1998) with slight modifications. For AA determination, a purified extract of sample in methanol/water at 5:95 (v/v), 0.5 g L−1 citric acid and 0.5 g L−1 ethylenediaminetetraacetic acid were used. For DHAA determination, 3 mL of the above extract was reacted with 1 mL of a solution of o-phenylenediamine (OPDA) in methanol/water at 5:95 (v/v) (332.72 mg OPDA per each 100 mL solution), which was kept at 4 °C in darkness for 40 min before analysis. The reverse-phase high-performance liquid chromatography (RP-HPLC) system was made up as follows: L-6200 pump (Merck-Hitachi, Darmstadt, Germany), 2050 Plus autosampler (Jasco Inc., Easton, UK), L-7420 UV detector (Merck-Hitachi) and a Gemini C18 column (300 × 4.6 mm, 5 μm) connected to a C18 reverse-phase guard column, both from Phenomenex, Torrance, CA, USA. The mobile phase used was methanol/water containing cetrimide and KH2PO4. The operating conditions were as follows: 100 μL injection volume, 260-nm (AA) or 340-nm (DHAA) detector wavelength and 0.9 mL min−1 flow rate.
Total phenols (mg gallic acid equivalents (GAE) 100 mL−1; ±2.5 mg 100 mL−1) were determined according to Singleton and Rossi (1965). The absorbance of a yellow compound formed from the reaction between a sample of the ethanolic extract and Folin-Ciocalteu reagent (containing phosphomolybdate and phosphotungstate) was measured at 765 nm. Total flavonoids (mg quercetin equivalents, QE, 100 mL−1; ±0.5 mg 100 mL−1) were determined according to the method of Chang et al. (2002). The absorbance of a yellow compound formed by reacting a sample of the methanolic extract with aluminium chloride, potassium acetate and water was measured at 415 nm. The principal sugars (sucrose, glucose and fructose) were determined by HPLC using the method described by Hellín et al. (2001). A water extract sample was directly injected into the HPLC system equipped with a L-7490 LaChrom refractive index detector (Merck-Hitachi) and a Carbosep CHO682 lead column (Transgenomic, Elancourt, France). The mobile phase used was pure water (MilliQ). The operating conditions were as follows: 20-μL injection volume, 0.4 mL min−1 flow rate and 80 °C temperature. The results were expressed as grams per 100 mL (±0.02 g 100 mL−1).
Quantitative Descriptive Analysis
The descriptors were generated using open discussion in two previous sessions. The retained descriptors are shown in Table 1. Six selected and trained assessors (ISO 8586–1:1993 and ISO 8586–146 2:1994) undertook the sensory analysis on 50 mL of multi-fruit smoothie. A non-structured scoring scale (Amerine et al. 1965) was used, where 0 meant the absence of the descriptor and 10 meant a high intensity of the descriptor. The sensory evaluation was separately performed for each sampling time in two sessions using one bottle of 250 mL of each treatment per session. A complete block design was used (Steel and Torrie 1983), where each taster assessed all the batches in each session. Eight sensory sessions per assessor were performed in total. The samples were coded using three random numbers and presented to the assessors, who balanced the first-order effects and the carry-over effects according to MacFie et al. (1989). The average score of the six assessors for each sample and session was recorded and used in the data analysis.
Data Analysis
Data was analysed by means of ANOVA using the GLM procedure of SAS 9.01 (SAS Institute Inc, Cary, USA). The model for microbiological, physico-chemical, enzymatic activities and nutritional data included the treatment (untreated, MH, HPP) and storage time (0AT, 7, 14 and 21 days) as fixed effects and the replicate as random effect. For the sensory data, the model included the treatment, storage time and taste session as fixed effects. Replicate was a random effect. The non-significant interactions (P > 0.05) were removed from the model. The mean differences between treatments and storage time were tested using the Tukey test (P < 0.05).
