Abstract
Anthracnose caused by the fungus Colletotrichum gloeosporioides is considered as one of the most devastating postharvest disease of papaya. The aim of this study was to evaluate the effectiveness of gaseous ozone as a potential antifungal preservation technique to overcome anthracnose disease of papaya during cold storage. Different concentrations of ozone (0 (control), 0.04, 1.6, and 4 ppm) were applied for various exposure durations (48, 96, and 144 h). Radial mycelia growth and conidial germination were evaluated in vitro after fungal exposure to the different levels and durations of ozone. Significant inhibition in radial mycelia growth of C. gloeosporioides was observed (p < 0.05) in all ozone treatments as compared to the control during 8 days of incubation at room temperature (25 ± 3 °C). Ozone treatment of papaya fruit with 1.6-ppm ozone for 96 h delayed and simultaneously decreased the disease incidence to 40 % whereas disease severity was rated at 1.7, following 28 days of storage at 12 ± 1 °C and 80 % relative humidity. The scanning electron microscopy showed that 4-ppm ozone caused disintegration of spore structure and did not affect the cuticular surface of fruit. Thus, ozone fumigation can reduce postharvest losses of papaya caused by anthracnose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papaya (Carica papaya L.) is a tropical fruit crop and is considered to be an important source of vitamins A and C. Papaya like many climacteric fruits undergoes a variety of physical and chemical changes after harvest (Shiota 1991). Climateric fruits exhibit a characteristic rise in ethylene production and respiration during ripening (Abeles et al. 1992). Softening and senescence associated with postharvest ripening makes these fruits vulnerable to several postharvest diseases.
A postharvest disease during storage and transportation significantly lowers the quality and value of this commodity. The incidence of postharvest diseases, especially anthracnose caused by Colletotrichum gloeosporioides, becomes a problem when fruits have 25 % or more skin yellowing (Alvarez and Nishijima 1987). This has been the main concern for fruit growers, distributors, and exporters in extending the storage life of papaya fruit while keeping the fruits at an acceptable quality.
Currently, there are several postharvest technologies adopted to control anthracnose in papaya that include the usage of prochloraz or propiconazole (Sepiah 1993), hot water treatment at 43–49 °C for 20 min (Couey et al. 1984), heat treatment in combination with fungicides (Couey and Farias 1979), edible coatings (Ali et al. 2010), and use of gamma rays in combination with UV-C irradiation (Cia et al. 2007). Synthetic fungicides and chemical agents particularly chlorine has been used in fresh produce, to disinfect the fruit surface. Nevertheless, studies show that chlorine and fungicides have limited ability to kill bacteria on fruit and vegetable surfaces (Sapers 1998), as the bacteria and fungus evolve to become more resistant. Environmental organizations have also expressed concern about the residual by-products of chlorine, such as trihalomethanes and other persistent chemical residues in wastewater (Graham et al. 1997). In addition, social concerns regarding the use of pesticides on fruit limits their use for disease control.
One approach to improve safety, quality, and shelf life is to identify alternative postharvest treatments that are safer, non-toxic, biodegradable, non-residual, and are highly effective as microbial agents while retaining the fruit’s nutritional value. Research and commercial applications have indicated that ozone has the potential to replace chlorine and fungicides. Ozone is a strong oxidant effective in controlling bacteria, molds, protozoa, and viruses (Kim et al. 2003). In 1997, ozone was granted “Generally Recognized as Safe (GRAS)” status (US-FDA 1997) and has since received full US-FDA approval (Kim et al. 1999). More recently, there has been interest in the evaluation of ozone treatments for processing and storage of fruits and vegetables (Palou et al. 2002; Perez et al. 1999). This gas was shown to have the potential for use as an antimicrobial agent to curb spoilage of fruits and vegetables in cold storage (Liew and Prange 1994; Barth et al. 1995; Sarig et al. 1996; Perez et al. 1999; Palou et al. 2002; Aguayo et al. 2006; Tzortzakis et al. 2007) and preserve ethylene-sensitive commodities (Skog and Chu 2001).
Most recent publication on effect of gaseous and aqueous ozone in the postharvest of papaya cv. Maradol-red has shown positive control of fungal pathogens growth such as Curvularia spp., Fusarium spp., Aspergillus spp., Sclerotium rolfsii, Phoma spp., Gliocladium spp., and Rhizoctonia solana (Bataller et al. 2012). To the best of our knowledge, in spite of its growing importance, there is no published work on the potential of ozone fumigation against anthracnose disease of papaya. Therefore, the present study was designed to investigate the efficacy of gaseous ozone on in vitro growth of C. gloeosporioides as well as in vivo control of papaya anthracnose.
