Abstract
The shelf life of Dictyophora rubrovolvata (D. rubrovolvata) has been strongly limited by autolysis, a natural process of self-degradation. Conventional heat treatment methods (hot water or hot air) are not suitable for D. rubrovolvata fruiting bodies because of their fragile and porous structure. Therefore, in this work, we attempted to use microwave treatment (MT/100 W, 75 W, 50 W) to delay autolysis and extend the shelf life of D. rubrovolvata fruiting bodies. The results showed that MT could delay the decrease in cellulose, chitosan, and β-1,3 glucan contents by inhibiting the corresponding enzyme activity and maintaining a high level of energy charge by delaying the decrease in ATP and ADP. Meanwhile, compared with the control group (CK), D. rubrovolvata fruiting bodies after MT had improvements in many qualities during storage (4 °C, 95% RH), including delayed deterioration of water migration, sensory evaluation, browning, shear force, ethanol, malondialdehyde (MDA), relative conductivity, and respiratory rate. Furthermore, D. rubrovolvata fruiting bodies after MT maintained contents of umami compounds compared to CK, which included free amino acids, 5′-nucleotides, and equivalent umami concentration (EUC). The electronic nose (E-nose) results showed that MT maintained a better flavor. Notably, the effect of low power (50 W) was better than that of high power (100 W, 75 W). Thus, microwaves could effectively regulate autolysis and energy metabolism of D. rubrovolvata fruiting bodies during the postharvest period. Therefore, microwaves can be applied as a pretreatment method, providing valuable insights regarding postharvest mushrooms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the edible mushrooms grown in plantations under forests is Dictyophora rubrovolvata (D. rubrovolvata), which has excellent edible value due to its unique flavor and texture, vitamins, minerals, amino acids, and polysaccharides (Zhuang & Sun, 2011). In addition, this mushroom possesses antitumor, liver protection, and hypoglycemic (Sun et al., 2017). Considering that Southwest China has a high forest coverage rate, such as in Sichuan, Guizhou, and Yunnan, planting D. rubrovolvata under the cover of a forest provides economic benefits. Throughout the “China’s 13th five-year plan” period, the cultivation of D. rubrovolvata has played a pivotal role in the development of the local edible mushroom industry. However, fresh D. rubrovolvata possesses a short postharvest life due to autolysis.
In fact, autolysis was the most serious problem of mushroom during the storage and transportation. It also occurs widely in mushroom species, such as Lentinula edodes, Agaricus bisporus, and Coprinus comatus. In previous studies, researchers focused on various hydrolases in mushroom cells, including cellulases, glucanases, and chitinase. During the autolysis process, once the membrane structure is destroyed, the independence of the organelle is broken, and its own enzyme is released. Therefore, enzymatic reactions can occur rapidly because of contact with the substrate. When autolysis occurs during postharvest storage of mushrooms, the intracellular activity of chitinase and β-(1,3)-glucanases is increased (Peng et al., 2020). Autolysis is also caused by metabolic disorders in mushrooms, which leads to a decrease in mitochondrial transmembrane potential, a decrease in energy charge level, and insufficient energy supply (Decros et al., 2019). Furthermore, it has been shown that the autolysis of mushroom was also closely related to energy metabolism. The sufficient energy supply was key to maintaining the physiological metabolism of postharvest mushrooms, which can contribute to the integrity of the cell membrane and reduce oxidative stress (Wang et al., 2019a, b). However, during postharvest storage, mushrooms mainly obtain energy by consuming nutrients, which often accelerated autolysis (Gong et al., 2020). In Yang et al.’s report, autolysis occurred because of downregulation of related proteins during autolysis which leaded to the reduction in ATP production and caused the disorders of energy metabolism. Therefore, it was necessary for mushroom to delay the autolysis and maintain the energy metabolism level after postharvest.
Currently, in the realm of postharvest storage, various methods are employed to extend the shelf life of mushrooms (Lurie & Pedreschi, 2014). Heat shock is one of the physical methods. Its underlying principle involves placing the fruiting bodies at elevated temperatures for a specific duration and subsequently rapidly cooling them. Hot air and hot water have been widely employed. Zhao et al. used hot air to Lentinula edodes by short-term partial dehydration (STPD). Consequently, the storage life of Lentinula edodes was prolonged, which can effectively inhibit the activity of polyphenol oxidase (PPO) and maintain the content of flavonoid compounds and flavor substances (Zhao et al., 2021). In Barron et al.’s work, Agaricus bisporus was bloped with hot water at 90 °C for 60 s. The results showed that hot water could effectively inhibit PPO activity and browning in Agaricus bisporus (Barrón-García et al., 2022).
In previous experiments, we found that D. rubrovolvata is prone to water absorption because of its fragile and porous structure. Therefore, following the application of hot water, D. rubrovolvata cannot be dried in a short time as it accelerates the deterioration of fruiting bodies. On the other hand, the moisture of D. rubrovolvata is readily evaporated by hot air, resulting in shrinkage and wilting of fruiting bodies. Microwave treatment (MT) is a new postprocessing method that warrants further development (Guzik et al., 2021). MT is more efficient than hot air and hot water and has been applied to fruits and vegetables. The studies investigated that microwave treatment could reduce peaches’ internal browning caused by chilling injury (Wang et al., 2023), delay the softening in persimmon (Chen et al., 2021), and inhibited yellowing and transpiration in bok choy (Song et al., 2018). In addition, Sisquella et al. (2013) found that the brown rot caused by Monilinia fructicola in nectarine fruits was decreased 30% by inhibiting the growth of pathogens after treated with microwave power of 17.5 kW for 50 s. Based on the reported studies, we hypothesized that MT may inhibit the activity of relevant autolytic enzymes and regulate the energy metabolism level of mushroom fruiting bodies. In this study, the effect of MT on the shelf life of D. rubrovolvata was investigated. The changes in sense, texture, physiology, flavor substances, autolytic substrates (cellulose, chitin, and glucan), and enzyme (cellulase, chitinase, and glucanase) activities were investigated. Furthermore, we studied the regulatory mechanism of MT on autolysis and energy metabolism. To the best of our knowledge, this technology has rarely been reported for prolonging the postharvest life of mushrooms.
Materials and Methods
Mushroom and Chemicals
Dictyophora rubrovolvata fruiting bodies were harvested on 3 October 2022, picked from Meiweixian Farm (Guiyang County, Guizhou Province, 106.71°E, 26.59°W) which were selected as experimental samples by uniform in size and without mechanical damage, autolysis or browning, and transported to the lab within 1 h. Experimental fruiting bodies of D. rubrovolvata were selected based on their size, shape, and absence of visible disease symptoms and mechanical injury. Analytical grade or higher purity reagents and chemicals were used throughout the experiment and were purchased from Aladdin Chemical Reagent (Shanghai, China).
