Abstract
Ozone is a potent oxidizing agent which has the potential to extend fruit storage life by inhibiting disease development. Its oxidative action may also affect fruit quality. Fruit of bell pepper were exposed to 0 (control), 1, 3, 5, 7 and 9 ppm ozone at 12 °C and 95 % relative humidity (RH) for 3 days, and their quality was monitored during an additional 24 days of storage at 12 °C. Treatment with 7 and 9 ppm ozone significantly affected quality by enhancing fruit ripening as shown by higher rates of respiration and colour change and lower titratable acidity than that in untreated fruit. The treatments also increased membrane permeability, reduced firmness, enhanced weight loss and decreased ascorbic acid content of the fruit. Exposure to lower ozone concentrations (1 and 3 ppm) had no effect on fruit respiration, colour, titratable acidity and firmness. However, ascorbic acid concentration was increased. This could be due to the activation of fruit defence system that produces a high level of ascorbic acid to neutralize the oxidative activity caused by ozone. Fruit exposed to 3 ppm ozone were also rated by sensory evaluation as having better fruit flavour after 19 days of storage than that of fruit treated with 7 or 9 ppm ozone. Treatment of 3 ppm ozone also reduced anthracnose incidence. This shows that exposure to 3 ppm ozone has the potential to increase fruit ascorbic acid content, reduce disease incidence and maintain fruit physico-chemical quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bell pepper (Capsicum annum L.) is a non-pungent or sweet pepper that belongs to the same species as pungent peppers, such as jalapeno, cayenne and serrano peppers. Bell pepper lacks the capsaicin gene which gives the pungency trait in some peppers (Bosland & Votava 2000). Bell pepper contains high levels of antioxidants, such as vitamins A, C and E, as well as other vitamins, including vitamins B1 (thiamine), B2 (riboflavin), B3 (niacin) and K (Bosland & Votava 2000). Vitamin C or ascorbic acid, the major antioxidant in bell pepper, may be up to six times the amount in oranges, and a medium-sized bell pepper can provide 180 % of the recommended daily allowance (RDA) of vitamin C (Bosland & Votava 2000).
Bell pepper is one of the most important vegetables with a growing demand due to its wide use in the fast food industry as well as consumer motivation to consume vegetable as a healthy diet (Castro et al. 2011). It is commonly used in salad and pizza for its nutritive benefit, natural flavour and colour. Its production is increasing annually with a total production of 30 million tonnes valued at USD 25 billion in 2011 (FAOSTAT 2012). China is the largest producer with 15 million tonnes in 2011 (FAOSTAT 2012).
Postharvest management of bell pepper is challenged by anthracnose, the major disease affecting bell pepper. Such diseases have caused substantial losses both in the field and postharvest around the world (Mahasuk et al. 2009). Currently, these diseases are controlled with synthetic fungicides such as Maneb (Lewis Ivey et al. 2004); however, there are hazards associated with their use and alternative safe treatments are needed.
Ozone, O3, is a naturally occurring gas with a potential to be an alternative postharvest treatment to replace synthetic fungicides. It was approved by the US Food and Drug Administration (FDA) for food treatment and storage in 2001 (FDA 2001). It oxidizes microorganisms’ cell membranes, nucleic acid and intracellular enzymes, hence inhibits microbial growth on fruit (Khadre et al. 2001). This has been demonstrated on a variety of crops such as orange (Palou et al. 2003), kiwi fruit (Minas et al. 2010) and grape (Gabler et al. 2010). In comparison to synthetic fungicides, ozone decomposes to oxygen and, hence, leaves no harmful residues on fruit. The activity of ozone on disease development and its lack of harmful residue emphasizes the possibility of ozone as an alternative treatment.
The oxidative environment achieved with ozone application may also affect the physiology of fruit and vegetables and consequently influence their physical and chemical qualities. For example, in carrot, exposure to 10 ppm ozone for 10 min reduced its respiration rate during storage, hence prolonged the storage life (Chauhan et al. 2011). The ozone treatment, however, caused transient increase in respiration rate of tomato (Rodoni et al. 2010). The variation in response is possibly related to antioxidant systems which can counteract the oxidative stress caused by ozone. Therefore, this study focused on the effect of ozone on the physiology as well as the physical and chemical qualities of pepper fruit and their correlation with ascorbic acid content, the major antioxidant in bell pepper.