Materials and Methods
Pathogen, Plant Materials, and Ozone Fumigation System
Papaya fruit showing typical anthracnose symptoms or lesions of rot were examined under an optical microscope. Small pieces of tissue were excised from the border of an actively growing lesion and surfaces were sanitized with 1.0 % sodium hypochlorite (NaOCl) for 2–3 min, followed by washing in two changes of sterile distilled water. The tissues were dried on a sterilized filter paper, plated on potato dextrose agar (PDA) (Difco Brand, USA), and incubated at 25 ± 2 °C for 8 days. Once mycelial growth was observed, the colonies were reisolated on fresh PDA plates to obtain pure cultures. The isolates were identified based on their morphological and cultural characters (Barnett and Hunter 1972). Cultures identified as C. gloeosporioides were maintained on PDA slants for further use.
Mature green papaya fruit with color index 2 (green with trace of yellow) were obtained on the day of harvest from the local exporter, Malaysian Agrifood Corporation Berhad, Puchong, Selangor, Malaysia. Fruit of the cultivar “Golden Frangi” of uniform size (400–500 g), shape, maturity, and free from any mechanical injury or insect or pathogen infection were selected for the experiment. Fruits were washed with distilled water and air-dried at ambient temperature (25–28 °C) before ozone treatment with or without inoculation.
The fumigation system, comprised of a chamber constructed from 5-mm-thick polycarbonate (112.0 cm length × 47.5 cm width × 42.5 cm height) equipped with four 12-V fans positioned directly below the sample platform to ensure a well-mixed atmosphere, was established in the postharvest laboratory, School of Biosciences, University of Nottingham, Malaysia campus (Fig. 1). Ozone was introduced to the chamber by an ozone generator (Model MedKlinn Professional Series, MedKlinn International Sdn. Bhd., Malaysia) and the ozone concentration was controlled automatically by on OEM-2 sensor (Eco-Sensor, MedKlinn International Sdn. Bhd., Malaysia ). The ozone concentration in the chamber was recorded via the ozone analyzer (Model IN-2000L2-LC, IN USA Incorporated, USA). The chamber was maintained at 25 °C and 70 % relative humidity (RH) in a controlled environment laboratory with the aid of a portable dehumidifier (Edenaire, Model ED 50106S, Enmark (M) Sdn. Bhd., Malaysia), 24-h air conditioned, and monitored with the aid of temperature/humidity sensors (Model U14-001, HOBO LCD Data Logger, Onset Computer Corporation, USA).
In Vitro Antifungal Ozone Assay Against C. gloeosporioides
Antifungal Assay
The in vitro antifungal activity of ozone was determined based on inhibition of radial mycelia growth and conidia germination of C. gloeosporioides using the poisoned food technique (Kiran, et al. 2010). Mycelia plugs (5 mm) obtained from 8-day-old cultures of C. gloeosporioides were transferred to the center of petri dishes containing 20 ml of PDA. Subsequently, the lids were left opened under aseptic conditions in order to allow exposure to ozone-enriched atmospheres with a concentration of 0, 0.04, 1.6, or 4 ppm for exposure durations of 48, 96, or 144 h. A replicate of 40 petri dishes were placed in a single layer of the chamber. Each chamber was prepared as a replicate with combination of concentration and period of exposure. There are four replications for each ozone treatment.
Radial Mycelia Growth
Daily radial measurements of mycelia growth were recorded until the fungus reached the edge of the control plate. The percentage inhibition in radial growth was recorded after 8 days of incubation according to the formula described by Sivakumar et al. (2002).