Microwave Equipment and Treatments
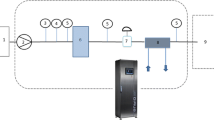
As shown in Fig. 1A, we constructed a microwave system consisting of a solid-state microwave source, a circulator, a directional coupler connected to a microwave power meter, and a multi-mode microwave resonant cavity with a rotational plate (Fig. 1B). The solid-state microwave source (WSPS-2450-1k-CCWA, Wattsine, Chengdu, China) can provide an adjustable power supply at 2.45 GHz. The circulator separates the input and reflected microwave powers, while the directional coupler, paired with a microwave power meter (NRX, R&S, Germany), allows for real-time monitoring of these powers. The microwave processing system we built boasts three remarkable advantages compared to a domestic microwave oven. First, the power transmitted from the source to the multi-mode resonant cavity can be continuously increased up to 1 kW, with real-time detection ensuring precise control. Additionally, the rectangular resonant cavity’s larger size allows for processing a greater volume of the sample at once. Lastly, the rotational plate in the cavity forces the sample to rotate at a consistent speed of 4 rpm during microwave treatment, which improves heating uniformity and ensures consistent quality of microwave treatments. Fig. S1 shows the temperature distribution of the heated sample D. rubrovolvata; it can be seen that the temperature difference does not exceed 5 °C across the entire heating area. Based on the temperatures at the sample points in the thermal image, the coefficient of variation (COV) value is calculated to be approximately 0.0573, which is considerably low (Chen et al., 2016). In microwave engineering, such a low COV value (< 0.4) indicates that the heating uniformity of this microwave equipment is quite high and the rotation is indeed effective. Besides, the low temperature prevents the microwave heating from damaging the normal physiological metabolism of fruits and vegetables.
Firstly, the preliminary experiment was set for choosing MT parameters by shear force and sensory evaluation. To choose the best conditions and explore the mechanisms, the MT parameters chosen by the preliminary experiment were further refined. The specific experiments were arranged as follows:
Preliminary Experiment
D. rubrovolvata was divided into seven groups. Thirty fruiting bodies were selected randomly from each group, placed in a cylindrical glass container, and then put into the microwave equipment, with three replicates performed for each group. The MT power was set to 50 W, 75 W, and 100 W, and the processing time was 30 s and 60 s, respectively. After cooling at 4 °C for 4 h, the fruiting bodies were packaged in PE20 modified atmosphere packaging. The untreated group was considered the control group (CK). All groups were stored at 4 °C and 95% relative humidity (RH) for 7 days. The changes in shear force and sensory evaluation could most directly reflect in the physiological quality of D. rubrovolvata during storage. Therefore, the shear force and sensory evaluation were performed on 7 days. According to the shear force (Fig. S2) and sensory evaluation (Fig. S3), the shear force of the treatment with microwave at 50 W for 60 s and 75 W for 30 s was 4.13 N and 4.33 N, which were significantly lower than other groups (P < 0.05), and the shear force was 32 and 35 points, which were significantly higher than other groups (P < 0.05). These results showed that these two MT conditions can effectively delay the rise in shear force and maintain the sensory evaluation of the D. rubrovolvata.
Formal Experiment
Chen et al. (2021) reported that high-power short-time (HPST) treatments were more effective in maintaining the postharvest quality of persimmons than low-power long-time (LPLT) treatments. To further investigate the effect of MT on fruiting bodies, a formal experiment was designed, and the microwave power and treatment duration were optimized. The experiments were set in a microwave at 100 W for 15 s (M1), 75 W for 30 s (M2), 75 W for 60 s (M3), 50 W for 60 s (M4), and 50 W for 90 s (M5) based on our per-experimental results (date is shown in Figs. S2 and S3). The method was consistent with the previous experiment. Thirty fruiting bodies from each group were randomly chosen and arranged in a cylindrical glass vessel. Subsequently, they were placed inside the microwave equipment and performed three repetitions per group. All groups were stored at 4 °C and 95% RH for 12 days. Subsequent physiological and biochemical assays were performed at 0, 3, 6, 9, and 12 days.
Sensory Qualities, Browning Degree (BD), Shear Force, and Ethanol
Based on the methods of Sun et al. (2017), with minor modifications, a sensory assessment was conducted. To conduct the sensory evaluation, 15 trained panelists were selected. Table 1 shows the sensory evaluation standard for D. rubrovolvata. The control group was fresh fruiting bodies (full score = 50). The overall acceptability of mushroom quality was judged by a sensory score greater than 30 (Wang et al., 2021).
The method of Liu et al. referred to the determination of browning degree with slight modification (Liu et al., 2019). Five grams of fruiting body sample was accurately weighed and ground with 20 mL of 0.2 mol L−1 sodium phosphate buffer (pH 6.8). After resting at 4 °C for 15 min, the sample was centrifuged at 4 °C and 10,000 g for 15 min. The absorbance of the supernatant was measured at 420 nm by an ultraviolet‒visible spectrophotometer.
Shear force was measured according to the method of Dong et al. (2022). Shear force was measured using a texture analyzer (EZ-Test, Shimazu, Japan). The P/2 N probe was selected; the posttest speed was 10 mm/s; the test speed was 3 mm/s, and the test distance was 60 mm.
According to Ventura-Aguilar et al.’s (2017) report, the ethanol content of fruiting bodies was determined. The sample to be tested was weighed and placed in a 20-mL headspace vial, which was then sealed. The sample was incubated at 80 °C for 30 min before injection. The temperature of the injection needle was maintained at 85 °C, and the injection volume was 100 µL. Each set of samples was tested in triplicate. The amount of ethanol was calculated as mg kg−1.
Low-Field Nuclear Magnetic Resonance (LF-NMR) Transverse Relaxation Measurements, Malondialdehyde (MDA) Content, Determination of Membrane Permeability, and Respiratory Rate
LF-NMR was measured according to the method of Cheng et al. (2020). D. rubrovolvata fruiting bodies were placed into the LF-NMR (MesoMR23-060H-I, Pneumax, Shanghai) spectrometer for detection and repeat the process three times.
The determination of MDA was based on the method of Yang et al. (2023). Samples were homogenized in ice bath with 5 mL of trichloroacetic acid, followed by centrifugation at 10,000 g and 4 °C for 20 min. A volume of 2 mL of the supernatant was taken and mixed with 2 mL of thiobarbituric acid solution and heated in a boiling water bath for 20 min. After a further centrifugation at 10,000 g for 20 min, the supernatant was collected. The absorbance is measured using a spectrophotometer at wavelengths of 450 nm, 532 nm, and 600 nm, respectively. The amount of MDA was calculated as mmol kg−1.
The relative degree of electrolyte leakage was measured according to the method of Zhu et al. (2024). Samples were added 40 mL of deionized water and shaked for 30 min. Measure the electrical conductivity immediately (P0). Then, place the mixture in a boiling water bath for 10 min and measure the electrical conductivity again after cooling (P1). The relative degree of electrolyte leakage was calculated using the formula P0/P1 × 100 and expressed as a percentage.
The respiratory rate was measured by the method of Ye et al. (2023). D. rubrovolvata fruiting bodies were placed in sealed jars at 25 °C for 2 h. The gas in the jars was measured by a headspace analyzer (CheckPoint 3, Ametek, Denmark). The respiratory rate was calculated as mg CO2 kg−1 h−1.
Contents of Cellulose, Chitin, and Glucan
Determination of cellulose was used as described previously by Yang et al. (2019). The experimental sample was centrifuged at 4000 g for 10 min after ground with 10 mL ethanol. A colorimetric anthrone assay was used to determine the cellulose content of the precipitate after digestion with 60% sulfuric acid (H2SO4) for 30 min. The amount of cellulose was calculated as g kg−1.