Results and Discussion
Microbiological Analysis
Initial levels of AMB and PSY were ca. 4.4 and 2.5 log cfu g−1, while YM and coliforms were 3.4 and 2.3 log cfu g−1, respectively (Table 2). The application of HPP and MH treatment resulted in significant reductions to the counts of AMB (2.9 ± 1.2 and 0.8 ± 0.9 log units, respectively). Variability in reductions after processing is probably related to differences in the endogenous microbiota of the raw matter used in each of the three independent experiments. The inhibitory effect on PSY, YM and coliforms was higher than on AMB, and both treatments reduced the counts of three different groups of microorganisms below the limit of detection.
During subsequent refrigerated storage (21 days at 4 ± 1 °C), counts of AMB, PSY, YM and coliforms maintained at the level achieved after stabilization treatment (Table 2). Due to their physico-chemical properties, mainly acidity (pH = 3.6), the developed multi-fruit smoothie can be considered a refrigerated food with extended shelf life from the microbiological quality point of view. In acidic fruit products submitted to an appropriate hygienization treatment, shelf life is usually more determined by sensory than microbiological quality. The efficiency of both thermal treatments and HPP in acidified foods is widely recognized as low pH not only enhances inactivation during treatment but also inhibits outgrowth of cells sublethally injured by heat or pressure. In this regard, it has been shown for different acidic fruit juices treated at 350 MPa that the levels of aerobic mesophilic bacteria and/or yeast and moulds decreased below the detection limit and no growth was observed during refrigerated storage (Varela-Santos et al. 2012; Lavinas et al. 2008).
From the food safety point of view, in acid fruit smoothies, E. coli O157:H7 would be the pertinent pathogen to consider in the validation of control measures such as the thermal treatment or the HPP for which a performance criterion of 5-log reduction is recommended (FDA 2001). According to the time-temperature profile registered during MH, the accumulated lethality (\( {P}_{60}^{5.9}=16,272\Big) \) rendered more than 9500 log reductions. Therefore, the applied treatment produced a safe product according to the FDA recommendation for fruit juices (FDA 2001).
In relation to the HPP, inactivation kinetic parameters for E. coli O157:H7 are less known. According to previous studies performed by Erkmen and Dogan (2004) about E. coli HPP inactivation in orange juice, the inactivation rate at 350 MPa was 1.24 log min−1; therefore, a 5-min treatment would render a satisfactory 6.2-log reduction. However, considering that those studies were not performed with an O157:H7 strain, additional studies would be required.
Instrumental Colour and Physico-chemical Analyses
The effect of HPP and MH treatments coupled to a storage of 21 days on different physical and physico-chemical parameters is presented in Table 3. Stabilization treatments and storage time did not produce substantial changes on the TSS, pH, TA and TIS of the smoothies. A similar observation on TA was done by Landl et al. (2010) on apple purée pasteurized at 400 MPa. In contrast, the transmittance of the upper juice was not affected by the HPP and MH treatments (day 0AT) but significantly increased from 4.8 % at day 7 to 16.3 % at day 21, showing a gradual clarification process. On the contrary, the transmittance of the upper juice of the MH samples remained mostly unchanged. For the absolute viscosity, at day 0, MH samples presented a slightly higher value (5.1 cP) with respect to the HPP (3.9 cP) and untreated (3.9 cP) samples. During storage, absolute viscosity of MH samples remained at the same level while gradually diminished from 3.9 at day 0 to 3.0 cP at day 21 and remained during the whole storage period inferior to the one measured for MH samples.
For colour parameters, small differences were found between treatments in L* values at day 0, with a minimum of 37.0 for HPP samples and a maximum of 41.5 for MH samples. For a* values, differences between treatments were observed at 7, 14 and 21 days. Also, a* values (reddish) tended to decrease during storage for MH samples and remained stable for HPP samples. MH produced a small increase in hue angle (Fig. 1) that maintained during the whole storage period and that can be associated with rust-brown tonalities (which agrees with the sensory results). Browning index (B.I.) was similar for treated and untreated samples at day 0 and at day 7 (Table 3). At days 14 and 21, the B.I. slightly increased in MH and decreased in HPP smoothies, indicating a difference (P < 0.05) between the effects of both treatments. In general, colour changes observed are in agreement with other studies. On a fruit smoothie, Keenan et al. (2010) showed modifications in colour parameters (L* and a*) between untreated samples and HPP samples (450 MPa for 5 min) while colour of thermally treated samples remained unaffected during storage. More recently, Yu et al. (2013) reported an important effect on a* values in HPP Chinese bayberry juice (400 MPa for 5 min). In this case, a* value of the control always stood at the highest indicating the most reddish of the juice. Sadilova et al. (2009) indicated that thermal treatment enhanced the formation of degradation products that can explain colour loss. In view of the results, oxidation and clarification phenomena would have a stronger contribution to the deterioration of pressurized smoothies than browning or acidification, at least in the time period studied. From this point of view, the lesser stability of pressurized fruit derivatives may limit their shelf life compared with the equivalent products pasteurized by mild heating (Oey et al. 2008).