where,
- R1:
-
Radial growth in control
- R2:
-
Radial growth in treatment
Conidial Germination Test
The in vitro conidial germination inhibition test was carried out using the cavity slide technique (Cronin et al. 1996). An aliquot of 10 μL of a freshly harvested conidial suspension of C. gloeosporioides (adjusted to 105 conidia ml−1 using a hemocytometer) was pipetted into the cavity and exposed to ozone concentrations of 0.04, 1.6, or 4 ppm for 24 h in the dark. Cavity slides without exposure to ozone served as controls. After 24-h incubation, the conidia were inactivated by adding 10 μl 2 % sodium azide to each cavity. Approximately 100 conidia were observed for germination in each treatment. A conidium was said to be germinated when its germ tube was half the length of the conidium. The percentage inhibition in germination was calculated:
where,
- Gr:
-
Number of spore germination in the treatment
- Gc:
-
Number of spore germination in the control
In Vivo Antifungal Ozone Assay Against C. Gloeosporioides
Pathogen Inoculum Preparation for In Vivo Bioassay
Pathogen inoculum was prepared by pouring 10-ml sterilized distilled water onto a 2-week-old culture of C. gloeosporioides. The conidia were dislodged from the surface with a sterile bent glass rod. The conidial suspension obtained was filtered through a double layer of sterilized muslin cloth. The conidial counts were adjusted to 105 conidia ml−1 using a hemocytometer (Sivakumar et al. 2002). Surface-sterilized papaya fruit were inoculated artificially by dipping in a liter of conidial suspension of C. gloeosporioides with 0.2 % (v/v) Tween-80 for 1 min. The fruit were then allowed to air dry for 1 h, followed by ozone treatments according to the various concentrations and periods of exposure. A replicate of 30 fruits were placed in a single layer of the chamber. Each chamber was prepared as a replicate with combination of concentration and period of exposure. Fruits were removed from a single chamber after 48, 96, and 144 h. There are four replications for each ozone treatment.
Disease Incidence
For each treatment, 40 fruits were packed in four corrugated cardboard boxes (34.0 cm length × 30.0 cm width × 16.5 cm height) and stored at 12 ± 1 °C and 80–85 % RH for 28 days. Disease incidence (DI) was recorded based on the anthracnose symptoms on fruit surfaces. The effect of ozone on disease incidence and severity for 40 fruits from each treatment were evaluated at 7-day intervals for 28 days during cold storage. Disease incidence was expressed as the number of fruit showing anthracnose out of the total number of fruits in each treatment (Sivakumar et al. 2002).
Disease Severity
Disease severity was scored at 7-day intervals as per the method of Sivakumar et al. (2002) with some modification. A rating of 1 was scored when there was no sign of anthracnose disease on the fruit surface. A rating of 2 was scored when up to a quarter (1–25 %) of the fruit surface showed anthracnose symptom. If 26 to 50 % of the fruit surface exhibited anthracnose symptom, a rating of 3 was scored. If anthracnose symptom appeared on 51 to 75 % of the fruit surface, then a rating of 4 was scored. A rating of 5 was recorded when more than 76 % of the fruit surface was infected with anthracnose disease.
Scanning Electron Microscopy
SEM on Spore Structure
Four spore samples from each treatment were stained directly on the glass slides in preparation to be scanned using an electron microscope (Model Quanta 400 FESEM, FEI Company, USA). The spores were mounted on aluminum stubs and viewed and photographed under the scanning electron microscope. Any changes in spore structure and shape were visualized. The results obtained were compared with the spores that did not receive any treatment that were designated as “control samples.”
SEM on Fruit Surfaces
Images of four fruit from each treatment were viewed under the scanning electron microscope. Samples of 5 mm2 of papaya surfaces were taken from the equatorial section of each fruit (16 samples in total). The samples were mounted on aluminum stubs and viewed and photographed under the scanning electron microscope. Changes to the cuticular surface of papayas were visualized.
Statistical Analysis
Treatments were arranged in a randomized complete block design. Experimental data were analyzed using analysis of variance procedure in SAS 9.1 software and means were separated using Duncan’s Mutiple Range Test (at p < 0.05). All percentage data were arcsine transformed before analysis. There were four replications per treatment. The entire experiment was repeated twice, and data were pooled before analysis.
Results
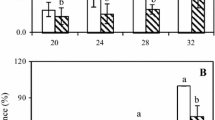
Mycelia growth of C. gloeosporioides was significantly (p < 0.05) affected by all ozone treatments as compared to the control following the 8-day incubation period. In vitro inhibition was significantly related to ozone concentration and exposure time. However, after 144 h of ozone exposure, ozone concentrations do not appear significantly different because 0.04-ppm treatment has a similar inhibition of mycelia growth as the 4-ppm treatment. Maximum inhibition of 91.6 % in mycelia growth was observed at 144 h with 4-ppm ozone exposure followed by 87.5 and 87.2 % inhibition of mycelia growth when treated with 0.04 and 1.6 ppm, respectively (Fig. 2). Mycelia plugs that were exposed for 96 h to ozone at concentrations of 0.04 ppm (36.3 % inhibition) and 1.6 ppm (67.1 % inhibition) significantly (p < 0.05) suppressed the mycelia growth compared to the control, but these were not as effective as the 4 ppm (89.4 % inhibition) treatment (Fig. 2). Continuous exposure of 4-ppm ozone for 144 h proved to be the best treatment that inhibited the mycelia growth by up to 91.6 %. This was followed by the 4-ppm ozone treatment for 96 h and 4-ppm ozone treatment for 48 h, which inhibited mycelia growth by 89.4 and 63.2 %, respectively. Moreover, the 0.04 and 1.6 ppm for 144-h ozone treatments had greater inhibition than the 4 ppm for 48-h treatment. Growth in the control plates was almost 12 times higher than in the 4-ppm treatment at the end of the incubation period for 144 h. There was no effect of ozone on mycelia growth when plates were exposed to the gas for less than 24 h (data not presented), while longer exposure of 48 h or more inhibited mycelia growth (Fig. 2).
