Abstract
The effect of microwave and oil bath heating on the evolution of Maillard reaction including pH, color and degree of glycation, and flavor compounds was investigated. Maillard reaction was induced by heating glucose-ammonium system for 10–30 min at 100–120 °C. Results indicated that microwave heating was more effective than traditional oil bath heating in Maillard reaction. The more decrease of pH values, higher A420, lower whiteness and chroma, and stronger glycation were recorded in the model system heated by microwave. Moreover, microwave heating did not affect the contents of pyrazines extracted by dichloromethane but affect the formation of D-limonene extracted by ethyl acetate from model system. In addition, 2-hydroxy-3-methyl-2-cyclopentene-1-one, 3-methyl-2-pyrazinyl-methanol, and 6-methyl-2-pyrazinyl-methanol were firstly reported as flavor compounds in the glucose-ammonium system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maillard reaction (MR) plays an important role in food chemistry due to the variety of compounds generated throughout the reaction. These compounds were known as Maillard reaction products (MRPs). Many food properties, such as taste, flavor, color, and even risk of safety, are contributed to by Maillard reaction products, and thus, the control of such non-enzymatic browning reactions is an essential technological task to ensure food quality (Wegener et al. 2017). MR has been proved to widely exist in various food products including baked food, beers, spirits, wines, beverages, sauces, and jam (Sengar and Sharma 2014; Wu et al. 2015). MR system is very complex, and it involves a number of intermediate complexes (Guan et al. 2011a), such as the intermediate products, and finally browning products (BPs) (Benjakul et al. 2005).
MR is an effective way to enhance food color and flavor, especially in cooking meat products. Hence, more and more model reactions between sugars and peptides were researched in order to avoid the complexity of meat heat processing. Influence of L-pyro-glutamic acid on the color formation was reported in the process of D-glucose model reactions with L-glutamine and L-alanine (Wegener et al. 2017). They used the absorption of 420 nm to monitor the color change and found that the typical amino-carbonyl reaction was slowed down due to a fast occurring cyclization of L-glutamine to pyro-glutamic acid. The absorption of 460 nm (Monti et al. 2000) and Colour Flex spectro-colorimeter (Abdelhedi et al. 2017) were used to determine the color in the lactose and lysine or Nα-acetyl-lysine model systems and smooth-hound viscera protein-sucrose conjugates, respectively. For many years, volatiles were found in many foods and beverages, such as spirit drinks (Charapitsa et al. 2013), beef (Kerth 2016), dark and milk chocolate (Liu et al. 2015), soy sauce (Feng et al. 2014), and so on. Some researchers have also reported that volatiles can generate from different Maillard reactions, including the model system of L-ascorbic acid with L-glutamic acid/L-aspartic acid (Tan and Yu 2012), of lysine-containing dipeptides (Van Lancker et al. 2010), of whey protein, hydrolyzed whey protein, amino acids, and glucose (Lee et al. 2012; Scalone et al. 2015), and so on. Most of these flavor compounds containing in pyrazines, furans, hydrocarbons, and esters were identified using gas chromatography-mass spectrum (GC-MS) (Guan et al. 2011a), GC-olfactory-MS (GC-O-MS) (Luo et al. 2017) and headspace solid-phase microextraction-GC-MS (HS-SPME-GC-MS) (Scalone et al. 2015), and characterized using electronic nose (EN) (Gao et al. 2017), odor activity value, and omission test (Feng et al. 2015).
Different factors are involved in the MR evolution. It is well known that the reaction parameters, such as temperature, time, pH, surroundings, zwitterion, reactant concentration, and ratios, have prominent effects on MRP formation (Schumacher and Kroh 1994). In the past two decades, different treating methods including ultrasound (Guan et al. 2010a; 2011a; Abdelhedi et al. 2017), ultrafiltration (Guan et al. 2011b), pressure (Guan et al. 2011c), pulsed electric (Guan et al. 2010b), and different reactant types including various proteins and peptides (Karangwa et al. 2016) on the Maillard reaction have been proven to affect Maillard reaction. Recently, microwave heating (MWH), a rapid and effective technology for food thermal processing (Huang and Sites 2010; Pu and Sun 2017; Guo et al. 2017), was widely used in food industries. And it was reported that MWH was more effective to retain the 4-(methylthio)-3-butenyl isothiocyanate (MTBITC) of Chinese radish (Han et al. 2016) and reduce the cooking loss of meat (Li et al. 2017). Several researches have been reported to discuss the effect of MWH on different Maillard reaction system. Shaman et al. (2007) reported that MWH has no any significant non-thermal effects on Maillard reaction, protein denaturation, polymer solubility, mutagenesis of bacteria, and mutarotation equilibrium of α/β-D-glucose. However, Guan et al. (2011) showed that MWH strongly influenced the kinetic of soy protein isolate-saccharide graft reaction. The glycation extent of ovalbumin was confirmed by high-resolution mass spectrometry to have the significant differences between MW and conventional heating (Wang et al. 2013). Nasrollahzadeh et al. (2017) also concluded that MWH was more effective on the reduction of free amino groups and increase in absorbance at 420 nm more than conventional heating and can obtain higher values in solubility, emulsion activity, and foam capacity on the bovine serum albumin-maltodextrin conjugates.