Changes in Enzymatic Activities and Antioxidant Status
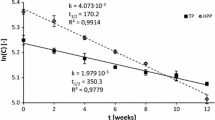
The effects of the stabilization treatments and storage time on enzymatic activities and antioxidant status of multi-fruit smoothies are shown in Table 4. Generally, HPP at 350 MPa resulted less effective than MH in inactivating spoiling enzymes. On pressurized smoothies, PPO remained as active as in untreated samples during all the storage time. On the contrary, MH treatment practically inactivated the enzyme, which remained inactive during storage. At day 0, POD enzyme was partially inactivated by the MH treatment while the HPP had no effect on it. During storage, POD activity of MH samples remained at 20 % while in HPP samples slightly decreased to 80 % at day 21. PME activity of HPP samples was similar to the untreated ones and was around six times higher than in the mild heated smoothies. PME activity was not affected by storage time. PME, PPO and POD are often inactivated by MH treatment but are highly resistant to HPP under commercially feasible conditions in fruit derivatives (Terefe et al. 2014), although few shelf life studies have been performed on multi-fruit smoothies. Keenan et al. (2012) found no clear evolution of PPO activity in multi-fruit smoothies kept under refrigeration for up to 10 h.
Antioxidant status assay was coherent with observed enzymatic activities. The pressurized smoothies had higher DPPH values and lower FRAP values than the MH smoothies at all sampling days. The results, in accordance to a similar shelf life study carried out by Keenan et al. (2010), also showed a higher reduction in the antioxidant capacity in the pressurized smoothies, suggesting that oxidative enzymes may contribute to the reduction of the antioxidant capacity, while thermal inactivation of these enzymes would help to stabilize their antioxidant capacity.
Nutritional Quality
The effects of the stabilization treatments and storage time on nutritional quality are shown in Table 5. Most of the vitamin C present in fruit smoothies corresponded to dehydroascorbic acid (DHAA). Fruit processing favours the oxidation of ascorbic acid to DHAA catalysed by ascorbate oxidase enzyme, although direct oxidation may also occur (Greenway and Ongomo 1990).
At day 0, even if MH samples presented lower values for ascorbic acid (AA), DHAA and vitamin C than HPP and untreated samples, the difference was not significant (P > 0.05). During storage, a regular decrease could be observed in both sample sets. For AA, a similar reduction of 70 % was observed for both HPP and MH samples all along the 21 days. For DHAA, reductions of 64 and 60 % were observed in HPP and MH samples, respectively. A similar trend was observed for vitamin C with a reduction of 65 % for HPP samples and 61 % for MH samples. Paradoxically, the application of high pressure did not destroy vitamin C but higher reductions in their levels were observed during subsequent storage. This fact could be correlated with the higher activity of PME, PPO and POD and lower antioxidant capacity of HPP smoothies, although vitamin C can also be degraded by non-enzymatic pathways, in particular, in fruit derivatives treated by heating (Perera 2007). Keenan et al. (2012) reported similar results regarding ascorbic acid in fruit smoothies stored at 4 °C for up to 10 h. In addition, Landl et al. (2010) reported a strong reduction of AA, DHAA and vitamin C in apple purée submitted to MH treatment (75 °C for 10 min) and HPP (400 MPa for 5 min).