The determination method of chitin was referred to Yang et al. (2022) with slight modification. The sample was hydrolyzed by 37% HCl at 110 °C for 4 h. Hydrolysates were then mixed with 2% resorcinol and 75% H2SO4. After cooling to room temperature, the mixture was heated in a boiling water bath for 30 min, and the absorbance was measured at 500 nm. The amount of chitin was calculated as g kg−1.
In addition, the glucan content in fruiting bodies was determined using a glucan assay kit (Megazyme International, Ireland). The amount of glucan was calculated as g kg−1.
Contents of Free Amino Acids, 5′-Nucleotides, Equivalent Umami Concentration (EUC), and Analysis of Electronic Nose
The composition of free amino acids within the D. rubrovolvata fruiting bodies was analyzed employing a modified version of the technique introduced by Liu et al. (2021), with slight adjustments. A lyophilized sample was mixed with 6 mol/L HCl acid to initiate the procedure. After introducing nitrogen into the bottle for 1 min, the bottle was sealed and hydrolyzed at 110 °C for 22 h. As a result of hydrolysis, the supernatant was collected by centrifugation at 5000 g for 10 min. This supernatant was then combined with H2SO4. After standing in darkness at 25 °C for 30 min, the resulting mixture was centrifuged for 10 min at 5000 g. Following filtration using a 0.22-mm filter membrane, the filtrate was analyzed using an amino acid analyzer (A388pro, Membrapure, Germany). The amount of free amino acids was calculated as g kg−1.
5′-Nucleotides were extracted from the sample and quantified following the method outlined by Zhang et al. (2019), with minor adjustments. Freeze-dried mushroom samples were combined with 25 mL of distilled water and subjected to boiling water immersion for a duration of 10 min. Following this step, the mixture was centrifuged (1000 g, 15 min) and then filtered through a 0.45-mm membrane. RIGOL L-3000 (Puyuan, Beijing, China) was used for high-performance liquid chromatography analysis of the extraction solution. The amount of free amino acids was calculated as g kg−1.
Monosodium glutamate (MSG) was used to standardize umami sensory effects between compounds using the EUC value, which corresponded to the umami intensity of a mixture of amino acids and 5′-nucleotides. The EUC value is calculated according to the method of Mau (2005) and expressed by g MSG kg−1.
Electronic noses (Portable Electronic Nose Pen3, Airsense, German) have ten sensors which can detect fresh, salty, sour, sweet, and bitter samples. In order to detect and analyze its taste characteristics, the electronic nose system was used after volatilizing the mushroom gas.
Activities of Cellulase, Chitinase, and Glucanase
Mushrooms were ground in an ice bath with 0.1 mol/L precooled phosphate buffer (pH 7.0) and centrifuged for 20 min at 12,000 g (4 °C). For enzyme activity analysis, the supernatant was collected as a crude enzyme extract solution.
The determination of cellulase followed the method described by Nguyen and Nguyen (2021) with slight modification. One cellulose activity unit was defined as the amount of enzyme that produced 1.0 g of glucose by hydrolyzing sodium carboxymethylcellulose (CMC) per minute at 37 °C. As a result, the crude enzyme extract solution and CMC were heated at 37 °C for 1 h. The methods of Jiang et al. (2010) were based on the determination of glucose. Into the extract, 0.5 mL of laminarin solution was added and incubated in a 40 °C water bath for 30 min as a blank control group. Then, 3 mL of DNS reagent was added, and the mixture was placed in a boiling water bath for 15 min for color development. Immediately after the color development was completed, the solution was cooled and made up to a volume of 25 mL with distilled water. The absorbance was measured at a wavelength of 550 nm. The methods of Wang et al. (2018) were based on the determination of chitinase. The mixture of 0.5 mL of acetic acid-sodium acetate buffer and 0.5 mL of colloidal chitin suspension was incubated in a 37 °C water bath for 1 h. Subsequently, 3 mL of DNS reagent was added, and the mixture was boiled for 15 min to develop color. After cooling, the absorbance of both groups was measured at a wavelength of 520 nm. The units of all enzymes were represented by U g−1. All the contents of chemical components and enzymes are based on fresh weight.
Contents of ATP, ADP, AMP, and Energy Charge
The contents of adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) were determined according to Li et al. (2018) and by a RIGOL L-3000 high-performance liquid chromatography system (RIGOL L-3000, Puyuan, Beijing, China) equipped with a Diamonsil C18 column. The supernatants were collected after 5.0 g frozen samples were homogenized in 10 mL of 0.6 mol/L perchloric acid and centrifuged for 20 min at 4 °C. The supernatants were then neutralized to pH 6.4–6.8 with 1 M KOH and incubated at 37 °C for 24 h. The composition of mobile phase A included 0.03 mol/L K2HPO4 and 0.02 mol/L KH2PO4, whereas mobile phase B consisted of methanol. A gradient elution schedule was employed, starting with 100% A at 0 min, changing to 80% A and 20% B at 7 min, then to 75% A and 25% B at 9 min, and finally reaching 100% B at 10 min. The flow rate was maintained at 1 mL/min, and the volume injected was set at 10 µL. The amount of ATP, ADP, and AMP were calculated as g kg−1. Energy charge (EC) was calculated as follows:
Statistical Analysis
Data were presented as mean ± SE (standard error). Duncan’s multiple range tests (P < 0.05) were used to analyze data differences using SPSS software version 20.0 (SPSS Inc., USA).
Results
Changes in Sensory Qualities, Browning Degree (BD), Shear Force, and Ethanol Contents
Sensory evaluation is an important index with which to evaluate the quality of mushrooms during postharvest storage. As illustrated in Fig. S4 and 2A, the quality of fruiting bodies declined with storage time during the storage period. Among them, M1 represents 100 W for 15 s, M2 represents 75 W for 30 s, M3 represents 75 W for 60 s, M4 represents 50 W for 60 s, M5 represents 50 W for 90 s, and the untreated group was considered the control group (CK). Compared with the CK, the overall sensory scores of the fruiting bodies after MT were significantly higher than those of CK, except for M3, and all groups. M4 showed the highest overall sensory scores on 12 days (Fig. 2A). According to the report of Wang et al. (2021), other groups were not accepted by consumers except for M4 and M2 (overall sensory scores < 30).
Effect of MT on overall sensory scores (A), browning degree (BD) (B), shear force (C), and ethanol content (D) of D. rubrovolvata fruiting bodies during storage. Among them, M1 represents 100 W for 15 s, M2 represents 75 W for 30 s, M3 represents 75 W for 60 s, M4 represents 50 W for 60 s, M5 represents 50 W for 90 s, and the untreated group was considered the control group (CK). The vertical bars represent the standard errors of the means (n = 3). Different lettering indicates statistically significant differences (P < 0.05) between groups, while identical lettering indicates no difference between groups
Similar to Agaricus bisporus (Li et al., 2019), D. rubrovolvata fruiting bodies are gradually browned after harvest. From Fig. 2B, BD increased with the extension of storage time. However, MT effectively inhibited BD in comparison with CK. On 12 days, the BD values of all MT samples were significantly lower than that of CK, while the BD values of M1 and M3 were significantly higher than those of M4, M5, and M2 (P < 0.05). This may be because MT could effectively reduce enzyme activity, which inhibited enzymatic browning (Baltacıoğlu et al., 2015).