Materials and Method
Fruit Material
Fruit of bell pepper (Capsicum annuum cv. ‘Zamboni’) were harvested at the physiologically mature, green stage from a commercial farm in Cameron Highlands, Pahang, Malaysia. Fruit of uniform size (≈150 g/fruit), free of physical damage and fungal infection were selected, rinsed three times with distilled water and air dried to remove surface water.
Ozone Exposure
Ozone fumigation was conducted in airtight polycarbonate chambers (112 × 47.5 × 42.5 cm) equipped with 12-V fans. The ozone concentration was controlled using an ozone sensor (model: OEM-2 Eco-Sensor, Inc.) calibrated against an ozone analyser (model: IN2000-L2-LC, In USA, Inc.) (Ong et al. 2013). The chambers were placed in a temperature-controlled room equipped with a charcoal ozone scrubber. Bell pepper fruit (20 fruits per chamber) were exposed to ozone at concentrations of 0 (control), 1, 3, 5, 7 and 9 ppm for 3 days at 12 °C and 95 % relative humidity (RH). These concentrations and exposure time were selected based on preliminary work that identified treatment conditions to obtain high-quality fruit. Following the ozone exposure, fruit were transferred to identical control chambers (0 ppm ozone) and stored for an additional 24 days at 12 °C and 95 % RH.
Determination of Physical Quality
Fruit colour at two opposite sides of the fruit equator was measured using a colourimeter (model: MiniScan XE Plus, HunterLab, USA), which was calibrated with standard black and white tiles. Twenty fruits were used per replicate. Values for L*, a * and b* were recorded every 3 days, and values for chroma (C*) and hue angle (h°) were calculated using the following equations (Xing et al. 2011):
Fruit weight loss was determined by weighing the fruit on a digital balance (model: GF-6100, A&D Co. Ltd., Japan) before the ozone treatment and every 3 days until the end of the storage period. Twenty fruits were used per replicate and the same fruits were used throughout the experiment. Percentage of weight was then calculated.
Fruit firmness was determined using an Instron texture analyser (Instron 2519-104, MA), equipped with an 8-mm plunger tip. Fruit pieces, 2.5 × 2.5 cm, were cut from the equatorial region, and firmness was determined by applying force with a constant speed of 20 mm min−1. Twenty fruits were used per replication and two fruit pieces were sampled per fruit. Sampling was done every 3 days and a maximum force required to penetrate through the fruit was expressed in Newton (N).
Membrane permeability was determined according to the method of Xing et al. (2011) with modifications. Briefly, ten fruit discs, including epidermis (≈0.059 cm3), were sampled using a 0.5-cm cork borer, washed with distilled water, dried on filter paper, placed in a flask with 30 ml distilled water, shaken at 25 °C on an orbital shaker (model: S1500, Bibby Scientific Limited, UK) for 30 min and boiled for 10 min. Electrical conductivity of the solution was measured using a conductivity metre (model: Eutech Cond 6+, Thermo Fisher Scientific Inc., USA) before and after boiling, and percentage leakage was calculated. Sampling was done every 3 days and 20 fruits were used per replication.
Determination of Chemical Quality
Soluble solid concentration (SSC) was determined using a Palette digital refractometer (model: PR-32α, Atago Co, Ltd. Japan). Ten grammes of fruit tissue from equatorial region were homogenized in distilled water, centrifuged (model: 5810 R, Eppendorf, UK) at 5500 rpm for 5 min and SSC of the supernatant was determined using the refractometer calibrated with distilled water. Sampling was done every 3 days and 20 fruits were used per replication. The reading was recorded and multiplied by the dilution factor.
Titratable acidity (TA) was determined based on malic acid content according to the method of Fox et al. (2005). Two drops of phenolphthalein were added to the supernatant from the SSC analysis and titrated with 0.1 % NaOH until the solution turned pink. The malic acid content was determined by the measuring volume of NaOH used and multiplying it by the dilution factor and molecular weight of the malic acid (134.09 g mol−1). Sampling was done every 3 days and 20 fruits were used per replication.