In contrast, the total content of phenols and flavonoids were similar in both types of smoothies at day 0. Flavonoids content remained stable for up to 21 days of storage for both treatments. The same can be stated for total phenol content of MH samples while HPP smoothies presented a significant reduction (26 %) during storage. This phenomenon was also observed by Keenan et al. (2010) who found a phenol partial degradation in both pressurized and heated fruit smoothies kept under refrigeration for up to 30 days. Phenolic compounds are responsible for antioxidant capacities in fruit, some of them being more resistant than vitamin C to fruit processing (Kalt 2006). These facts might partially explain why the total content of phenols of MH samples did not decrease during storage.
The effect of stabilization treatments and storage on sucrose, glucose and fructose is presented in Fig. 2. At day 0, HPP and untreated samples present similar sucrose, glucose and fructose contents. During storage of HPP samples, between day 0 and day 7, sucrose content dropped drastically from 0.7 g mL−1 to undetectable level while glucose and fructose remained stable over time. On the contrary, MH samples presented a different pattern; at day 0, sucrose content was four times higher, while glucose (−27 %) and fructose (−56 %) contents were lower in comparison with the values reported for HPP smoothies. During storage, values remained stable. The higher glucose and fructose contents, observed during storage, suggest that total sucrose hydrolysis took place in the pressurized smoothies.
In HPP samples, the sucrose inversion was relevant at day 7; in contrast, the levels of sucrose remained constant in the MH-treated smoothies during storage, which indicates that beta-fructosidase enzymes were inactivated by the MH treatment (80 °C for 7 min). Butz et al. (2003) found similar results in orange, lemon and carrot mixed juices processed by mild heating and high pressure.
Effects of Processing and Storage Time on the Sensory Attributes of the Fruit Smoothie
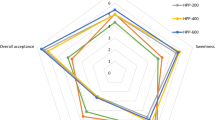
The results of the Quantitative Descriptive Analysis (QDA) for untreated (fresh), HPP and MH samples are shown in Table 6 (odour, appearance and mouthfeel attributes) and Table 7 (taste, flavour and overall sensory quality).
The odour of the smoothies was described using four attributes: overall intensity, banana, strawberry and cooked odour. The results of these attributes divide the samples into two groups: untreated-HPP and MH. Untreated and HPP smoothies were characterized by higher intensity of banana and strawberry odour and lower intensity of the off-odour cooked fruit (Table 6). These results were consistent with those obtained for orange-lemon-carrot mixed juice by Fernández-García et al. (2001)), where no differences in aroma were found between non-treated and pressurized (500 MPa; 5 min) juices and Picouet et al. (2015) in carrot juices (600 MPa; 5 min).
The colour of fruit and vegetable juices is an important attribute in consumer preferences and has been used as an indicator of the sensory and nutritional quality of food during processing treatment and subsequent storage. Regarding the appearance attributes, after the preservation treatment (0AT), HPP samples showed no significant differences regarding those untreated. Dede et al. (2007) and Picouet et al. (2015) reported that for many fruit products, the HP processing was positively valued for the preservation of the fresh colour. Our results are consistent with this finding because on overall, the colour of the HPP samples was most similar to the untreated ones at the beginning of the storage time. Throughout the shelf life, a general deterioration of the colour has been evidenced by a decrease of the coral colour intensity and, conversely, an increase of the rust-brown intensity. These changes were clearer in HPP samples and could be related to the higher activity of POD and PPO enzymes (Zabetakis et al. 2000; Liavoga and Matella 2012). In addition, these results were in agreement with those of Keenan et al. (2010) which stated that thermally processed smoothies exhibited lower colour change than their HPP counterparts. However, at the end of storage, the colour of HPP smoothies was more similar to the untreated product than MH-treated ones.
Significant differences regarding sliminess were observed between HPP- and MH-processed samples. The former samples were scored with lower intensity of sliminess when compared with the latter ones, but none of them showed differences with the untreated ones. This result agrees with the higher viscosity showed by MH samples (Table 3). This fact can be related to the higher activity of PME enzyme (Table 4) which is involved in the breakdown of pectin network surrounding the cellulose backbone of the cell wall and thus producing low viscous products (Giovane et al. 2004). Furthermore, HPP samples had lower content of sucrose (Fig. 2) than the MH samples (Fig. 2), and this fact could also be related to the viscosity and sliminess attributes. It is known that sucrose might protect PME against denaturation by reducing the water activity of the medium (Chakraborty et al. 2014). In contrast, the MH samples were the grittiest.