Despite the above studies, detailed information on the effects of MWH on the simple Maillard reaction model system is still not available. Thus, this study aims to investigate the effects MW heating on the pH value, color changes, and volatile compounds of MRPs produced from a glucose-ammonium model system. Sequentially, it might provide some academic bases and references for application of microwave heating in Maillard reaction and caramel-related food products.
Materials and Methods
Reagents
Methanol (HPLC grade, ≥ 99.9%, CAS No. 67-56-1) was obtained from Merck (Damstadt, Germany). Ammonium hydroxide (HPLC grade, 35%, CAS No. 1336-21-6) was obtained from Thermo Fisher Scientific Co., Ltd (USA). Glucose was purchased from Shanghai Bio Science & Technology Co., Ltd (Shanghai, China). Ultrapure water was produced by ultrapure water system (UPHW-I-90T, Chengdu Ultrapure Technology Co., Ltd.). C7-C30 n-alkanes were purchased from Sigma-Aldrich Chemical Co. Ltd. (St. Louis, MO).
Preparation of Alkaline Glucose-Ammonium System
In this experiment, 18.016 g glucose and 7.70 mL ammonium were mixed in 1000 mL ultrapure water to prepare the alkaline model reaction system. The solutions were at an initial pH value of about 10.65 and an initial concentration of 100 mmol L−1 of glucose at room temperature. This model system is designed to simulate the preparation of class III caramel and the alkaline model systems are easier to generate pyrazines.
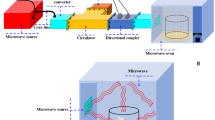
Ten milliliters of model reaction solution was loaded into the screw-capped Pyrex tubes for oil-bath (DF-101S, Gongyi YUHUA Instrument Co., Ltd., Zhengzhou, China) and microwave digestion polytetrafluoroethylene vessels from microwave-assisted system (MARS, CEM Corporation, USA) heating, respectively. The optic fiber thermometer (MARS, CEM Corporation, USA) and temperature sensors (PT100, Gongyi YUHUA Instrument Co., Ltd., Zhengzhou, China) were used to monitor the temperature of the samples heated by microwave oven and oil bath, respectively. Because the Maillard reaction is easy to conduct in the alkaline systems, too high temperature is easier to generate solid brown products and thus affect the properties of caramel color and results’ analysis. The reaction parameters were set as follows: (1) the heating temperature was 100, 110, and 120 °C, (2) the preheating time was all set 5 min to reach required temperature, and (3) the total heating time was 10, 20, and 30 min. After the reaction, all samples were cooled immediately to room temperature with iced water. All samples were performed for nine times.
Measurements of pH, Intermediate Products, and Browning Intensity
In order to monitor the pH change during reaction, the pH values were measured using a FE20-pH meter (METTLER TOLEDO, USA) calibrated with buffer solutions at pH 4.0 and 10.0.
Intermediate products (A294) and browning intensity (A420) of the model system were measured using a UV-1810 spectrophotometer (PERSEE instruments Co., Ltd, Beijing, China) based on a previously described methodology (Guan et al. 2011a). Before UV-Vis tests, the model system was diluted to the appropriate multiple by ultrapure water due to the upper limit (4 is the maximum value for the UV-Vis) of UV-Vis scale. Samples were diluted to multiples of 10, 20, and 40 by ultrapure water to test the absorbance at 294 and 420 nm, respectively.
Color Measurement of the MRPs
The color of studied model systems was determined by Chroma Meter (CR-400, Komica Minolta Investment Ltd. Tokyo, Japan), readably calibrated using a white standard plate (L* = 92.84, a* = −1.27, b* = 0.45). The result of different reaction samples were displayed as L*, a*, and b* values which indicated lightness, redness, and yellowness, respectively (Kim et al. 2015). All samples were determined in triplicate. The following equations were used to calculate the whiteness (W*), chroma (C*), and difference in color (△E*) (Abdelhedi et al. 2017):
where L0, a0, and b0 are the color of the reaction samples at t = 0.
Determination of Glucose
High-performance anion exchange chromatography (HPAEC) was used for glucose measurement. The HPAEC system is as follows: chromatogram controller, ICS-5000 (Dionex, USA); pump, DIONEX SP (Dionex, USA); chromatographic column, CarboPac PA1 (4 × 250 mm, Dionex, USA); a pulsed ampere-metric detector (Dionex, USA) and a CarboPac PA1 guard column (4 × 50 mm, Dionex, USA). The glucose was eluted using mobile phase A (100 mM NaOH in ultrapure water) and mobile phase B (ultrapure water) in a isocratic program with a flow of 1.0 mL min−1: 0–25 min, 16% A + 84% B. All reacted samples were diluted 1000-fold and filtered through 0.45-μm membrane filter (Ameritech Technology Co., Ltd. Tianjin, China) before HPAEC analysis. Data are presented as means ± SD of triplicates.