Chlorophyll concentration was determined according to the method of Xing et al. (2011). One gramme of fruit tissue from the equatorial region was homogenized with 10 ml of 80 % acetone, centrifuged at 5500 rpm for 5 min and absorbance of the supernatant was measured at 646.6, 663.6 and 750 nm using a UV–vis spectrophotometer (Varioskan Flash Multimode Reader, Thermo Fisher Scientific, USA). Sampling was done every 3 days and 20 fruits were used per replication. Absorbance at 646.6 and 663.6 nm was corrected by subtracting with absorbance at 750 nm, and the chlorophyll content was calculated using the following equation and expressed as microgrammes per gramme fresh weight of fruit sample.
Determination of Respiration and C2H4 Production
Respiration and C2H4 production by fruit was determined according to the method of Forney et al. (2007) with modifications. Briefly, two fruits were sealed in a 500-ml container and incubated for 1 h at room temperature (25–26 °C), after which 1 ml of head space gas was sampled and injected into a gas chromatograph (model: Clarus 500, Perkin Elmer Inc, USA) equipped with a stainless steel column, 30 m × 0.530 mm (Porapak R 80/100), and a thermal conductivity detector (TCD). Helium was used as a carrier gas at a flow rate of 20 ml min−1 and temperature of the oven, injector and TCD was set at 60, 100 and 200 °C, respectively. One millilitre of CO2 gas standard (Scotty Gases, USA) was used for calibration. The CO2 production or respiration was expressed as millilitre per kilogramme per hour. Fruit respiration rate was measured every 3 days and the same fruit was used throughout the experiment. Ten sets of fruit were used per replication.
Fruit C2H4 production was quantified by injecting 1 ml of the head space gas into a gas chromatograph (model: Clarus 500, Perkin Elmer Inc, USA) equipped with a stainless steel column, 30 m × 0.530 mm (Porapak T, 100/120), and a flame ionization detector (FID). Nitrogen was used as a carrier gas and temperature of the oven, injector and FID was set at 150, 200 and 200 °C, respectively. One milliltre of C2H4 gas standard (Scotty Gases, USA) was used for calibration. The ethylene production was expressed as microlitre per kilogramme per hour. Fruit respiration rate was measured every 3 days and the same fruit was used throughout the experiment. Ten sets of fruit were used per replication.
Determination of Ascorbic Acid Content
Ascorbic acid concentration was determined according to the method of Ranggana (1986). For each fruit, 10 g of fruit tissue from the equatorial region was homogenized with 90 ml of 3 % HPO3 + 8 % glacial acetic acid, filtered using Whatman filter paper (pore size 11 μm) and 100 ml of filtrate were titrated with 2,6-dichlorophenol indophenol (DCPIP) dye (containing 2.49 mM NaHCO3) until pink colour persisted for 15 s. Sampling was done every 3 days and 20 fruits were used per replication. Standardization of DCPIP dye was determined prior to analysis by titrating 1 g l−1 ascorbic acid (dissolved in 3 % HCl) against the prepared DCPIP dye to obtain the dye factor (dye factor = 0.5 per titre value). Ascorbic acid content was calculated using the following equation and expressed in milligrammes of ascorbic acid in 100 g of fruit sample.
Sensory Evaluation
Sensory evaluation of bell pepper was performed at day 19 of storage using a hedonic scale rating (Whangchai et al. 2006). Fruit tissues (5 cm × 5 cm) from the equatorial region were sampled and coded in random numbers, and 20 panelists were asked to evaluate the criteria by allotting values; 1-extreme dislike, 3-dislike, 5-acceptable, 7-good and 9-excellent. The samples were rated based on appearance, colour, texture, aroma, flavour and overall acceptability.