The effect of MT on shear force during storage is shown in Fig. 2C. The shear force of fruiting bodies provided an upward trend; two main factors contributed to this, which were the loss of water and fast softening of fruiting bodies (Zivanovic & Buescher, 2004). The results indicated that MT inhibits the rise in shear force compared with CK (8.09 N). Likewise, M4 (6.03 N) exhibited significant inhibition of the increase in shear force compared with the other groups (P < 0.05).
Ethanol is a byproduct of anaerobic respiration in plants, which could disrupt the normal metabolism of mushroom cells (Ventura-Aguilar et al., 2017). Mature fruiting bodies exhibit ethanol odors of their own. As demonstrated in Fig. 2D, the ethanol content gradually increased with time, leading to a decline in quality. However, the accumulation of ethanol was inhibited by MT. Notably, among all MT, M4 exhibited the strongest inhibition of the rise in ethanol content, while that of M1 was the weakest. This phenomenon could result from MT reducing the anaerobic respiratory rate by inactivating respiratory pathway-related enzymes (Zhang et al., 2016; Song et al., 2018).
Changes in Low-Field Nuclear Magnetic Resonance (LF-NMR) Transverse Relaxation, Malondialdehyde (MDA) Content, Relative Conductivity, and Respiratory Rate
Cell integrity is disrupted when mushroom fruiting bodies undergo autolysis, leading to moisture loss and a reduction in free water (Gui et al., 2022). In the study of Cheng et al. (2019), the relaxation times of T21 (bound water), T22 (immobile water), and T23 (free water) of mushrooms were detected, but in our study, only bound water and free water were detected, which was consistent with our previous research (Xia et al., 2023). It can be seen from Fig. 3A that the T23 peak area of D. rubrovolvata fruiting bodies for each group was lower than that of each initial sample. Among them, the T23 peak area of M4 was significantly 45.17% higher than that of CK (P < 0.05), which showed that M4 could effectively maintain cell integrity. However, there was no significant difference between M3 and CK (P > 0.05), which indicated that the effect of M3 was poor in the maintenance of cellular integrity.
Effect of microwaves on the content of low-field nuclear magnetic resonance (LF-NMR) transverse relaxation (A), malondialdehyde (MDA) content (B), relative conductivity (C), and respiratory rate (D) in D. rubrovolvata during storage at 4 °C. The vertical bars represent the standard errors of the means (n = 3). Different lettering indicates statistically significant differences (P < 0.05) between groups, while identical lettering indicates no difference between groups
MDA is generally considered a measure of the degree of lipid peroxidation. In Fig. 3B, the MDA content showed an upward trend, which indicated that lipid peroxidation occurred during storage. However, the MDA content of fruiting bodies after MT was significantly lower than CK (P < 0.05), especially in M4 and M2, which was reduced by 34.71% and 26.46%, respectively.
Cell membrane permeability is commonly evaluated by relative conductivity. It could be seen from the data in Fig. 3C that the relative conductivity of all samples gradually increased with the prolongation of storage time; however, the increasing rate in the CK was significantly higher than that of MT (P < 0.05). After storage, M4 significantly inhibited the increase in relative conductivity compared with the other groups (P < 0.05). M4 exhibited the lowest relative conductivity, with only a 21.64% change, which was consistent with the respiration results, while the relative conductivities of M1, M2, M3, and M5 were 32.95%, 32.36%, 43.32%, and 38.33%, respectively, which were higher than that of M4.
The respiration rate is related to water migration and cellular metabolism. Autolysis occurred concomitantly with an increase in the respiration rate (Gui et al., 2022). Therefore, the main purpose of mushroom preservation is to inhibit the postharvest respiration rate (Shen et al., 2019). From the data in Fig. 3D, the respiratory rate exhibited a trend of first increasing and then decreasing over 12 days. The respiration rate of D. rubrovolvata fruiting bodies after MT was significantly lower than that of CK (P < 0.05). It is worth noting that the respiratory peak of CK was the highest (18.89 mg kg−1 h−1), while those of M4 (10.29 mg kg−1 h−1) and M2 (11.46 mg kg−1 h−1) were significantly lower than those of the other groups (P < 0.05). In addition, M4 inhibited the respiration rate compared with the other groups, while there was no significant difference between M1 and CK on 12 days (P > 0.05).
Contents of Cellulose, Chitin, and Glucan and Activities of Cellulase, Chitinase, and Glucanase
As shown in Fig. 4A, C, and E, the contents of cellulose, chitin, and glucan gradually decreased with prolonged storage time, while the reduction in content was inhibited by MT, which was consistent with the changes in MDA content and relative conductivity. The contents of cellulose, chitin, and glucan in CK were 58.75 g kg−1, 20.85 g kg−1, and 36.14% on 3 days; 54.13 g kg−1, 16.77 g kg−1, and 33.54% on 6 days; 50.02 g kg−1, 13.65 g kg−1, and 30.14% on 9 days; and 46.99 g kg−1, 8.11 g kg−1, and 20.41% on 12 days, respectively. However, the contents of cellulose, chitin, and glucan in M4 were significantly higher than those in the other groups (P < 0.05). Especially on 12 days, they were 23.26%, 29.01%, and 128.23% higher than those in the CK. Hence, MT could delay fruiting body softening by maintaining the components of the cell wall.
Effect of microwaves on the content of cellulose (A), chitin (C), and glucan (E) and the activities of cellulase (B), chitinase (D), and β-1,3 glucanase (F) in D. rubrovolvata during storage at 4 °C. The vertical bars represent the standard errors of the means (n = 3). Different lettering indicates statistically significant differences (P < 0.05) between groups, while identical lettering indicates no difference between groups
As shown in Fig. 4B, D, and F, the activity of cellulase and chitinase displayed a tendency to first increase and then decrease, while β-1,3-glucanase had a continuous increasing trend. The activities of cellulase on 3 days and 6 days and chitinase on 3 days in M1 were not significantly different from those in CK (P > 0.05), but the enzymatic activities of the other MT groups were significantly lower than those in CK (P < 0.05). In contrast, M3 significantly elevated the activity of β-1,3-glucanase compared with CK (P < 0.05) on 3 days. After 12 days of storage, D. rubrovolvata fruiting bodies after MT had lower activities of cellulase, chitinase, and β-1,3 glucanase compared with CK. In particular, those in M4 and M2 were significantly lower than that in CK (P < 0.05). Therefore, MT could inactivate cellulase, chitinase, and β-1,3 glucanase, which was helpful in inhibiting autolysis, especially under M4 and M2.
Content of Free Amino Acids, 5′-Nucleotides, Equivalent Umami Concentration (EUC), and Analysis of Electronic Nose
In Tab. S1, alanine acid (Ala), serine acid (Ser), threonine acid (Thr), and phenylalanine acid (Phe) all showed higher contents in fruit bodies at harvest, and Ala, Ser, and Thr were sweet amino acids. Thus, the sweet amino acid was the main amino acid of D. rubrovolvata. In Fig. 5A, the total free amino acid content increased by hydrolyzing proteins on 6 days. The accumulation of total free amino acids was inhibited after MT. This indicated that MT was likely to inhibit protein hydrolase activity, with M4 being the most efficient compared with CK. The content of free amino acids in fruiting bodies decreased along with serious quality deterioration at the end of storage and was lower than that in the initial sample. However, MT could inhibit the reduction of total free amino acid. In addition, the total free amino acid contents of the M4 and M2 samples were 17.57 g kg−1 and 15.21 g kg−1, respectively, which were significantly higher than that of CK on 12 days (P < 0.05).