Determination of Flavor Compounds
Pretreatment of MRPs for Flavor Compounds Measurement
It is well known that different extracting solvent may leads to significant deviation of the results. In this paper, the following procedure was used to treat the samples for the determination of pyrazine compounds: 20 mL MR solution was mixed with 10 mL dichloromethane on a Vortex mixer (XW-80A, Shanghai Precision and Scientific industrial Co., Ltd, Shanghai, China) for 3 min and ultrasonically extracted with ice water in the ultrasonic system (KQ3200DA, Kunshan Ultrasonic Instruments Co., Ltd, Kunshan, China) for 20 min. The mixtures were centrifuged at 8000 r min−1 for 20 min at 4 °C using a high-speed refrigerated centrifuge (centrifuge 5810R, Eppendorf, Germany), and the dichloromethane layer (lower layer) was collected by a 15-mL glass tube. For other aroma compounds, the extract solvent of dichloromethane was replaced by ethyl acetate. And the ethyl acetate layer (upper layer) was collected by a 15-mL glass tube.
After dehydrated with anhydrous sodium sulfate for all extracts, 1.0 mL extract was filtered through 0.22-μm nylon membrane filter and stored in the refrigerator at 4 °C until GC-MS analysis.
GC-MS Conditions
GC-MS was performed with an Agilent 6890 N gas chromatograph (GC) coupled with a 5975C mass selective detector (MS, Agilent Technologies, Inc., CA, USA), an Agilent 7683 B auto-sampler and a Chemstation recorder. The analytical conditions were modified primarily based on the method of Guan et al. (2011a). The GC was equipped with a DB-5MS capillary column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, Inc., CA, USA). Injection mode was split-less and the injection volume was 5 μL. The flow rate of helium gas (> 99.999%) was 1.0 mL min−1. The oven temperature was programmed as follows: started at 50 °C (held for 5 min), raised to 250 °C at 8 °C min−1 ramp (held for 10 min), in a total running time of 40 min. The temperatures of front inlet, ion source, and transfer line were set at 240, 230, and 250 °C, respectively. The electron-impact (EI) ionization mode (70 eV) was used in the mass spectrometry. The quantification was performed in full scan, and the monitoring range was 35–500 m/z. The aroma compounds were identified with NIST08 and Wiley275 libraries and by their linear retention index (LRI) from our experiment and other literature.
Statistical Analysis
Statistical analyses were carried out using Minitab 15 (Minitab Inc., State College, PA, USA) and statistical product and service solutions (SPSS) 13.0 (IBM SPSS Inc., NY, USA) analytical software. For all data, different letters in the same system with different treatments indicate statistically significant differences (p < 0.05).
Results and Discussion
pH Change of the MRPs
The pH change of MRPs subjected to MWH and OBH was monitored to evaluate the MR evolution. As shown in Fig. 1, with the increasing time and temperature, the pH values of system heated by two methods were gradually decreased. The pH changed slightly at the temperature of 100 °C regardless of the reaction time. However, significant changes (p < 0.05) in pH value appeared as the heating temperature was increased to 110 and 120 °C at all reaction times. These results indicated that the NH4+ was reacting constantly with glucose, and then, some organic acids including formic acid, acetic acid, and so on (Van Boekel and Martins 2002; Davidek et al. 2006) was generated during reaction. Hence, these reaction products lead to the decrease of pH value. The result was in agreement with some previous reports, which was performed with sugar-amino acid systems under hydrothermal method (Morales and Jimenez-Perez 2001; Vhangani and Van Wyk 2013).
Meanwhile, the results also revealed that MWH did more produce MRPs and reduce the pH value of glucose-ammonium model system than OBH (p < 0.05 in all case by variance analysis using SPSS 13.0). The minimum pH values at heating temperatures of 100, 110, and 120 °C were 9.49 ± 0.01, 9.08 ± 0.05, and 8.19 ± 0.08 for OBH; 8.83 ± 0.06, 8.28 ± 0.05, and 6.83 ± 0.04 for MWH, respectively. In addition, the differences of pH between MW and OBH (Fig. 1) were increasing with the increasing temperature. Report (Guan et al. 2011a) has shown that ultrasound at higher intensities could reduce the pH values of Maillard reaction system. However, no satisfying explanation could be found for this result, but it is obvious that two reasons may be responsible for the reduction of pH value. On one hand, the microwave digestion tube has a higher bulk than oil bath digestion tube, thus the ammonia gas from the heating model system must be volatilized into the cavity and then lead to the decrease of pH value. And on the other hand, there might be the presence non-thermal effect during MWH, thus more NH4+ participates in the reaction and more organic acids are produced for microwave heating.
