Abstract
The incorporation of different flours into wheat bread may pursue different objectives, such as increasing the nutritional quality of the products or the recovery of flour with little use in the industry. The “mesquite flour” (MF) is rich in sugar, fiber, and protein and is an interesting additive to wheat flour in baking. In this study, we used crude and thermally processed bread dough formulations of wheat flour (WF), replacing 15, 25, and 35% with mesquite flour. Furthermore, each formulation was tested for two levels (0.01 and 0.1%) of the enzyme transglutaminase (TG). Dough rheology was studied by small amplitude oscillatory compression tests, and the microstructure was analyzed by laser confocal microscopy using fluorescein isothiocyanate and rhodamine B as fluorophores. It was concluded that the incorporation of mesquite into the dough resulted in changes in the structure, as evidenced by the increase in tan δ, microscopic observations (loss of the filamentary and cross-linked gluten structure), and by the increase in the gelatinization temperature. The addition of TG led to dissimilar effects on doughs, depending on the formulation (wheat/mesquite content), but most encouraging results indicate the recovery of the structure, evidenced by a reduction in tan δ and the generation of a more filamentary structure in the dough with a higher content of mesquite flour. However, the effect of TG addition on “processed dough” was attenuated and the viscoelastic matrix of gluten did not recover.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incorporation of different flours into wheat bread may pursue different objectives, such as increasing the nutritional quality of the products or the valorization of flour with little use in the industry (Barba de la Rosa, Frias-Hernández, Olalde-Portugal, and González Castañeda 2006; Hefnawy, El-Shourbagy, and Ramadan 2012; Ribotta, Arnulphi, León, and Añón 2005). Composite breads play an important role in the functional food market due to the increasing demand for healthy food. The development of new functional foods often requires the total or partial replacement of components in traditional foods (Roberfroid 1999).
Leguminous pods are known by their high content in protein, fiber, minerals, and polyphenol compounds and the high quality of their lipids; therefore, they have been the target of numerous studies for incorporation into different foods (Messina 1999). Mesquite flour (MF) is obtained by milling the whole pods of Prosopis spp., a leguminous tree present in South and North America, Africa, and Asia. Due to its high sugar content, MF can be used as coffee and chocolate substitute as well as in sweet bakery products (Sciammaro, Ferrero, and Puppo 2015). MF is also rich in fiber and protein, and consequently, MF is an interesting ingredient to blend with wheat flour in bread making (Bigne, Puppo, and Ferrero 2016a; Bravo, Grados, and Saura-calixto 1998; Felker, Takeoka, and Dao 2013).
However, the application of new ingredients can lead to unwanted modifications of product properties, and consequently, the incorporation of quality improvers is necessary. In baking and other food industries, a wide range of quality improvers are used. They are intended to improve the final quality of the product, facilitate processing steps, improve any attribute that may be diminished for reasons related to the raw materials, and extend the life of the product, among others (Cauvain and Young 2001). The most common additives used in baking are redox agents, emulsifiers, hydrocolloids, and different types of enzymes, such as proteases, lipases, α-amylases, and transglutaminases (Caballero, Gómez, and Rosell 2007; Collar, Andreu, Martínez, and Armero 1999; Faergemand and Krog 2003; Wieser 2003). The latter are cross-linking enzymes able to catalyze the bonding reaction of type ε-(γ-glutamyl)-lysine between inter- or intraprotein residues of lysine and glutamine. Moreover, transglutaminases have been reported to increase the cross-linking of the gluten network, leading to a reinforcement of the matrix and providing greater strength in doughs (useful in weak doughs) and improving crumb uniformity on breads (Caballero et al. 2007; Roccia, Ribotta, Ferrero, Pérez, and León 2012). However, enzymes require optimal conditions (pH and temperature) in order to ensure their functionality. Optimal activity of the microbial transglutaminase was reported at a pH between 5 and 8 and approximately 50 °C; nevertheless, the enzyme showed activity in a wider range of pH and at temperatures below 10 °C (Ando et al. 1989; Yokoyama, Nio, and Kikuchi 2004).
In previous work, Bigne et al. (2016a) and Bigne, Puppo, and Ferrero (2016b) described the disruptive effect of the components of MF on the gluten matrix, leading to loss of cohesiveness and springiness in composite bread doughs. The objective of the present study is to evaluate the effect of a transglutaminase enzyme on the rheology and microstructure of the composite (wheat/mesquite) doughs, before and after thermal processing, searching for the recovery of those properties lost by the addition of MF to the formulation.