Determination of Disease Incidence
Anthracnose incidence on bell pepper was evaluated by inoculating 50 μl of 1 × 105 spore ml−1 Colletotrichum capsici spore suspension onto a 5.0-mm wound. The wound was artificially prepared using a cork borer at the equatorial area of bell pepper. The spore suspension was prepared by agitating 8-day-old C. capsici culture with sterile distilled water, and detached fungal hyphae fragments were removed by filtering the suspension through a double layer of cheese cloth (average pore size ≈ 150 μm). Concentration of the spore suspension was estimated using a 0.0025-mm2 Neubauer Improved haemocytometer (Hirschmann EM Techcolor, Germany) and adjusted to 1 × 105 spores ml−1. Inoculated fruits were subjected to ozone at concentrations of 0 (control), 1, 3, 5, 7 and 9 ppm for 3 days at 12 °C and 95 % RH and moved to identical control chambers (0 ppm ozone) for an additional 21 days at 12 °C and 95 % RH. Twenty fruits were used per replicate. Percentage of disease incidence at the end of storage period was calculated as below:
Statistical Analysis
The experiments were carried out with a completely randomized design (CRD) with three replicates per treatment. Replicates were placed in different chambers. Same fruit was used for colour, weight loss, respiration and ethylene production rate experiments, while another set of fruit was used for destructive experiment such as firmness, membrane permeability, TA, SSC and chlorophyll and ascorbic acid contents. The experiment was repeated thrice and data was analysed separately to check for homogeneity. The data were then merged during analysis using statistical analysis software (SAS, version 9.1.3, SAS Institute Inc., USA). Analysis of variance (ANOVA) was performed with P < 0.05 significance level, and differences in data means were analysed using Duncan’s multiple range test (DMRT). Data are presented with standard errors generated using SAS.
Results and Discussion
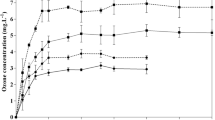
Treatment of green pepper fruit with 1 and 3 ppm ozone had no effect (P > 0.05) on fruit colour (Table 1), firmness (Fig. 1), TA (Fig. 2) and respiration (Fig. 3). Similarly to control, epidermis colour of the fruit gradually changed from green to yellow and then reddish during storage. In addition, firmness, titratable acidity and respiration of the fruit also decreased at the same rate with the control during storage. The reduction in these attributes is a progression of ripening as observed in other studies (Jin-Hua et al. 2007; Lim et al. 2007). This shows that the treated fruit had similar ripening progression with the control fruit, and quality of the fruit was maintained comparable to the control.
Exposure to 1 and 3 ppm ozone also had no effect on fruit membrane permeability (P > 0.05). This shows that the oxidative stress of ozone had no effect on membrane integrity or lipid peroxidation. This indicates a balanced oxidative status in the cell which could be due to activation of fruit defence system to neutralize the oxidative activity of ozone and its induced reactive oxygen species (ROS) such as ·OH, ·OOH and H2O2, hence maintain redox status in the cells. This is evident by the significant increase in ascorbic acid concentration in fruit treated with 1 (12.1 %) and 3 (25.6 %) ppm ozone (Fig. 4), immediately after exposure. The stimulating effect of oxidative stress on ascorbic acid level was also observed in pepper treated with 15 mM of H2O2 (Bayoumi 2008). This could be due to activation of ROS scavenging genes as a fruit’s second line of defence (Asghari & Aghdam 2010). This involves reduction of monodehydroascorbate (MDA) and dehydroascorbate (DHA) through ascorbate-glutathione cycle (Halliwell-Asada pathway) which involves enzymes such as monodehydroascorbate reductase (MDAR) and dehydroascorbate reductase (DHAR) (Forney 2003; Apel & Hirt 2004). This increases the level of reduced ascorbate to neutralize oxidative action of ozone and its induced reactive compounds, hence maintained the cell redox potential and fruit ripening progress. This also increases ascorbic acid content in the fruit which increases value of the fruit in terms of vitamin content.
Exposure to high concentrations of ozone (7 and 9 ppm) significantly affected fruit physiology, as seen from the significant increase in respiration rate following ozone exposure (Fig. 3). The increase was, however, transient where no significant difference (P >0.05) was observed in CO2 production during storage. A similar transient increase in respiration rate was observed in tomatoes exposed to 10 ppm ozone (Rodoni et al. 2010) and carrot exposed to 1 ppm ozone in combination with 1-MCP (Forney et al. 2007). The transient increase was in response to oxidative stress of ozone and its induced free radicals during ozone treatment (Tiwari et al. 2002). The increase in fruit respiration was also associated with a significant decrease in TA (Fig. 2) on fruit exposed to high ozone concentration. This could be a result of greater utilization of organic acids as a respiratory substrate (Kays 1999) and/or be a result of ozone-enhanced ripening.
