Abstract
This study examined the effect of the incorporation of flesh fibre concentrate (FFC) from apple, pear, and date pomaces on wheat bread dough performance and bread quality. The nutritional composition and techno-functional properties (water-holding capacity, oil-holding capacity, swelling capacity) of FFC were determined beforehand. Dough performance was evaluated by farinograph, alveograph and visco-amylograph. Bread quality was assessed by physical (weight, specific volume, and color) and textural (hardness and elasticity) parameters. Digital imaging analysis was also performed in order to better understand the observed effects. Results showed that the addition of FFC in wheat flour significantly improved (P < 0.05) dough properties inducing an increase of water absorption (from 55 to 60 %), of stability (from 4 to 31 min),of tenacity (from 83 to 116 mmH2O) , a reduction of extensibility (from 69 to 29 mm), of softening (from 60 to 20 BU), of breakdown (from 34 to 25 BU) and of setback (from 103 to 93 BU) in comparison to the control dough (without fibre). The formulation containing FFC produced loaves that had various colors (crust, 0 < ΔE* < 10 and crumb, 0 < ΔE* < 20; ΔE* corresponding to color variation), a comparable specific volume (2.7 vs 2.9 cm3/g for control) and a more aerated internal crumb structure compared to the control. During storage of breads at 20 °C, there was no significant difference (P < 0.05) between enriched and control bread crumb and crust texture profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, there is a growing demand from consumers for baked products with lower caloric density and higher levels of dietary fibre (DF). The latter is considered as an exceptional ingredient with a lot of beneficial effect on human health (Sun-Waterhouse et al. 2008; Tsatsaragkou et al. 2012; Mahawar et al. 2012). According to the Association of Official Analytical Chemists (1995) dietary fibres are defined as “the polysaccharides and remnants of plant materials that are resistant to hydrolysis (digestion) by human digestive enzymes” (DeVries et al. 1999). High dietary fibre intake is generally believed to reduce the risk of coronary heart disease (Sun-Waterhouse et al. 2008), prostate and laryngeal cancer (Pelucchi et al. 2004). In addition to the physiological benefits provided by high fibre foods, studies have shown that dietary fibres can also impart some functional properties to foods, e.g. increase water-holding capacity, oil-holding capacity, emulsification and/or gel formation. DF’s beneficial effects and properties, justify its addition in food products such as bakery products, jam, soup and meat etc. In fact, many authors illustrated that dietary fibres incorporated into food products can modify textural properties, avoid syneresis, stabilize high fat food and emulsions and improve shelf-life (Elleuch et al. 2011).
However, there is often insufficient fibres consumption in the daily diet, which is partly attributed to the fact that insufficient fibres are incorporated into popular product categories such as bakery products (Sun-Waterhouse et al. 2008). According to the United States Department of Agriculture, the recommendations for DF intake range from 25 to 30 g/day. Therefore, the development of enriched bread with higher fibre content is one of the efficient ways to increase fibre intake (Goesaert et al. 2005).
Many researchers have studied the effect of increasing fibre content in bakery products (De Escalada et al. 2010). Wittig de Penna et al. (1998) used lupinus fibre in muffins. Wheat bran, corn bran, oat bran and soy hulls were used by Jeltema and Zabic (1979)) in cakes. Furthermore, many forms of DF have been used in bread making such as wheat bran, oat bran (Park et al. 1997), corn bran (Abdul-Hamid and Luan 2000), date seed fibre (Borchani et al. 2011), apple fibre (Mahawar et al. 2012), carob fibre (Tsatsaragkou et al. 2012), psyllium husk fibre (Chen et al. 1988), field pea hull (Collar et al. 2007), and hazelnut (Anil 2007). To the best of our knowledge, there are no reports on the use of apple, pear and date fibre from cooked fruit by-products as a source of DF in bread making. Cooked fruits have specific flavors, generated from non-enzymatic browning reactions, including caramelized sugar aromas. In Belgium, the production of “Liège syrup”, a very popular fruit-concentrate spread similar to apple butter, generates annually one thousand tons of by-products from cooked apples, pears and dried dates (Aguedo et al. 2012; Rabetafika et al. 2013).
The aim of the present study is to evaluate the potentiality of apple, pear and date fibres, from cooked fruits by-products of “Liège syrup” manufacturing, as a bakery ingredient. Thus, two sets of experiments were carried out, one to determine the composition and techno-functional properties (water-holding capacity, oil-holding capacity, swelling capacity) of flesh fibre concentrate (FFC) and the other to evaluate the impact of FFC addition on physicochemical properties of dough (e.g. water absorption, development time, stability, deformation energy, tenacity, extensibility, breakdown etc.) and bread quality (e.g. weight loss, specific volume, color, crumb structure and texture, etc.).
Material and Methods
Material
The by-products of cooked apple, pear and dried date, came from the production of “Liège syrup” from the Siroperie Meurens (Aubel, Belgium) in autumn 2011. During the industrial process, apples (mainly French “Granny-Smith” and Belgian “Jonagold” and “Jonagored” cultivars) and pears (Belgian and French “Conference” cultivars) were cooked separately during one hour. Sun-dried dates (“Deglet noor” from Tunisia) were heated in water during 3 h and were then pitted; the ensuing pulp was then cooked during 2 h. After cooking, the different fruit were filter-pressed, yielding solid by-products: 100 kg of fruit yielded approximately 12 kg of by-products. Approximately 10 kg of fresh by-products were randomly collected from two different batches (each weighing several hundred kilogrammes) and rapidly stored at −20 °C for later analysis (Aguedo et al. 2012).
Wheat commercial flour of standard quality provided by Ceres group (BELGIQUE) was used for dough preparation. Flour composition was: proteins, 11.7 %; lipids, 1.2 %; moisture, 13.2 % and ash, 0.6 %.
Sample Preparation
For all analyses, the samples were desugared, oven-dried and ground, in order to avoid microbial spoiling during storage. Desugaring process was performed as follows; pomaces were stirred at room temperature during 15 min in distilled water at a ratio 1/10 (w/v; pomace/water). The mixtures were then filtered on 100-μm nylon filter to separate the insoluble residues. These operations of extraction and filtration were repeated twice (Rabetafika et al. 2013). Residues of desugared fruit pomaces were then oven-dried. Drying experiments were carried out in a laboratory scale drier with an air velocity of 2.0 ± 0.1 m/s. The drying cabinet (Memmert tcp 800, Schutzart, Germany) was equipped with an electrical heater, a fan and temperature indicators. The grinding was carried out with a laboratory hammer mill Retsch with 1-mm mesh sieve (Haan, Germany).
