Abstract
The effect of aqueous ozone at different doses on fungal incidence, microstructure, mechanical properties, and weight loss of blueberry fruit (Vaccinium corymbosum, cv. O’Neal) throughout 20 days of storage at 4 ± 1 °C was studied. Fruits were exposed to 10 and 18 mg O3 L-1 in a bubble column for different periods of time ranging from 10 to 30 min. Native mycobiota and Botrytis cinerea incidences were reduced by ozone exposure. After 15 days of storage, exposure to 18 mg O3 L-1 for 10 and 20 min reduced the percentage of infected fruit in ~ 34 and 40 %, respectively, when compared with untreated blueberries. Slight but no significant differences among ozone treatments were observed for native mycobiota and B. cinerea incidence. Stiffness, rupture force, and mechanical work required to break the fruit epidermis were not significantly affected by ozone treatments. The effect of ozone treatment on weight loss of blueberries was dose-dependent and was partially correlated with microstructural changes induced by ozone: disruptions in outer tangential and epidermal cell walls and alteration of the cuticle. Exposure times beyond 15 min significantly increased weight loss and did not achieve greater fungal inhibition with respect to 10- and 15-min ozonized fruit. Thus, the ozone dose to be selected would be limited by the negative effect on weight loss. Exposure to 18 mg O3 L-1 for 10 min would be the most suitable treatment to reduce fungal decay without causing an excessive loss of weight along cold storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High bush blueberries are one of the most popular berries among consumers because of their health-promoting compounds, mainly anthocyanins, flavonoids, ascorbic acid and polyphenols, and attractive appearance and flavor (Fava et al. 2006; Alwi and Ali 2015; Contigiani et al. 2018; Piechowiak et al. 2018). Blueberry fruit are traditionally eaten fresh or as a primary component in juices, muffins, cakes, jams, yogurts, and others. The main genotypes harvested in Argentina are O’Neal and Misty, being O’Neal predominant in the province of Buenos Aires. Ninety percent of the Argentinean production is exported as fresh fruit to the USA, the European Union, and minor proportion to Asia and Canada (Argentinean Blueberry Committee n.d.; Greco et al. 2012). The blueberry exporting industry is profitable owing to counter-seasonality: harvesting begins in November when in the northern hemisphere there is a lack of fresh fruit.
Postharvest decay, fruit softening, physiological disorders, and water loss are the major factors limiting marketing and consumer’s acceptability of fresh blueberries (Chun et al. 2013; Duan et al. 2011; Greco et al. 2012; Moggia et al. 2016; Skurtys et al. 2011; Vicente et al. 2007). Fungi associated with blueberry diseases are mainly Alternaria tenuissima, Botrytis cinerea, Colletotrichum acutatum, and Colletotrichum gloeosporioides (Chun et al. 2013; Greco et al. 2012). In particular, B. cinerea is a ubiquitous plant pathogen that causes grey mold disease contributing to major economic losses to growers, processors, marketers, and consumers. Infection by B. cinerea is primarily latent with symptoms appearing only during cool storage. Its chemical control with synthetic fungicides is discouraged due to the ability of this necrotizing fungus to develop fungicide resistance and health and environmental concerns for the possible toxicity of fungicide residues (Mengiste et al. 2010). Consequently, the search for alternative safe and effective decontamination (pathogenic and non-pathogenic human microorganisms) strategies for obtaining high-quality fresh berries constitutes an ongoing challenge to the scientific community. A number of chemical, physical, and biological approaches (among others UV-C irradiation, pulsed light, modified atmosphere packaging, ozonation, and cold plasma) have been studied in the last years for controlling postharvest fruit decay during cold storage
(Duan et al. 2011; Alzamora et al. 2000; Misra et al. 2014; Xu and Liu 2017). Particularly, ozone is a cost-effective and eco-friendly sanitizer with a broad spectrum of antimicrobial activities due to its powerful oxidizing effect (Horvitz and Cantalejo 2014). Its rapid decomposition to harmless oxygen leaves no residues in food and environment (Muthukumarappan et al. 2000). In 2002, the Food and Drug Administration has approved the use of this agent for postharvest processing of fruit and vegetables. Ozone (mainly in gaseous phase) has been tested in berries as a postharvest treatment against native microflora and/or artificially inoculated microorganisms, such as Escherichia coli O157: H7, Salmonella, Listeria innocua, Alternaria, and Penicillium spp. (Bialka and Demirci 2007; Pangloli and Hung 2013; Alexandre et al. 2011; Contigiani et al. 2018). Results about ozone effectiveness are somehow contradictory (Brodowska et al. 2018; Horvitz and Cantalejo 2014). Different operative conditions and type of berries employed in these studies (ozone concentration and exposure time—not always correctly characterized, delivery system, gaseous or aqueous phase, pH, temperature, size and dimensions of the reactor, pressure, presence of contaminants and catalysts, type of target microorganisms, initial level of contamination, physiological state of the microorganisms, storage conditions, etc.) could explain these discrepancies since all of them influence ozone effectiveness. Moreover, several studies reported that, in certain doses, ozone triggers reactive oxygen species (ROS) accumulation in fruit and vegetables as a natural response to abiotic stressful condition (Alwi and Ali 2015; Contigiani et al. 2018; Piechowiak et al. 2018; Sandermann et al. 1998). As a result of the accumulation of ROS, plant organs induce the synthesis of phytoalexins (isoflavonoids, stilbenephytoalexins) and other antioxidant and antimicrobial systems. There are scarce studies dealing with the effect of gaseous ozone on blueberries (Concha-Meyer et al. 2015; Piechowiak et al. 2018; Kim and Hung 2012). Piechowiak et al. (2018) reported an inhibitory effect of ozone in total counts of mesophilic bacteria and fungi and an increase in the antioxidant capacity of blueberries. On contrary, Concha-Meyer et al. (2015) did not observe an inhibitory effect in total counts of yeasts and molds in blueberries stored under ozone atmosphere, although ozone reduced the weight and firmness losses of fruit. On the other hand, the studies are even more limited when ozone is used in the aqueous phase (Bialka and Demirci 2007; Pangloli and Hung 2013). Deeper knowledge about the impact of ozone doses on quality parameters, microstructure, mycoflora, and decay incidence are needed to design an adequate postharvest treatment. In particular, postharvest softening and weight loss reduce the quality and limit blueberry commercialization for fresh consumption (Chen et al. 2015; Giongo et al. 2013). Therefore, the aim of this work was to evaluate the effect of aqueous ozone at different doses on fungal incidence, microstructure, mechanical properties, and weight loss of blueberry fruit (Vaccinium corymbosum, cv. O’Neal) throughout storage at 4 ± 1 °C.