Intermediate Products and Browning Intensity Measurement of MRPs
The Maillard reaction is generally characterized by reactant consumption, formation of initial, intermediate, and complex brown products. Ajandouz et al. (2001) demonstrated that the uncolored intermediate products (UIPs) were the precursors of BPs in non-enzymatic browning reaction, and thus, the absorption at 294 nm was used as an indicator of the production of UIPs. These UIPs produced by glycosylation (Hong et al. 2015) included aldehydes, ketones, and so on (Yu et al. 2017). The BPs of the final stage colored and monitored using their absorption at 420 nm were reported by Yu et al. (2012). In general, the UV absorption of a reaction solution at 294 nm can be used to predict the beginning of color change of the reaction, while the absorption at 420 nm is usually used to determine the generation of BPs which can reflect the browning degree of the Maillard reaction (Lertittikul et al. 2007; Rufián-Henares et al. 2002; O’Charoen et al. 2015).
The evolutions of the absorption of glucose-ammonium hydroxide reaction solutions at 294 nm with different reaction time and heating methods at different temperature are displayed in Fig. 2 (1-1, 2-1, and 3-1). As can be seen, the absorptions of both heating methods at 294 nm were increasing with the increasing heating time and temperature. The analysis of variance studies indicated that the absorptions of reaction solution at 294 nm with MWH were higher than that of oil bath heating at temperature of 100 and 110 °C; however, there was no significant difference of absorption at 294 nm between MW and OBH at temperature of 120 °C. In other words, the differences of absorptions between MW and OBH were reducing with the increasing temperature and were negligible when the heating temperature rises to 120 °C. It could be concluded that the MWH did slightly affect the formation rate of UIPs, did not affect the amount of UIPs at the intermediate stage. These findings are in conformity with the report by MeiPner and Erbersdobler (1996).
Figure 2 (1-2, 2-2, and 3-2) reveals the absorbance of glucose-ammonium reaction solutions at 420 nm varying with different reaction time and heating methods at different temperature, from which the following conclusions can be drawn: (1) the model systems heated by MW and OB have similar absorption at 420 nm with reaction time at 100–120 °C; (2) the absorption of reaction solutions at 420 nm increase with the increase of reaction time and temperature; and (3) as the trends of absorption at 294 nm, the absorptions of reaction solutions at 420 nm with MWH were significant higher than that of OBH at temperature of 100, 110, and 120 °C (p < 0.05 in all case). Because the MW oven is designed to achieve the rapid heating and its heating time is generally required less than 30 min, the long heating time was not designed to inspect the further influences. Moreover, as presented in Fig. 2, the absorptions of reaction solutions at 294 and 420 nm heated by MW had larger standard deviations (notable error bars) than that of the OBH. These results might be mainly caused by the inhomogeneity of the microwave heating, which provided poor repeatability (Han et al. 2016; Li et al. 2017). Even so, the variance analysis performed between the absorption at 420 nm of reaction solutions heated by MWH and OBH still revealed that MWH can promote the Maillard reaction, especially to promote the formation of BPs in the glucose-ammonium model system.
Color Measurement of MRPs
The lightness (L*), redness (a*), and yellowness (b*) changes of the MRPs heated at 100, 110, and 120 °C for 10, 20, and 30 min by two methods are recorded using the chroma meter. Table 1 shows the whiteness (W*), chroma (C*), and color difference (△E*) of the corresponding MRPs calculated by the formulas in section of Color Measurement of MRPs from Materials and Methods. With the increasing temperature and time, the W* decreased gradually for both heating methods, and this decrease was more pronounced in the MWH samples rather than OBH (p < 0.05). This result indicated that the incessant formation of BPs has weakened the lightness or whiteness during the Maillard reaction and microwave heating can promote the formation of BPs.
The markedly increasing △E* values were observed with the increase of reaction time and temperature. Results manifested that MWH can slightly promote the color change in the glucose-ammonium system. The C* values illustrates the following trends for both heating methods: (a) with the increase in reaction time, the trend of the C* values were found to increase first, then decrease and (b) the maximum C* values were at 20 min of reaction time and 100 °C of reaction temperature. When the heating temperature reaches to 110 °C, the C* values were decreased with the reaction time. In addition, this decrease was more obvious in the MWH samples than that of OBH. The minimum C* values at heating temperatures of 110 and 120 °C were 6.27 ± 0.25 and 2.19 ± 0.30 for OBH and 5.22 ± 0.99 and 0.66 ± 0.16 for MWH, respectively. According to the Eq. (2) in the section of Color Measurement of MRPs from Materials and Methods, the C* values was depended on the a* and b* values, which represent red/green and blue/yellow color of samples, respectively. Our data showed the increase of a* and increase first, then decrease of b* over time of heating, which indicates that (1) the redness increased, and (2) the blueness decreased first (because of the increasing yellowness), and then increased of samples through Maillard reaction (data not shown). Similar findings were obtained by Nasrollahzadeh et al. (2017) after bovine serum albumin-maltodextrin conjugates heating for 120 min at 90 °C. Since the mixture of blue and red color induces formation of BPs, the observed whiteness (W*) and chroma (C*) of the MRPs changes in this paper demonstrated the occurring of the MR stages, characterized by the formation of BPs, which was relatively promoted by MWH. This result is consistent with the results of A420.
Changes of Glucose Contents
The HPAEC peaks were identified to be glucose by comparing the retention times between them and standard compounds. The regression equations for glucose standards are y = 4.1595x + 1.68 (R2 = 0.9989), where x = glucose concentration, mg L−1; y = peak area of glucose standards.