The particle size of these preparations expressed as D(0.5) was determined by laser diffraction using a laser particles size analyzer (Mastersizer-Malvern 2000, United kingdom). D(0.5) corresponds to the mean particle size for which 50 % of the sample volume is below. Bulk density was determined in accordance with Sabanis et al. (2009).
All analytical determinations were performed in triplicate. Values were expressed as the mean ± standard deviation.
Physico-Chemical Analysis of FFC
Dry Matter, Ash, Lipid and Water Activity
Dry matter (DM) was determined by drying samples at 106 °C to constant weight (Association of Official Analytical Chemists 1995). Total ash content (525 °C for 3 h, expressed as percent of DM), lipids were analyzed according to AOAC methods (Association of Official Analytical Chemists 1995). Water activity (a w) was measured using Aqualab Cx-2 instrument (Decagon, Pullman, USA) at 20 °C (Bchir et al. 2012a).
Protein Determination: Dumas Method
Protein concentration was determined using a Dumas Elementar Rapid N cube 161 15054 (Donaustrasse, Germany). Up to 1 g solid sample material was wrapped in paper and pressed into pellets. The wrapped samples were loaded onto a carousel with 60 positions. Samples are individually transferred to the combustion tube via the well ball valve (a rotary valve that has a ball closure). Nitrogen determination is based on the quantitative combustion digestion of the sample at approximately 900 °C in excess oxygen (Saint-Denisa and Groupy 2004). The bound nitrogen is transformed into molecular nitrogen and nitric oxides. The analysis gases are transferred with CO2 as carrier gas via a catalytic post combustion zone onto a reduction zone. At this point, the conversion of the nitric oxides into nitrogen at hot tungsten takes place. Furthermore, the excess oxygen is bound. After a two-stage drying phase, the gas mixture flows to the thermoconductivity detector via an electronic flow controller. A connected PC calculates the nitrogen concentration in the sample from the thermoconductivity detector signal of the N2 in the CO2 and from the sample weight. A factor of 6.25 was used for conversion of nitrogen to crude protein (Bchir et al. 2012a).
Dietary Fibre Determination
Insoluble dietary fibres (IDF) including neutral dietary fibre (NDF) such as cellulose, hemicelluloses and lignins were analysed according to the Van Soest and Wine (1967) method, and AOAC 973.18 method (Association of Official Analytical Chemists 1995) using selective solubilisation in neutral and acid detergent solutions of the insoluble fibre. Klason lignin was determined as the insoluble residue after treatment with H2SO4 72 % of the insoluble acid detergent.
Pectin content was quantified by the determination of the amount of galacturonic acid in samples. Analyses were performed by high-performance anion exchange chromatography coupled with pulsed amperometric detector (HPAEC-PAD) using a Dionex ICS-3000 system equipped with a PA100 anion exchange column (4 × 250 mm) and a PA100 guard column (4 × 50 mm). Elutions were performed at 35 °C at constant flow of 1 ml/min with solvent A (100 mM NaOH), solvent B (600 mM CH3COONa +100 mM NaOH), and solvent C (500 mM NaOH) as following: 100 % A during 5 min, then a linear 2 min gradient up to 25 % B and 75 % A maintained during 7 min, then a linear 0.5 min gradient up to 50 % B and 50 % A maintained during 9.5 min, then up to 100 % A in 0.5 min A and maintained during 9.5 min (Rabetafika et al. 2013).
Free Sugars Determination
HPAEC-PAD analysis was performed to quantify free sugars namely glucose, fructose and saccharose. Free sugars were extracted with water before injection on the Dionex ICS-3000 system equipped with a PA100 anion exchange column (4 × 250 mm) and a PA100 guard column (4 × 50 mm). Elutions were performed at 35 °C at constant flow of 1 ml/min with solvent A (100 mM NaOH), solvent B (600 mM CH3COONa +100 mM NaOH), solvent C (500 mM NaOH), and solvent D (distilled water) as following: 50 % solvent A + 50 % solvent D during 8 min, linear gradient up to 100 % solvent A during 4 min, 100 % A during 3 min, linear gradient up to 30 % B and 70 % A during10 min, 30 % B and 70 % A during 3 min, up to 50 % B and 50 % C during 0.1 min, 50 % B and 50 % C during 9.9 min, up to 50 % D and 50 % A during 0.1 min, 50 % D and 50 % A during 10 min (Rabetafika et al. 2013).
Techno-Functional Properties
Water-Holding Capacity
The water-holding capacity (WHC) was determined using a modified method described in the literature (Mac Connell et al. 1974). 1 g of sample was stirred with 15 ml of distilled water in a 50-ml centrifuge tube and soaked overnight at room temperature. The mixture was then centrifuged at 15,000×g for 20 min. The free water was discarded and the absorbed water weighed. WHC was expressed as gramme of water per gramme of dry powder.
Oil-holding Capacity
The oil-holding capacity (OHC) was measured using a method described in the literature (Fermenia et al. 1997). One gramme of samples was added to 15 ml of sunflower oil in a 50-ml centrifuge tube. The content was left overnight at room temperature. After that the tubes were centrifuged at 15,000×g for 20 min. The free oil was decanted and the absorbed oil was weighed. OHC was expressed as gramme of oil per gramme of dry powder.
Swelling Capacity
The swelling capacity (SWC) was determined according to Guillon and Champ (2000). One gramme of sample was weighed in a glass cylinder and left to swell overnight at room temperature after adding 15 ml of distilled water. SWC was expressed as milliliter of swollen sample per gramme of dry initial matter.
Bread Making Procedure
Four formulations were tested: control bread (without fibre), breads enriched with date, pear, and apple fibre. FFC fractions were incorporated in a bread formula in order to obtain loaf with 2 % of fibre. Breads were prepared by mixing dough constituents and water. First, wheat flour enriched by dietary fibre and yeast (10 % of flour weight) were mixed in a Kenwood (Serial, KM 336, Germany) device during 2 min. The mixing rate was fixed on the ± 248 rpm. Then, the margarine (25 % of flour weight) was divided in small pieces and added to the mix. This blend was mixed again during 2 min at 248 rpm. Next, water was added little by little in one minute. During this operation, the mixing rate was fixed at ± 305 rpm. After 2.5 min of mixing at 305 rpm, salt (15 % of flour weight) was added and the mixing rate was reduced to 248 rpm. After mixing, a first fermentation took place. The dough was placed in a Salva fermentation room (IVERPAN-87, Toulouse, France) during 45 min (30 °C, ±85%HR). Next, the dough was divided in 4 parts and these parts were rolled by dough rounder (Brabender, Duisburg, Germany) to obtain four dough bowls of ± 400 g. Each bowl was put in a previously oiled mould. For the second fermentation, the moulds with the dough bowls were placed in the fermentation room (30 °C, ± 85%HR). The fermentation time was controlled using a fermentation measurer. 25 ± 0.3 g of dough were rolled by hand and put in the fermentation measurer composed of a plunger and graduated cylinder. The plunger of the measure was placed at 2.4 cm. The second fermentation was stopped when the plunger reached the 4-cm graduation. Dough volume was recorded every 10 min until the end of the second fermentation.