Materials and Methods
Materials
Wheat flour (WF) 000 type (according to the Argentinean Food Code), provided by Molino Campodónico SA (Argentina), with the following composition determined in our laboratory (mean ± SD g/100 g), was used: proteins, 12.11 ± 0.03; lipids, 1.32 ± 0.04; moisture, 13.74 ± 0.11; ash, 0.70 ± 0.01; and total dietary fiber, 3.74 ± 0.03. For the determinations of protein, moisture, ash, and total dietary fiber, AACC Methods 46-12, 44-19, 8-1, and 32-05, respectively, were used (AACC 2000), while for lipid extraction, a Soxhlet device was employed. MF (MF) was provided by local producers (Baguala) from Santiago del Estero (Argentina). Its composition, as determined in our laboratory, was (% w/w) as follows: proteins, 11.13 ± 0.04; lipids, 1.13 ± 0.04; moisture, 6.01 ± 0.03; ash, 3.59 ± 0.04; and total dietary fiber, 26.77 ± 0.95. Other ingredients used in dough formulation were distilled water, margarine (Flora), sodium chloride (Panreac), and a commercial transglutaminase enzyme, Probind TX (100 units/g, according to producer’s information), provided by BDF Ingredients (Spain). For microscopic measurements, rhodamine B, RodB (Sigma Aldrich), and fluorescein isothiocyanate, FITC (MERCK), were used as fluorophores.
Methods
Experimental Design
A two-factor design was employed, varying the replacement of WF with MF (0, 15, 25, and 35% w/w), and three-addition levels of transglutaminase enzyme were used for these formulations: 0, 0.01, and 0.1 g/100 g of mixture of flours (without TG, TG 0.01, and TG 0.1, respectively). The water content in each formulation was optimized according to the farinograph absorption, as determined in previous work (Bigne et al. 2016b). The formulations are summarized in Table 1.
Dough Preparation
Doughs (85 g per batch) were prepared by mixing in a two-blade counter-rotating Polylab QC batch mixer (Haake Thermo Scientific, Germany) at room temperature (25 °C) and 63 rpm. Mixing speed was selected taking into account the speed value in standard farinograph assays (Brabender 2009). First, all the dry ingredients were mixed, and then, the optimum amount of water (Table 1) was gradually added while mixing. Once all the ingredients were added, the torque and temperature were recorded. The optimum mixing time for each dough was determined as described below. After mixing, doughs were left to rest for 10 min covered by a plastic film to prevent superficial dehydration before carrying out the tests. It is worth mentioning that the protocol for mixing wheat flour in an internal mixer is given by the ICC-Standard Method No. 173 (Moreira et al. 2012). However, this protocol involves a heating stage up to 90 °C. Unfortunately, the application of such high-temperature stage over several minutes would drive to the thermo-inactivation of transglutaminase, which has led to the selection of the above-described simpler and milder protocol. From the torque vs time curves, optimal mixing times were calculated as the time necessary to reach the maximum torque value (maximum dough consistency). These times were calculated for formulations without added enzyme and used to prepare the doughs with enzyme. The assays were performed in triplicate.
Dough Viscoelastic Characterization
Dough materials were characterized by small amplitude oscillatory compression (SAOC) measurements with an RSA3 controlled strain rheometer (TA Instruments, USA) using two smooth parallel plates of 15 mm diameter under auto tension mode. Samples were coated with Dow Corning high vacuum grease to minimize dehydration during the assays. Strain sweep SAOC tests were performed in order to establish the linear viscoelastic range. All the systems studied had the same thermorheological history before performing any rheological test. Values of the elastic (E′) and viscous (E″) moduli as well as the loss tangent, tan δ (relation between E″ and E′), were recorded. All measurements were performed in triplicate.
Strain Sweep Tests
Strain sweep tests were performed in triplicate between 0.001 and 2% at constant frequency (1 Hz) and different temperatures (25, 50, and 80 °C) in order to find the linear viscoelastic range required for the frequency sweep and temperature ramp tests.
Frequency Sweep Tests
Frequency sweep tests (to obtain the so-called mechanical spectra) were performed in triplicate between 0.016 and 16 Hz, at 25 °C and at a constant strain within the linear viscoelastic range (ranging from 0.01 to 0.04% depending on the sample). The same test was carried out for doughs before and after the thermal treatment (which simulates cooking) as described below.
Thermal Treatment
The cooking process was simulated in situ in the rheometer, and the linear viscoelastic properties of the doughs were monitored in three different stages: (i) the first step was carried out at a constant heating rate (5 °C/min) and at 1 Hz (6.23 rad/s) from 25 to 85 °C; (ii) then, a cooling stage was carried out at a constant cooling rate (10 °C/min) and at 1 Hz from 85 to 25 °C; and finally, (iii) frequency sweep tests were performed on the “thermally processed dough” as previously described.
Dough Microstructural Characterization
After mixing for the optimal time, the crude doughs were left to rest for 10 min before being prepared for analysis by two different microscopy techniques.