Effect of MT on the content of free amino acids (A), 5′-nucleotides: 5′-inosine monophosphatem (5′-IMP), 5′-guanosine monophosphate (5′-GMP), 5′-xanthosine monophosphate (5′-XMP), and 5′-adenosine monophosphate (5′-AMP) (B), equivalent umami concentration (EUC) (C), electronic nose (D), and principal component analysis PCA (E) in D. rubrovolvata during storage at 4 °C. The vertical bars represent the standard errors of the means (n = 3). Different lettering indicates statistically significant differences (P < 0.05) between groups, while identical lettering indicates no difference between groups
5′-Nucleotides were also important umami substances which were consisted of 5′-inosine monophosphatem (5′-IMP), 5′-guanosine monophosphate (5′-GMP), 5′-xanthosine monophosphate (5′-XMP), and 5′-adenosine monophosphate (5′-AMP). As shown in Fig. 5B, MT maintained the contents of 5′-nucleotides compared with CK during 6 days of storage. At the end of storage, the decrease in 5′-nucleotides of fruiting bodies was inhibited after microwave treatment, except for M3. M4 was the highest among them, which was 1.21 g kg−1 and 39.91% lower than the initial group (fruiting bodies on 0 day), while the CK was only 0.44 g kg−1, which was 77.78% lower than the initial group. Thus, the trend of umami nucleotides was consistent with that of free amino acids (Fig. 5A), and MT could also inhibit the decrease in umami nucleotides, especially in M4. In contrast, M3 decreased nucleotides faster than CK during the end of storage, especially in 5′-XMP. The EUC value was based on the synergistic effect of monosodium glutamate (MSG)-like components and 5′-nucleotides (Zhang et al., 2019). Figure 5C shows the EUC value for D. rubrovolvata fruiting bodies, which decreased gradually during storage. The EUC value of fruiting bodies after MT significantly increased compared with CK on 6 days (P < 0.05). On 12 days, the EUC values of M4 and M2 were 194.41 g MSG kg−1 and 128.32 g MSG kg−1, respectively, which were significantly higher than that of CK (P < 0.05). However, there were no significant differences in M1, M4, and M5 compared with the CK group (P > 0.05). From the above results, it could be inferred that M4 and M2 could inhibit the loss of umami taste.
The radar map of Fig. 5D illustrates that there were more significant responses for sensors when D. rubrovolvata fruiting bodies underwent autolysis (Table S1, Fig. S4). The samples under different MTs showed similar profiles in the radar images, which indicated that there were some similarities between each group. At the end of 12 days of storage, the response values and shapes of the radar plot in each group were significantly lower than the initial value (P < 0.05). Notably, the response value and profiles of the radar map in M4 and M2 were close to the initial sample (0 day, CK), while M1 was close to CK on 12 days. The results showed that M4 and M2 could effectively maintain the flavor substances. This result was further confirmed using PCA analyses (Fig. 5E).
Changes in ATP, ADP, AMP, and Energy Charge
As depicted in Fig. 6A–C, the content of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) gradually declined, while the adenosine monophosphate (AMP) content increased during the storage period, which led to a decrease in energy charge (Fig. 6D). On 6 days, there was no significant difference between MT and CK (P > 0.05). This was probably because autolysis did not occur. On 12 days, the ATP and ADP contents of M4 were significantly higher than those of the other groups, which were 1.52 times and 1.60 times higher than those of CK (P < 0.05). MT also inhibited the increase in AMP. In addition, the energy charge of D. rubrovolvata fruiting bodies after MT was significantly higher than that after CK (P < 0.05). Among them, the M4 and M2 groups increased by 17.19% and 10.17%, respectively, compared with the CK group. Therefore, M4 and M2 effectively delayed the decrease in ATP and ADP and the increase in AMP, thereby maintaining the energy charge.
Effect of microwaves on changes in ATP (A), ADP (B), AMP (C), and energy charge (D) in D. rubrovolvata during storage at 4 °C. Here, the vertical bars indicate the standard error calculated from the results of three experimental runs. Where shown, different letters represent significant differences across groups at the same storage time (P < 0.05)
Correlation Analysis
The correlation among the different parameters of D. rubrovolvata fruiting bodies was analyzed with Pearson’s correlation analysis. As shown in Fig. 7, autolysis was closely correlated with energetic metabolism, umami substances (free amino acids, 5′-nucleotides, and EUC), sensory index (sensory qualities, browning degree, shear force, and ethanol), and physiological indicators (moisture migration, MDA, relative electrical conductivity, and respiratory rate). The cellulose, chitin, and glucan contents had positive correlations with ATP content, energy charge, sensory qualities, water migration, umami substances, and EUC in D. rubrovolvata fruiting bodies. The activities of cellulase, chitinase, and glucanase had positive correlations with ATP and AMP content, browning degree, and ethanol content in the samples.
Correlation matrix of the relationship among the different parameters of D. rubrovolvata fruiting bodies. Dots with large and dark blues indicate strong positive correlations, whereas dots with large and dark reds indicate strong negative correlations. Asterisks represent significant differences (P < 0.05)
Discussion
Heat shock is a common method for preserving fruit, vegetables, and mushrooms. In previous studies, both hot air and hot water were employed in the fresh-keeping of mushrooms (Lurie & Pedreschi, 2014; Zhao et al., 2021). Indeed, D. rubrovolvata fruiting bodies, which have fragile and porous structures, are affected by hot air and hot water susceptibly. MT heat shock is an innovative heat method that has been confirmed to improve the postharvest quality of fruits and vegetables (Chen et al., 2021). However, its effect on mushrooms during postharvest storage is currently unknown. In this study, we investigated the feasibility of using MT to maintain the postharvest quality of fruiting bodies by regulating autolysis and energy metabolism. Our results suggested that compared with CK, MT could inhibit autolytic enzymes and regulate energy metabolism. In addition, we found a strong correlation between autolysis and energetic metabolism (Fig. 7). By correlation analysis (Fig. 7), a close connection was observed between the sensory index (Fig. 2) and physiological indicators (Fig. 3) with energy metabolism (Fig. 6). The results indicated that an increase in respiration rate accelerated metabolic processes which triggered autolysis. Autolysis further leaded to the destruction of cell structure which caused the increase in MAD and relative electrical conductivity and resulted in disorders in energy metabolism. In appearance, D. rubrovolvata fruiting bodies exhibited browning, increased shear force and ethanol content, and decreased sensory quality. These changes directly reflect the negative impact of autolysis on the quality of D. rubrovolvata, which highlighted the importance of controlling the autolysis and maintaining energy metabolism balance to extend its shelf life.
The increase in BD, shear force, and ethanol content were the main deterioration characteristics of fruiting bodies during storage (Cheng et al., 2022). Our analysis suggested that sensory evaluation declined with the extension of storage time, as indicated by the increase in shear force, browning degree, and ethanol content (Fig. 2). This observation was consistent with the results established by Wang et al. (2019a, b), substantiating that in the case of shiitake mushrooms, the degradation of quality gained momentum as storage time lengthened, as evidenced by the escalation in browning degree. It is worth noting that MT delayed the increase in BD, shear force, and ethanol content by inhibiting the respiratory rate and moisture migration over 12 days (Figs. 2 and 3). According to a study by Wang et al. (2022), the process of autolysis was strongly associated with a decrease in the permeability of the membrane. Therefore, MT could also delay the accumulation of MDA and the rise in relative conductivity (Fig. 3). Among MT, M4 maintained a better physiological state than the other groups, with no occurrence of autolysis and browning phenomena. However, M1 had the weakest effect because high power could destroy the D. rubrovolvata fruiting body cells and lead to quality reduction.
