Abstract
Purpose of review
Appropriate management of asymptomatic patients with severe aortic stenosis (AS) is increasingly debated given recent improvements in options for aortic valve replacement (AVR). The goal of this review is to provide an updated approach to evaluation and management of patients with asymptomatic severe AS and to discuss the rationale for early AVR.
Recent findings
Registry data, retrospective studies, and one small randomized controlled clinical trial suggest a mortality benefit to AVR before symptom onset, although larger randomized trials are needed given potential biases of observational data. Other promising approaches to risk stratification of asymptomatic adults with severe AS include cardiac biomarkers (such as serum B-type natriuretic peptide levels), left ventricular global longitudinal strain, and myocardial fibrosis detected on cardiac magnetic resonance imaging.
Summary
Routine close clinical follow-up, periodic imaging, patient education, and shared decision-making are essential in caring for asymptomatic patients with severe AS but there is not yet enough evidence to support early AVR in most patients. Ongoing clinical trials and evaluation of biomarkers will illuminate whether intervention before symptom onset will improve the length or quality of life in adults with severe AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disease overview
The prevalence of calcific aortic stenosis (AS) increases with age, with severe AS affecting up to 3.4% of people 75 years of age or older [1, 2]. Moderate AS is even more common and typically progresses to severe AS within 5 to 10 years. At the time of diagnosis, 30–50% of patients with severe AS are asymptomatic [3, 4]. Morbidity and mortality are closely linked to AS disease severity and the emergence of symptoms in patients who initially are asymptomatic [5]. Generally, the goals of aortic valve replacement (AVR) for severe AS are to prolong life, reduce symptoms, and prevent heart failure long-term. Although AVR is clearly indicated in patients with symptoms from valve obstruction, the balance of risks and benefits is less clear in patients who are asymptomatic. After all, patients without symptoms will not have symptomatic benefit with AVR. The hypothesis that asymptomatic patients with severe AS may benefit from AVR before symptom onset is often referred to as “early AVR.”
In addition to procedural morbidity and mortality, a bioprosthetic AVR carries the long-term risk of valve degeneration, often requiring reintervention, especially in adults under 65 years of age. If a mechanical prosthesis is implanted, the risks of bleeding and thrombosis must be considered in addition to the inconvenience and life-style limitations with long-term vitamin-K antagonist anticoagulation. Thus, AVR is recommended primarily for symptomatic patients with severe AS because this population most clearly derives benefit from an intervention that justifies the short and long-term risks [6,7,8]. AVR for asymptomatic patients with severe AS is not routinely recommended unless the patient has a low left ventricular (LV) ejection fraction, very severe AS, rapid disease progression, symptoms provoked on exercise testing, or is undergoing other cardiac surgery [6, 8] (Table 1). However, some data suggest a possible mortality benefit with early surgery [4, 9,10,11,12,13]. Therefore, determining whether patients and which patients may benefit from earlier intervention is increasingly debated with several randomized trials now underway aimed at addressing these questions.
In this review, we will discuss the definition of “severe” AS, consider the challenges in ensuring patients are truly asymptomatic, and review current guidelines for intervention. We then go on to present the research rationale for early AVR and emerging parameters to identify patients most likely to benefit from early intervention. We also discuss the current clinical management of patients with asymptomatic severe AS, with practical guidance, including a framework for shared decision-making regarding the risks and considerations related to valve intervention. Lastly, we will summarize the ongoing trials testing the hypothesis that early AVR might be beneficial for prolonging life and reducing long-term adverse outcomes.
Defining severe aortic stenosis
Severe AS might best be defined as the degree of valve obstruction that results in symptom onset in each patient. However, there is no single numerical measure of AS severity that reliably predicts symptoms in all patients. In addition, there is evidence of adverse effects of valve obstruction on the left ventricle, even before the onset of symptoms. Our current definition of severe AS is derived from natural history studies showing that the rate of symptom onset corresponds closely with the degree of valve obstruction, with symptom onset occurring within a short time period once aortic velocity reaches 4 m/s or higher [14,15,16,17,18].