Confocal Scanning Laser Microscopy (CSLM)
A non-covalent labeling with FITC and RodB was performed. Fluorophore solutions were prepared in water (FITC 0.01% and RodB 0.001%). A portion of fresh dough was spread on a slide and immediately covered with the solution of fluorophores. Then, the samples were kept in the dark for 1 h. Excess fluorophore was removed with water, and the prepared samples were kept in the dark until carrying out the microscopic observations. A confocal microscope LSM 7 DUO (ZEISS, Germany) was used to obtain micrographs of different fields with various magnifications. The excitation wavelength was 488 nm for FITC and 561 nm for RodB, while filters for emission were set between 493 and 548 nm for FITC and between 566 and 703 nm for RodB.
Scanning Electron Microscopy (SEM)
Portions of fresh dough (2 mm diameter and 1 mm thick) were cut and stored in 2.5% v/v glutaraldehyde solution, at 4 °C. Dehydration of the samples was performed by washing with consecutive solutions of acetone at increasing concentrations (0, 25, 50, 75, and 100% v/v). Samples were dried at the critical point and coated with gold particles. A scanning electron microscope Jeol JSM-6360 LV (Jeol, Japan) was employed to obtain images of different fields at various magnifications. Control wheat and 35% replaced doughs without and with (0.01 and 0.1%) enzyme were analyzed.
Statistical Analysis
ANOVA was performed to determine significant effects of treatments when necessary, and the LSD test was applied to compare mean values. The software Statgraphics Centurion XV version 2.15.06 (USA) was used to perform the analysis.
Results and Discussion
Dough Preparation (Optimal Mixing Times)
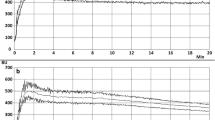
Figure 1 shows the evolution of torque and temperature as a function of mixing time for the control and 15, 25, and 35% composite doughs (Fig. 1a–d, respectively). It can be observed that after an induction period during which the torque remained constant (which takes 15–20 min), there was an apparent increase in torque up to a maximum value, as a consequence of mixing, due to the development of the gluten matrix. This maximum torque value was followed by a decrease that tended to reach a plateau value. This decrease is indicating a weakening of the dough due to the rupture of linkages in the gluten network (Skerritt, Luch, and Bekes 1999). Temperature profiles showed an increase that may be associated with the mechanical energy dissipated during mixing. In any case, the maximum temperature value achieved (until optimal consistency of the mass) was below 32 °C for all doughs. This moderate temperature indicates that the enzyme would not undergo thermal denaturation during this stage, according to information available from the enzyme producer and enzyme stability reported by Ando et al. (1989).
No significant differences were found in optimal mixing times (defined as the time necessary to reach the maximum torque value) between the control dough and the doughs with 15 and 25% of MF, since the mean values (±SD) were 19.3 ± 0.2, 20.1 ± 1.2, and 20.9 ± 1.5 min, respectively. The optimal time for the dough with maximum replacement of WF was significantly longer (28.5 ± 1.0 min). In a previous work (Bigne et al. 2016b), it was reported that farinograph development time was the longest for the maximum level of replacement with MF. The increase in optimal development time when increasing the replacement with MF could mainly be attributed to the presence of sugar, proteins, and fiber in the MF. Calderón Domínguez, Neyra Guevara, Farrera Rebollo, Arana Errasquín, and Mora Escobedo (2003) reported longer mixing times to reach the same mixing development stage in sweet-yeasted doughs, compared to bread doughs, and linked this behavior with the presence of water-soluble components (like sugar) that compete for water with flour. Ribotta et al. (2005) suggested that soy flour proteins interfere with gluten formation in composite doughs by both direct (interactions between soy and gluten proteins) and indirect (water competition) effects and reported longer development times for WF-soy flour doughs. The same effect in development time was also reported by Sabanis and Tzia (2009) when adding soy flour to WF in bread formulations. Addition of different soluble and insoluble fibers to wheat dough also increases development time. This effect is probably due to competition for water and interactions with gluten proteins as reported by several authors (Laurikainen and Ha 1998; Peressini and Sensidoni 2009; Sudha, Vetrimani, and Leelavathi 2007).
Dough Characterization
Frequency Sweep Tests
Figure 2 shows the mechanical spectra obtained by means of frequency sweep tests for control dough (Fig. 2a) and dough with 35% of MF (Fig. 2c) without enzyme and with 0.01 and 0.1% of enzyme addition, data for 15 and 25% WF replacement not shown.
Mechanical spectra (frequency sweep test) after the mixing process obtained for the control dough (a) and dough replaced with 35% of mesquite flour (c) and different concentrations of enzyme transglutaminase, TG (without enzyme and with 0.01 and 0.1% of enzyme addition), and the corresponding mechanical spectra after thermal treatment for control (b) and 35% replaced dough (d)
The wheat control and composite wheat-mesquite doughs exhibited a viscoelastic behavior in the frequency range assayed, with higher E′ than E″ values, indicating the prevalence of elastic contributions over the viscous ones. An important dependence of both moduli on frequency was observed, which is a characteristic of weak gel-like structures (Steffe 1996).