The test on inhibition of conidial germination was also carried out to confirm the efficacy of ozone treatments. The ozone treatments significantly (p < 0.05) inhibited conidia germination as compared to the control after 24 h. All ozone treatments at 0.04, 1.6, and 4 ppm totally inhibited conidia germination by 100 %. However, if the spores were incubated following the ozone treatments, they were able to germinate and the ozone treatment was merely inhibitory.
As shown in Fig. 3b, there was a significant (p < 0.05) delay in anthracnose disease development in both non-inoculated and artificially inoculated papaya fruit, after 96 h of ozone exposure during the 28 days of storage (12 °C, 80 % RH). The disease incidence and severity in the controls increased gradually from week to week and reached 100 % (scale 4.1 and 4.4 in non-inoculated and inoculated fruit, respectively), after 28 days of storage. The symptoms were visible as early as the first week of storage and most papayas were spoiled due to severe disease infection. Different concentrations of ozone and exposure durations not only delayed the onset of anthracnose disease but also maintained the freshness of papaya during the first 2 weeks of storage and later showed minimal symptom of anthracnose. The highest fungistatic effect was observed in artificially inoculated papayas treated with 1.6-ppm ozone for 96 h. In non-inoculated fruits, disease incidence of 40 % and disease severity scale of 1.7 was observed after 28 days of storage when treated with 1.6-ppm ozone for 96 h. Meanwhile, the lowest disease severity scale of 2.4 in inoculated fruits was also obtained with 96 h of exposure to 1.6-ppm ozone, after 28 days of incubation at 12 °C and 80 % RH (Table 1).
In the present study, fruit exposed to 0.04 and 1.6-ppm ozone for up to 144 h showed no phytotoxic effects. Some surface browning appeared on papaya treated with 4-ppm ozone continuously for 144 h (after 7 days of storage at 12 ± 1 °C). No surface oxidation effects were observed on fruit exposed to 4 ppm continuously for less than 96 h.
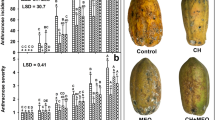
Scanning electron micrographs illustrate a more detailed picture of the inactivation of fungal spores subjected to ozone exposure. The scanning electron microscopy (SEM) images of the C. gloeosporioides spores exposed to ozone fumigation reveal that 4-ppm ozone exposure (Fig. 4d) altered the spore structure, compared to treatment with 0.04-ppm (Fig. 4b) and 1.6-ppm ozone (Fig. 4c). No apparent erosion of the spore coat was observed with 0.04-ppm and 1.6-ppm ozone exposure. There was regularity, smoothness, and integrity in unexposed (control) spores and those spores exposed to 0.04- and 1.6-ppm ozone. However, spores in the 0.04- and 1.6-ppm ozone treatments appeared smaller in width than the control spores. Even though no apparent structural damage was visualized, the spore inner structure may have been considerably weakened. The SEM micrographs show damage to the spore external structure in those subjected to 4-ppm ozone. These micrographs clearly show that the structure of C. gloeosporioides spores was affected. Some spores were seriously deformed or had lost their original shape and extrusion of their inner material was also observed (Fig. 4d). Some of the spores had disintegrated, showing fragments of their initial structure.
The SEM photomicrograph of control samples of the papaya cuticles illustrates the typical surface morphology of papaya fruit (Fig. 5a). The SEM images of papaya fruit treated with 0.04-, 1.6-, and 4-ppm ozone are illustrated in Fig. 5b, c, and d, respectively. It can be observed that cuticles of fruit treated with 0.04- and 1.6-ppm ozone had similar wax covering as those in the control sample (Fig. 5a). Nevertheless, the 4 ppm treated fruit (Fig. 5d) appears to be smoother, less granular than the control or the 0.04- and 1.6-ppm-treated fruit. This indicates that these ozone treatments below 4 ppm did not affect this layer. These results revealed no major variation in cuticle morphology between control and treated fruits below 4-ppm ozone. No external wax accumulation or wax crystals could be detected.