Finally, the breads were baked in a Salva bakery oven at 230 °C during 15 min. Steam was injected into the oven at the beginning of the baking. After baking, breads were removed from their moulds and allowed to cool during 2 h at room temperature (∼25 °C). The volume and the weight of the breads were measured before packing them in bakery paper bags. The bread making was performed in duplicate, and control breads without fibre addition were also prepared each time that breads with different fibre fractions were processed.
Dough Characteristics
The effect of added FFC on dough rheology during mixing was determined by a farinograph (Brabender, Duisburg, Germany), and alveograph (Chopin AL 87, France) and viscoamylograph (Brabender PT-100, Germany).
Farinograph
Regarding the farinograph the following method 54-30-02 (American Association of Cereal Chemists AACC 2000) was used. The determined parameters were: water absorption or percentage of water required to yield dough consistency of 500 BU (Brabender units), dough development time (DDT; time to reach maximum consistency in minutes), stability (S; time when dough consistency remains at 500 BU), degree of softening (DS; 12 min after maximum), Quality index (QI; time interval between the points, where water is added and the point where the curve presents a decrease of 30 BU). Measurements were performed in triplicate. Values were expressed as the mean ± standard deviation.
Alveograph
The following method 54-30-02 (American Association of Cereal Chemists AACC 2000) was used to determine the alveograph test. The monitored parameters were the deformation energy (W), tenacity or resistance to extension (P), dough extensibility (L) and curve configuration ratio (P/L ratio) of dough, with or without fibres. Measurements were performed in quintuplicate. Values were expressed as the mean ± standard deviation.
Viscoamylograph
Enriched dough and control dough properties were evaluated following the method of Wiesenborn et al. (1994) using a viscoamylograph (Brabender PT-100, Germany). Enriched flour suspension was heated to 95 °C at a rate of 1.5 °C/min, held at this temperature for 15 min, cooled to 50 °C at the same rate and held at this second temperature for another 15 min. Amylogram was used to calculate maximum viscosity, breakdown and setback in BU. Viscosity at the initial gelatinization temperature corresponds to 0 BU (Torre-Gutiérrez et al. 2008).
On other hand, dough yield was also calculated by dividing dough weight (gramme) by flour weight multiplied by one hundred. Measurements were performed in triplicate. Values were expressed as the mean ± standard deviation.
Bread Quality Evaluation
Weight, Volume, Specific Volume and Weight Loss Determination
Weight and volume were measured 2 h after removal of bread loaves from the oven. Loaf volume was determined by seed displacement in a loaf volume meter and specific volume was calculated by dividing volume by loaf weight (cubic centimeter per gramme).Weight of breads before and after cooking was measured using a precision weighing scale; the weight loss was calculated using the Eq. (1) (Borchani et al. 2011).
Surface Color Measurement
Colors of crust and crumb were determined as the CIELAB coordinates (L*, a*, b*) using a colorimeter Mini Scan MS/Y-2500 with a lamp (D 65) lamp (HunterLab, Reston, VA). In this coordinate system, L* value is a measure of lightness, ranging from 0 (black) to +100 (white), a* value ranges from −100 (greenness) to +100 (redness) and b* value ranges from −100 (blueness) to +100 (yellowness) (Bchir et al. 2012b). The total color difference (∆E*) was determined by using Equation (2) (Romano et al. 2008):
Where L*, a*, b* and L0 *, a0 *, b0 * are the CIELAB coordinates of enriched and control breads, respectively. Two analyses were performed on crust and crumb at 3 cm from the edge. The spot size corresponds to 4 cm.
Texture Analysis
The loaves were cut perpendicular to their axis in 15-mm thick slices, using an electric knife (Krups, France), and texture was taken at 2.5 cm of the crust. Texture analysis were carried out using a texture profile analyzer (TA.XT2; Stable Micro Systems, UK), with 7-mm (diameter) cylinder probe for crust and 12.7-mm (diameter) spherical probe for the crumb. The operating conditions of the instrument were as follows: 2-mm/s pre-test speed, 2-mm/s test speed, 2-mm/s post-test speed, 0.10-N trigger force and 10-mm distance of penetration and 60-s time of compression. The hardness and elasticity of crust and crumb were the means of 6 single slices. Two analyses were performed on each slice at 2.5 cm from the edge. Hardness [Newton] was taken as the force in compression which corresponded to the end of the compression, while the elasticity was calculated by dividing the value of hardness (Newton) after 60 s by the value the value of initial hardness.
Digital Imaging
To acquire the digital image of the bread and the cut surface of the bread crumb for visual evaluation, a digital camera was used (Sony corp; DSC-W320; CHINA).
Statistical Analyses
Statistical analyses were carried out using statistical software (SPSS for windows version 11.0). The data sets were subjected to analysis of variance using the general linear model option (Duncan test) in order to investigate differences among samples (P < 0.05).
Results and Discussion
Characteristics of Apple, Pear And Date Pomaces
Composition and physicochemical properties of apple, pear and date pomaces are shown in Table 1. Results showed predominance of fibre in all pomaces especially insoluble fibre, followed by protein, fat, ash, and free sugar. Pomaces were desugared to obtain a flesh fibre concentrate (FFC). Larrauri (1999) reported that desugared pomaces contained more dietary fibre (DF) such as cellulose and hemicelluloses rich in hydroxyl group. In addition, washing the pomaces, particularly with hot water, dramatically increased the techno-functional capacities owing to the sugar released (Larrauri 1999).
In fact, Table 1 shows a high total dietary fibre content, particularly for IDF reaching 78, 81 and 89 g/100 g DM, for apple, date, and pear pomaces respectively. Cellulose represented the major component of insoluble dietary fibre in apple and pear FFC, whereas for date FFC it was lignin. Both pear and apple characteristics were higher to those observed in the literature. Indeed, DF in unprocessed apple pomace from juice manufacturing was reported to be only 51.1 % (Sudha et al. 2007). Grigelmo-Miguel and Martin-Belloso (1999)) and Martin-Cabrejas et al. (1995) found that concentrate pear pomaces were constituted with 36.1 to 43.9 % DF. However, DF content in the date FFC is within the range of Borchani et al. (2011) results, corresponding to 89 g/100 g DM. Total DF content of pear, apple and date FFC was higher than those of other fruit DF concentrates reported for grape fruit, lemon orange, and mango (28–71 g/100 g DM) (Figuerola et al. 2005; Vergara-Valencia et al. 2007). Soluble dietary fibre protein and free sugar are present in low amounts in pomaces. This is due to their solubilisation in water during the desugaring.