In current guidelines, severe AS is defined as a maximum aortic valve velocity (Vmax) ≥ 4 m/s or mean aortic valve gradient (mean ΔP) ≥ 40 mmHg. Typically, aortic valve area (AVA) is 1.0 cm2 or less (or aortic valve area indexed to body surface area (AVAi) ≤ 0.6 cm2/m2) but may be higher with mixed stenosis and regurgitation or with a high cardiac output. A high aortic velocity (or pressure gradient) alone is adequate for diagnosis of severe AS, regardless of valve area calculations. In addition to high-gradient severe AS, some patients with severe AS have a calcified immobile valve with a small AVA but a lower velocity and gradient due to a low transaortic flow rate. Low-gradient, low-flow severe AS may be associated with a low LV ejection fraction (< 50%) or with a normal ejection fraction but low transaortic stroke volume (< 35 ml/min/m2) [6, 19].
Identification of symptoms is critical in the evaluation of patients with severe AS. The initial symptoms are reduced exercise tolerance and exertional dyspnea; frank angina, syncope, and heart failure are end-stage symptoms. Even if mild symptoms are present, candidacy for AVR is pursued. However, determining exercise tolerance and the presence of mild symptoms is challenging in many older patients. Patients and providers alike may have difficulty in recognizing the early onset of subtle symptoms, particularly in an elderly population, because of limited mobility and patient adaptation to decreased exercise tolerance [20]. Conversely, there are many alternate causes for reduced exercise capacity or exertional dyspnea in older adults. For patients with unclear exercise tolerance or ambiguous symptoms, exercise tolerance testing allows the assessment of physiological changes and symptoms with exercise. An abnormal exercise test—fall in blood pressure, decreased exercise tolerance, symptoms of severe AS, marked ST-depression, or ventricular arrhythmias—predicts symptom onset and cardiac events at 1 year [21,22,23]. Therefore, exercise testing is reasonable in selected patients to confirm the absence of symptoms and exclude high-risk features in patients with severe AS who deny overt symptoms [6, 8]. Of course, given the demographics of patients with severe AS, many patients are unable to undergo exercise testing secondary to limited exercise capacity, comorbidities, frailty, or advanced age [24]. In the USA, only about 30–40% of patients with severe asymptomatic AS undergo exercise treadmill testing [11, 25, 26]. Whether this is an appropriate level of exercise testing or whether more exercise testing would improve clinical outcomes has not been studied.
Current indications for AVR in patients with asymptomatic severe aortic stenosis
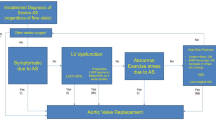
American and European guidelines for evaluation and management of asymptomatic patients with severe AS are shown in Table 1 [6,7,8]. Indications for valve replacement include LVEF < 50%, abnormal exercise tolerance test, rapidly progressing valve obstruction (ΔVmax > 0.3 m/s/year), or if a patient is already planned to undergo cardiac surgery for another reason. Surgical aortic valve replacement (SAVR) rather than transcatheter aortic valve implantation (TAVI) is recommended in asymptomatic patients requiring intervention. The European guidelines also specify that valve replacement should be considered for patients with a markedly elevated BNP or pulmonary artery systolic pressure > 60 mmHg and low surgical risk. Generally, patients with very severe AS and low surgical risk are recommended to undergo SAVR, rather than TAVI, although the definitions for very severe AS differ slightly between European and American guidelines. In the European guidelines, the definition of very severe AS is an aortic Vmax ≥ 5.5 m/s whereas the breakpoint in the American guidelines is an aortic Vmax ≥ 5 m/s or mean ΔP ≥ 60 mmHg. Both guidelines likely will be updated soon with possible changes in the recommendations for the timing of AVR and the choice between SAVR versus TAVI. Fig. 1
Rationale for early aortic valve replacement
Prevention of left ventricular dysfunction
A key element underlying the rationale for early AVR in asymptomatic patients with severe AS is the concept that removing the high afterload due to valve obstruction might prevent end-organ LV damage and thus reduce long-term symptoms and adverse outcomes from diastolic and systolic heart failure. Advanced cardiac imaging demonstrates that subclinical LV damage occurs early in the disease course of adults with AS [27]. Many of these changes persist or fail to fully resolve after valve replacement [28]. Chronic pressure overload leads to LV myocyte hypertrophy which contributes to reduced coronary flow reserve and chronic ischemia [29]. Apoptosis of ischemic myocytes with subsequent irreversible replacement fibrosis leads to changes in LV myocardial longitudinal function, even when global ejection fraction is maintained. These subclinical LV myocardial changes contribute to diastolic dysfunction, pulmonary hypertension, heart failure, and cardiac death [30]. With early AVR, the hope is to prevent these LV changes and eventual heart failure.