Discussion
Ozone treatments have excellent potential to control anthracnose disease caused by C. gloeosporioides in papayas. Mycelia growth and conidia germination were clearly affected at various concentrations and periods of ozone exposure. Among all the ozone treatments tested, the 4-ppm ozone treatment was found to be the most effective in controlling fungal growth and spore germination in vitro. However, 96 h of 1.6-ppm ozone exposure showed the best suppression of disease incidence and severity in vivo. Ozone treatments at lower concentrations also showed some fungistatic effects in vitro when the exposure period was prolonged to 144 h. This is mainly because the development of C. gloeosporioides mycelia required 8 days or 192 h reaching the edge of control plate. It explained that ozone concentration do not appear significantly different on the inhibition of mycelia growth after 144-h exposure.
Ozone with its fungitoxic and fungistatic activity could be considered as a suitable alternative to fungicides for controlling anthracnose in papayas. In vitro inhibition was directly related to the ozone concentration. These results are in agreement with the findings of Tzortzakis et al. (2008), who showed that ozone was highly inhibitory to mycelia growth and spore production of Alternaria alternata and Colletotrichum coccodes in tomato fruits, and that the inhibitory effect was directly related to the concentration and duration of ozone exposure. The study by Ozkan et al. (2011) also confirmed that the inhibitory effect of ozone on spore germination of Penicillium digitatum, Penicillium italicum, and Botyris cinerea of table grapes was higher with an increase in the concentration of ozone and exposure time.
On the other hand, the same relationship with ozone concentration and exposure time was not apparent in the in vivo study. Fruit treated with 4-ppm ozone had increased fruit decay, indicating that higher doses of ozone (4 ppm) reduced the disease resistance to anthracnose. This could be due to degradation of structural components and membrane structures. Papayas treated with 4-ppm ozone continuously for 144 h developed distinct surface browning as a result of surface oxidation from excessive use of ozone. Kim et al. (1999) stressed that ozone may promote oxidative spoilage. Ozone has been shown to change the surface color of some fruits and vegetables such as carrots (Liew and Prange 1994) and broccoli florets (Lewis et al. 1996). In contrast, treatments with lower concentrations of ozone showed no phytotoxic effects and had better physical appearance (uniform color with less fungal spoilage and blemishes).
Several studies have revealed that ozone exposure prevents microbial growth and thus extends the shelf life of treated produce. Barth et al. (1995) observed that treatment of blackberries with ozone concentrations as low as 0.3 ppm effectively suppressed fungal development, and similar findings were reported by Sarig et al. (1996) for grapes, Liew and Prange (1994) for carrots, Aguayo et al. (2006) for tomatoes, Palou et al. (2002) for peaches, and Baranovskaya et al. (1979) for beetroot, potato, and onions.
Successful applications of gaseous ozone treatment include the work of Ewell (1940) on strawberries, raspberries, and grapes where the shelf life was doubled by applying 2–3-ppm ozone. Kuprianoff (1953) prolonged apple shelf life by several weeks, while Baranovskaya et al. (1979) prolonged potato shelf life to as long as 6 months with 3-ppm ozone.
Exposure to 1.6-ppm ozone delayed the onset of disease incidence by up to 21 days in papayas during storage, while in control fruit the anthracnose symptoms appeared within 7 days of storage. The actual mechanism of action of ozone is not clear and needs to be further investigated. However, anthracnose is a latent infection and begins with the germination of conidia, and therefore ozone may have had fungistatic effects (Ranasinghe et al. 2005). Furthermore, ozone has been shown to induce natural resistance to disease caused by B. cinerea, a type of latent infection in carrot (Forney et al. 2007). Krause and Weidensaul (1978) reported that exposure to low ozone concentrations did not kill but reduced the subsequent virulence of conidia in B. cinerea.
Reports describing the doses of ozone required to inactivate fungal conidia are few and conclusions differ (Ozkan et al. 2011). It is generally reported that ozone may delay the appearance of disease symptoms of infected fruit. Several studies have reported reductions in spore production and viability following ozone treatment (Krause and Weidensaul 1978; Palou et al. 2002; Aguayo et al. 2006; Karaca and Velioglu 2007; Tzortzakis et al. 2007), but spore production can resume when treated fruit are removed from the ozone-enriched atmosphere (Smilanick 2003). Reports on other microbes indicate that very high ozone doses may be required for control. Currier et al. (2001) reported that exposure to 9,000 ppm of ozone for 15 h was required to kill the spores of Bacillus globigii var. niger.