In order to compare the effects of the MWH on the glucose degradation, the retention rates of glucose are shown in Fig. 3. The retention rates of glucose were calculated by the following equation:
where ω is the retention rate of glucose, %; C t is the content of glucose after reacting, mg; and C0 is the content of glucose in the model system at t = 0, mg. The minimum retention rates at heating temperatures of 100, 110, and 120 °C were 42.2 ± 0.3, 32.5 ± 0.5, and 27.4 ± 0.5% for OBH and 33.4 ± 2.7, 22.2 ± 2.1, and 19.4 ± 2.1% for MWH, respectively.
Figure 3 reveals the following trends for the two heating methods: (1) with the increase in heating time, the retention rate of the glucose was decreased gradually; and (2) with the increase in heating temperature, the retention rate of the glucose was reduced. The extent of retention rate of the glucose was similar at 100, 110, and 120 °C for the two heating methods. Nevertheless, for MWH, the retention rates of the glucose were all lower than that of the oil bath heating methods. By performing the variance analysis for the results, a result-oriented conclusion can be obtained that the MWH can promote the greater Maillard reaction than that of OBH (p < 0.05). This might be explained by the distinctiveness of the microwave heating principle. In oil bath heating, the heat is predominantly transferred by conduction from the outside surface of the product to its interior whereas MWH is achieved by the generation of internal thermal energy. In addition, glucose can be transformed into mannose and fructose under heating, so the content of glucose can reduce by this path.
Flavor Compounds
GC/MS Analysis of Dichloromethane Extracts
GC/MS analysis is a rapid and simple method to identify the main aromatic compounds of the MRPs. Eleven main volatiles in the dichloromethane extracts from the browned glucose-ammonium solutions heated by two methods were identified. Their mass spectra were compared with the spectra from the library for confirmation, and the LRIs and matching degrees are displayed in Table 2 (Guan et al. 2011a; Tan and Yu 2012; Van Boekel and Martins 2002; Van Lancker et al. 2010). Both methods heated at different temperature and time contained pyrazine, alkanes, esters, ketone, and amide.
At the heating temperature of 100 °C for 30 min, the amounts of alkanes and amide were identified. They constituted more than 50% of the total extracted. On the contrary, no ester and pyrazine was identified during this time and temperature and they began to generate at the temperature of 110 °C for 20 min and 120 °C for 30 min, respectively. The result indicated that pyrazine were more likely to be formed at higher reaction temperature and longer reaction time. In general, the main flavor compounds were identified as 2,3-dimethyl-pyrazine A), 2,6-dimethyl-pyrazine B), 2-hydroxy-3-methyl-2-cyclopentene-1-one C), 3-methyl-2-pyrazinyl-methanol, D) 6-methyl-2-pyrazinyl-methanol E), 2-methylpropyl-pyrazine F), 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester G), and (Z)-13-docosenamide H). Among them, A), B), and F) were generally formed in many Maillard reaction model system, such as L-ascorbic acid-L-glutamic acid/L-aspartic acid (Tan and Yu 2012), sugar-peptides (Van Lancker et al. 2012), glucose-glycine (Ames et al. 2001), and so on. In addition, A) and B), as two of the most common flavor compounds, are used for preparation of a series of essence of coffee, chocolates, meats and nuts and meats, coca, nuts, popcorn, potato, bread, coffee, chocolates, cream, and tobacco, respectively. C), E), and F) were firstly reported in the glucose-ammonium model system; especially C) is widely used for preparation of essences of creamy hard sugar, smoky, and apricot.
No significant differences were observed between MWH and OBH on the contents of pyrazine compounds (p > 0.05) (data not shown). The formation mechanism of pyrazines must be responsible for this result. The formation pathway of pyrazines proposed by Yu et al. (2013) indicated that the pyrazines are generated from the degradation products of UIPs (Fig. 4). As analyzed previously in the results of A294 and A420, MWH mainly promotes the formation of BPs, but not affects the amount of UIPs in the Maillard reaction. Hence, MWH may affect the formation rate of pyrazines and do not affect the amounts of pyrazines.
GC/MS Analysis of Ethyl Acetate Extracts
As shown in Fig. 5, four main volatiles in the ethyl acetate extracts from the browned glucose-ammonium solutions heated by MW and OB were listed with their retention time, LRI, structural formula, matching degrees, and CAS No. Two poly-dimethyl-siloxane-like compounds (Fig. 5, i and ii) identified from GC column bleeding were negligible. Compared with the dichloromethane extracts, only four volatiles were identified in the ethyl acetate extracts, and G) and H) were identified in both dichloromethane and ethyl acetate extracts. D-limonene I) and 1,2-benzenedicarboxylic acid, bis (2-methylpropyl) ester J) were only identified in the ethyl acetate extracts.