Exposure to 7 and 9 ppm also resulted in significant reduction in ascorbic acid level (Fig. 4) which may be a result of high oxidative stress from possible ozone-induced generation of ROS that could perturb the antioxidant-oxidative stress equilibrium (Jin-Hua et al. 2007). This could be a toxicity symptom of ozone on bell pepper. This negative effect of ozone was also observed on fresh-cut pineapple and guava where their ascorbic acid contents were reduced by 46.44 and 67.13 %, respectively, after a reaction with 0.76 ppm ozone (Alothman et al. 2010). High oxidative stress of the ozone dosage perturbed the equilibrium of ascorbate-glutathione cycle where oxidized form of ascorbate exceeded the reduced form (Apel & Hirt 2004). This reduced ascorbic acid content and resulted in its depletion.
The low level of ascorbic acid in fruit may act as a pro-oxidant where it produced H2O2 in a reaction with oxygen (Fry 1998). The H2O2 then produced hydroxyl radical (OH·) from its reaction with traces of metal compounds such as Fe2+ and Cu2+ through Haber-Weiss reaction (Gaetke & Chow 2003; Sakihama et al. 2002; Apel & Hirt 2004). Hydroxyl radical is a highly reactive radical, and its accumulation increased fruit oxidative stress and led to subsequent oxidative chain reactions such as lipid and DNA degradation (Sakihama et al. 2002; Apel & Hirt 2004).
The high oxidative stress in fruit treated with 7 and 9 ppm ozone is evident from the increase in cell electrolyte leakage (Fig. 1). This could be a result from oxidative action of oxidant from ozone exposure as well as Haber-Weiss reaction which led to lipid peroxidation and cell damage. This reduced cell membrane integrity and increased moisture loss. This contributed to increase fruit weight loss and reduction in fruit firmness (Fig. 1). Relation between cell membrane integrity and fruit weight loss was also observed by Maalekuu et al. (2006).
The high oxidative stress also enhanced ripening as indicated by the increased rate of titratable acidity loss and change in colour (L*, C* and h° values) (Table 1). Colour lightness, chroma and hue angle of fruit treated with high ozone concentrations were significantly different from that of the control fruit at the end of the storage period, reflecting an increase in yellow and reddish hues of the treated fruit. The increase in colour change could also be due to oxidation of chlorophyll molecules by ozone or ozone-induced ROS. This is supported by the significant reduction in chlorophyll content of fruit exposed to high ozone concentration (Fig. 2). Similar oxidation effect of ozone was observed on bell pepper treated with 300 mW cm2 UV-C for more than 5 min (Sakaldaş & Kaynaş 2010). This could be due to the oxidizing effect of free radicals such as superoxide radical (O2 −) which was reported to involve in chlorophyll a degradation (Sakaki et al. 1983; Jin-Hua et al. 2007).
The enhanced colour change observed could be a phytotoxic symptom of ozone on bell pepper. This symptom differs from phytotoxic symptoms observed on papaya (Ong et al. 2013) and carrot (Hildebrand et al. 2008) which resulted in epidermis browning after exposure to 4 ppm ozone for 6 days and 0.05 ppm ozone for 2 months, respectively. The epidermis browning could be a result from necrosis of cells near to lenticels which are the main entry of ozone into the cells (Forney 2003). In contrast to bell pepper with less lenticels (Torlak et al. 2013), ozone has no selective entry into the cells; hence, oxidation activity occurred evenly on the epidermis and resulted in the enhanced colour change. Besides, epidermis of bell pepper is covered by a surface cuticle layer (Maalekuu et al. 2006). The cuticle layer could be oxidized by ozone. However, it could also act as a physical barrier against oxidation activity which reduced susceptibility of bell pepper to ozone, compared to other commodity without cuticle layer. Besides, in comparison to other commodity, the cuticle layer may also reduce the effect of ozone on other attributes such as weight loss and respiration which correlated with firmness and titratable acidity of the fruit.
Exposure to ozone had no effect on fruit SSC (Fig. 2). An increase in SSC was observed during ripening due to the biosynthesis of sugars, but the changes were comparable to the control. The application of other oxidizing agent was also reported to have no effect on bell pepper SSC as observed from application of 50 ppm ClO2 solution on bell pepper (cv. Longrum) (Jin-Hua et al. 2007) for 40 days and application of 14 kJ m−2 UV-C on bell pepper (cv. Zafiro) (Vicente et al. 2005). No significant effect was also reported on SSC of white pepper treated with 15 mM H2O2 for 30 min (Bayoumi 2008) and mango exposed to 2.2 ppm ozone for 45 min (Barbosa-Martinez et al. 2002). These results suggested that the oxidative agents did not affect synthesis of polysaccharides in bell pepper.