BNP levels
There are emerging methods and indices which show promise in detecting subclinical LV dysfunction which are not yet routine clinical practice but may be recommended in the future and can be used in individual cases when needed to identify patients that might benefit from early interventions. In patients with AS and a preserved LV ejection fraction, B-type natriuretic peptide (BNP) elevation is associated with heart failure hospitalization, need for AVR, and increased risk of mortality [31,32,33,34]. Elevations in BNP greater than or equal to three times the upper limit of normal for age and sex may be most predictive of adverse outcomes [32]. Notably, BNP is influenced by a patient’s comorbidities, age, and sex; thus, if measured, clinicians must be reasonably sure that elevations are secondary to valvular disease rather than an alternative etiology. In European guidelines, markedly elevated BNP and low surgical risk for a patient with severe asymptomatic AS is an indication to consider valve replacement. Although it was not included in the 2017 American guidelines, an update is in progress and there remains significant clinical interest in using this biomarker for risk stratifying patients with asymptomatic severe AS.
Global longitudinal strain
Two-dimensional echocardiographic LV strain imaging also has prognostic ability in aortic stenosis. In patients with asymptomatic severe AS and a normal LV ejection fraction, LV global longitudinal strain (GLS) is reduced compared with patients without AS, supporting the concept that myocardial damage occurs before symptom onset [35]. In addition, impaired LV GLS in asymptomatic patients with severe AS is associated with an increased risk of mortality, suggesting this might be a useful biomarker for identifying which patients might benefit from earlier AVR [36,37,38]. However, it is challenging to identify a specific absolute strain value reliable enough to be considered a trigger for valve replacement. Additionally, technical differences among vendors in the measurement of GLS, as well as intra- and interobserver variability in recording and measuring the data, further contribute to the lack of reliability in using GLS for decision-making in patients with severe AS. It is hoped that 3D echocardiographic strain imaging might avoid some of the limitations of 2D strain imaging, with preliminary studies suggesting that 3D GLS is a predictor of major adverse events including death, ventricular arrhythmias, and hospital admission in patients with AS [39].
Myocardial fibrosis
LV myocardial fibrosis on cardiac magnetic resonance imaging (CMR) is another promising approach to risk stratification of asymptomatic patients with severe AS. Late gadolinium enhancement (LGE) identifies regions of replacement fibrosis, with a mid-wall LGE pattern identifying myocardial scarring distinct from infarct, and is associated with an increased risk of mortality in patients with AS [40,41,42,43,44]. Mid-wall LGE is present in the ventricle of patients with severe AS, even when ejection fraction is normal, and remains unchanged after AVR suggesting that subclinical myocardial fibrosis is irreversible, at least in the short-term [45]. Unfortunately, despite the association of mid-wall LGE with mortality in patient with AS, it is difficult to define a specific level of LGE that might justify early valve replacement. Variability between studies contributes to this uncertainty with differing approaches to the quantification of the amount of myocardium affected (absolute or relative) as well as some studies simply reporting the presence or absence of LGE. CMR T1 mapping is also abnormal in patients with AS. Interstitial fibrosis, secondary to myofibroblast infiltration and extracellular expansion, accompanies the earlier pathological changes of myocyte hypertrophy and ischemia and can be detected with T1 mapping [45].
Similar to LGE, T1 mapping also predicts mortality after AVR [28]. Interstitial fibrosis, as identified by T1 mapping, appears reversible and thus has the potential to detect reversible subclinical LV dysfunction [46]. In contrast, LGE identifies replacement fibrosis, which remains unchanged even after AVR [28]. Currently, LGE and T1 mapping are not commonly used for clinical decision-making in patients with AS but given their prognostic ability, they remain the focus of ongoing research.