Materials and Methods
Plant material
Organic blueberries (V. corymbosum L.) cv. O’Neal at harvest maturity stage (100% blue, approximately 65 days after anthesis; pH = 3.5 ± 0.5; 12.5 ± 1.0 °Brix) were collected in Suipacha, province of Buenos Aires, Argentina. Blueberries that showed mechanical injuries or fungal development were discarded. Fruit with similar color, shape, and uniform in size and weight (diameter, 1.77 ± 0.07 cm; weight, 2.0 ± 0.5 g; n = 60) were sorted, randomly distributed in plastic boxes, and kept under refrigeration at 4 ± 1 °C until processed, within a day. While picking, transporting, and preparing the berries for ozone treatments and posterior analysis during cold storage, special care was taken to avoid rubbing off the wax layer.
Ozone Treatment
The ozone equipment setup used in this work was previously described by Garcia Loredo et al. (2015). Briefly, ozone was generated by means of a corona discharge equipment model UTK-O-4 (UNITEK, Mar del Plata, Argentina). Pure oxygen was used as feed gas at a flow rate of 5 L min-1. The ozone generator’s exit was connected to a bubble column (diameter, 0.1 m; height, 0.24 m) with an inbuilt diffuser that sparged gaseous ozone into the liquid phase. The column was filled with 1.5 L of distilled water and ozone-containing gas was continuously injected from the bottom of the column. The electrical power supplied to the generator was set to provide an ozone concentration in the inlet gas of 10 ± 2 mg O3 L-1 or 18 ± 2 mg O3 L-1, evaluated according to the iodometric methodology described by Rakness et al. (1996). Sixty blueberries were immersed into the distilled water and exposed for 10 and 20 min to10 mg O3 L-1 and for 10, 15, 20, and 30 min to 18 mg O3 L-1. After treatments, blueberries were dried by rolling them on a paper towel, packed in air-permeable polypropylene boxes (30 blueberries per tray), and stored up to 20 days at 4 ± 1 °C.
Ozone Concentration in Water
Ozone concentration in the liquid phase was determined by the indigo colorimetric method according to Bader and Hoigné (1982). Measurements were carried out in the distilled water of the column with or without blueberries in order to evaluate the residual ozone concentration at which blueberries were exposed after the reaction of ozone with ozone demanding material. Three replicates were measured for each condition.
Decay by Native Mycobiota
Fungal development was daily evaluated by visual inspection along with storage at 4 ± 1 °C according to Contigiani et al. (2018) and Duarte-Molina et al. (2016). Those fruit that showed macroscopic mycelial development, regardless of the severity of the infection, were considered as decayed. Results were expressed as percentage decayed fruit according to Eq. (1):
Ozonized fruits were compared with an untreated control (i.e., fresh fruit without any treatment) and with washing controls. These washing controls consisted of blueberries immersed into the column and exposed to the same conditions as those used for ozone treatments but with the ozone generator turned off. Oxygen was bubbled in the column for 10 min (control O2 10 min), 15 min (control O2 15 min), 20 min (control O2 20 min), and 30 min (control O2 30 min). Four replicates of 30 blueberries each (n = 120) were analyzed for each condition.
Decay by Botrytis cinerea
B. cinerea BAFC 3003 strain was provided by BAFC Culture Collection (Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina). Isolates were incubated on malt extract agar (MEA) during 14 days at 25 °C ± 1; then, conidia were harvested by washing the culture in a Tween 80 detergent solution (0.05 % v/v) in peptone water (0.1 % w/v) and shook gently on a vortex mixer. The final cell suspension was counted by a Neubauer camera (Exacta, Germany), being approximately 107 conidia/mL.
Blueberries were washed with a hypochlorite solution (200 mg L-1) for 2 min and rinsed three times with sterile water to decontaminate the berry’s surface. Fruits were immediately dried with a paper towel. Then, each berry was inoculated in a laminar airflow cabinet with 10 μL of the conidium suspension using a spot-inoculation method. A small injury was produced at the point of attachment of the pedicel to the berry and then a small drop (10 μL) of inoculum was applied with a micropipette. Inoculated blueberries were kept overnight in the laminar flow cabinet at 21 ± 2 °C to promote fungus adhesion.
Finally, inoculated blueberries were exposed to ozone treatments (10 mg O3 L-1 for 20 min and 18 mg O3 L-1 for 10 and 20 min). B. cinerea incidence in inoculated blueberries stored at 4 ± 1 °C was evaluated daily by visual inspection as previously described for native mycobiota. Ozone-treated fruit was compared against untreated inoculated fruit (control) and inoculated berries bubbled with oxygen (washing controls). Three replicates of 20 blueberries each (n = 60) were analyzed for each condition.
Light Microscopy Observations
Preparation of fruit samples was performed as previously described by Fava et al. (2011). Briefly, sections (≈ 3 mm) of control and treated blueberries were fixed in glutaraldehyde solution (3% w/w) and then in 0.1 M potassium phosphate buffer (pH = 7.4) during 48 h at room temperature. After rinsing three times with distilled water, samples were treated with OsO4 solution (1.5 % w/w) at room temperature and dehydrated in a graded acetone series before embedding in low-viscosity Spurr resin. Sections (1–2-μm thick) of the Spurr-embedded tissue were cut on a Sorvall MT2-B Ultracut microtome and stained with toluidine blue (1% w/w) and basic fuchsine (1% w/w) solutions. Samples were observed under a Zeiss AxiosKop 2 light microscope (Carl Zeiss AG, Jena, Germany). Images were captured with a Canon EOS 1000D camera (Canon, Tokyo, Japan) and analyzed with the Axio Vision 4.8.2 Software package (Carl Zeiss AG, Jena, Germany).