In the absence of enzyme TG, an increasing MF proportion in the formulation led to the increase of the elastic and viscous moduli, although the values (compared at 0.1, 1, and 10 Hz) were only significantly (p < 0.05) higher for dough with 35% of replacement with MF, compared to wheat control dough. The increase of dynamic moduli (G′, G″, G*) when increasing MF level in composite WF-MF doughs was previously described by Bigne et al. (2016b).
The incorporation of 0.01% of enzyme to the control dough led to a significant increment in the dynamic moduli (p < 0.05); nevertheless, when using the maximum concentration of enzyme (0.1%), this effect partially reverted, and no significant differences were found in modulus values compared to the control dough without TG (p > 0.05) (Fig. 3a). The effect of different doses of TG on glutenin macropolymer was studied by Steffolani, Pérez, Ribotta, Puppo, and León (2008). These authors reported the formation of a more viscous glutenin macropolymer with behavior between that of a macromolecular solution and a cross-linked macromolecular dispersion at the highest doses used.
Mean values of E′ and tan δ at 1 Hz (6.23 rad/s) for the control dough and doughs with different replacements of WF with MF (15, 25, and 35%) and different concentrations of enzyme TG (without enzyme and with 0.01 and 0.1% of enzyme addition), before (a, c) and after (b, d) the thermal treatment. Different letters indicate significant differences (p < 0.05) between doughs in each group (same level of replacement with MF)
In the composite doughs (with 15, 25, and 35% MF), the incorporation of transglutaminase did not lead to the marked changes observed in the E′ and E″ moduli for the wheat control one (Fig. 3a). In fact, in the doughs with 35% of MF, the spectra appeared almost overlapped, indicating a slight or no effect of the enzyme on the E′ and E″ moduli in the frequency range evaluated (Fig. 2c).
The values of tan δ measured at 1 Hz (tan δ1) for doughs with different levels of MF and transglutaminase were between 0.28 and 0.39 and are shown in Fig. 3c. The reported values for wheat bread doughs were generally lower than 0.5, indicating a marked predominance of the elastic character over the viscous one (Gómez 2011). The results from our assays were in agreement with this value.
No significant differences were found between mean values of the control dough and doughs with 15, 25, and 35% of MF when no enzyme was added (p > 0.05), indicating an approximately constant balance between elastic and viscous components for doughs with different levels of MF. Enzyme addition at both concentrations tended to lower the values of tan δ (higher elastic character of the matrix) for the doughs with and without MF. This effect could be associated with a certain degree of cross-linking between proteins promoted by the enzyme The same trend on tan δ of glutenin macropolymer was found by Steffolani et al. (2008) when increasing TG levels in dough formulation.
Microscopic Characterization of Doughs
The micrographs obtained by CSLM for the control and composite doughs with different levels of enzyme TG are shown in Fig. 4.
The structures stained in green color correspond to those labeled with FITC, which showed affinity for starch and gluten protein network, as previously reported by Correa, Ferrer, Añón, and Ferrero (2014), while the red structures correspond to those labeled with RodB, which have affinity for proteins as reported by Kim et al. (2008) for complex protein/polysaccharide systems.
When analyzing doughs without enzyme, it was found that the addition of MF altered the gluten matrix (which can be seen in green-yellowish color by labeling with both FITC and Rod B), leading to a progressive loss of the highly cross-linked and filamentary structure when the replacement of WF with MF was increased (Fig. 4a, d, g, j). The analysis also revealed no gluten film formation when MF was incorporated into dough and the presence of mesquite particles of protean character, stained red by Rod B, some of them surrounded by the filaments of gluten or adjacent to the latter. These results are in accordance with those reported by Roccia, Ribotta, Pérez, and León (2009) for a mixture of wheat gluten and soy protein studied by SEM. These authors observed that the mixture had a more porous structure with soy protein globular aggregates immersed into the gluten network.
In the wheat control dough, the enzyme modified the structure. When a lower concentration (0.01%) was used, the presence of gluten films diminished, while the fibers of gluten thickened (Fig. 4b). The upper concentration of transglutaminase (0.1%) considerably incremented protein aggregation, and the loss of the filamentous gluten network was evident (Fig. 4c). These observations can be associated with the transglutaminase action mainly on the high molecular weight glutenin, richer in lysine than the other gluten proteins (Autio et al. 2005).
Although in composite doughs, no gluten films were observed, the doughs with 15 and 25% of MF kept a filamentous protein network. These doughs showed (like the control dough) the formation of thicker filaments with the addition of the enzyme to the formulation. By applying 0.1% TG (Fig. 4f, i), the observed structures were similar to those with the lower level of enzyme (Fig. 4e, h).