In dichloromethane extracts, there are no significant differences between MW and OBH on the contents of flavor compounds. However, the amounts of I) was identified at the heating temperature of 110 °C for 30 min for MWH, while for OBH, the amounts of I) was identified at the heating temperature of 120 °C for 20 min. The amounts of I) heated by MW were more than that of the OBH method (p < 0.05) at the same reaction temperature and time according to the Fig. 5. It is obvious that the microwave heating can improve the generation of I) in the ethyl acetate extracts. There is no satisfying explanation could be found for this observation, but the differences of heating principle must be responsible for the promotion of I). In microwave heating, electromagnetic energy converts into thermal energy, and thus, it is a kind of energy conversion rather than heat transfer. Microwave radiations can interact with polar molecules and charged ions and result in molecular frictions and ion migration within food matrix (Chandrasekaran et al. 2013). Hence, MW may be used to promote the formation of D-limonene in Maillard reaction. D-limonene is used for preparing essences of white lemon and citrus; thus, MW can be recommended for heating some foods related to Maillard reaction to enhance lemon flavor.
Conclusion
Effect of microwave and oil bath heating on Maillard reaction was investigated from glucose-ammonium system in present study. Results showed that microwave heating can promote the decrease of pH values, increase in A420, and degradation of glucose. Furthermore, microwave process decreased significantly whiteness and chroma and affected the formation of D-limonene in the glucose-ammonium system. Because of the slightly influence in A294, microwave heating did not affect the contents of pyrazines. In addition, three flavor compounds, such as 2-hydroxy-3-methyl-2-cyclopentene-1-one, 3-methyl-2-pyrazinyl-methanol, and 6-methyl-2-pyrazinyl-methanol were firstly reported in the glucose-ammonium system.
References
Abdelhedi, O., Mora, L., Jemil, I., Jridi, M., Toldra, F., Nasri, M., & Nasri, R. (2017). Effect of ultrasound pretreatment and Maillard reaction on structure and antioxidant properties of ultrafiltrated smooth-hound viscera proteins-sucrose conjugates. Food Chemistry, 230, 507–515.
Ajandouz, E. H., Tchiakpe, L. S., Dalle Ore, F., Benajiba, A., & Puigserver, A. (2001). Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. Journal of Food Science, 66(7), 926–931.
Ames, J. M., Guy, R. C. E., & Kipping, G. J. (2001). Effect of pH, temperature, and moisture on the formation of volatile compounds in glycine/glucose model systems. Journal of Agricultural and Food Chemistry, 49(9), 4315–4323.
Benjakul, S., Lertittikul, W., & Bauer, F. (2005). Antioxidant activity of Maillard reaction products from a porcine plasma protein-sugar model system. Food Chemistry, 93(2), 189–196.
Chandrasekaran, S., Ramanathan, S., & Basak, T. (2013). Microwave food processing—a review. Food Research Interational, 52(1), 243–261.
Charapitsa, S. V., Kavalenka, A. N., Kulevich, N. V., Makoed, N. M., Mazanik, A. L., Sytova, S. N., Zayats, N. I., & Kotov, Y. N. (2013). Direct determination of volatile compounds in spirit drinks by gas chromatography. Journal of Agricultural and Food Chemistry, 61(12), 2950–2956.
Davidek, T., Devaud, S., Robert, F., & Blank, I. (2006). Sugar fragmentation in the Maillard reaction cascade: Isotope labeling studies on the formation of acetic acid by a hydrolytic beta-dicarbonyl cleavage mechanism. Journal of Agricultural and Food Chemistry, 54(18), 6667–6676.
Feng, Y. Z., Cai, Y., Su, G. W., Zhao, H. F., Wang, C. X., & Zhao, M. M. (2014). Evaluation of aroma differences between high-salt liquid-state fermentation and low-salt solid-state fermentation soy sauces from China. Food Chemistry, 145, 126–134.
Feng, Y., Su, G. W., Zhao, H. F., Cai, Y., Cui, C., Sun-Waterhouse, D., & Zhao, M. M. (2015). Characterisation of aroma profiles of commercial soy sauce by odour activity value and omission test. Food Chemistry, 167, 220–228.
Gao, L. H., Liu, T., An, X. J., Zhang, J. L., Ma, X. R., & Cui, J. M. (2017). Analysis of volatile flavor compounds influencing Chinese-type soy sauces using GC-MS combined with HS-SPME and discrimination with electronic nose. Journal of Food Science and Technology-Mysore, 54(1), 130–143.
Guan, J. J., Zhang, T. B., Hui, M., Yin, H. C., Qiu, A. Y., & Liu, X. Y. (2011). Mechanism of microwave-accelerated soy protein isolate-saccharide graft reactions. Food Research International, 44(9), 2647–2654.
Guan, Y. G., Wang, J., Yu, S. J., Xu, X. B., & Zhu, S. M. (2010a). Effects of ultrasound intensities on a glycin-maltose model system-a means of promoting Maillard reaction. International Journal of Food Science and Technology, 45(4), 758–764.
Guan, Y. G., Lin, H., Han, Z., Wang, J., Yu, S. J., Zeng, X. A., Liu, Y. Y., Xu, C. H., & Sun, W. W. (2010b). Effects of pulsed electric field treatment on a bovine serum albumin-dextran model system, a means of promoting the Maillard reaction. Food Chemistry, 123(2), 275–280.