Mechanical Properties
Puncture test was performed at 25 ± 1 °C using an Instron Testing Machine model 3345 (Instron, Canton, MA, USA) with a flat-end cylindrical probe (diameter, 0.003 m), a cross-head speed of 0.050 m min-1, and a 5-N load cell. Each sample was penetrated 0.010 m in the equatorial side (perpendicular to the abscission zone). Four mechanical parameters were computed from the force-displacement curve: the maximum force required to break the fruit epidermis (FR); the probe displacement at the epidermis rupture point (DR); the mechanical work needed to break the epidermis (W), estimated by the area under the curve up to the epidermis rupture point; and the stiffness (Stiff), calculated as the slope of the initial linear portion of the force/displacement curve. Thirty berries were analyzed for each condition at 0, 5, 10, and 15 days of storage.
Weight Loss
Untreated and treated blueberries were weighted at 0, 5, 10, and 15 days of storage on an analytical balance (Adventurer, Ohaus Corp., USA) with an accuracy of ± 0.0001 g. Results were expressed as the percentage of weight loss compared to the weight of fruit sample at day 0, according to Eq. (2).
where WL (%) is the percentage of weight loss, W0 is the initial weight of blueberry sample, and Wt is the weight at time t. Thirty fruits were evaluated for each condition.
Statistical analysis
Ozone treatment effects along storage time on the percentage of weight loss of blueberries were compared by a general linear mixed model (GLMM). The model included treatments (between-subject factor), time (within-subject factor), and individual fruits (random factor). Mean values were compared by LSD Fisher post hoc tests. The Akaike information criterion (AIC) was used for choosing the best-fitting model as a minimal adequate one. The GLMM analysis was conducted by using the lme function (lme4 package, R Development Core Team 2014). When variances were not homogeneous, the variance structure of the residual was corrected using VarExp, VarIdent, or VarPower option (nlme package). Multivariate analysis of variance (MANOVA) was used to detect differences in the mechanical properties. Hotelling corrected by Bonferroni tests was performed in case of finding significant differences. Prior to performing the analyses, multivariate outliers were detected by the Mahalanobis distance and removed from the data set and assumptions of normal distribution and homogenous variance among groups were verified. The effect of ozone treatment and storage time on decay incidence data were analyzed by a two-way analysis of variance (ANOVA). Mean values were compared by Tukey’s post hoc tests.
Results and Discussion
Ozone Concentration in Water
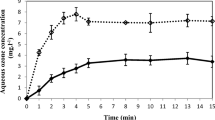
The concentration of ozone in the distilled water of the bubble column with or without blueberries is presented in Fig. 1. Final aqueous ozone concentration was lower when blueberries were immersed in the distilled water (3.0 ± 0.1 and 5.1 ± 0.1 mg O3 L-1 for gaseous ozone concentrations at the column inlet of 10 mg O3 L-1 and 18 mg O3 L-1, respectively) than in free-fruit water (3.7 ± 0.1 and 6.7 ± 0.2 mg O3 L-1 for gaseous ozone concentrations at the column inlet equal to 10 mg O3 L-1 and 18 mg O3 L-1, respectively). This could be associated with the interaction between ozone and organic matter (i.e., ozone-consuming compounds) present in the fruit matrix. Regardless of the presence or absence of fruit, the stabilization time was approximately 5 min for both ozone concentrations assayed.
Aqueous ozone concentration in distilled water bubbled with ozone for different times. Values are the mean of samples (n = 3), and vertical bars represent standard deviation. Water with 60 blueberries (straight line); water without fruit (broken line). Gaseous ozone concentration at the column inlet: 10 mg O3 L-1 (black circle); 18 mg O3 L-1 (black square)
Fungal Incidence
The effect of ozone treatments on decay produced by native mycobiota along storage is shown in Fig. 2a. The percentage of infected fruit increased with storage time in all samples, but decay was reduced by ozone exposure. Statistical analysis showed no significant interaction between “treatment” and “storage time” (F60, 165 = 1.33; p = 0.11) but main effects of both factors were significant (F10, 165 = 20.09; p < 0. 0001and F6, 165 = 148.0; p < 0.0001, respectively).
Fungal decay incidence in ozonized (a) and control and washing control (b) blueberries stored during 15 days at 4 ± 1 °C. Control (untreated fruit) (white circle). Washing controls: O2 10 min (white square); O2 15 min (white up-pointing triangle); O2 20 min (white diamond); O2 30 min (down-pointing triangle). Ozonized fruit: 10 mg O3 L-1 10 min (black circle); 10 mg O3 L-1 20 min (black square); 18 mg O3 L-1 10 min (black up-pointing triangle); 18 mg O3 L-1 15 min (black diamond); 18 mg O3 L-1 20 min (black down-pointing triangle); and 18 mg O3 L-1 30 min (multiplication sign). Values are the means of four replicates (n = 120), and vertical bars represent standard deviation
While little decay (less or equal than 10%) was observed in ozonized berries held at 4 ± 1 °C for 15 days, decay increased up to 21% in control fruit. At 20 days of storage, a 54–70% or a 38–48% reduction in average decay incidence compared with untreated fruit was observed when blueberries were exposed to 18 mg O3 L-1 or 10 mg O3 L-1, respectively. The greatest ozone concentration assayed appeared to be more efficient in reducing fungal infection, although differences were not significant (Online Resource 1). Fruit treated with 10 mg O3 L-1 for 10 min showed a delay in the infection onset, but at the end of the storage (20 days), they presented a higher percentage of infected fruit (15.8 %) than those exposed to 18 mg O3 L-1 for 10 min (10.0 %). Different exposure times at 18 mg O3 L-1 resulted in slight and non-significant differences in the percentage of infected fruit, indicating that treatment times beyond 10 min did not achieve greater fungal inhibition.