The mushroom samples could be evaluated objectively using electronic noses, and the free amino acids and umami nucleotides were the main umami substances of the mushrooms (Xia et al., 2021). The content of umami substances was lost, and odor compounds were released by autolysis. In this study, the electronic nose profiles of MT were close to the initial sample (0 day, CK), suggesting that MT helped delay changes in odor. Our study suggested a trend that the content of free amino acids initially increased and then subsequently declined as the storage period was extended. The increase on 6 days could be attributed to the fact that proteins could be broken down into free amino acids by proteinases. However, the decrease in free amino acids on 12 days was connected to the deterioration of fruiting body quality. The content of umami nucleotides and the value of EUC decreased during storage. The alterations observed in the aforementioned flavor compounds align with the research findings reported by Xia et al. (2023). Notably, MT could effectively delay the decrease in the content of umami substances, especially in M4, which was consistent with the E-nose and PCA results.
Cellulose, chitin, and glucans within the cell wall of mushrooms play key roles in maintaining the cell structure (Yang et al., 2022). Once they are destroyed, cells are damaged by osmotic pressure and undergo autolysis (Zhang et al., 2019). The increase in glucanase, cellulase, and chitinase activities in mushrooms could cause polysaccharides and glycosaminoglycans to turn into monosaccharides, which will lead to autolysis (Liu et al., 2021). In our study, the activities of cellulase, chitinase, and glucanase were gradually activated during storage, which resulted in the autolysis of D. rubrovolvata fruiting bodies and a decline in the content of cellulose, chitin, and glucans (Fig. 4). The same phenomenon is also observed in Lentinula edodes and Coprinus comatus (Li et al., 2022; Yang et al., 2022). Interestingly, MT inhibited the enzyme activities of cellulase, chitinase, and glucanase and delayed the decrease in cellulose, chitosan, and β-1,3 glucan contents. The thermal effect of MT could passivate autolytic enzymes, which inhibited autolysis, especially in M4. However, among all MT, M1 showed the weakest inhibition of autolysis, while it instead increased glucanase activity compared with CK on 3 days. Therefore, MT, such as 50 W or 75 W, could reduce the degree of autolysis, but higher powerful MT, such as 100 W, would show the weakest passivation of the enzyme. Moreover, the correlation between shear force and the activities of cellulase, chitinase, and β-1,3 glucanase showed that these enzymes also played a significant role in leading to the softening of samples. According to findings by Zhao et al. (2021), MT treatment reduced the cellulase activity of persimmon by 36.8% compared with CK and delayed fruit softening on 9 days. These findings were similar to those we found. Here, it was not observed that MT could passivate enzymes, which could lead to the inhibition of autolysis.
There have been reports in the literature that reducing energy loss and keeping energy charge in mitochondria are crucial for delaying the autolysis of mushroom quality (Li et al., 2021a, b; Yang et al., 2022). Increasing evidence has shown that metabolism disturbances observed in mushrooms could be due to an insufficient energy supply. There were studies found that Coprinus comatus and Volvariella volvacea were prone to autolysis after postharvest. Proteomic and transcriptomic analyses have revealed significant changes in carbon metabolism during autolysis which highlighted a key role in energy metabolism (Gong et al., 2022; Yang et al., 2022). Further study found that chilling stress disrupted physiological metabolic processes mediated by downregulate related proteins which leaded to disorder in energy metabolism and promoted autolysis. Consequently, ATP production diminished, leading to insufficient energy supply, cell senescence, and ultimately apoptosis. These findings showed that autolysis could cause disorder in energy metabolism, reduce ATP content, and lead to the decrease in the quality of mushroom. In the present study, a gradual decrease in ATP and ADP contents was observed, which converted into AMP and led to a decrease in the energy charge level (Fig. 6A–D). This corresponded to the results in Coprinus comatus and Volvariella volvacea reported by Yang et al. (2022) and Gong et al. (2022). As shown in Fig. 6, MT significantly delayed the decline in ATP, ADP, and energy charge (P < 0.05). Additionally, we found that M4 and M2 had better effects than M1, M3, and M5, which indicated that MT was beneficial for the maintenance of energy metabolism in fruiting bodies. According to the correlation analysis (Fig. 7), a higher energy charge level could contribute to delaying the deterioration of quality and autolysis.
Conclusions
In summary, in the present study, we developed a novel microwave treatment tool that showed better effectiveness in delaying D. rubrovolvata fruiting body autolysis during the postharvest period. MT (M4/50 W, 60 s) effectively delayed autolysis and decreased the sensory qualities and umami substances of D. rubrovolvata fruiting bodies by thermal and nonthermal effects. MT delayed autolysis by inhibiting cellulase, chitinase, and glucanase enzyme activity. MT maintained a relatively higher energy charge by delaying the decrease in ATP and ADP. Therefore, the delay of postharvest autolysis in the D. rubrovolvata after MT may be attributed to the regulation of energy metabolism. These findings have important implications for the mushroom industry, especially in the D. rubrovolvata fruiting bodies industry. Future research could explore the applicability of MT to other mushroom species and deeper into the underlying mechanisms.
Data Availability
On reasonable request, the data are available from the corresponding author on reasonable request.