Guan, Y. G., Zhang, B. S., Yu, S. J., Wang, X. R., Xu, X. B., Wang, J., Han, Z., Zhang, P. J., & Lin, H. (2011a). Effects of ultrasound on a glycin-glucose model system—a means of promoting Maillard reaction. Food and Bioprocess Technology, 4(8), 1391–1398.
Guan, Y. G., Shi, W. H., Yu, S. J., & Xu, X. B. (2011b). Ultrafiltration of caramel color solutions reduces 5-hydroxymethyl-2-furaldehyde. Journal of Membrane Science, 380(1–2), 9–12.
Guan, Y. G., Yu, P., Yu, S. J., Xu, X. B., Shi, W. H., & Sun, W. W. (2011c). Effects of pressure on the glucose-ammonium sulphite caramel solutions. Food Chemistry, 127(2), 596–601.
Guo, Q. S., Sun, D. W., Cheng, J. H., & Han, Z. (2017). Microwave processing techniques and their recent applications in the food industry. Trends in Food Science & Technology, 67, 236–247.
Han, Z., Li, H., Yu, X. C., & Sun, D. W. (2016). Effects of low temperature cooking on the retention of 4-(methylthio)-3-butenyl isothiocyanate (MTBITC) of Chinese white radish (Raphanussativus L.) Food and Bioprocess Technology, 9(10), 1640–1647.
Hong, X., Meng, J., & Lu, R. R. (2015). Improvement of ACE inhibitory activity of casein hydrolysate by Maillard reaction with xylose. Journal of the Science of Food and Agriculture, 95(1), 66–71.
Huang, L., & Sites, J. (2010). New automated microwave heating process for cooking and pasteurization of microwaveable foods containing raw meats. Journal of Food Science, 75(2), E110–E115.
Karangwa, E., Murekatete, N., Habimana, J. D. D., Masamba, K., Duhoranimana, E., Muhoza, B., & Zhang, X. M. (2016). Contribution of crosslinking products in the flavor enhancer processing: the new concept of Maillard peptide in sensory characteristics of Maillard reaction systems. Journal of Food Science and Technology-Mysore, 53(6), 2863–2875.
Kerth, C. (2016). Determination of volatile aroma compounds in beef using differences in steak thickness and cook surface temperature. Meat Science, 117, 27–35.
Kim, J., Her, J., & Lee, K. (2015). Formation and reduction of carcinogenic furan in various model systems containing food additives. Food Chemistry, 189(SI), 108–113.
Lee, S. E., Chung, H., & Kim, Y. S. (2012). Effects of enzymatic modification of wheat protein on the formation of pyrazines and other volatile components in the Maillard reaction. Food Chemistry, 131(4), 1248–1254.
Lertittikul, W., Benjakul, S., & Tanaka, M. (2007). Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein-glucose model system as influenced by pH. Food Chemistry, 100(2), 669–677.
Li, H., Sun, D. W., Han, Z., & Yu, X. C. (2017). Effects of low temperature cooking methods and holding times on selected quality attributes of cooked pork longissimus dorsi. Journal of Food Process Engineering, 40(6), e12585. https://doi.org/10.1111/jfpe.12585.
Liu, J. B., Liu, M. Y., He, C. C., Song, H. L., Guo, J., Wang, Y., Yang, H. Y., & Su, X. X. (2015). A comparative study of aroma-active compounds between dark and milk chocolate: relationship to sensory perception. Journal of the Science of Food and Agriculture, 95(6), 1362–1372.
Luo, D. S., Chen, J., Gao, L., Liu, Y. P., & Wu, J. H. (2017). Geographical origin identification and quality control of Chinese chrysanthemum flower teas using gas chromatography-mass spectrometry and olfactometry and electronic nose combined with principal component analysis. International Journal of Food Science and Technology, 52(3), 714–723.
MeiPner, K., & Erbersdobler, H. F. (1996). Maillard reaction in microwave cooking: comparison of early Maillard products in conventionally and microwave-heated milk. Journal of the Science of Food and Agriculture, 70, 307–310.
Monti, S. M., Borrelli, R. C., Ritieni, A., & Fogliano, V. (2000). A comparison of color formation and Maillard reaction products of a lactose-lysine and lactose-N-alpha-acetyllysine model system. Journal of Agricultural and Food Chemistry, 48(4), 1041–1046.
Morales, F. J., & Jimenez-Perez, S. (2001). Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chemistry, 72(1), 119–125.
Nasrollahzadeh, F., Varidi, M., Koocheki, A., & Hadizadeh, F. (2017). Effect of microwave and conventional heating on structural, functional and antioxidant properties of bovine serum albumin-maltodextrin conjugates through Maillard reaction. Food Research International, 100(Pt 2), 289–297.
O’Charoen, S., Hayakawa, S., & Ogawa, M. (2015). Food properties of egg white protein modified by rare ketohexoses through Maillard reaction. International Journal of Food Science and Technology, 50(1), 194–202.