In the dough with 35% of MF, no filamentous structure was observed, and a high degree of protein aggregation was evident (Fig. 4j). The addition of TG seemed to increase, at least partially, the presence of protein filaments, although these structures were thicker than those observed for the doughs with a lower MF percentage. The protein matrix generated by adding the enzyme was comparable to that observed in the doughs with lower MF content.
Figure 5 shows the images obtained by SEM and CSLM for the control dough and 35% MF without TG (w/o TG) and with 0.01 and 0.1% of enzyme addition (TG 0.01 and 0.1%) obtained with higher magnifications than in Fig. 4. With respect to the control system, observations by the two microscopic techniques were consistent and showed the formation of gluten films (GF) and filamentous (FG) structures (Fig. 5a). This is indicative of a well-developed gluten network. By incorporating the enzyme at the lowest concentration (Fig. 5b), the ability to form gluten films remained, although these structures appeared to be less closed compared to the dough without TG. The most important change in the structure was observed with the highest concentration of enzyme (Fig. 5c), where a marked increase in protein aggregation was verified; nevertheless, by electronic microscopy, it was possible to distinguish areas where films of gluten had formed, although to a much lower extent.
On the other hand, SEM and CSLM micrographs obtained for the doughs with 35% of MF showed the effect of TG addition on the microstructural characteristics of the composite WF-MF dough. In the SEM micrographs, some components appear like irregular structures, apparently of fibrous nature, that would correspond to fragments of mesquite pods (MC). The formation of gluten films for dough with 35% of MF could be observed only in some reduced areas in the absence of TG (Fig. 5d), as shown by SEM. With the addition of the enzyme at the lowest concentration (Fig. 5e), a more pronounced presence of thick filamentous structures was observed. However, the highest level of enzyme seemed to increase protein aggregation without the formation of filamentous structures (Fig. 5f). The presence of starch granules (SG) as rounded structures embedded in the matrix could be seen for both wheat and composite wheat-mesquite doughs.
Both microscopy techniques allowed verifying important changes in gluten matrix formation when adding MF to the bread formulation. These changes were due to the presence of majority components from MF (fiber and sugars) that modify water availability for gluten development and can have disruptive effects on the matrix (in the case of insoluble fiber particles).
Thermal Treatment
The objective of this test was to study the rheological behavior of the doughs during heating to 85 °C (close to the temperature reached inside the bread during baking) and subsequent cooling.
Figure 6 depicts the typical response of E′ and tan δ for the wheat control dough (Fig. 6a) and doughs where WF has been replaced with MF in different proportions, namely 15% (Fig. 6b), 25% (Fig. 6c), and 35% (Fig. 6d), during increasing and decreasing temperature ramps. The curves corresponding to doughs without (w/o TG) and with 0.01 and 0.1% of transglutaminase addition (TG 0.01 and 0.1%, respectively) are also shown.
Doughs with different mesquite contents showed a somewhat different behavior when subjected to heating and cooling. However, in general, in the first part of the heating stage (from 20 to 50 °C, approximately), the elastic modulus decreased and tan δ increased. This fact can be associated with the greater mobility of the matrix by the increase in the kinetic energy of the system, related to thermal agitation and the breaking of interactions (electrostatic and hydrogen bond interactions are reduced) with temperature, and, as a consequence, the decrease of viscosity (Doǧan 2002). At the middle part of the heating ramp (from 45 to 60 °C, depending on dough formulation), E′ began to increase and, consequently, tan δ decreased, reinforcing the prevalence of the elastic character over the viscous one. This behavior may be associated with the partial starch gelatinization, which leads to an increase in water absorption by the granules and to the leaching of amylose, in addition to the denaturation of proteins (Campos, Steffe, and Ng 1997). It was verified that the increase in E′ started at significant higher temperatures as the content of MF increased in the doughs; nevertheless, no differences were found at such temperatures between doughs with different levels of enzyme addition and the same MF level. Those temperatures were 47.66 ± 3.58 (°C), 53.31 ± 1.18 (°C), 55.46 ± 1.75 (°C), and 57.47 ± 1.56 (°C) for the control dough and composite doughs with 15, 25, and 35% of replacement with MF, respectively. The same effect was observed in gelatinization temperatures, calculated as the inflection point in the E′ increasing curve. The gelatinization temperatures were 72.68 ± 0.42 (°C), 76.56 ± 0.49 (°C), 78.61 ± 0.45 (°C), and 80.27 ± 0.80 (°C) for the control dough and doughs with 15, 25, and 35% of replacement with MF. This effect was also in agreement with DSC results reported by Bigne et al. (2016a) for wheat-mesquite doughs, where significant increments in the onset and peak temperatures associated with the gelatinization peak were observed when the content of MF was increased. The shifting effect to higher temperatures could be related to the sugar content in the dough, as reported by Salvador, Sanz, and Fiszman (2006) in a study on wheat doughs added with sucrose levels of 10–20 g/100 g of flour. These sugar contents are similar to those provided by the MF in the formulations of the present work (between 7.5 and 23 g/100 g of WF or 6.4 and 14.9 g/100 g of total flour). Besides, these authors indicated that the effect should not only be caused by the reduction of available water for gelatinization due to the increase in the concentration of sugars, but also by specific interactions between sugars, the leached amylose and the intact granules. Such effect in gelatinization temperature was also observed by Bchir, Rabetafika, Paquot, and Blecker (2014) in wheat doughs enriched with fiber from different sources and related this to the high water-holding capacity of fiber.