Fungal decay in washing controls is shown in Fig. 2b. No significant differences were observed between washing controls and untreated fruit, except for control O2 30 min, which presented a lower percentage of infected fruit than the control (Online Resource 1). When comparing 10-min and 20-min ozone treatments with their corresponding washing controls, inhibition of fungal infection was significantly greater than in only washed fruit. When the fruit was treated for 30 min, fungal decay was greater in the ozone-exposed samples, although these differences were not significant (Online Resource 1). Therefore, ozone did have an antifungal activity per se beyond the washing effect mainly for short exposure times.
Infection by B. cinerea mold in ozonized, only washed, and untreated inoculated blueberries during storage is presented in Fig. 3. Ozone exposure beyond 20 min was not assayed considering the effect observed in native mycobiota and other quality factors, described in the following sections, which indicated that 30 min of treatment would be excessive to preserve the freshness of blueberries. Incidence of B. cinerea decay showed a similar pattern than native mycobiota one. All samples (treated and untreated) showed an increase in the percentage of infected fruit; nevertheless, it was lower in ozonized fruit. Reduction in decay incidence was more notorious up to 10-day storage and at the highest ozone concentration assayed. No significant interactions between “treatment” and “storage time” were observed (F30, 84 = 1.56; p = 0.06); however, all factors were statistically significant (F5, 84 = 52.78; p < 0. 0001 for treatment; F6, 84 = 106.10; p < 0. 0001 for storage). Average decay incidence in the untreated fruit group increased from about 13% at 5 days to 53% at 15 days of storage. A significant inhibitory effect of ozone at 18 mg O3 L-1 on B. cinerea growth was observed regardless the exposure time (Online Resource 2): average decay incidence was 0–2% and 17% after 5 days and 10 days of storage, respectively. The most effective ozone concentration to inhibit B. cinerea decay would appear to be18 mg O3 L-1, although differences with the treatment at 10 mg O3 L-1 20 min were not statistically significant. Washing controls did not significantly differ from untreated inoculated control. All ozone treatments resulted statistically different from the corresponding washing control.
B. cinerea decay incidence in ozonized and control inoculated blueberries stored 15 days at 4 ± 1 °C. Control (untreated) (white circle). Washing controls: O2 10 min (white square); O2 20 min (white diamond). Ozonized fruit: 10 mg O3 L-1 20 min (black diamond); 18 mg O3 L-1 10 min (black up-pointing triangle); and 18 mg O3 L-1 20 min (black down-pointing triangle). Values are the means of three replicates (n = 60), and vertical bars represent standard deviation
Literature reporting the effect of ozone on native microbiota of blueberry or on artificially inoculated microorganisms is scarce. Pangloli and Hung (2013) evaluated the effect of ozonized water (1.5 mg O3 L-1) in the reduction of E. coli O157:H7 previously inoculated in blueberries (106 CFU/mL). They reported 2.3–3.5 log-reductions on the surface of the fruit washed for 1 and 5 min, respectively. Also, when blueberries were washed with potable water, reductions of 1.9–2.7 log CFU/g were obtained, indicating an effect of ozone per se. Similarly, Bialka and Demirci (2007) assayed the effect of aqueous ozone (8.9 mg O3 L-1) treatments in blueberries inoculated with E. coli O157:H7 and Salmonella (106 CFU/mL). Fruits were subjected to different treatment times (2–64 min) and temperatures (20 and 4 °C). At 20 °C, similar reductions of E. coli than those reported by Pangloli and Hung (2013) were achieved after 16 min of treatment, even when the ozone concentration used was almost 6 times higher.
Alexandre et al. (2012) studied the effect of ozone washings (0.3 and 2 mg O3 L-1; 1, 2, and 3 min, 15 °C) on total mesophilic organisms of strawberries and found the greatest microbial reductions (2.3 ± 0.4 log-cycles) when using the highest ozone concentration (2 mg O3 L-1) for 3 min. They also reported significant differences between ozone-treated fruit and its corresponding washing control. Contigiani et al. (2018) observed a significant effect of ozonized water washing (3.5 mg O3 L-1, 5 min) on fungal spoilage of strawberries throughout storage at 5 °C. In general, fungal incidence was ~ 22–25% lesser than in untreated fruit after 12 days of storage. Ong et al. (2013) evaluated the effect of ozone (1.6 mg O3 L-1, 96 h) on papaya anthracnose caused by Colletotrichum gloeosporioides. They reported that ozone delayed and simultaneously decreased the disease incidence to 40% in papaya fruit with respect to the control after 28 days of storage at 12 ± 1 °C and 80% RH.
Microscopic Features
Blueberry is a “soft fruit” characterized by a well-developed outer tangential wall (OTW) that can be divided into four main layers from outer to inner, as summarized by Lyshede (1982) and Jeffree (2006): (1) the epicuticular waxes, amorphous, and/or crystalline; (2) the cuticle, consisting in the cuticle proper, composed of almost exclusively cutin (a polyester matrix of polyhydroxylated C16 and C18 fatty acids), and the cutinized layer, constituted by a cellulose skeleton incrusted with cutin, wax and pectin (composition similar to that of primary cell walls); (3) the pectic layer, composed mainly of pectic polysaccharides; and (4) the cellulose layer, consisting in interacting networks of cellulose and hemicelluloses, pectic polysaccharides, extensine, and other minor constituents (Carpita and Gibeaut 1993; Fava et al. 2006; Konarska 2015a, 2015b). In particular, the skin (outer surface layers plus epidermal and subepidermal tissues) confers mechanical strength to the fruit, while the cuticle is determinant in controlling water loss by non-stomatal diffusion due to the cuticle hydrophobic nature (Chu et al. 2018; Lara et al. 2014; Schreiber and Schonherr 2009). Waxes are considered the major compounds accounting for their role as an impermeable barrier.