The major factor determining spore resistance to biocidal agents appears to be the spore coat (Komanapalli and Lau 1996; Young and Setlow 2004). This was supported with TEM micrographs by Kim et al. (2003), where aqueous ozone treatments caused damage to the surface layer as well as to the outer and inner coats. Such damage facilitates the action of ozone on the cortex, and finally causes spore inactivation through intracellular damage (Kim et al. 2003; Young and Setlow 2004). Besides, ozone as a biocidal agent may have several targets such as proteins, enzymes, and even DNA (Swadeshi et al. 1986). Mahfoudh et al. (2010a) working on Bacillus atrophaeus and Bacillus pumilus spores observed severe alterations to spore integrity, although the morphology of a minority of them was apparently unaffected. The progressive degradation of these structures involves changes in permeability and cell integrity, and is often followed by cell lysis (Broadwater et al. 1973; Kim and Yousef 2000; Khadre and Yousef 2001; Thanomsub et al. 2002). Recent findings by Mahfoudh et al. (2010b) suggest that ozone molecules diffuse through the spores and then react (oxidation process) with targets that are essential to spore survival such as water. Besides, the presence of water in the core of the spore can initiate chemical reactions with ozone and their reaction by-products may provide further oxidant species such as radicals and oxidant molecules which participate in the spore inactivation process.
Scanning electron micrographs of Kechinski et al. (2011) revealed that cuticles of papaya fruits treated with 4-ppm ozonized water for 2 min had the same wax covering as those in the control samples. Thus, ozone application did not affect the fruit’s cuticular surface or wax arrangement on fruit. According to Forney (2003), harvested fruits and vegetables have closed stomata that limits the penetration of ozone into harvested plant tissues.
Conclusion
The demand for safe and good quality foods with high nutrients by the consumer has prompted research to discover and evaluate novel antimicrobial agents as an alternative postharvest technology. Many studies have proven that ozone is a safe and effective antimicrobial agent in many food processing applications. It is concluded from the present study that ozone exposure at the concentrations of 1.6 and to a lesser extent 4 ppm for 96 h is a safe and effective method for treating papayas to control anthracnose disease. Ozone treatment inhibited mycelia growth and spore germination effectively and delayed the onset of decay of artificially inoculated fruits. Conversely, ozone treatment did not affect the wax arrangement on the fruit surface. Overall, the results of this study signify that ozone fumigation has excellent potential to combat spoilage caused by C. gloeosporioides.
References
Abeles, F. B., Morgan, P. W., & Salveit, M. E. (1992). Ethylene in plant biology (2nd ed., p. 414). London: Academic.
Aguayo, E., Escalona, V. H., & Artes, F. (2006). Effect of cyclic exposure to ozone gas on physicochemical, sensorial and microbial quality of whole and sliced tomatoes. Postharvest Biology and Technology, 39, 169–177.
Ali, A., Muhammad, M. T. M., Sijam, K., & Siddiqui, Y. (2010). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry, 124, 620–626.
Alvarez, A. M., & Nishijima, W. T. (1987). Postharvest diseases of papaya. Plant Disease, 71, 681–686.
Baranovskaya, V. A., Zapolskii, O. B., Ovrutskaya, L. Y., Obodovskaya, N. N., Pschenichnaya, E. E., & Yushkevich, O. I. (1979). Use of ozone gas sterilization during storage of potatoes and vegetables. Koshervnaya I Ovoshchesushhil’naya Prom, 4, 10–12.
Barnett, H. L., & Hunter, B. B. (1972). Illustrated genera of imperfect fungi (p. 24). USA: Burgess Publishing Co.
Barth, M. M., Zhou, C., Mercier, J., & Payne, F. A. (1995). Ozone storage effects on anthocyanin content and fungal growth in blackberries. Journal of Food Science, 60, 1286–1288.
Bataller, M., González, J. E., Veliz, E., & Fernández, L. A. (2012). Ozone applications in the post-harvest of papaya (Carica papaya L.): an alternative to Amistar fungicide. Ozone: Science & Engineering: The Journal of the International Ozone Association, 34(3), 151–155.
Broadwater, W. T., Hoen, R. C., & King, P. H. (1973). Sensitivity of three selected bacterial species to ozone. Applied Microbiology, 26, 391–393.