During the cooling stage, the elastic modulus continuously increased in all cases, probably due to hydrogen bonding formation. However, this increase was not pronounced, and tan δ remained virtually constant.
As previously mentioned, the addition of TG enzyme did not significantly alter the temperatures at which the rapid increase in E′ started or the gelatinization temperature. On the other hand, with the addition of TG to the formulations without MF and with 15% replacement with MF, qualitatively higher values of E′ were observed along almost the entire temperature range tested. This TG-induced effect only took place when a certain level of gluten was present, since it could not be detected for the doughs with a higher MF content (25 and 35%).
After cooling to 25 °C, frequency sweep tests in the linear viscoelasticity range were performed for evaluating the viscoelastic characteristics of the thermally processed doughs. The mechanical spectra for control and 35% replaced doughs are shown in Fig. 2b, d, where a typical gel-like behavior can be observed, with higher values of E′ than E″, both of them showing a moderate dependence on frequency. The dynamic moduli showed values one order of magnitude higher than those observed from tests for the doughs before heat treatment (Fig. 2a, c). The dependence of the moduli on frequency was lower than the one for the crude doughs, indicating a clear reinforcement of the matrix structure obtained as a consequence of the cooking process. The values of E′ measured at 1 Hz for doughs with different concentrations of MF and enzyme can be seen in Fig. 3b. According to the mechanical spectra shown in Fig. 2b, the enzyme seemed to have some effect on the formulations without mesquite, increasing the E′ and E″ moduli, although the differences were not significant (p > 0.05). In the composite formulations, the mechanical spectra were almost overlapped for the doughs with different levels of TG. Therefore, no significant differences in the mechanical spectra were detected between doughs with different amounts of MF or with different levels of enzyme TG after the thermal treatment.
The values obtained for tan δ (at 1 Hz) for the doughs with different contents of MF and different levels of transglutaminase after heating/cooling are shown in Fig. 3d.
By comparing Fig. 3c, d, it can be observed that the values of tan δ obtained for all samples were significantly lower for the thermally processed doughs, indicating that these systems exhibited a decrease in viscosity and an increased elastic character. Tan δ ranged from values between 0.28 and 0.39 for crude doughs, to values between 0.13 and 0.18 for the thermally processed doughs, suggesting the increase in the elastic character for the latter. Partial starch gelatinization and protein denaturation caused by heating can explain this effect. The range of values of tan δ for thermally processed doughs was lower than the values for the crude doughs. This can be related to the influence of gelatinized starch on the rheology of these systems, as indicated by Campos et al. (1997). However, heat-induced gelation of the protein fraction may also contribute significantly to the viscoelastic response to heat treatment found for the dough samples studied. In fact, the viscoelastic behavior shown in Fig. 6 is similar to that typically obtained for thermal protein gelation (Cordobés et al. 2004).
The two-way analysis of variance indicated that both the MF level and enzyme level had significant effects on the values of tan δ and that the interaction between the two factors was also significant. As a general trend, enzyme addition decreased tan δ in the doughs. The lowest level of enzyme did not modify tan δ for the control dough, but this parameter decreased significantly when the highest concentration of enzyme was used. In the composite doughs, the enzyme also decreased tan δ values, except for the dough with 25% MF and the highest level of enzyme.
Conclusions
The replacement of WF by MF in dough formulation gave rise to significant changes in the rheology, microstructure, and thermal properties of doughs. The main effects caused by increasing addition of MF were as follows: higher optimal development times, increased elastic and viscous moduli, and higher gelatinization temperatures. Besides, gluten matrix was progressively worsened by MF addition as observed by microscopy assays. These effects would be associated with the presence of MF components that are capable of competing with gluten proteins for the available water and also could interfere with gluten network development. The thermally processed doughs showed much higher values of the elastic modulus and much lower values of tan δ than the raw doughs, as a result of the partial starch gelatinization and protein gelation.