Light microscopy (LM) images of ozonized (18 mg O3 L-1; 20 and 30 min of exposure) and untreated blueberry tissues at 0, 10, and 15 days of storage are shown in Fig. 4. Micrographs in Fig. 4a, b show a general and a detailed view respectively of the skin or epicarp (E) and part of the mesocarp (M) of untreated fresh fruit. The epicarp was composed of one stratum of epidermal cells and a collenchymatous hypodermis with two or three subepidermal cell layers. Epidermal cells (EP) were organized in a continuous monolayer of strongly attached quadrangular cells with asymmetric cell walls, while hypodermal cells, round in shape, appeared more loosely attached. Anthocyanins (An), flavonoids responsible for the blue color, were observed to accumulate in epidermal and hypodermal cells of blueberry skin as clumps with indefinite or spherical form in vacuoles or positioned closely appressed to tonoplast. Outer tangential epidermal wall (OTW) was observed undulated with a darker cuticle proper layer, a lighter reticulated cutinized layer, and a well-stained cellulose layer. OTW appeared thicker than inner tangential walls (ITW) and radial walls (RW). The mesocarp (M) exhibited irregular turgid parenchymatic cells with parietal cytoplasm, separated by intercellular spaces and with few cell-to-cell contact points. Stone cells (micrographs not shown) and vascular bundles were also visualized in the mesocarp.
LM images of transverse sections of ozonized and control blueberry tissues. a, b, f, i Day 0; c, g, j Day 10; d, e, h, k, l Day 15. a–e Control (untreated). f–h 18 mg O3 L-1 20 min. i–l 18 mg O3 L-1 30 min. E, epicarp; M, mesocarp; An, anthocyanins; EP, epidermis; OTW, outer tangential wall; ITW, inner tangential wall; RW, radial walls; c, cuticle; EW, epicuticular waxes; arrow, cracks in OTW and epidermis cell wall; asterisk, detachment of subepidermal cells. Scale, a–d and F–l, 100 μm; e, 20 μm
Immediately after treatment, fruit subjected to ozone washing during 20 min and 30 min showed a severe compaction of epidermal and hypodermal (first layer) cells (Fig. 4f), or a contraction of epidermal cells with a notorious detachment of these from the hypodermis, where cells looked turgid and without morphological modifications (Fig. 4i). Ozone treatment also slightly increased the separation between cells in the mesocarp and some cells showed folding of walls and very irregular shapes (micrographs not shown).
Slight differences were observed in the microstructure of untreated tissues after 10 days under cold storage (Fig. 4c). Epidermal cells appeared with numerous vacuoles and a dense cytoplasm between transverse sections of fruits which exhibited well-defined epicarp and mesocarp. OTW organization was maintained although the transition between cuticle layers was less abrupt. After 15 days of storage, flattening of epidermal and subepidermal cells was detected (Fig. 4d). Neither detachment of the skin from the fruit flesh nor an increase in the separation between epidermis and hypodermal layers was observed (Fig. 4d). OTW appeared densely stained. In some samples, cells of epicarp appeared integer, with dark-stained inclusions of different sizes and irregular shapes. In others, dark-stained OTW appeared with many microcracks and various epicarp cells exhibited broken walls and plasmalemma (Fig. 4e). Cold storage induced important changes in the epicarp structure of ozonized tissues. Intercellular spaces of mesocarp (micrographs not shown) and separation between hypodermal layers increased (Fig. 4g, h, j–l). Epidermal and subepidermal cells appeared flattened. Some subepidermal cells showed disrupted membranes and some others appeared with folded walls and/or with incipient plasmolysis. OTW, including cuticle, was lightly stained and in some parts the different layers could not be distinguished (Fig. 4k, l). Various rupture episodes were detected in the OTW; these microcracks extended from the cuticle to the walls of epidermal cells. These structure modifications would indicate an effect of ozone or free radical species formed during the auto decomposition of ozone, as •OH, HO2•, •O2, and •O3, on OTW components. This behavior would be supported by the findings of Muthukumarappan et al. (2016) and Wang et al. (1999). These authors reported that ozone caused depolymerization of macromolecules (such as pectin and cellulose) and degradation of carbohydrates because of its high oxidant power.
Mechanical Properties
Typical force-displacement curves from the puncture test of treated and untreated berries at day 0 are shown in Fig. 5. All samples exhibited a similar mechanical pattern. Epidermis of both, treated and untreated blueberry fruit, contributed to an average between 70 and 80% of the firmness before the rupture point. Mean values of mechanical parameters (FR, DR, W, and Stiff) are shown in Table 1. There was not a significant effect of ozone treatment in the mechanical parameters extracted from the force-displacement curves at day 0. Overall, treated and untreated fruit showed almost constant values of FR and a very small increase in DR and/or W values during storage, but these differences were not significant. Neither relationship between ozone concentration nor exposure time and mechanical parameters was observed, nor was a clear trend of softening throughout storage detected.
Typical penetration force-displacement curves for ozonized and control blueberry fruit (0 days). Control (untreated fruit) (straight line). Ozonized fruit: 10 mg O3 L-1 20 min (black circle); 18 mg O3 L-1 15 min (black square); 18 mg O3 L-1 20 min (black up-pointing triangle); and 18 mg O3 L-1 30 min (dotted line)
These results did not seem to have a relationship with observed changes in blueberry microstructure. It is well known that the organization and composition of the OTW as well as the turgor pressure within individual cells, the cell wall rigidity, and the cell-cell adhesion (determined by the integrity of the middle lamella and the plasmodesmata) are the most important structural factors that determine the rheological characteristics of berries (Alzamora et al. 2008; Jackman and Stanley 1995; Lara et al. 2014). It would be expected that the alterations in the OTW (disruptions in the OTW and in the cell walls of epidermis; less stained cuticle and cellulose layer; greater separation between epicarp layers) induced by ozone and/or storage were reflected in lower FR and DR values. However, the maintenance of these penetration parameters could partially be ascribed to opposing structure effects: contraction of epidermis versus microcracks in the OTW and cell walls. The non-significant effect of ozone treatment and/or cold storage on evaluated mechanical parameters could also be associated with the high biological variability within and between berries, either fresh or ozonized, translated in very different penetration curves within control fruit samples with a given ozonization process, and consequently in large standard deviations associated with mechanical data (Online Resource 3) (Alzamora et al. 2008). In addition, the four mechanical parameters studied did not represent the whole force-displacement curve. In particular, they did not take into account the region corresponding to probe displacement after the rupture of the epidermis, related to subepidermis and flesh properties. Despite the notorious variability, force values for subepidermis and flesh penetration were clearly greater for control berries than for ozonized ones (arrows, Online Resource 3). The detachment of the epidermis from deeper hypodermis layers and mesocarp and the increased in cellular spaces would be translated into a lower resistance to probe penetration in the tissues beneath the epidermis of ozonized fruit compared with that of control blueberry.