However, FFC might be considered as a potential ingredient for dietetic food products. Protein content in FFC was high (5.7–6–10.7 g/100 g DM, pear, apple and date, respectively) compared with other fibres such as mango fibre (4.28 g/100 g DM) (Vergara-Valencia et al. 2007), lemon fibre (6.7 g/100 g DM) and grape fruit fibre (4.42 g/100 g DM) (Figuerola et al. 2005). FFC contained a significant low amount of ash (0.9–1.4–2.1 g/100 g DM, respectively), compared to that determined in fibre from Citrus sinensis peel (3.3 g/100 g DM) (Chau and Huang 2003), lemon (3.4 g/100 g DM), orange (3.9 g/100 g DM), and grapefruit (3.2 g/100 g DM) (Figuerola et al. 2005). In addition, date, pear, and apple FFC presented a low content of lipid (2.6–3.7–2.5 g/100 g DM, respectively), lower than those reported in the commercial grape fibre (6.9 g/100 g DM) (Saura-Calixto 1998), peel of Citrus sinensis (22.2 g/100 g DM) (Chau and Huang 2003) and apple fibre (3.12 g/100 g DM) (Figuerola et al. 2005).
Physical properties of FFC are studied due to their importance in relation to the functionality and nutritional quality of food fibre. Three parameters have been widely studied in food functionality: the WHC, the OHC and the SWC (Sudha et al. 2007).
Date and pear FFC had respectively a water-holding capacity of 5.7 and 4.9 mL/g DM, lower than those reported in the literature (5.9, 6.7 ml/g DM, for date and pear pomaces, respectively). Whereas WHC for apple FFC were higher than those found for concentrated apple pomace (Liberty cultivars) by Figuerola et al. (2005). Oil-holding capacity is another important parameter, ranging between 2.0 and 2.2 g/g for both apple and pear pomaces, which were higher than 0.6–1.45 g/g, values reported by Figuerola et al. (2005) and Pourfarzad et al. (2013). A similar oil-holding capacity of date fibre (2.1 g oil/g dry sample) was observed by Borchani et al. (2011) (2.3 g oil/g DM). However, SWC were comparable or lower than those found in the literature for different FFC (Martin-Cabrejas et al. 1995; Figuerola et al. 2005; Sudha et al. 2007).
These differences could be related to the amount of insoluble dietary fibre in various varieties but also to the different fibre particle size. In fact, Sangmark and Noomhorm (2003) and Oarrott and Thrall (1978) reported that higher particle size of dietary fibre is associated with higher WHC and OHC. A decrease of the hydration properties was obtained after particle size reduction with carrot, cabbage fibres (Cadden 1987), ashgourd and radish fibres (Gupta and Premavalli 2010). The variation of WHC was explained by a structural modification like the surface area, porosity of fibres and fibre density (Cadden 1987; Lario et al. 2004).
L*, a*, b* values of pomaces reflected the modification of FFC colors after cooking and desugaring process. Different pomaces have a low L*, a*, b* values (28, 9, 9; 36, 6, 17; 35, 6, 13, for date, pear and apple, respectively). Pear and apple pomaces have a less dark color compared to date pomace. Taken into account the operating condition, the coloration may result from enzymatic and non enzymatic browning.
In addition, water activities of different pomaces were under 0.650, inhibiting bacteria, fungi, and yeast development (Bchir et al. 2012a) and inducing a better conservation of pomaces. Concerning the bulk density of powders, date had the higher value followed by apple and pear.
Physicochemical and nutritional characteristics of dietary fibres showed an interesting potentiality of fibre to be used in food formulation as ingredients.
Effects of Flesh Fibre Concentrate on Dough Properties
Table 2 summarises the farinograph, alveograph and viscoamylograph results of the control dough (without fibre addition) and those of dietary fibre (date, pear, and date FFC) supplemented dough.
Farinograph Analysis
Results showed that the addition of FFC promoted differences on the dough mixing behavior. In fact, a significant (P < 0.05) increase in the water absorption with added FFC-enriched dough compared with the control can be noticed. This result agreed with those found by other authors with different kinds of fibre and hydrocolloids (Abdel-Kader 2000; Rosell et al. 2010). This result was expected due to the important number of hydroxyl groups existing in the fibre structure, which allows more water interactions through hydrogen bonding (Gómez et al. 2003). These results explained an improvement of the dough yield (Table 3). The higher water absorption was observed in date and apple FFC-enriched dough.
Dough development time (DDT) and stability are indicators of the flour strength, higher values suggesting stronger doughs (Rosales-Juarez et al. 2008). DDT corresponding to the time required for dough development, or to reach a dough consistency equivalence of 500 BU, did not vary as a function of fibre additions. Similar results were obtained by Borchani et al. (2011). The use of FFC significantly increased the stability of the dough (19, 29, 31 min, respectively, for pear, date and apple) compared to the control (4 min; P < 0.05; Table 2), independently of fibre source. This can be explained by higher interactions between FFC, water and flour protein (gluten) (Borchani et al. 2011). Our results are in accordance with the results reported by Anil (2007) and Laurikainen et al. (1998), who found an increase of the stability with fibre addition. Apple and date FFC exhibited a higher effect on dough stability than the dough supplemented with pear FFC. This could be related to the structure and the composition of the added fibre. The addition of the FFC reduced three times the DS parameter compared to the control dough. This result was in concordance with those reported by Stojceska and Ainsworth (2008) and Anil (2007), which found a decrease of softening degree regarding addition of hazelnut fibre. The highest softening value (60 BU) was observed in the control sample because the antioxidants did not have any effect on the control dough. It is worth noting that phenolic substances and antioxidants are present in different FFC (Aguedo et al. 2012). Due to their richness in phenolic compounds, FFC could be considered a good source of natural antioxidants that may have contributed to strict doughs (Anil 2007; Borchani et al. 2011). Compared with the control dough, FFC addition increased significantly (P < 0.05) the QI of dough. These results can be explained by the interaction between FFC and gluten (Chen et al. 1988).
Alveograph Analysis
The effects of FFC addition on the alveograph parameters of wheat flour dough are shown in Table 2. Dough resistance to deformation or tenacity (P) gives information about the handling characteristics of the dough (dough’s ability to retain gas). Likewise, the extensibility of dough (L) is an indicator of the dough handling characteristics, particularly the capacity of extending without breaking down. The addition of FFC always increased dough tenacity (P) and reduced dough extensibility (L). The increase of P values is likely due to the interaction between fibre structure and flour protein (Wang et al. 2002) or the bad hydratation of doughs enriched with fibre. The resulting effect on P and L becomes evident in the P/L ratio value, (which gives information about the elastic resistance and extensibility balance of flour dough and summarizes the effect of P and L parameters) which always showed an increase (∼3 versus 1 in the control). This increase was not moderate and showed significant differences among fibres from different origins. This could be related to the different composition of the added fibre (Table 1). Wang et al. (2002) revealed that an increase of the P/L parameter might be caused by the high content of cellulose present in the fibre, which promotes a strong interaction among fibre and flour protein.