References
Barrón-García, O. Y., Nava-Álvarez, B., Gaytán-Martínez, M., Gonzalez-Jasso, E., & Morales-Sánchez, E. (2022). Ohmic heating blanching of Agaricus bisporus mushroom: Effects on polyphenoloxidase inactivation kinetics, color, and texture. Innovative Food Science and Emerging Technologies, 80, 103105. https://doi.org/10.1016/J.IFSET.2022.103105
Baltacıoğlu, H., Bayındırlı, A., Severcan, M., & Severcan, F. (2015). Effect of thermal treatment on secondary structure and conformational change of mushroom polyphenol oxidase (PPO) as food quality related enzyme: A FTIR study. Food Chemistry, 187, 263–269. https://doi.org/10.1016/j.foodchem.2015.04.097
Chen, F., Warning, A. D., Datta, A. K., & Chen, X. (2016). Thawing in a microwave cavity: Comprehensive understanding of inverter and cycled heating. Journal of Food Engineering, 180, 87–100. https://doi.org/10.1016/j.jfoodeng.2016.02.007
Chen, Y., Zhang, X., Luo, Z., Sun, J., Li, L., Yin, X., Li, J., & Xu, Y. (2021). Effects of inside-out heat-shock via microwave on the fruit softening and quality of persimmon during postharvest storage. Food Chemistry, 349(3), 129161. https://doi.org/10.1016/j.foodchem.2021.129161
Cheng, M., Wang, J., Zhang, R., Kong, R., Lu, W., & Wang, X. (2019). Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. International Journal of Biological Macromolecules, 141, 259–267. https://doi.org/10.1016/j.ijbiomac.2019.08.215
Cheng, S., Ranran, L., Yang, H., Wang, S., Lin, R., & Tan, M. (2020). Characterisation of moisture migration of shiitake mushroom (Lentinula edodes) during storage and its relationship to quality deterioration. International Journal of Food Science & Technology, 55(5), 2132–2140. https://doi.org/10.1111/ijfs.14456
Cheng, Y., Wei, Y., Zhang, M., & Wang, H. (2022). Effect of micro-perforated film packing on physicochemical quality and volatile profile of button mushroom (Agaricus bisporus) during postharvest shelf-life. Journal of Food Processing and Preservation, 46(7), e16648. https://doi.org/10.2139/ssrn.3899830
Decros, G., Baldet, P., Beauvoit, B., Stevens, R., Flandin, A., Colombié, S., Gibon, Y., & Pétriacq, P. (2019). Get the balance right: ROS homeostasis and redox signalling in fruit. Frontiers in Plant Science, 10, 1091. https://doi.org/10.3389/fpls.2019.01091
Dong, S., Guo, J., Yu, J., Bai, J., Xu, H., & Li, M. (2022). Effects of electron-beam generated X-ray irradiation on the postharvest storage quality of Agaricus bisporus. Innovative Food Science & Emerging Technologies, 80, 103079. https://doi.org/10.1016/j.ifset.2022.103079
Gong, M., Li, Z., Wan, J., Chen, M., & Bao, D. (2020). Chilling stress reduced protein translation by the ubiquitination of ribosomal proteins in volvariella volvacea. Journal of Proteomics, 215, 103668. https://doi.org/10.1016/j.jprot.2020.103668
Gong, M., Wang, Y., Su, E., Zhang, J., Tang, L., Li, Z., & Bao, D. (2022). The promising application of a β-glucosidase inhibitor in the postharvest management of Volvariella volvacea. Postharvest Biology and Technology, 185, 111784. https://doi.org/10.1016/j.postharvbio.2021.111784
Gui, H., Zhao, M., Zhang, S., Yin, R., Hu, C., Fan, M., & Li, L. (2022). Active antioxidant packaging from essential oils incorporated polylactic acid/poly (butylene adipate co terephthalate)/ thermoplastic starch for preserving straw mushroom. Foods, 11(15), 2522. https://doi.org/10.3390/foods11152252
Guzik, P., Zając, M., & Migdał, W. (2021). Microwave applications in the food industry: An overview of recent developments. Critical Reviews in Food Science and Nutrition, 62(29), 7989–8008. https://doi.org/10.1080/10408398.2021.1922871
Jiang, T., Wang, Q., Xu, S., Jahangir, M. M., & Ying, T. (2010). Structure and composition changes in the cell wall in relation to texture of shiitake mushrooms (Lentinula edodes) stored in modified atmosphere packaging. Journal of the Science of Food and Agriculture, 90(5), 742–749. https://doi.org/10.1002/jsfa.3876
Li, B., Ding, Y., Tang, X., Wang, G., Wu, S., Li, X., & Tang, X. (2019). Effect of L-arginine on maintaining storage quality of the white button mushroom (Agaricus bisporus). Food and Bioprocess Technology, 12, 563–574. https://doi.org/10.1007/s11947-018-2232-0
Li, D., Li, L., Xiao, G., Limwachiranon, J., Xu, Y., Lu, H., Yang, D., & Luo, Z. (2018). Effects of elevated CO2 on energy metabolism and γ-aminobutyric acid shunt pathway in postharvest strawberry fruit. Food Chemistry, 265, 281–289. https://doi.org/10.1016/j.foodchem.2018.05.106
Li, D., Wang, D., Fang, Y., Li, L., Lin, X., Xu, Y., Chen, H., Zhu, M., & Luo, Z. (2021a). A novel phase change coolant promoted quality attributes and glutamate accumulation in postharvest shiitake mushrooms involved in energy metabolism. Food Chemistry, 351, 129227. https://doi.org/10.1016/j.foodchem.2021.129227
Li, Y., Ding, S., Kitazawa, H., & Wang, Y. (2022). Storage temperature effect on quality related with cell wall metabolism of shiitake mushrooms (Lentinula edodes) and its modeling. Food Packaging and Shelf Life, 32, 100865. https://doi.org/10.1016/J.FPSL.2022.100865
Li, Y., Ding, S., Xiang, T., Kitazawa, H., Sun, H., & Guo, Y. (2021b). Effects of light irradiation on the textural properties and energy metabolism of postharvest shiitake mushrooms (Lentinula edodes). Journal of Food Processing and Preservation, 45(12), e16066. https://doi.org/10.1111/jfpp.16066
Liu, J., Liu, S., Zhang, X., Kan, J., & Jin, C. (2019). Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biology and Technology, 147(1), 39–47. https://doi.org/10.1016/j.postharvbio.2018.09.004
Liu, Q., Cui, X., Song, Z., Kong, W., Kang, Y., Kong, W., & Ng, T. B. (2021). Coating shiitake mushrooms (Lentinus edodes) with a polysaccharide from Oudemansiella radicata improves product quality and flavor during postharvest storage. Food Chemistry, 352, 129357. https://doi.org/10.1016/j.foodchem.2021.129357
Lurie, S., & Pedreschi, R. (2014). Fundamental aspects of postharvest heat treatments. Horticulture Research, 1(1), 176–182. https://doi.org/10.1038/hortres.2014.302014
Mau, J. L. (2005). The umami taste of edible and medicinal mushrooms. International Journal of Medicinal Mushrooms, 7, 125–199. https://doi.org/10.1615/IntJMedMushr.v7.i12.120
Nguyen, G., & Nguyen, T. (2021). Effect of extraction conditions (temperature, pH and time) by cellulase on chemical properties of dried oyster mushroom (Pleurotus sajor-caju) extract. Food Research, 5(3), 351–358. https://doi.org/10.26656/fr.2017.5(3).613
Peng, Y., Li, T., Jiang, H., Gu, Y., Chen, Q., Yang, C., & Zhang, X. (2020). Postharvest biochemical characteristics and ultrastructure of Coprinus comatus. PeerJ, 8, e8508. https://doi.org/10.7717/peerj.8508
Shen, X., Zhang, M., Devahastin, S., & Guo, Z. (2019). Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biology and Technology, 149, 159–165. https://doi.org/10.1016/j.postharvbio.2018.11.023
Sisquella, M., Viñas, I., Teixidó, N., Picouet, P., & Usall, J. (2013). Continuous microwave treatment to control postharvest brown rot in stone fruit. Postharvest Biology and Technology, 86, 1–7. https://doi.org/10.1016/j.postharvbio.2013.06.012