Exposure to ozone also had no effect on fruit ethylene production. Ethylene production in treated fruit was comparable to the control even though a slight increase was observed in fruit exposed to 7 and 9 ppm ozone, immediately after treatment. This suggests that ozone treatments have no significant effect on the fruit ethylene production, possibly due to the non-climacteric nature of bell pepper and its low ability to produce ethylene. This finding is similar to the effect of ozone on peaches as a slight increase in ethylene production was observed after exposure to 0.3 ppm ozone for 3 weeks (Palou et al. 2002). The increase is comparable to the control and transient (Palou et al. 2002).
Fruit sensory quality was not affected by the ozone treatments as panelists had no preference between treated and control fruit in terms of appearance, colour, texture, aroma and overall acceptability (Table 2). Fruit treated with 3 ppm ozone, however, received the highest rating for flavour implying that the treatment may have improved fruit flavour. This fruit flavour was preferred by the panelists compared to 9 ppm treated fruit, which received the lowest rating for flavour as well as all other attributes. The 9 ppm treated fruit may have been preferred less by the panelists due to the enhanced ripening which produced a partially coloured fruit (Fox et al. 2005) with a soft texture (Yuen & Hoffman 1993).
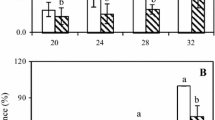
Treatment with 1 and 3 ppm ozone which maintained fruit physico-chemical quality similar to control also reduced anthracnose incidence (Fig. 5). This shows the ozone dosage inhibited fungal development without jeopardizing fruit physical and chemical qualities. Lower disease incidence was observed on fruit exposed to 7 ppm ozone. This could be due to stronger antifungal effect of ozone on spore germination (Barbosa-Martinez et al. 2002) and/or mycelia growth (Antony-Babu & Singleton 2009). The ozone dosage, however, enhanced fruit ripening as indicated by enhanced colour change and reduction in firmness and titratable acidity. Exposure to a higher ozone dosage, 9 ppm ozone showed higher progress of fruit ripening which resulted in fruit with softer a texture and higher sugar content. This favoured fungal development, hence resulted in higher disease incidence compared to 7 ppm ozone, regardless higher antifungal effect was expected. Similar situation was reported in strawberry treated with high UV-C dose (4.0 kJ m−2) which has a higher disease development compared to strawberry exposed to lower dose of UV-C (0.5 kJ m−2) (Nigro et al. 2000).
The potential of ozone treatment demonstrated in this study provides an option to replace hazardous fungicides as a postharvest treatment. The ozone generator used in this study can be switched off prior to chamber opening. This prevents direct contact with ozone, hence minimizes its risk. Besides, the chamber was designed to be airtight to prevent ozone contamination in the atmosphere.
Conclusion
This study has shown that application of ozone influenced ascorbic acid content in bell pepper which affects their quality. Application of 1 and 3 ppm ozone enhanced fruit ascorbic acid content which improved fruit nutritive quality and maintained fruit quality similar to the control. The treatment also reduced anthracnose incidence on bell pepper, hence reduced fruit loss during storage. Meanwhile, exposure to 7 and 9 ppm ozone showed a degradation in fruit quality due to the excess of oxidative stress. By considering the effect of ozone on fruit physico-chemical quality as well as on disease inhibition, treatment of 3 ppm ozone is recommended for postharvest treatment of bell pepper during cold storage. The treatment exhibited dual action on fruit quality which increased fruit ascorbic acid content as well as reduced fungal development. Therefore, this shows the potential of ozone to be used as postharvest treatment of bell pepper, hence can be an option to replace hazardous fungicides.
References
Alothman, M., Kaur, B., Fazilah, A., Bhat, R., & Karim, A. A. (2010). Ozone-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innovative Food Science and Emerging Technologies, 11, 666–671.
Antony-Babu, S., & Singleton, I. (2009). Effect of ozone of spore germination, spore production and biomass production in two Aspergillus species. Antonie van Leeuwenhoek, 96, 413–422.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55(1), 373–399.