Chen et al. (2015) studied the changes in blueberry’s firmness during refrigerated storage at 5 and 10 °C. Fruit stored at 5 °C showed loss of firmness only after 28 days of storage. This loss of firmness was associated with the increase in total water-soluble pectin and the disassembly of primary cell walls and middle lamella structures. These findings are in agreement with the results presented in this study since up to 15 days of storage, a decrease in FR values was not observed.
Weight Loss
Weight loss of control and ozonized blueberries throughout cold storage is shown in Fig. 6. No significant interaction between “treatment” and “storage time” was observed (F8, 250 = 0.93; p = 0.49) but the main effects of each factor were statistically significant. All berries showed a significant decline in weight (F2, 250 = 389.6; p < 0.0001) from day 0 to day 15. Ozone treatments significantly increased weight loss in comparison with untreated fruit (F4, 125 = 32.05; p < 0.0001). For the same exposure time, the highest the ozone concentration, the highest the loss of weight is.
Weight loss (%) for ozonized and control blueberry fruit throughout storage at 4 ± 1 °C. Control (untreated fruit) ( ). Ozonized fruit: 10 mg O3 L-1 20 min (
). Ozonized fruit: 10 mg O3 L-1 20 min ( ); 18 mg O3 L-1 15 min (
); 18 mg O3 L-1 15 min ( ); 18 mg O3 L-1 20 min (
); 18 mg O3 L-1 20 min ( ); and 18 mg O3.L-1 30 min (
); and 18 mg O3.L-1 30 min ( ). At the same storage day, means followed by different lowercase letters indicate significant differences (p < 0.05) between treatments. Means followed by different uppercase letter indicate significance differences (p < 0.05) throughout storage time
). At the same storage day, means followed by different lowercase letters indicate significant differences (p < 0.05) between treatments. Means followed by different uppercase letter indicate significance differences (p < 0.05) throughout storage time
Several studies have mentioned that higher levels of ozone may lead to damages in the cuticle and epidermal tissues and oxidation of epicuticular waxes due to the great oxidant power of ozone (Lara et al. 2014; Tzortzakis et al. 2007; Palou et al. 2002). Weight loss has a close relationship with the cuticle integrity, cuticular material, and epicuticular waxes, which are known to be a protective barrier against water loss (Heredia 2003; Järvinen et al. 2010; Lara et al. 2014). There are no published reports about weight loss of blueberries treated with aqueous ozone. Concha-Meyer et al. (2015) reported a significant reduction in weight loss rates of blueberries stored at 12 °C under ozone enriched atmosphere when compared with those of control. Similarly, Tzortzakis et al. (2007) observed higher weight losses when tomato fruits were exposed to high concentrations of gaseous ozone; however, tomatoes were found to be unaffected by low-level ozone treatment. Some studies carried out in “Casselman” plums treated with gaseous ozone reported significant differences in cuticular waxes and cuticle thickness compared with control fruit. They suggested that the ozone effect in the cuticle and waxes contributed to the weight loss observed (Crisosto et al. 1993).
In this study, blueberry’s weight loss was affected by aqueous ozone treatment; the effect was dose-dependent and increased throughout storage. Changes in blueberry OTW microstructure due to ozone treatment and/or cold storage shown in Fig. 4 would partially support water loss results. The cuticle in 20-min and 30-min ozonized berries stored for 10 days was observed altered, stained very little, and with numerous cracks. Accordingly, cuticle water permeability as well as the pathway for water transport would increase. These cracks became wider and deeper as the storage period increased to 15 days, accelerating water loss. Incidence of cuticle cracking in untreated fruit was more heterogeneous and was more evident at 15 days of storage, resulting in a lower water loss than in ozonized fruit.
Conclusions
Ozone treatment at18 mg O3 L-1 was effective for diminishing the infection by B. cinerea and native mycobiota in blueberries (cv. O’Neal), without affecting the mechanical parameters: the maximum force required to break the fruit epidermis; the probe displacement at the epidermis rupture point and the mechanical work needed to break the epidermis and stiffness, calculated as the slope of the initial linear portion of the force/displacement curve, compared with untreated fruit. Weight loss of blueberries was ozone dose-dependent; exposure times greater than 15 min significantly increased water loss throughout storage at 4 ± 1 °C. Therefore, ozone treatments would be conditioned by the weight loss of the fruit, which showed a notorious increase at the highest ozone concentration and exposure times beyond 20 min. Changes in blueberry microstructure (disruptions in the OTW and in the cell walls of the epidermis; less stained cuticle and cellulose layer; greater separation between epicarp layers) partially explained weight loss. Ozone treatment at 18 mg O3 L-1 for 10 min could be an alternative for reducing fungal decay of blueberries without causing an excessive weight loss during refrigerated storage.
References
Argentinean Blueberry Committee (n.d.), Estadísticas. Hectáreas por región. https://www.argblueberry.com/home/estadisticas/. Accessed 14 Dic 2018.
Alexandre, E., Brandão, T., & Silva, C. (2012). Efficacy of non-thermal technologies and sanitizer solutions on microbial load reduction and quality retention of strawberries. Journal of Food Engineering, 108(3), 417–426.