When adding the transglutaminase enzyme, a significant reduction of tan δ was found in doughs with 15 and 25% of MF, since the enzyme addition generally increased gluten protein aggregation in doughs. After the thermal treatment, the doughs with enzyme exhibited lower values of tan δ, except for the highest content of MF. From the above results, it can be concluded that higher levels of transglutaminase do not improve the rheological behavior of composite dough, since it does not succeed in forming an adequate gluten network, as evidenced by a more aggregated protein matrix. However, at a low level, it has a certain positive effect on composite dough rheology, though it is not enough to compensate the loss of quality produced in the viscoelastic gluten matrix by MF addition.
References
AACC International. (2000). Approved methods of the American Association of Cereal Chemists (10th ed.). St. Paul, MN: American Association of Cereal Chemists.
Ando, H., Adachi, M., Umeda, K., Matsuura, A., Nonaka, M., Uchio, R., Tanaka, H., & Motoki, M. (1989). Purification and characteristics of a novel transglutaminase derived from microorganisms. Agricultural and Biological Chemistry, 53(10), 2613–2617.
Autio, K., Kruus, K., Knaapila, A., Gerber, N., Flander, L., & Buchert, J. (2005). Kinetics of transglutaminase-induced cross-linking of wheat proteins in dough. Journal of Agricultural and Food Chemistry, 53(4), 1039–1045.
Barba de la Rosa, A. P., Frias-Hernández, J. T., Olalde-Portugal, V., & González Castañeda, J. (2006). Processing, nutritional evaluation, and utilization of whole mesquite flour (Prosopis laevigata). Journal of Food Science, 71(4), S315–S320.
Bchir, B., Rabetafika, H. N., Paquot, M., & Blecker, C. (2014). Effect of pear, apple and date fibres from cooked fruit by-products on dough performance and bread quality. Food and Bioprocess Technology, 7(4), 1114–1127.
Bigne, F., Puppo, M. C., & Ferrero, C. (2016a). Fibre enrichment of wheat flour with mesquite (Prosopis spp.): effect on breadmaking performance and staling. LWT - Food Science and Technology, 65, 1008–1016.
Bigne, F., Puppo, M. C., & Ferrero, C. (2016b). Rheological and microstructure characterization of composite dough with wheat and mesquite (Prosopis spp.) flours. International Journal of Food Properties, 19(2), 243–256.
Brabender VA (2009). Standards and Methods. Duisburg, Germany. Retrieved from http://www.brabender.com/fileadmin/dateien/gb/download/nahrungsmittel/download/Veroeffentlichungen/E_Standards_and_Methods_2009_2.pdf
Bravo, L., Grados, N., & Saura-calixto, F. (1998). Characterization of syrups and dietary fiber obtained from mesquite pods (Prosopis pallida L.). Journal of Agricultural and Food Chemistry, 8561(97), –6.
Caballero, P. A., Gómez, M., & Rosell, C. M. (2007). Improvement of dough rheology, bread quality and bread shelf-life by enzymes combination. Journal of Food Engineering, 81(1), 42–53.
Calderón Domínguez, G., Neyra Guevara, M., Farrera Rebollo, R., Arana Errasquín, R., & Mora Escobedo, R. (2003). Structural and farinographic changes during mixing of a yeast sweet dough. Food/Nahrung, 47(5), 312–319.
Campos, D. T., Steffe, J. F., & Ng, P. K. W. (1997). Rheological behavior of undeveloped and developed wheat dough. Cereal Chemistry, 74(4), 489–494.
Cauvain, S., & Young, L. (2001). Improvers. In baking problems solved (1st ed., pp. 52–62). Cambridge: Woodhead Publishing Limited.
Collar, C., Andreu, P., Martínez, J. C., & Armero, E. (1999). Optimization of hydrocolloid addition to improve wheat bread dough functionality : a response surface methodology study. Food Hydrocolloids, 13, 467–475.
Cordobes Carmona, F., Partal Lopez, P., & Guerrero Conejo, A. (2004). Rheology and microstructure of heat-induced egg yolk gels. Rheologica Acta, 43(2), 184–195.
Correa, M. J., Ferrer, E., Añón, M. C., & Ferrero, C. (2014). Interaction of modified celluloses and pectins with gluten proteins. Food Hydrocolloids, 35, 91–99.
Doǧan, İ. S. (2002). Dynamic rheological properties of dough as affected by amylases from various sources. Food/Nahrung, 46(6), 399–403.
Faergemand, M., & Krog, N. (2003). Using emulsifiers to improve food texture. In B. M. Mckenna (Ed.), Texture in food. Cambridge: Woodhead Publishing Limited.
Felker, P., Takeoka, G., & Dao, L. (2013). Pod Mesocarp flour of north and south American species of leguminous tree Prosopis (mesquite): composition and food applications. Food Reviews International, 29(1), 49–66.
Gómez, A. V. (2011). Efecto de distintos emulsificantes sobre la microestructura de la masa y su relación con la calidad de productos de panificación (PhD Thesis). Facultad de Cs. Exactas, Universidad Nacional de La Plata, Argentina.