Asghari, M., & Aghdam, M. S. (2010). Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends in Food Science & Technology, 21(10), 502–509.
Barbosa-Martinez, C., Ponce de Leon-Garcia, L., & Sepulveda-Sanchez, J. (2002). Effects of ozone, iodine and chlorine of spore germination of fungi isolated from mango fruits. Mexical Journal of Phytopatology, 20(1), 60.
Bayoumi, Y. A. (2008). Improvement of postharvest keeping quality of white pepper fruits (Capsicum annuum, L.) by hydrogen peroxide treatment under storage conditions. Acta Biologica Szegediensis, 52(1), 1–15.
Bosland, P. W. & Votava, E. J. (2000). Peppers: vegetable and spice capsicums. Wallingford,Oxfordshire, CABI.
Castro, S. M., Saraiva, J. A., Domingues, F. M. J., & Delgadillo, I. (2011). Effect of mild pressure treatments and thermal blanching on yellow bell peppers (Capsicum annuum L.). LWT - Food Science and Technology, 44(2), 363–369.
Chauhan, O. P., Raju, P. S., Ravi, N., Singh, A., & Bawa, A. S. (2011). Effectiveness of ozone in combination with controlled atmosphere on quality characteristics including lignification of carrot sticks. Journal of Food Engineering, 102(1), 43–48.
FAOSTAT (2012). Food and Agriculture Organization of the United Nations. Available at http://faostat3.fao.org/faostat-gateway/go/to/home/E. Accessed 29 October 2013.
Food and Drug Administration (2001). Secondary direct food additives permitted in food for human consumption. Federal Register, 66(123), 33830.
Forney, C. F. (2003). Postharvest response of horticultural products of ozone. In D. M. Hodges (Ed.), Postharvest oxidative stress in horticultural crops (pp. 13–54). New York: Food Products Press.
Forney, C. F., Song, J., Hildebrand, P. D., Fan, L., & McRae, K. B. (2007). Interactive effects of ozone and 1-Methylcyclopropene on decay resistance and quality of stored carrots. Postharvest Biology and Technology, 45, 341–348.
Fox, A. J., Del Pozo-Insfran, D., Lee, J. H., Sargent, S. A., & Talcott, S. T. (2005). Ripening-induced chemical and antioxidant changes in bell peppers as affected by harvest maturity and postharvest ethylene exposure. HortScience, 40(3), 732–736.
Fry, S. C. (1998). Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochemical Journal, 332, 507–515.
Gabler, F. M., Smilanick, J. L., Mansour, M. F., & Karaca, H. (2010). Influence of fumigation with high concentrations of ozone gas on postharvest gray mold and fungicide residues on table grapes. Postharvest Biology and Technology, 55(2), 85–90.
Gaetke, L. M., & Chow, C. K. (2003). Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology, 189(1–2), 147–163.
Hildebrand, P. D., Forney, C. F., Song, J., Fan, L., & McRae, K. B. (2008). Effect of a continuous low ozone exposure (50 nL L−1) on decay and quality of stored carrots. Postharvest Biology and Technology, 49(3), 397–402.
Jin-Hua, D. U., Mao-run, F. U., Miao-miao, L. I., & Wei, X. I. A. (2007). Effects of chlorine dioxide gas on postharvest physiology and storage quality of green bell pepper (Capsicum frutescens L. var. Longrum). Agricultural Sciences in China, 6(2), 214–219.
Kays, S. J. (1999). Preharvest factors affecting appearance. Postharvest Biology and Technology, 15(3), 233–247.
Khadre, M. A., Yousef, A. E., & Kim, J. G. (2001). Microbiological aspects of ozone applications in food: a review. Journal of Food Science, 66(9), 1242–1252.
Lewis Ivey, M. L., Nava-Diaz, C., & Miller, S. A. (2004). Identification and management of Colletotrichum acutatum on immature bell peppers. Plant Disease, 88(11), 1198–1204.
Lim, C. S., Kang, S. M., Cho, J. L., Gross, K. C., & Woolf, A. B. (2007). Bell pepper (Capsicum annuum L.) fruits are susceptible to chilling injury at the breaker stage of ripeness. HortScience, 42(7), 1659–1664.