A statistical difference (P < 0.05) was observed in Table 2 between the control and the enriched FFC dough concerning W (the deformation energy). Indeed, all the FFC tested led to a decrease (up to 15 %) in the deformation energy (W). Similar results were obtained by Gómez et al. (2003) showing that the addition of wheat fibre and cocoa fibre decreased W by 23 and 10 %, respectively. However, other works showed that the addition of fibres contributed to a great improvement of the deformation energy. The influence on W depended on the fibre sources. In fact, Wang et al. (2002) found that the addition of fibres promotes a remarkable reduction of the proteolytic degradation, being practically neutralized by inulin and carob fibre.
Figure 1 exposes the properties of gas retention within the FFC-enriched dough that were controlled by measuring the volume of dough during fermentation using the growth indicator. The control dough revealed a faster rate than the enriched FFC dough. This might be due to an impediment of gas retention in the dough caused by an excessive amount of fibre (Sangnark and Noomhorm 2004). However, the volume of control dough tended to be stable after 80 min of fermentation, nevertheless the volumes of enriched dough continued to increase. This might be caused by the greater water absorption of FFC, affecting yeast action and gluten structure during dough development.
Viscoamylograph Analysis
Brabender viscoamylograph was used to measure changes in the viscosity of dough during heating and cooling, with continuous stirring. During heating, the viscosity increased with the temperature from 0 BU (at initial gelatinization temperature) to 112.5 at 122.0 BU (at 95 °C) due to swelling of the starch granules. This was followed by a decrease in the viscosity caused by the rupture and fragmentation of granules after 15 min at 95 °C (Table 2). During cooling at 50 °C, viscosity increased due to the starch molecules re-association to form a gel. In fact, amylose molecules aggregate to form a network with remnants of starch granules (Borchani et al. 2011). Many authors observed similar dough behavior (Anil 2007 ; Rosales-Juarez et al. 2008).
Viscoelastic properties are closely linked to gluten performance while pasting/gelling profiles are strongly linked to starch and so to flour formulation (Mahawar et al. 2012). Table 2 shows a decrease of all dough peak viscosity when adding FFC. Similar results were observed by Rosell et al. (2010) showing a decrease of peak viscosity values with the addition of apple pomace in the dough. This fact is attributed to the dilution of starch and gluten. Indeed, fibre substitution of flour provoked a gluten dilution effect and an interruption of starch-gluten matrix (Angioloni and Collar 2009). Moreover, lipids content were reported to be highly correlated with peak viscosity of starch measured by the Brabender viscoamylograph (Kim et al. 1995). Various lipids decrease the starch viscosity (Mahawar et al. 2012). Lipids and phospholipids form a complex with the amylose and long branch chains of amylopectin in the cereal starches, resulting in the reduction of swelling and amylose leaching inhibition.
In addition, gelatinization temperature of enriched dough increased due to the techno-functional properties of fibre. In fact, the high water-holding capacity of fibre induces a reduction of the water availability for starch, involving an increase in gelatinization temperature. Rosell et al. (2010) showed that the incorporation of beet fibre into the dough matrix induces the disruption of the viscoelastic system leading to weaker doughs, and it greatly competes for water with starch affecting pasting and gelling.
During heating (95 °C) and cooling (50 °C) apple FFC-enriched dough, had the highest viscosity followed by date and pear FFC-enriched dough. This could be due to the higher content of pectin in apple FFC (Table 1). However no significant differences (P < 0.05) among the viscosities were observed. Similar results were observed by Sudha et al. (2007) and showed the importance of pectin in gel formation.
Fibre-enriched doughs have lower breakdown, and setback values compared to control dough. These low values suggest that enriched dough would have high paste stability in mechanical processes, as it is the case with red and white sweet potato starches (Osundahunsi et al. 2003). These are vital aspects to consider when incorporating these FFC into a food production process.
Effects of Flesh Fibre Concentrate on Bread Quality Evaluation
Dough Yield and Physical Properties of Breads
The effect of FFC addition on dough yield and physical properties of breads is shown in Table 3. Compared with the control, the addition of fibre significantly increased (P < 0.05) dough yields which is in agreement with the results obtained by Gómez et al. (2003), Bouaziz et al. (2010) and De Escalada et al. (2013). This trend could be explained by greater water absorption by fibres. The increase of the dough absorption is important from an economic point of view. Hence, the addition of FFC in bread formulation could give an interesting value addition to date, pear and apple flesh.
Regarding specific bread volume, Table 3 shows a slight decrease of this parameter as consequence of FFC addition. However, the decrease was not significantly different (P < 0.05) from control and enriched breads, it is probably due to the low quantity of added fibres (below to 3 %). Gómez et al. (2003) revealed that the reduction of loaf volume was more evident when the fibre addition was increased from 3 to 5 %. This means that the addition of FFC to bread formulas did not produce an undesirable diminishing of loaf volume. A reduction of the specific volume was previously reported for fibre addition (Chen et al. 1988; Abdel-Kader 2000). Gómez et al. (2003) studied the effect of cellulose, cocoa, coffee, pea, orange and wheat fibres addition up to 50 g/kg flour. They stated that the main problem of dietary fibre addition in baking is the important reduction of loaf volume. This effect is attributed to the interaction between fibre and gluten and could be due to the dilution of gluten, which led to a decrease in the gas retention capacity (Chen et al. 1988).
Dough enriched with different FFC had similar yield and physical properties close to control breads. A significant difference (P < 0.05) was observed between control and fortified breads concerning breads weight loss. This might be due to the water retention during baking and to fibre additions. Generally, the breads weight loss before and after cooking is in direct relation with the water loss. In fact, the quantity of water necessary for dough hydratation depends on the fibre addition during baking (Table 2). The increase of water absorption with added fibre involves a higher amount of water loss during baking enhancing bread weight loss. Hence, breads weight loss before and after cooking was strongly influenced by added fibre. Our results are in concordance to those reported by Bouaziz et al. (2010).