Cia, P., Pascholati, S. F., Benato, E. A., Camili, E. C., & Santos, C. A. (2007). Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biology and Technology, 43, 366–373.
Couey, H. M., & Farias, G. (1979). Control of postharvest decay of papaya. HortScience, 14, 719–721.
Couey, H. M., Alvarez, A. M., & Nelson, M. G. (1984). Comparison of hot water spray and immersion treatment for control of postharvest decay of papaya. Plant Disease, 68, 436–437.
Cronin, M. J., Yohalem, D. S., Harris, R. F., & Andrews, J. H. (1996). Putative mechanism and dynamics of inhibition of apple scab pathogen Venturia inequalis by compost extracts. Soil Biology and Biochemistry, 28, 1241–1249.
Currier, R., Toracco, D., & Cross, J. (2001). Deactivation of clumped and dirty spores of Bacillus globigii. Ozone Science and Engineering, 23, 285–294.
Ewell, A. W. (1940). Ozone and its applications in food preservation. Refrigeration application data book. In Sect. II. ‘Cold storage practice’ (2nd ed., pp. 199–203). Menasha: American Society of Refrigeration Engineering.
Forney, C. F. (2003). Postharvest response of horticultural products to ozone. In D. M. Hodges (Ed.), Postharvest oxidative stress in horticultural crops (pp. 13–54). New York: Food Products Press.
Forney, C. F., Song, J., Hildebrand, P. D., Fan, L., & McRae, K. B. (2007). Interactive effects of ozone and 1-methylcyclopropene on decay resistance and quality of stored carrots. Postharvest Biology and Technology, 45, 341–348.
Graham, D. M., Pariza, M., Glaze, W. H., Newell, G. W., Erdman, J. W., & Borzelleca, J. F. (1997). Use of ozone for food processing. Food Technology, 51, 72–75.
Karaca, H., & Velioglu, S. Y. (2007). Ozone applications in fruit and vegetable processing. Food Review International, 23, 91–106.
Kechinski, C. P., Cândida, R. S. M., Pâmela, V. R. G., Caciano, P. Z. N., Lígia, D. F. M., Isabel, C. T., et al. (2011). Effects of ozonized water and heat treatment on the papaya fruit epidermis. Food and Bioproduct Process. doi:10.1016/j.fbp.2011.01.005.
Khadre, M. A., & Yousef, A. E. (2001). Sporicidal action of ozone and hydrogen peroxide: a comparative study. International Journal of Food Microbiology, 71, 131–138.
Kim, J. G., & Yousef, A. E. (2000). Inactivation kinetics of foodborne spoilage and pathogenic bacteria by ozone. Journal of Food Science, 65, 521–528.
Kim, J. G., Yousef, A. E., & Dave, S. (1999). Application of ozone for enhancing the microbiological safety and quality of foods: a review. Journal of Food Protection, 62(9), 1071–1087.
Kim, J. G., Yousef, A. E., & Khadre, M. A. (2003). Ozone and its current and future application in the food industry. Advances in Food and Nutrition Research, 45, 167–218.
Kiran, B., Lalitha, V., & Raveesha, K. A. (2010). Screening of seven medicinal plants for antifungal activity against seed borne fungi of maize seeds. African Journal of Basic & Applied Sciences, 2(3–4), 99–103.
Komanapalli, I. R., & Lau, B. H. S. (1996). Ozone-induced damage of Escherichia coli K-12. Applied Microbiology and Biotechnology, 46, 610–614.
Krause, C. R., & Weidensaul, T. C. (1978). Effects of ozone on the sporulation, germination and pathogenicity of Botrytis cinerea. Phytopathology, 68, 195–198.
Kuprianoff, J. (1953). The use of ozone in cold storage of fuits. Zeitschrift Kaltetechnik, 10, 1–9.
Lewis, L., Zhuang, H., Payne, F. A., & Barth, M. M. (1996). Beta-carotene content and color assessment in ozone-treated broccoli florets during modified atmosphere packaging. Institute of Food Technologists annual meeting, book of abstracts (pp. 99).
Liew, C. L., & Prange, R. K. (1994). Effect of ozone and storage temperature on post-harvest diseases and physiology of carrots (Daucus carota L.). Journal of the American Society for Horticultural Science, 119, 563–567.
Mahfoudh, A., Barbeau, J., Moisan, M., Leduc, A., & Seguin, J. (2010). Biocidal action of ozone-treated polystyrene surfaces on vegetative and sporulated bacteria. Applied Surface Science, 256, 3063–3072.