Maalekuu, K., Elkind, Y., Leikin-Frenkel, A., Lurie, S., & Fallik, E. (2006). The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biology and Technology, 42(3), 248–255.
Mahasuk, P., Khumpeng, N., Wasee, S., Taylor, P. W. J., & Mongkolporn, O. (2009). Inheritance of resistance to anthracnose (Colletotrichum capsici) at seedling and fruiting stages in chili pepper (Capsicum spp.). Plant Breeding, 128(6), 701–706.
Minas, I. S., Karaoglanidis, G. S., Manganaris, G. A., & Vasilakakis, M. (2010). Effect of ozone application during cold storage of kiwifruit on the development of stem-end rot caused by Botrytis cinerea. Postharvest Biology and Technology, 58, 203–210.
Nigro, F., Ippolito, A., Lattanzio, V., Venere, D. D., & Salerno, M. (2000). Effect of ultraviolet-C light on postharvest decay of strawberry. Journal of Plant Pathology, 82(1), 29–37.
Ong, M. K., Kazi, F. K., Forney, C. F., & Ali, A. (2013). Effect of gaseous ozone on papaya anthracnose. Food and Bioprocess Technology, 6(11), 2996–3005.
Palou, L., Crisosto, C. H., Smilanick, J. L., Adaskaveg, J. E., & Zoffoli, J. P. (2002). Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biology and Technology, 24, 39–48.
Palou, L., Smilanick, J. L., Crisosto, C. H., Mansour, M., & Plaza, P. (2003). Ozone gas penetration and control of the sporulation of Penicillium digitatum and Penicillium italicum within commercial packages of oranges during cold storage. Crop Protection, 22(9), 1131–1134.
Ranggana, S. (1986). Handbook of analysis and quality control for fruit and vegetable products. New Delhi: McGraw-Hill.
Rodoni, L., Casadei, N., Concellon, A., Alicia, A. R. C., & Vicente, A. R. (2010). Effect of short-term ozone treatments on tomato (Solanum lycopersicum L.) fruit quality and cell wall degradation. Journal of Agricultural and Food Chemistry, 58, 594–599.
Sakaki, T., Kondo, N., & Sugahara, K. (1983). Breakdown of photosynthetic pigments and lipids in spinach leaves with ozone fumigation: role of active oxygens. Physiologia Plantarum, 59(1), 28–34.
Sakaldaş, M., & Kaynaş, K. (2010). Biochemical and quality parameters changes of green sweet bell peppers as affected by different postharvest treatments. African Journal of Biotechnology, 9(48), 8174–8181.
Sakihama, Y., Cohen, M. F., Grace, S. C., & Yamasaki, H. (2002). Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology, 177(1), 67–80.
Tiwari, B. S., Belenghi, B., & Levine, A. (2002). Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiology, 128(4), 1271–1281.
Torlak, E., Sert, D., & Ulca, P. (2013). Efficacy of gaseous ozone against Salmonella and microbial population on dried oregano. International Journal of Food Microbiology, 165(3), 276–280.
Vicente, A. R., Pineda, C., Lemoine, L., Civello, P. M., Martinez, G. A., & Chaves, A. R. (2005). UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biology and Technology, 35(1), 69–78.
Whangchai, K., Saengnil, K., & Uthaibutra, J. (2006). Effect of ozone in combination with some organic acids on the control of postharvest decay and pericarp browning of longan fruit. Crop Protection, 25(8), 821–825.
Xing, Y., Li, X., Xu, Q., Yun, J., Lu, Y., & Tang, Y. (2011). Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annum L.). Food Chemistry, 124, 1443–1450.
Yuen, C. M. C., & Hoffman, H. (1993). New capsicum varieties: storage suitability and consumer preference. Food Australia, 45(4), 184–187.
Acknowledgments
This study was supported by MedKlinn International Sdn. Bhd. and the School of Biosciences, The University of Nottingham Malaysia Campus.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alwi, N.A., Ali, A. Dose-dependent Effect of Ozone Fumigation on Physiological Characteristics, Ascorbic Acid Content and Disease Development on Bell Pepper (Capsicum annuum L.) During Storage. Food Bioprocess Technol 8, 558–566 (2015). https://doi.org/10.1007/s11947-014-1419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-014-1419-2