Regarding the flavour attributes (Table 7), the MH-processed samples had the lowest fresh fruit flavour (interpreting fresh fruit as higher intensity of banana, strawberry and overall flavour intensity) and the highest cooked off-flavour, which shows the detrimental effect of the MH treatment on both odour and flavour attributes. According to Farkas and Hoover (2000) and Barbosa-Cánovas and Rodriguez (2002), high-pressure levels that are normally used in the food industry cannot disrupt covalent bonds, which maintain unchanged colour, aroma and flavour compounds that are responsible for the sensory quality of food. Throughout the shelf life, MH samples remained stable from a flavour point of view while HPP samples increased in acid taste and decreased in sweet taste. The increase in acid taste may be related to the fact that HP processing is acidic in nature (Chakraborty et al. 2014), and this raise could modify the perception of sweet taste of the samples, as pointed out by Pangborn (1961)).
Flavour results can be related to the decrease of the overall sensory quality of HPP samples through the shelf life because most people naturally tend to prefer a sweet taste to an acidic one. Other basic tastes beside sweet (sour, salty, bitter and umami) are more complex to acquire and develop a preference (Sijtsema et al. 2012). Furthermore, regarding the overall sensory quality, no significant differences were observed between untreated (fresh) and HPP smoothies at 0AT (Table 7), and these samples had the highest score. Throughout the storage time, the overall sensory quality of HPP samples significantly decreased while no changes for the MH smoothies were observed. However, overall sensory quality of HPP samples was higher than that of the MH ones. HP-induced enzyme inactivation is a very complex phenomenon, and most times, the application of high pressure without heat treatment is insufficient to achieve complete inactivation of the oxidative enzymes (Chakraborty et al. 2014) which are responsible for the deterioration of colour, flavour and nutritional value of fruit juices (Liavoga and Matella 2012) and smoothies (Keenan et al. 2010) and negatively affect their hedonic score.
Conclusions
The lower stability of the HPP multi-fruit smoothies during storage is confirmed by the fact that HPP is unable to reduce oxidative enzyme activities and changes in instrumental colour and physico-chemical parameters were observed. On the nutritional aspect, the degradation of vitamin C was mostly similar in both treatments while MH samples present higher concentration in sucrose. However, HPP better preserved the original colour and flavour of the multi-fruit smoothies, and overall, HPP could be an effective alternative to thermal processing for the production of a high-quality multi-fruit smoothie although it should be taken into account that the residual enzyme activity is likely to limit the shelf life of the product.
References
Amerine, M. A., Pangborn, R. M., & Roessler, E. B. (1965). Principles of sensory evaluation of food. In Food science and technology monographs (pp. 338–339). New York: Academic Press.
Andrés, V., Villanueva, M. J., & Tenorio, M. D. (2016). The effect of high-pressure processing on colour, bioactive compounds, and antioxidant activity in smoothies during refrigerated storage. Food Chemistry, 192, 328–335.
Barba, F. J., Esteve, M. J., & Frigola, A. (2012). High pressure treatment effect on physicochemical and nutritional properties of fluid foods during storage: a review. Comprehensive Reviews in Food Science and Food Safety, 11(3), 307–322.
Barbosa-Cánovas, G. V., & Rodriguez, J. J. (2002). Update on non-thermal food processing technologies: pulsed electric field, high hydrostatic pressure, irradiation and ultrasound. Food Australia, 54, 513–520.
Briz, T., Sijtsema, S. J., Jasiulewicz, A., Kyriakidi, A., Guàrdia, M. D., Van Der Berg, I., & Van Der Lans, I. A. (2009). Barriers to fruit consumption: driving forces behind consumer behaviour. Scripta Horticulturae, 8, 7–18.
Butz, P., Fernández-García, A., Lindauer, R., Dieterich, S., Bognár, A., & Tauscher, B. (2003). Influence of ultra high pressure processing on fruit and vegetable products. Journal of Food Engineering, 56, 233–236.