Mahfoudh, A., Moisan, M., Seguin, J., Barbeau, J., Kabouzi, Y., & Keroack, D. (2010). Inactivation of vegetative and sporulated bacteria by dry gaseous ozone. Ozone Science and Engineering, 32(3), 180–198.
Ozkan, R., Smilanick, J. L., & Karabulut, O. A. (2011). Toxicity of ozone gas to conidia of Penicillium digitatum, Penicillium italicum and Botyris cinerea and control of gray mold on table grapes. Postharvest Biology and Technology, 60, 47–51.
Palou, L., Crisosto, C. H., Smilanick, J. L., Adaskaveg, J. E., & Zoffoli, J. P. (2002). Effects of continous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biology and Technology, 24, 39–48.
Perez, A. G., Sanz, C., Rios, J. J., Olias, R., & Olias, J. M. (1999). Effects of ozone treatment on postharvest strawberry quality. Journal of Agricultural and Food Chemistry, 47, 1652–1656.
Ranasinghe, L., Jayawardena, B., & Abeywickrama, K. (2005). An integrated strategy to control postharvest decay of Embul banana by combining essential oils with modified atmosphere packaging. International Journal of Food Science and Technology, 40, 97–103.
Sapers, G. M. (1998). New technologies for safer produce-chemical based treatments and decontamination by washing. In Proceedings Conference on Fresh Fruit and Vegetables: Food Safety Challenges. Chicago: National Centre for Food Safety Technology. May 12–14.
Sarig, P., Zahavi, T., Zutkhi, Y., Yannai, S., Lisker, N., & Ben-Arie, R. (1996). Ozone for control of postharvest decay of table grapes caused by Rhizopus stolonifer. Physiological and Molecular Plant Pathology, 48, 403–415.
Sepiah, M. (1993). Efficacy of propiconazole against fungi causing postharvest diseases on Eksotica papaya. In: Proceedings of the International Postharvest Conference on Handling Tropical Fruits, (pp. 53). Chiangmai, Thailand.
Shiota, H. (1991). Volatile components of pawpaw fruit (Asimia triloba). Journal of Agricultural and Food Chemistry, 39, 1631–1635.
Sivakumar, D., Hewarathgamagae, N. K., Wijeratnam, R. S. W., & Wijesundera, R. L. C. (2002). Effect of ammonium carbonate and sodium bicarbonate on anthracnose of papaya. Phytoparasitica, 30, 1–7.
Skog, L. J., & Chu, C. L. (2001). Effect of ozone on qualities of fruits and vegetables in cold storage. Canadian Journal of Plant Science, 81, 773–778.
Smilanick, J. L. (2003). Postharvest use of ozone on citrus fruit (pp. 1–6). USA: Packinghouse Newsletter 199.
Swadeshi, K., Miura, K., Ohtsuka, E., Ueda, T., Shinriki, N., & Ishizaki, K. (1986). Structure and sequence-specificity of ozone degradation of supercoiled plasmid DNA. Nucleic Acids Research, 14, 1159–1169.
Thanomsub, B., Anupunpisit, V., Chanphetch, S., Watcharachaipong, T., Poonkhum, R., & Srisukonth, C. (2002). Effects of ozone treatment on cell growth and ultrastructural changes in bacteria. The Journal of General and Applied Microbiology, 48, 193–199.
Tzortzakis, N. G., Singleton, I., & Barnes, J. D. (2007). Deployment of low level ozone-enrichment for the preservation of chilled fresh produce. Postharvest Biology and Technology, 43, 261–270.
Tzortzakis, N. G., Singleton, I., & Barnes, J. D. (2008). Impact of low-level atmospheric ozone-enrichment on black spot and anthracnose rot of tomato fruit. Postharvest Biology and Technology, 47, 1–9.
United States Food and Drug Administration (US-FDA). (1997). Substances generally recognized as safe, proposed rule. Federal Register, 62, 18937–18964.
Young, S. B., & Setlow, P. (2004). Mechanism of Bacillus subtilis spore resistance to and killing by aqueous ozone. Journal of Applied Microbiology, 96, 1133–1142. B.
Acknowledgments
We would like to thank MedKlinn International Sdn. Bhd. for providing financial and technical support for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ong, M.K., Kazi, F.K., Forney, C.F. et al. Effect of Gaseous Ozone on Papaya Anthracnose. Food Bioprocess Technol 6, 2996–3005 (2013). https://doi.org/10.1007/s11947-012-1013-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-1013-4