Song, L., Luo, H., Cheng, X., Yan, F., Yang, Z., & Yu, Z. (2018). Effects of microwave treatment on physiology and quality of minimally processed bok choy (Brassica campestris L.) during storage at 5 ℃. Journal of Food Measurement and Characterization, 12, 913–922. https://doi.org/10.1007/s11694-017-9707-y
Sun, L. P., Bao, C. J., Chang, W. D., & Zhuang, Y. L. (2017). Preparation, characterisation, antioxidant and antiglycation activities of the novel polysaccharides from the pileus of Dictyophora rubrovolvata. International Journal of Food Science and Technology, 52(1), 161–170. https://doi.org/10.1111/ijfs.13262
Ventura-Aguilar, R. I., Colinas-León, M. T., & Bautista-Baños, S. (2017). Combination of sodium erythorbate and citric acid with MAP, extended storage life of sliced oyster mushrooms. LWT-Food Science and Technology, 79, 437–444. https://doi.org/10.1016/j.lwt.2017.01.053
Wang, K., Zhu, G., Li, Y. L., Chen, S. Q., Rashid, A., Wang, X. T., & Wu, X. Y. (2023). Non-thermal effects of microwave irradiation alleviates postharvest chilling injury of peach fruit by retarding phenolic accumulation and enhancing membrane stability. Food Chemistry, 411, 135448. https://doi.org/10.1016/j.foodchem.2023.135448
Wang, T., Yun, J., Zhang, Y., Bi, Y., Zhao, F., & Niu, Y. (2021). Effects of ozone fumigation combined with nano-film packaging on the postharvest storage quality and antioxidant capacity of button mushrooms (Agaricus bisporus). Postharvest Biology and Technology, 176(1), 111501. https://doi.org/10.1016/J.POSTHARVBIO.2021.111501
Wang, X., Huang, X., Zhang, F., Hou, F., Yi, F., Sun, X., & Liu, Z. (2022). Characterization of chitosan/zein composite film combined with tea polyphenol and its application on postharvest quality improvement of mushroom. Food Packaging and Shelf Life, 33, 100869. https://doi.org/10.1016/J.FPSL.2022.100869
Wang, Y., Ji, D., Chen, T., Li, B., Zhang, Z., Qin, G., & Tian, S. (2019a). Production, signaling, and scavenging mechanisms of reactive oxygen species in fruit-pathogen interactions. International Journal of Molecular Sciences, 20(12), 2994. https://doi.org/10.3390/ijms20122994
Wang, Y., Mo, Y., Li, D., Xiang, C., Jiang, Z., & Wang, J. (2019b). The main factors inducing postharvest lignification in king oyster mushrooms (Pleurotus eryngii): Wounding and ROS-mediated senescence. Food Chemistry, 301, 125224. https://doi.org/10.1016/j.foodchem.2019.125224
Wang, Y., Niu, X., Guo, X., Yu, H., Liu, Z., Zhang, Z., & Yuan, S. (2018). Heterologous expression, characterization and possible functions of the chitin deacetylases, Cda1 and Cda2, from mushroom Coprinopsis cinerea. Glycobiology, 28(5), 318–332. https://doi.org/10.1093/glycob/cwy007
Xia, R., Wang, L., Xin, G., Bao, X., Sun, L., Xu, H., & Hou, Z. (2021). Preharvest and postharvest applications of 1-MCP affect umami taste and aroma profiles of mushrooms (Flammulina velutipes). Lwt, 144, 111176. https://doi.org/10.1016/J.LWT.2021.111176
Xia, Z. Q., Wang, R., Ma, C., Li, J. K., Lei, J. Q., Ji, N., & Chen, T. J. (2023). Effect of controlled atmosphere packaging on the physiology and quality of fresh-cut Dictyophora rubrovolvata. Foods, 12(8), 1665. https://doi.org/10.3390/FOODS12081665
Yang, B., Han, Y., Gao, H., Liu, R., Xu, F., Liu, R., & Chen, H. (2023). Application of melatonin delays lignification in postharvest water bamboo shoots in association with energy metabolism. Postharvest Biology and Technology, 196, 112149. https://doi.org/10.1016/j.postharvbio.2022.112149
Yang, H., Zheng, Z., Zhou, H., Qu, H., & Gao, H. (2022). Proteomics reveals the mechanism underlying the autolysis of postharvest Coprinus comatus fruiting bodies. Journal of Agricultural and Food Chemistry, 70(4), 1346–1357. https://doi.org/10.1021/acs.jafc.1c07007
Yang, W., Wu, Y., Hu, Q., Pei, F., & Mariga, A. M. (2019). Preharvest treatment of Agaricus bisporus with methyl jasmonate inhibits postharvest deterioration. LWT, 106, 158–163. https://doi.org/10.1016/j.lwt.2019.02.069
Ye, L., Niu, Y., Wang, Y., Shi, Y., Liu, Y., Yu, J., & Luo, A. (2023). Effect of X-ray irradiation on quality, cell ultrastructure and electrical parameters of postharvest kiwifruit. Innovative Food Science & Emerging Technologies, 89, 103483. https://doi.org/10.1016/j.ifset.2023.103483
Zhang, R. Y., Hu, D. D., Zhang, Y. Y., Goodwin, P. H., Huang, C. Y., Chen, Q., & Zhang, J. X. (2016). Anoxia and anaerobic respiration are involved in “spawn-burning” syndrome for edible mushroom Pleurotus eryngii grown at high temperatures. Scientia Horticulturae, 199, 75–80. https://doi.org/10.1016/j.scienta.2015.12.035
Zhang, Z., Zhang, X., Xin, G., Gong, X., Wang, Y., Wang, L., & Sun, B. (2019). Umami taste and its association with energy status in harvested Pleurotus geesteranus stored at different temperatures. Food Chemistry, 279, 179–186. https://doi.org/10.1016/j.foodchem.2018.12.010
Zhao, X., Wang, Y., Zhang, Z., Sun, L., Wei, Y., Bao, X., & Xin, G. (2021). Postharvest short-time partial dehydration affects shiitake mushroom (Lentinus edodes) storage quality and umami taste. Scientia Horticulturae, 287, 110274. https://doi.org/10.1016/J.SCIENTA.2021.110274
Zhu, B., Liu, Y., Brennan, M., Brennan, C., Qin, Y., Li, L., & Chen, H. (2024). Application of antimicrobial nanocomposite film packaging on the postharvest quality and specific spoilage organisms of mushrooms (Russula virescens). Food Control, 155, 110056.
Zhuang, Y., & Sun, L. (2011). Nutritional characteristics of proteins from the volva and pileus in cultivated mushroom Dictyophora rubrovolvata. International Journal of Food Sciences and Nutrition, 62(4), 392–396. https://doi.org/10.3109/09637486.2010.539552
Zivanovic, S., & Buescher, R. (2004). Changes in mushroom texture and cell wall composition affected by thermal processing. Journal of Food Science, 69(1), SNQ44–SNQ49. https://doi.org/10.1111/j.1365-2621.2004.tb17885.x
Funding
This work was supported by the Guizhou Provincial Foundation for Excellent Scholars Program, China (No.GCC[2023]063), the Academic Seedling Cultivation and Free Exploration Innovation Special Project of the Guizhou Provincial Department of Science and Technology, China, and the Guizhou Province College Students’ Innovation Training Project (202210976092).
Author information
Authors and Affiliations
Contributions
Xu Zhang: investigation, experiment, formal analysis, writing—review and editing. Rui Wang: project administration, conceptualization, methodology, writing—review and editing, funding acquisition. Wencong Zhang: device, methodology. Cunkun Chen: formal analysis. Chao Ma: formal analysis. Ning Ji: formal analysis. Nanxin Zhang: experiment, data analysis. Jiqing Lei: investigation, formal analysis. Yiming Tian: experiment, data Analysis. All authors have read and agreed to the published version of the manuscript. Panpan Zhang: experiment, data analysis.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Wang, R., Zhang, W. et al. Microwave Treatment for Dictyophora rubrovolvata in Regulating Postharvest Autolysis and Energy Metabolism. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03388-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03388-y