Chakraborty, S., Kaishik, N., Srinivasa, P., & Misrha, H. N. (2014). High-pressure inactivation of enzymes: a review on its recent applications on fruit purees and juices. Comprehensive Reviews in Food Science and Food Safety, 13, 578–596.
Chang, C. C., Yang, M. H., Wen, H. M., & Chern, J. C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis, 10, 178–182.
Dede, S., Alpas, A., & Bayindirli, H. (2007). High 410 hydrostatic pressure treatment and storage of carrot and tomato juices: antioxidant activity and microbial safety. Journal of the Science of Food and Agriculture, 87, 773–782.
Erkmen, O., & Dogan, C. (2004). Kinetic analysis of Escherichia coli inactivation by high hydrostatic pressure in broth and foods. Food Microbiology, 21, 181–185.
European Food Safety Authority (2008). Concise Database summary statistics – Total population. http://www.efsa.europa.eu/en/datexfoodcdb/datexfooddb.htm
Farkas, D. F., & Hoover, D. G. (2000). High pressure processing. Journal of Food Science, 65, 47–64.
FDA (2001) Hazard Analysis and Critical Control Point (HAACP); Procedures for the Safe and Sanitary Processing and Importing of Juice https://www.federalregister.gov/articles/2001/01/19/01-1291/hazard-analysis-and-critical-control-point-haacp-procedures-for-the-safe-and-sanitary-processing-and
Fernández-García, A., Butz, P., Bognàr, A., & Tauscher, B. (2001). Antioxidative capacity, nutrient content and sensory quality of orange juice and an orange lemon-carrot juice product after high pressure treatment and storage in different packaging. European Food Research and Technology, 213, 290–296.
Gil, M. I., Ferreres, F., & Tomas-Barberan, F. A. (1998). Effect of modified atmosphere packaging on the flavonoids and vitamin C content of minimally processed Swiss chard (Beta vulgaris subspecies cycla). Journal of Agricultural and Food Chemistry, 46, 2007–2012.
Giovane, A., Servillo, L., Balestrieri, C., Raiola, A., D’Avino, R., Tamburrini, M., Ciardiello, M., & Camardella, L. (2004). Pectin methylesterase inhibitor. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1696(2), 245–152.
González-Hidalgo, I., Bañón, S., & Ros, J. M. (2012). Evaluation of table olive by-product as a source of natural antioxidants. Food Science and Technology, 47, 674–681.
Greenway, G. M., & Ongomo, P. (1990). Determination of L-ascorbic acid in fruit and vegetable juices by flow injection with immobilised ascorbate oxidase. Analyst, 115, 1297–1299.
Hellín, P., Ros, J. M., & Laencina, J. (2001). Changes in high and low molecular weight carbohydrates during Rhizopus nigricans cultivation on lemon peel. Carbohydrate Polymers, 45, 169–174.
Hurtado, A., Picouet, P. A., Jofré, A., Guàrdia, M. D., Ros, J. M., & Bañón, S. (2015). Application of high pressure processing for obtaining “fresh-like” fruit smoothies. Food and Bioprocess Technology, 8, 2470–2482.
Kalt, W. (2006). Effects of production and processing factors on major fruit and vegetable antioxidants. Journal of Food Science, 70(1), 11–19.
Keenan, D. F., Brunton, N. P., Gormley, T. R., Butler, F., Tiwari, B. K., & Patras, A. (2010). Effect of thermal and high hydrostatic pressure processing on antioxidant activity and colour of fruit smoothies. Innovative Food Science & Emerging Technologies, 11, 551–556.
Keenan, D. F., Röβle, C., Gormley, R., Butler, F., & Brunton, N. P. (2012). Effect of high hydrostatic pressure and thermal processing on the nutritional quality and enzyme activity of fruit smoothie. LWT--Food Science and Technology, 45, 50–57.
Landl, A., Abadias, M., Sárraga, C., Viñas, I., & Picouet, P. A. (2010). Effect of high pressure processing on the quality of acidified Granny Smith apple purée product. Innovative Food Science & Emerging Technologies, 11, 557–564.
Lavinas, F. C., Miguel, M. A. L., Lopes, M. L. M., & Valente Mesquita, V. L. (2008). Effect of high hydrostatic pressure on cashew apple (Anacardium occidentale L.) juice preservation. Journal of Food Science, 73(6), 273–277.