Pino, J. A., Mesa, J., Munoz, Y., Marti, M. P., & Marbot, R. (2005). Volatile components from mango (Mangifera indica L.) cultivars. Journal of Agricultural and Food Chemistry, 53(6), 2213–2223.
Pu, Y. Y., & Sun, D. W. (2017). Combined hot-air and microwave-vacuum drying for improving drying uniformity of mango slices based on hyperspectral imaging visualization of moisture content distribution. Biosystems Engineering, 156, 108–119.
Rufián-Henares, J. A., García-Villanova, B., & Guerra-Hernández, E. (2002). Furosine content, loss of o-phthaldialdeyde reactivity, fluorescence and colour in stored enteral formula. International Journal of Dairy Technology, 55(3), 121–126.
Scalone, G. L. L., Cucu, T., De Kimpe, N., & De Meulenaer, B. (2015). Influence of free amino acids, oligopeptides, and polypeptides on the formation of pyrazines in Maillard model systems. Journal of Agricultural and Food Chemistry, 63(22), 5364–5372.
Schumacher, D., & Kroh, L. W. (1994). The effect of Maillard reaction products on enzyme reactions. Zeitschrift fur Ernahrungswissenschaft, 35(3), 213–225.
Sengar, G., & Sharma, H. K. (2014). Food caramels: a review. Journal of Food Science and Technology-Mysore, 51(9), 1686–1696.
Shaman, A., Mizrahi, S., Cogan, U., & Shimoni, E. (2007). Examining for possible non-thermal effects during heating in a microwave oven. Food Chemistry, 103(2), 444–453.
Tan, Z. W., & Yu, A. N. (2012). Volatiles from the Maillard reaction of L-ascorbic acid with L-glutamic acid/L-aspartic acid at different reaction times and temperatures. Asia-Pacific Journal of Chemical Engineering, 7(4), 563–571.
Van Boekel, M. A. J. S., & Martins, S. I. F. S. (2002). Fate of glycine in glucose–glycine reaction: A kinetic analysis. International Congress Series, 1245, 289–293.
Van Lancker, F., Adams, A., & De Kimpe, N. (2010). Formation of pyrazines in Maillard model systems of lysine-containing dipeptides. Journal of Agricultural and Food Chemistry, 58(4), 2470–2478.
Van Lancker, F., Adams, A., & De Kimpe, N. (2012). Impact of the N-terminal amino acid on the formation of pyrazines from peptides in Maillard model systems. Journal of Agricultural and Food Chemistry, 60(18), 4697–4708.
Varlet, V., Knockaert, C., Prost, C., & Serot, T. (2006). Comparison of odor-active volatile compounds of fresh and smoked salmon. Journal of Agricultural and Food Chemistry, 54(9), 3391–3401.
Vhangani, L. N., & Van Wyk, J. (2013). Antioxidant activity of Maillard reaction products (MRPs) derived from fructose–lysine and ribose–lysine model systems. Food Chemistry, 137(1), 92–98.
Wang, H., Tu, Z. C., Liu, G. X., Liu, C. M., Huang, X. Q., & Xiao, H. (2013). Comparison of glycation in conventionally and microwave-heated ovalbumin by high resolution mass spectrometry. Food Chemistry, 141(2), 985–991.
Wegener, S., Kaufmann, M., & Kroh, L. W. (2017). Influence of L-pyroglutamic acid on the color formation process of non-enzymatic browning reactions. Food Chemistry, 232, 450–454.
Wu, X. L., Kong, F. S., Huang, M. H., & Yu, S. J. (2015). Effects of pH on the formation of 4(5)-Methylimidazole in glucose/ammonium sulfate and glucose/ammonium sulfite caramel model reactions. Food Research International, 76(3), 661–665.
Yu, A. N., Tan, Z. W., & Shi, B. (2012). Influence of the pH on the formation of pyrazine compounds by the Maillard reaction of L-ascorbic acid with acidic, basic and neutral amino acids. Asia-Pacific Journal of Chemical Engineering, 7(3), 455–462.
Yu, A. N., Tan, Z. W., & Wang, F. S. (2013). Mechanistic studies on the formation of pyrazines by Maillard reaction between L-ascorbic acid and L-glutamic acid. LWT-Food Science and Technology, 50(1), 64–71.
Yu, A. N., Zhou, Y. Y., & Yang, Y. N. (2017). Kinetics of browning and correlations between browning degree and pyrazine compounds in L-ascorbic acid/acidic amino acid model systems. Food Chemistry, 221, 1678–1684.
Acknowledgements
All authors acknowledge the National Science Foundation of China (Grant No. 31771931), Science and Technology Planning Project of Guangdong Province of China (No. 2014B020205001 and No. 2013B051000015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, H., Wu, CJ. & Yu, SJ. Impact of Microwave-Assisted Heating on the pH Value, Color, and Flavor Compounds in Glucose-Ammonium Model System. Food Bioprocess Technol 11, 1248–1258 (2018). https://doi.org/10.1007/s11947-018-2093-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2093-6