Alexandre, E., Santos-Pedro, D., Brandão, T., & Silva, C. (2011). Influence of aqueous ozone, blanching and combined treatments on microbial load of red bell peppers, strawberries and watercress. Journal of Food Engineering, 105(2), 277–282.
Alwi, N. A., & Ali, A. (2015). Dose-dependent effect of ozone fumigation on physiological characteristics, ascorbic acid content and disease development on bell pepper (Capsicum annuum L.) during storage. Food and Bioprocess Technology, 8(3), 558–566.
Alzamora, S., Tapia, M. S., & López-Malo, F. (2000). Overview. In S. M. Alzamora, M. S. Tapia, & A. López-Malo (Eds.), Minimally processed fruits and vegetables (pp. 1–9). Maryland: EEUU Aspen Publishers Inc.
Alzamora, S. M., Viollaz, P. E., Martínez, V. Y., Nieto, A. B., & Salvatori, D. (2008). Exploring the linear viscoelastic properties structure relationship in processed fruit tissues. In G. F. Gutiérrez-López, G. V. Barbosa-Cánovas, J. Welti-Chanes, & E. Parada-Arias (Eds.), Food Engineering: Integrated Approaches (pp. 155–181). New York: EE.UU: Springer New York.
Bader, H., & Hoigné, J. (1982). Determination of ozone in water by the indigo method: a submitted standard method. Ozone Science and Engineering, 4(4), 169–176.
Bialka, K. L., & Demirci, A. (2007). Decontamination of Escherichia coli O157:H7 and Salmonella enterica on blueberries using ozone and pulsed UV-Light. Journal of Food Science, 72(9), M391–M396.
Brodowska, A. J., Nowak, A., & Śmigielski, K. (2018). Ozone in the food industry: principles of ozone treatment, mechanisms of action, and applications: an overview. Critical Reviews in Food Science and Nutrition, 58(13), 2176–2201.
Carpita, N. C., & Gibeaut, D. M. (1993). Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal, 3(1), 1–30.
Concha-Meyer, A., Eifert, J. D., Williams, R. C., Marcy, J. E., & Welbaum, G. E. (2015). Shelf life determination of fresh blueberries (Vaccinium corymbosum) stored under controlled atmosphere and ozone. International Journal of Food Science, 2015, 9.
Contigiani, E. V., Jaramillo-Sánchez, G., Castro, M. A., Gómez, P. L., & Alzamora, S. M. (2018). Postharvest quality of strawberry fruit (Fragaria x Ananassa Duch cv. Albion) as affected by ozone washing: fungal spoilage, mechanical properties, and structure. Food and Bioprocess Technology, 11(9), 1639–1650.
Crisosto, C., Retzlaff, W., William, L., DeJong, T., & Zoffoli, J. (1993). Postharvest performance evaluation of plum (Prunus salicina Lindel., ‘Casselman’) fruit grown under three ozone concentrations. Journal of the American Society for Horticultural Science, 118(4), 497–502.
Chen, H., Cao, S., Fang, X., Mu, H., Yang, H., Wang, X., et al. (2015). Changes in fruit firmness, cell wall composition and cell wall degrading enzymes in postharvest blueberries during storage. Scientia Horticulturae, 188, 44–48.
Chu, W., Gao, H., Chen, H., Fang, X., & Zheng, Y. (2018). Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chemistry, 239, 68–74.
Chun, H. H., Kang, J. H., & Song, K. B. (2013). Effects of aqueous chlorine dioxide treatment and cold storage on microbial growth and quality of blueberries. Journal of Korean Society for Applied Biological Chemistry, 56(3), 309–315.
Duan, J., Wu, R., Strik, B. C., & Zhao, Y. (2011). Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biology and Technology, 59(1), 71–79.
Duarte-Molina, F., Gómez, P. L., Castro, M. A., & Alzamora, S. M. (2016). Storage quality of strawberry fruit treated by pulsed light: fungal decay, water loss and mechanical properties. Innovative Food Science and Emerging Technologies, 34, 267–274.
Fava, J., Alzamora, S. M., & Castro, M. A. (2006). Structure and nanostructure of the outer tangential epidermal cell wall in Vaccinium corymbosum L. (blueberry) fruits by blanching, freezing-thawing and ultrasound. Food Science and Technology International, 12(3), 241–251.
Fava, J., Hodara, K., Nieto, A., Guerrero, S., Alzamora, S. M., & Castro, M. A. (2011). Structure (micro, ultra, nano), color and mechanical properties of Vitis labrusca L. (grape berry) fruits treated by hydrogen peroxide, UV–C irradiation and ultrasound. Food Research International, 44(9), 2938–2948.
Garcia Loredo, A. B., Guerrero, S. N., & Alzamora, S. M. (2015). Inactivation kinetics and growth dynamics during cold storage of Escherichia coli ATCC 11229, Listeria innocua ATCC 33090 and Saccharomyces cerevisiae KE162 in peach juice using aqueous ozone. Innovative Food Science and Emerging Technologies, 29, 271–279.
Giongo, L., Poncetta, P., Loretti, P., & Costa, F. (2013). Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biology and Technology, 76, 34–39.
Greco, M., Patriarca, A., Terminiello, L., Fernández Pinto, V., & Pose, G. (2012). Toxigenic Alternaria species from Argentinean blueberries. International Journal of Food Microbiology, 154(3), 187–191.
Heredia, A. (2003). Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochimica et Biophysica Acta (BBA), 1620(1), 1–7.
Horvitz, S., & Cantalejo, M. J. (2014). Application of ozone for the postharvest treatment of fruits and vegetables. Critical Reviews in Food Science and Nutrition, 54(3), 312–339.
Jackman, R. L., & Stanley, D. W. (1995). Perspectives in the textural evaluation of plant foods. Trends in Food Science and Technology, 6(6), 187–194.
Järvinen, R., Kaimainen, M., & Kallio, H. (2010). Cutin composition of selected northern berries and seeds. Food Chemistry, 122(1), 137–144.