Li, R., Wang, Y., Wang, S., & Liao, X. (2015). A comparative study of changes in microbiological quality and physicochemical properties of N-2-infused and N-2-degassed banana smoothies after high pressure processing. Food and Bioprocess Technology, 8(2), 333–342.
Liavoga, A., & Matella, N. J. (2012). Enzymes in quality and processing of tropical and subtropical fruits. In M. Siddiq (Ed.), Tropical and subtropical fruits: postharvest physiology, processing and packaging (pp. 35–51). Oxford: Wiley-Blackwell.
Lobstein, T., Baur, L., & Uauy, R. (2004). Obesity in children and young people: a crisis in public health. Obesity Reviews, 5(suppl.1), 4–85.
Lopez-Malo, A., Palou, E., Barbosa-Canovas, G. V., Welti-Chanes, J., & Swanson, B. G. (1998). Polyphenoloxidase activity and color changes during storage of high hydrostatic pressure treated avocado puree. Food Research International, 31(8), 549–556.
MacFie, H. J., Bratchell, N., Greenhoff, H., & Vallis, L. V. (1989). Designs to balance the effect of order of presentation and first-order carry-over effects in hall test. Journal of Sensory Studies, 4, 129e149.
Mazzotta, A. S. (2001). Thermal inactivation of stationary-phase and acid-adapted Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in fruit juices (2001). Journal of Food Protection, 64(3), 315–320.
Nienaber, U., & Shellhammer, T. H. (2001a). High-pressure processing of orange juice: kinetics of pectinmethylesterase inactivation. Journal of Food Science, 66(2), 328–331.
Nienaber, U., & Shellhammer, T. H. (2001b). High-pressure processing of orange juice: combination treatments and a shelf life study. Journal of Food Science, 66(2), 332–336.
Nuñez-Mancilla, Y., Perez-Won, M., Uribe, E., Vega-Galvez, A., & Di Scala, K. (2013). Osmotic dehydration under high hydrostatic pressure: effects on antioxidant activity, total phenolics compounds, vitamin C and colour of strawberry (Fragaria vesca). LWT--Food Science and Technology, 52(2), 151–156.
Oey, I., Lille, M., Van Loey, A., & Hendrickx, M. (2008). Effect of high pressure processing on colour, texture and flavor of fruit and vegetable-based food products: a review. Trends in Foods Science and Technology, 19(6), 320–328.
Pangborn, R. M. (1961). Interaction of tastes. In M. A. Amerine, R. M. Pangborn, & E. B. Roessler (Eds.), Food Science and Technology. Principles of sensory evaluation of food (p. 125). New York: Academic Press.
Patazca, E., Koutchma, T., & Balasubramaniam, V. M. (2007). Quasi-adiabatic temperature increase during high pressure processing of selected foods. Journal of Food Engineering, 96, 568–574.
Patterson, M. F., McKay, A. M., Connolly, M., & Linton, M. (2012). The effect of high hydrostatic pressure on the microbiological quality and safety of carrot juice during refrigerated storage. Food Microbiology, 30, 205–212.
Perera, C. O. (2007). Minimal processing of fruits and vegetables. In M. S. Rhaman (Ed.), Handbook of Food Preservation (2nd ed., pp. 137–148). Roca Baton: CRC Press.
Picouet, P. A., Sárraga, C., Cofán, S., Beletti, N., & Guàrdia, M. D. (2015). Effects of thermal and high pressure treatments on carotene content, microbiological safety and sensory properties of acidified and of non-acidified carrot juice. LWT--Food Science and Technology, 62, 920–926.
Rastogi, N. K., Raghavarao, K. S. M. S., Balasubramaniam, V. M., Niranjan, K., & Knorr, D. (2007). Opportunities and challenges in high pressure processing of foods. Critical Reviews in Food Science and Nutrition, 47(1), 69–112.
Rodrigo, D., Sampedro, F., Silva, A., Palop, A., & Martinez, A. (2010). New food processing technologies as a paradigm of safety and quality. British Food Journal, 112(5), 467–475.