Crumb Structure and External Appearance of Breads
Bread as a solid “soft” (Clark 1991), like many other foodstuffs, consists, at a macroscopic level, of two phases: a fluid (air) and a solid (cell wall material) (Scanlon and Zghal 2001). Observation of control bread crumb cross-section (Fig. 2), showed that the solid phase was entirely connected and the air cells were isolated. The volume fraction of the phases and the nature of their connectivity determine the structure, and consequently the mechanical properties of the bread (Scanlon and Zghal 2001). Therefore, processing conditions and raw materials determine the structure of the bread. Indeed, in Fig. 2 a variation can be seen in the distribution of the air cells between the control bread and the fibres-enriched breads. The latter exhibited a number of non-uniformlarge gas cells, affecting the uniformity of the crumb structure, and subsequently the bread quality. So, there are more cells of larger diameter in fibre bread crumb than in the crumb of control breads. According to Correa et al. (2012), alveolus stabilization depends primarily on gluten–starch matrix and in a second instance on the liquid lamella that is surrounding the cell. So, a possible explanation for the increased size of alveolus in the presence of fibre could be related to the viscosity decrease of these lamellae and the destabilization of the gluten starch matrix. However, no significant difference was observed between crumb structures of enriched breads.
External appearance of enriched bread is also similar to that of the control. Close results were obtained by Sabanis et al. (2009), Bouaziz et al. (2010) and Farrera-Rebollo et al. (2012).
Crust and Crumb Color
The effects of FFC addition on the bread color are summarized in Table 4. The color of bread crust is an important parameter to determine its acceptability (Gómez et al. 2003; Rosales-Juarez et al. 2008). Shittu et al. (2008) reported that the crust color of bread depends on various factors such as the dough formulation (i.e. type of flour, type and quantity of ingredient used), baking temperature and time. The color difference, ΔE* (taking the control bread as reference) showed the influence of fibre additions on the breads color. Statistical analysis did not show a significant difference between the crust of the control bread and the date and pear FFC supplemented breads, probably induced by a low quantity of added fibres (2 %). Gómez et al. (2003) did not find any significant differences between the crust of the control bread and the crust of 2 % fibre (cellulose, pea, orange, wheat) supplemented bread. However, Fig. 2 shows that the crust of the breads enriched with date fibre had a darker color characterized by a low lightness (L*; Table 4). This fact could be due to the original color of date fibre. In fact, the original color of fibre can have some influence on the bread crust color, this is mainly associated to Maillard and caramelization reactions (Shittu et al. 2008)”.
In terms of crumb color, Table 4 shows a more important color variation (ΔE*) than for the crust. Besides, addition of fibre significantly increased crumb a* values and significantly decreased b* and L* values (P < 0.05). This difference was essentially related to the low lightness, L* in comparison with the control, as consequence of its darker color. The original color of ingredients can have some influence on the bread crumb. Gómez et al. (2003)) showed that bread crumb color is usually similar to the color of the ingredients because the crumb does not reach as high a temperature as the crust.
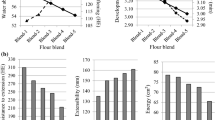
Crumb and Crust Texture
The effects of FFC supplementation on bread textures during storage are shown in Fig. 3a, b, c. During storage (from days 0 to 3), all breads showed an increase in hardness. Indeed, the crust hardness increased from 8.0 to ∼26.5 N for the control and the FFC containing breads (Fig. 3a). Crust hardness was not significantly different between FFC-enriched breads and control. This could be due to the lower (2 %) added fibre level and the origin of fibre. Amylose recrystallization plays an important role in the initial bread hardness and in the first stages of aging, because the formation of the amylose network induces bread hardening (Barcenas and Rosell 2006).
Figure 3b, c show the evolution of crumb hardness and elasticity of control and FFC-enriched breads during the storage. No significant difference was observed between FFC supplemented breads and the control regarding the crumb hardness and elasticity, for all fibres. Regarding the staling, breads showed a significant (P < 0.05) increase in crumb hardness during storage. The most notable difference in hardening was observed after 1 day of storage for all formulations. This effect, observed by many other authors (He and Hoseney 1990; Martin et al. 1991; Gómez et al. 2003; Hug-Iten et al. 2003), is due to the amylopectin recrystallization, the formation of complexes between starch and proteins, and the water redistribution among the bread constituents. Regarding elasticity, Fig. 3c reveals a slight decrease of this parameter during storage for both control and enriched breads, which confirms our previously results.
Conclusion
On the basis of obtained results, cooked fruit by-products are an excellent source of fibres (more than 70 %) which makes them a great potential ingredient for food applications, especially in improving dough performance. Indeed, the addition for FFC had pronounced effects on dough properties yielding; higher water absorption, stability, tenacity, and smaller extensibility, softening, breakdown and setback compared to the control dough (without fibre addition). Apple FFC-enriched dough had the best viscoelastic properties compared to the date or pear FFC-enriched dough.
The bread enriched with FFC had similar characteristics with the white wheat bread, since no significant differences were found on the specific volume of breads and on the crumb and crust texture. However, a slight difference on the breads crust and crumb color was observed compared to the control bread color. Moreover, slight differences were found between enriched bread with fibres from different origin (pear, apple and date).
These studies have shown the potential of developing fibre-rich breads in order to increase the added value of cooked fruit by-product. Therefore, FFC from cooked fruits by-products could be introduced in many other bakery (e.g. cereal bar, biscuit) or dairy (e.g. yogurt) products.
Abbreviations
- FFC:
-
Flesh fibre concentrate
- DF:
-
Dietary fibre
- DM:
-
Dry matter
- a w :
-
Water activity
- IDF:
-
Insoluble dietary fibre
- NDF:
-
Neutral dietary fibre
- WHC:
-
Water-holding capacity
- OHC:
-
Oil-holding capacity
- SWC:
-
Swelling capacity
- WA:
-
Water absorption
- BU:
-
Brabender units
- DDT:
-
Dough development time
- DS:
-
Degree of softening
- QI:
-
Quality index
- W:
-
Deformation energy
- P:
-
Tenacity
- L:
-
Extensibility
- ∆E*:
-
Color difference
- S:
-
Stability
References
American Association of Cereal Chemists (AACC). (2000). Approved methods of the AACC, (10th ed.). St. Paul, MN. Methods 54-30-02.
Abdel-Kader, Z. M. (2000). Enrichment of Egyptian “balady” bread. Part 1. Baking studies, physical and sensory evaluation of enrichment with decorticated caracted broadbeans flour (Vicia faba L.).Nahrung, 44, 418–421.
Abdul-Hamid, A., & Luan, Y. S. (2000). Functional properties of dietary fibre from defatted rice bran. Food Chem, 68, 15–19.
Aguedo, M., Kohnen, S., Rabetafika, N., Vanden Bossche, S., Sterckx, J., Blecker, C., Beauve, C., & Paquot, M. (2012). Composition of by-products from cooked fruit processing and potential use in food products. Journal of Food composition and analysis, 27(1), 61–69.