Jeffree, C. E. (2006). The fine structure of the plant cuticle. In Riederer, M., & Muller, C. (Eds.), Biology of the plant cuticle, Vol. 23 (, pp. 11-125). Germany: Blackwell Publishing.
Kim, C., & Hung, Y. (2012). Inactivation of E. coli O157:H7 on blueberries by electrolyzed water, ultraviolet light, and ozone. Journal of Food Science, 77(4), M206–M211.
Konarska, A. (2015a). Development of fruit quality traits and comparison of the fruit structure of two Vaccinium corymbosum (L.) cultivars. Scientia Horticulturae, 194, 79–90.
Konarska, A. (2015b). Morphological, anatomical, and ultrastructural changes in Vaccinium corymbosum fruits during ontogeny. Botany, 93(9), 589–602.
Lara, I., Belge, B., & Goulao, L. F. (2014). The fruit cuticle as a modulator of postharvest quality. Postharvest Biology and Technology, 87, 103–112.
Lyshede, O. B. (1982). Structure of the outer epidermal wall in xerophytes. In D. F. Cutler, K. L. Alvin, & C. E. Price (Eds.), The plant cuticle (pp. 87–98). London: Academic Press.
Mengiste, T., Laluk, K., & AbuQamar, S. (2010). Mechanisms of induced resistance against B. cinerea. In D. Prusky & M. Gullino (Eds.), Postharvest Pathology (pp. 13–30). Dordrecht: Springer Netherlands.
Misra, N. N., Moiseev, T., Patil, S., Pankaj, S. K., Bourke, P., Mosnier, J. P., et al. (2014). Cold plasma in modified atmospheres for post-harvest treatment of strawberries. Food and Bioprocess Technology, 7(10), 3045–3054.
Moggia, C., Graell, J., Lara, I., Schmeda-Hirschmann, G., Thomas-Valdés, S., & Lobos, G. A. (2016). Fruit characteristics and cuticle triterpenes as related to postharvest quality of highbush blueberries. Scientia Horticulturae, 211, 449–457.
Muthukumarappan, K., Halaweish, F., & Naidu, A. (2000). Ozone. In A. S. Naidu (Ed.), Natural Food Antimicrobial Systems (pp. 783–800). New York: CRC/ Press.
Muthukumarappan, K., Tiwari, B., O’Donnell, C. P., & Cullen, P. (2016). Ozone and CO2 processing: rheological and functional properties of food. In J. Ahmed, H. Ramaswamy, S. Kasapis, & J. Boye (Eds.), Novel Food Processing: Effects on Rheological and Functional Properties (pp. 127–146). New York, EE.UU: CRC/ Press, Taylor y Francis Group.
Ong, M. K., Kazi, F. K., Forney, C. F., & Ali, A. (2013). Effect of gaseous ozone on papaya anthracnose. Food and Bioprocess Technology, 6(11), 2996–3005.
Palou, L., Crisosto, C., Smilanick, J., Adaskaveg, J., & Zoffoli, J. (2002). Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biology and Technology, 24(1), 39–48.
Pangloli, P., & Hung, Y. (2013). Reducing microbiological safety risk on blueberries through innovative washing technologies. Food Control, 32(2), 621–625.
Piechowiak, T., Antos, P., Józefczyk, R., Kosowski, P., Skrobacz, K., & Balawejder, M. (2018). Impact of ozonation process on the microbiological contamination and antioxidant capacity of highbush blueberry (Vaccinum corymbosum L.) fruit during cold storage (pp 1-10). Ozone: Science and Engineering.
R Development Core Team. (2014). R: A language and environment for statistical computing. R foundation for statistical computing, Wien, Austria. http://www.R-proyect.org.
Rakness, K., Gordon, G., Langlais, B., Masschelein, W., Matsumoto, N., Richard, Y., et al. (1996). Guideline for measurement of ozone concentration in the process gas from an ozone generator. Ozone Science and Engineering, 18(3), 209–229.
Sandermann, H., Ernst, D., Heller, W., & Langebartels, C. (1998). Ozone: an abiotic elicitor of plant defence reactions. Trends in Plant Science, 3(2), 47–50.
Schreiber, L., & Schonherr, J. (2009). Water and solute permeability of plant cuticles (4th ed.). Berlin: Springer.
Skurtys, O., Velásquez, P., Henriquez, O., Matiacevich, S., Enrione, J., & Osorio, F. (2011). Wetting behavior of chitosan solutions on blueberry epicarp with or without epicuticular waxes. LWT - Food Science and Technology, 44(6), 1449–1457.
Tzortzakis, N., Borland, A., Singleton, I., & Barnes, J. (2007). Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biology and Technology, 45(3), 317–325.
Vicente, A. R., Ortugno, C., Rosli, H., Powell, A. L. T., Greve, L. C., & Labavitch, J. M. (2007). Temporal sequence of cell wall disassembly events in developing fruits. 2. analysis of blueberry (Vaccinium species). Journal of Agricultural and Food Chemistry, 55(10), 4125–4130.
Wang, Y., Hollingsworth, R. I., & Kasper, D. L. (1999). Ozonolytic depolymerization of polysaccharides in aqueous solution. Carbohydrate Research, 319(1-4), 141–147.
Xu, F., & Liu, S. (2017). Control of postharvest quality in blueberry fruit by combined 1-methylcyclopropene (1-MCP) and UV-C irradiation. Food and Bioprocess Technology, 10(9), 1695–1703.
Funding
The authors would like to acknowledge the financial support from Universidad de Buenos Aires (UBACYT 2014-7 Project 20020130100388BA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) of Argentina and from Banco Interamericano de Desarrollo (BID), and SENESCYT from Ecuador.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jaramillo-Sánchez, G., Contigiani, E.V., Castro, M. et al. Freshness Maintenance of Blueberries (Vaccinium corymbosum L.) During Postharvest Using Ozone in Aqueous Phase: Microbiological, Structure, and Mechanical issues. Food Bioprocess Technol 12, 2136–2147 (2019). https://doi.org/10.1007/s11947-019-02358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02358-z