Ros, J. M., Laencina, J., Hellín, P., Jordán, M. J., Vila, R., & Rumpunen, K. (2004). Characterization of juice in fruits of different Chaenomeles species. Lebensmitell-Wissenschaft und-Technologie, 37, 301–307.
Sadilova, E., Stintzing, F. C., Kammerer, D. R., & Carle, R. (2009). Matrix dependent impact of sugar and ascorbic acid addition on color and anthocyanin stability of black carrot, elderberry and strawberry single strength and from concentrate juices upon thermal treatment. Food Research International, 42, 1023–1033.
Sampedro, F., Fan, X., & Rodrigo, D. (2010). Case studies in novel food processing technologies: innovations in processing, packaging, and predictive modelling. In C. J. Doona, K. Kustin, & F. E. Deeherry (Eds.), High hydrostatic pressure processing of fruit juices and smoothies: research and commercial application (pp. 34–72). Cambridge: Woodhead Publishing. ISBN 978-1-84569-551-4.
Sijtsema, J., Reinders, M. J., Hiller, S. R. C. H., & Guàrdia, M. D. (2012). Fruit and snack consumption related to sweet, sour and salty taste preferences. British Food Journal, 114(7), 1032–1046.
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–153.
Steel, R. G. D., & Torrie, J. H. (1983). Principles and procedures of statistics (p. 632). New York: McGraw-Hill. ISBN 0-07-060926-8.
Stollewerk, K., Jofré, A., Comaposada, J., Arnau, J., & Garriga, M. (2012). The impact of fast drying (QDS process) and high pressure on food safety of NaCl-free processed dry fermented sausages. Innovative Food Science & Emerging Technologies, 16, 89–95.
Terefe, N. S., Buckow, R., & Versteeg, C. (2014). Quality-related enzymes in fruit and vegetable products: effects of novel food processing technologies, part 1: high-pressure processing. Critical Reviews in Food Science and Nutrition, 54, 24–63.
Ting, S. V., & Rouseff, R. L. (1986). Undesirable substances formed during processing and storage. In S. R. Tannenbaum & P. Walstra (Eds.), Citrus fruit and their product. Analysis and technology (pp. 175–182). New York: Marcel Dekker Inc.
Varela-Santos, E., Ochoa-Martinez, A., Tabilo-Munizaga, G., Reyes, J. E., Pérez-Won, M., Briones-Labarca, V., & Morales-Castro, J. (2012). Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innovative Food Science & Emerging Technologies, 13, 13–22.
Vervoort, L., Van der Plancken, I., Grauwet, T., Timmerman, R., Mastwijk, H. C., Matser, A., Hendrickx, M., & Van Loey, A. (2011). Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice part II: impact on specific chemical and biochemical quality parameters. Innovative Food Science & Emerging Technologies, 12(4), 466–477.
Wang, S., Lin, T., Man, G., Li, H., Zhao, L., Wu, J., & Liao, X. (2014). Effects of anti-browning combinations of ascorbic acid, nitrogen and carbon dioxide on the quality of banana smoothies. Food and Bioprocess Technology, 7, 161–173.
Yu, Y., Lin, Y., Zhan, Y., He, J., & Zhu, S. (2013). Effect of high pressure processing on the stability of anthocyanin, ascorbic acid and colour of Chinese bayberry juice during storage. Journal of Food Engineering, 119, 701–706.
Zabetakis, I., Leclerc, D., & Kajda, P. (2000). The effect of high hydrostatic pressure on the strawberry anthocyanins. Journal of Agricultural and Food Chemistry, 48(7), 2749–2754.
Acknowledgments
This study has been carried out with the financial support from the Spanish Government via the national project FRUITECH (INIA RTA2011-00038-C02-01). Authors are grateful to Marta Baret and Yolanda Beltrán (IRTA) for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Picouet, P.A., Hurtado, A., Jofré, A. et al. Effects of Thermal and High-pressure Treatments on the Microbiological, Nutritional and Sensory Quality of a Multi-fruit Smoothie. Food Bioprocess Technol 9, 1219–1232 (2016). https://doi.org/10.1007/s11947-016-1705-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-016-1705-2