Angioloni, A., & Collar, C. (2009). Gel, dough and fibre enriched fresh breads: relationships between quality features and staling kinetics. Journal of Food Engineering, 91, 526–532.
Anil, M. (2007). Using of hazelnut testa as a source of dietary fiber in breadmaking. J Food Eng, 80, 61–67.
Association of Official Analytical Chemists. (1995). Official methods of analysis of (15th ed.). Washington, DC
Barcenas, M. E., & Rosell, C. M. (2006). Different approaches for improving the quality and extending the shelf life of the partially baked bread: low temperatures and HPMC addition. Journal Food Engineering, 72, 92–99.
Bchir, B., Besbes, S., Karoui, R., Attia, H., Paquot, M., & Blecker, C. (2012a). Effect of air-drying conditions on physico-chemical properties of osmotically pre-treated pomegranate seeds. Food Bioprocess Technology, 5, 1840–1852.
Bchir, B., Besbes, S., Karoui, R., Paquot, M., Attia, H., & Blecker, C. (2012b). Osmotic dehydration kinetics of pomegranate seeds using date juice as an immersion solution base. Food Bioprocess Technology, 5, 999–1009.
Borchani, C., Masmoudi, M., Besbes, S., Attia, H., Derouanne, C., & Blecker, C. (2011). Effect of date flesh fiber concentrate addition on dough performance and bread quality. Journal of texture Studies, 42, 300–308.
Bouaziz, M., Ben Amara, W., Attia, H., Blecker, C., & Besbes, S. (2010). Effect of the addition of defatted date seeds on wheat dough performance and bread quality. J Texture Studies, 41, 511–531.
Cadden, A. M. (1987). Comparative effects of particle size reduction on physical structure and water binding properties of several plant fibers. J Food Sci, 52, 1595–1599.
Chau, C. F., & Huang, Y. L. (2003). Comparison of the chemical composition and physicochemical properties of different fiber prepared from de peel of Citrus sinensis L. Cv. Liucheng. J. Agric. Food Chem., 51, 2615–2618.
Chen, H., Rubenthaler, G. L., & Schanus, E. G. (1988). Effect of apple fiber and cellulose on the physical properties of wheat flour. J Food Sci, 53, 304–305.
Clark, A. H. (1991). Structural and mechanical properties of biopolymer gels. In E. Dickinson (Ed.), Food polymers, gels, and colloids (pp. 322–338). Cambridge: The Royal Society of Chemistry.
Collar, C., Santos, E., & Rosell, C. M. (2007). Assessment of the rheological profile of fibre-enriched bread doughs by response surfacemethodology. J. Food Eng., 78, 820–826.
Correa, M. J., Perez, G. T., & Ferrero, C. (2012). Pectins as breadmaking additives: effect on dough rheology and bread quality. Food Bioprocess Technology, 5, 2889–2898.
Escalada, D., Pla, M., Uribe, E., Fissore, L., & Gerschenson, A. M. (2010). Influence of the isolation procedure on the characteristics of fibre-rich products obtained from quince wastes. Journal of Food Engineering, 96, 239–248.
Escalada, D., Pla, M., Rojas, A. M., & Gerschenson, L. N. (2013). Effect of butternut (Cucurbita moschata Duchesne ex Poiret) fibres on bread making, quality and staling. Food Bioprocess Technology, 6, 828–838.
DeVries, J. W., Prosky, L., Li, B., & Cho, S. (1999). A historical perspective of defining dietary fiber. Cereal Foods World, 44, 367–369.
Elleuch, M., Bedigian, D., Roiseux, O., Besbes, S., Blecker, C., & Attia, H. (2011). Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem, 124, 411–421.
Farrera-Rebollo, R., Salgado-Cruz, R., Chanona-Perez, M., Gutierrez-Lopez, F. G., & Calderon, G. (2012). Evaluation of image analysis tools for characterization of sweet bread crumb structure. Food Bioprocess Technology, 5, 474–484.
Figuerola, F., Hurtado, M. L., Estevez, A. M., Chiffelle, I., & Asenjo, F. (2005). Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem, 91, 395–401.
Goesaert, H., Briijs, K., Veraverbeke, W. S., Courtin, C. M., Geruers, K., & Delcour, J. A. (2005). Wheat flour constituent: how they impact bread and how to impact their functionality. Trends Food Sci Technol, 16, 12–30.
Gómez, M., Ronda, F., Blanco, C. A., Pedro, A., & Apesteguia, A. (2003). Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol., 216, 51–56.
Grigelmo-Miguel, N., & Martin-Belloso, O. (1999). Characterisation of dietary fiber fromorange juice extraction. Food Res Int, 31, 355–361.
Guillon, F., & Champ, M. (2000). Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res Int, 33, 233–245.
Gupta, P., & Premavalli, K. S. (2010). Effect of particle size reduction on physicochemical properties of ashground (Benincasa hispida) and radish (Raphanus sativus) fibres. Int J Food Sci Nutr, 61(1), 18–28.
He, H., & Hoseney, R. C. (1990). Changes in bread firmness and moisture during long-term storage. Cereal Chemi., 67, 603–605.
Hug-Iten, S., Escher, F., & Conde-Petit, B. (2003). Staling of bread: role of amylose and amylopectin and influence of starchdegrading enzymes. Cereal Chem, 80(6), 654–661.
Jeltema, M., & Zabic, M. (1979). Fibre components-quantitation and relationship to cake quality. J Food Sci, 44, 1732–1735.
Kim, Y. S., Wiesenborn, D. P., Orr, P. H., & Grant, L. A. (1995). Differential scanning calorimetry as a tool for screening potato starch for novel properties. J Food Sci, 60(5), 1060–1065.
Lario, Y., Sendra, E., Garcia-Perez, J., Fuentes, C., Sayas-Barbera, E., Fernandez-Lopez, J., & Perez-Alvarez, J. A. (2004). Preparation of high dietary fiber powder from lemon juice by-products. Innov. Food Sci. Emerg., 5, 113–117.
Larrauri, J. A. (1999). New approaches in the preparation of high dietary fibre powders from fruit by-products. Trends Food Sci Technol, 10(1), 3–8.
Laurikainen, T., Harkonen, H., Autio, K., & Poutanen, K. (1998). Effects of enzymes in fibre-enriched-baking. J Sci Food Agric, 76, 239–249.
Mac Connell, A. A., Eastwood, A., & Mitchell, W. D. (1974). Physical characterization of vegetable foodstuffs that could influence bowel function. J Sci Food Agric, 25, 1457–1464.
Mahawar, M., Singh, A., & Jalgaonkar, K. (2012). Utility of apple pomace as a substrate for various products: a review. Food and Bioproducts Procesing, 90, 597–605.