Hefnawy, T., El-Shourbagy, G. A., & Ramadan, M. F. (2012). Impact of adding chickpea (Cicer arietinum L.) flour to wheat flour on the rheological properties of toast bread. International Food Research Journal, 19(2), 521–525.
Kim, E. H.-J., Petrie, J. R., Motoi, L., Morgenstern, M. P., Sutton, K. H., Mishra, S., & Simmons, L. D. (2008). Effect of structural and physicochemical characteristics of the protein matrix in pasta on in vitro starch digestibility. Food Biophysics, 3(2), 229–234.
Laurikainen, T., & Ha, H. (1998). Effects of enzymes in fibre-enriched baking. Journal of the Science of Food and Agriculture, 76, 239–249.
Messina, M. J. (1999). Legumes and soybeans: overview of their nutritional profiles and health effects. American Journal of Clinical Nutrition, 70(3).
Moreira, R., Chenlo, F., Torres, M. D., & Prieto, D. M. (2012). Technological assessment of chestnut flour doughs regarding to doughs from other commercial flours and formulations. Food and Bioprocess Technology, 5(6), 2301–2310.
Peressini, D., & Sensidoni, A. (2009). Effect of soluble dietary fibre addition on rheological and breadmaking properties of wheat doughs. Journal of Cereal Science, 49(2), 190–201.
Ribotta, P. D., Arnulphi, S. A., León, A. E., & Añón, M. C. (2005). Effect of soybean addition on the rheological properties and breadmaking quality of wheat flour. Journal of the Science of Food and Agriculture, 85(11), 1889–1896.
Roberfroid, M. B. (1999). What is beneficial for health? The concept of functional food. Food and Chemical Toxicology, 37, 1039–1041.
Roccia, P., Ribotta, P. D., Ferrero, C., Pérez, G. T., & León, A. E. (2012). Enzymes action on wheat–soy dough properties and bread quality. Food and Bioprocess Technology, 5(4), 1255–1264.
Roccia, P., Ribotta, P. D., Pérez, G. T., & León, A. E. (2009). Influence of soy protein on rheological properties and water retention capacity of wheat gluten. LWT-Food Science and technology, 42(1), 358–362.
Sabanis, D., & Tzia, C. (2009). Effect of rice, corn and soy flour addition on characteristics of bread produced from different wheat cultivars. Food and Bioprocess Technology, 2(1), 68–79.
Salvador, A., Sanz, T., & Fiszman, S. M. (2006). Dynamic rheological characteristics of wheat flour–water doughs. Effect of adding NaCl, sucrose and yeast. Food Hydrocolloids, 20(6), 780–786.
Sciammaro, L., Ferrero, C., & Puppo, M. C. (2015). Chemical and nutritional properties of different fractions of Prosopis alba pods and seeds. Journal of Food Measurement and Characterization. doi:10.1007/s11694-015-9282-z.
Skerritt, J. H., Hac, L., & Bekes, F. (1999). Depolymerization of the glutenin macropolymer during dough mixing: I. Changes in levels, molecular weight distribution, and overall composition. Cereal Chemistry, 76(3), 395–401.
Steffe, J. F. (1996). Rheological methods in food process engineering (2nd ed.). East Lansing, Michigan: Freeman Press.
Steffolani, M. E., Pérez, G. T., Ribotta, P. D., Puppo, M. C., & León, A. E. (2008). Effect of transglutaminase on properties of glutenin macropolymer and dough rheology. Cereal Chemistry, 85(1), 39–43.
Sudha, M. L., Vetrimani, R., & Leelavathi, K. (2007). Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chemistry, 100(4), 1365–1370.
Wieser, H. (2003). The use of redox agents. In S. Cauvain (Ed.), Bread making: improving quality. Cambridge: Woodhead Publishing Limited.
Yokoyama, K., Nio, N., & Kikuchi, Y. (2004). Properties and applications of microbial transglutaminase. Applied Microbiology and Biotechnology, 64(4), 447–454.
Acknowledgements
The authors also acknowledge CIITUS (Universidad de Sevilla) for providing full access and assistance to the Confocal Microscopy Service and Patricia Sarmiento from the Museum of Natural Sciences, UNLP, for her assistance in obtaining the SEM micrographs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by CONICET (PIP 0354, PIP 0289), UNLP (11/X517, 11/A230) and the program BEC.AR.
Rights and permissions
About this article
Cite this article
Bigne, F., Romero, A., Ferrero, C. et al. Rheological and Microstructural Study of Wheat Doughs Partially Replaced with Mesquite Flour (Prosopis alba) and Added with Transglutaminase. Food Bioprocess Technol 10, 819–830 (2017). https://doi.org/10.1007/s11947-017-1869-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1869-4