Martin, J., Gordon, M., & Fitzwater, E. (1991). The case for iron. American Society of Liminology and Occanography, 36, 1793–1802.
Martin-Cabrejas, M., Jaime, L., Karanja, C., Downie, A., Parker, M., Fransisco, J., Maina, G., Esteban, R., Smith, A., & Waldron, K. (1995). Modications to physicochemical and nutrional properties of hard-to-cook beans (Phaseolus vulgaris L.) by extraction cooking. J. Agri Food Chem, 47, 1174–1182.
Osundahunsi, O. F., Fagbemi, T. N., Kesselman, E., & Shimoni, E. (2003). Comparison of the physicochemical properties and pasting characteristics of flour and starch from red and white sweet potato cultivars. Journal of Agriculture and Food Chemistry, 51, 2232–2236.
Oarrott, M. E., & Thrall, B. E. (1978). Functional properties of various fibers physical properties. J Food Sci, 43, 759–766.
Pelucchi, C., Malvezzi, M., La Vecchia, C., Levi, F., Decarli, A., & Negri, E. (2004). Mesothelioma epidemic in Western Europe: an update. Br J Cancer, 90, 1022–1024.
Park, H., Seib, P. A., & Chung, O. K. (1997). Fortifying bread with a mixture of wheat fiber and psyllium husk fiber plus three antioxidants. Cereal Chem., 74, 207–211.
Pourfarzad, A., Mahdavian-Mehr, H., & Sedaghat, N. (2013). Coffee silverskin as a source of dietary fiber in bread-making: optimization of chemical treatment using response surface methodology. LWT- Food Sci Technol, 50, 599–606.
Rabetafika, H., N., Bchir, B., Aguedo, M., Paquot, M., & Blecker C. (2013). Effects of processing on the composition and physicochemical properties of dietary fibre from cooked fruit pomaces. Food Bioprocess Tech. doi:10.1007/s11947-013-1073-0.
Romano, R., Baranyai, L., Gottschalk, K., & Zude, M. (2008). An approach for monitoring the moisture content changes of drying banana slices with laser light backscattering imaging. Food Bioprocess Tech, 4, 410–414.
Rosales-Juarez, M., Mendoza, G. B., Guel, C. E., Bautista, F. L., Perez, J. C., Lopez, G. G., Rebollo, F. R., & Dominguez, C. G. (2008). Changes on dough rheological characteristics and bread quality as result of the addition of germinated and non germinated soybean flour. Food Bioprocess Technology, 1, 152–160.
Rosell, C. M., Santos, E., & Collar, C. (2010). Physical characterization of fiber-enriched bread doughs by dual mixing and temperature constraint using the Mixolab. Eur Food Res Technol, 231, 535–544.
Sabanis, D., Lebesi, D., & Tzia, C. (2009). Effect of dietary fibre enrichment on selected properties of gluten-free bread. LWT- Food Sci Technol, 42, 1380–1389.
Saint-Denisa, T., & Groupy, J. (2004). Optimization of a nitrogen analyser based on the Dumas method. Anal Chim Acta, 515, 191–198.
Sangmark, A., & Noomhorm, A. (2003). Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem, 80, 221–229.
Sangnark, A., & Noomhorm, A. (2004). Effect of dietary fibers fromsugarcane bagasse and sucrose ester on dough and bread properties. LWT- Food Sci Technol, 37, 697–704.
Saura-Calixto, F. (1998). Antioxidant dietary fiber product: a new concept and a potential food ingredient. J Agric Food Chem, 46, 4303–4306.
Scanlon, M., & Zghal, M. (2001). Bread properties and crumb structure. Food Res Int, 34, 841–864.
Shittu, T. A., Dixon, A., Awonorin, S. O., Sanni, L. O., & Mazia-Dioxon, B. (2008). Bread from composite cassava-wheat flour: effect of cassava genotype and nitrogen fertilizer on bread quality. Food Res Int, 41, 569–578.
Stojceska, V., & Ainsworth, P. (2008). The effect of different enzymes on the quality of high-fibre enriched brewer’s spent grain breads. Food Chem, 110, 865–872.
Sudha, M. L., Baskaran, V., & Leelavathi, K. (2007). Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem, 104, 686–692.
Sun-Waterhouse, D., Farr, J., Wibisono, R., & Saleh, Z. (2008). Fruit-based functional foods I: production of food-grade apple fibre ingredients. Int. J. Food Sci. Technol., 43, 2113–2122.
Torre-Gutiérrez, L., Chel-Guerrero, L., & Betancur-Ancona, D. (2008). Funtional properties of square banana (Musa balbisiana) starch. Food Chem, 106, 1138–1144.
Tsatsaragkou, K., Yiannopoulos, S., Kontogiorgi, A., Poulli, E., Krokida, M., & Mandala, I. (2012). Mathematical approch of structural and textural properties of gluten free bread anriched with carob flour. Journal of Cereal Science. doi:10.1016/j.jcs.2012.07.007.
Van Soest, P. J., & Wine, R. (1967). Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell wall constituents. Journal of the Association of Official Analytical Chemistry, 50, 50–55.
Vergara-Valencia, N., Granados-Péreza, E., Agama-Acevedo, E., Tovar, J., Ruales, J., & Bello-Pérez, L. A. (2007). Fibre concentrate from mango fruit: characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT- Food Sci Technol, 40, 722–729.
Wang, J., Rosell, C. M., & Benedito De Barber, C. (2002). Effect of the addition of different fibers on wheat dough performance and bread quality. Food Chem, 79, 221–226.
Wiesenborn, D. P., Orr, P. H., Casper, H. H., & Tacke, B. K. (1994). Potato starch behaviour as related to some hysical/chemical properties. J Food Sci, 59, 644–648.
Wittig de Penna, E., Bunger, A., Ibieta, A., Soto, D., Hernández, N., & López, L. (1998). Alimentos para el adulto mayor: muffins enriquecidos con fibra de lupino. In Programa Iberoamericano de Ciencia y Tecnología para el desarrollo, CYTED (Eds.), Temas en Tecnología de Alimentos. Vol. 2. Fibra Dietética (pp. 259–277). Mexico DF: Alfaomega Grupo Editor.
Acknowledgments
The authors gratefully acknowledge “Siroperie Meurens” for their cooperation, the Walloon Region of Belgium for the financial support of Wal-Aid Wagralim project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bchir, B., Rabetafika, H.N., Paquot, M. et al. Effect of Pear, Apple and Date Fibres from Cooked Fruit By-products on Dough Performance and Bread Quality. Food Bioprocess Technol 7, 1114–1127 (2014). https://doi.org/10.1007/s11947-013-1148-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1148-y