Reduction in mortality
The other major hypothesis underlying clinical trials of valve intervention before symptom onset in adults with severe asymptomatic AS is that early AVR will improve long-term survival. Asymptomatic patients with severe AS generally have a low risk of sudden cardiac death (< 1% per year) [47,48,49,50,51,52]. In data from one contemporary registry that included 861 asymptomatic patients with severe AS treated conservatively, cardiovascular death–free survival rate was 96% at 2 years, 87% at 4 years, and 71% at 8 years with nearly all patients undergoing AVR during the follow-up period [26]. There were 64 deaths during medical management, 50% were due to a cardiovascular cause but only 4 were classified as sudden death [26].
Several recent retrospective studies have shown a lower all-cause mortality with a strategy of early AVR in adults with severe asymptomatic AS compared with patients who did not undergo AVR [4, 9,10,11,12,13] (Table 2). Additionally, early AVR was associated with fewer heart failure hospitalizations in these studies. However, these observational data are limited by potential differences between those who did or did not undergo AVR and uncertainty about whether AVR was performed promptly once symptoms supervened. Additionally, observational studies can only show an association, not a cause-effect relationship.
The Randomized Comparison of Early Surgery versus Conventional Treatment in Very Severe Aortic Stenosis (RECOVERY) trial is the first randomized trial data for early treatment of asymptomatic patients with very severe AS, defined in this study as an aortic valve area 0.75 cm2 or less with either an aortic velocity of 4.5 m/s or greater or a mean gradient of 50 mmHg or greater [53•]. The primary endpoint of operative mortality or death from cardiovascular causes was 1% at both 4 and 8 years in the early-surgery group, compared with 6% at 4 years and 26% at 8 years in the conservative-care group (P = 0.003). The cumulative incidence of sudden death in the conservative care group was higher than expected—4% at 4 years and 14% at 8 years—with no sudden deaths in the surgical group. These data are very encouraging but may not be directly applicable to most patients with AS seen in our practices. The subjects in this randomized trial, were young (mean age 63.4 years), had a high prevalence of bicuspid aortic valves (54%), had very severe AS (mean Vmax 5.04 ± 0.44 m/s and mean AVA 0.64 ± 0.09 cm2), low surgical risk scores (EuroSCORE II 0.9%), and 50% received a mechanical AVR. On the other hand, the reduction in the risk of sudden death in this study should give us pause and certainly supports the current recommendations for AVR in asymptomatic adults with very severe AS.
Treatment
Approach to management of asymptomatic patients with severe AS
When evaluating a patient with asymptomatic severe AS, careful imaging and clinical evaluation are of utmost importance (Table 1). Transthoracic echocardiography at time of diagnosis and routinely thereafter is necessary to determine accurate valve hemodynamics and LV function. Clinical history to determine the presence of even the mildest symptoms is also equally important as symptom onset triggers referral for AVR. These symptoms may present as a slight decrease in exercise tolerance as opposed to the more dramatic symptoms of syncope. Generally, patients are seen every 6 months to 1 year to evaluate echocardiographic data, clinical history, and physical examination. Patient education centered around “red flag” symptom awareness, such as angina, exertional syncope, and dyspnea or simply a gradual reduction in exercise tolerance, takes place at each clinic visit. If the history is ambiguous, an exercise tolerance test may be considered to confirm the absence of symptoms. Additionally, evaluating novel markers such as BNP or GLS may be helpful to determine the presence of subclinical LV dysfunction. Clinicians also need to be alert to the indications for AVR in asymptomatic patients with severe AS to ensure optimal timing of intervention [6, 7]. Even though routine AVR is not recommended for most asymptomatic patients with severe AS, clinical decision-making in an individual patient also includes other clinical and nonclinical factors. For example, patients who need treatment for other conditions, such as cancer, may benefit from AVR to improve hemodynamics during chemotherapy. Similarly, AVR might be considered before symptom onset in a young woman considering pregnancy. Another example is the patient who lives in a remote location with likely long delays in access to clinical care when symptoms occur and thus may benefit from consideration of earlier AVR. AS is a progressive disease; once severe AS is present, hemodynamic progression and symptom onset are inevitable. Many factors enter the decision to consider AVR earlier in the disease course than recommended in guidelines. We recommend both active patient involvement in decision-making and referral to a Comprehensive Valve Center when this option is being considered [54].
Risks and considerations of valve replacement
When considering valve replacement, the risk of intervention must be balanced against the benefit. SAVR and TAVI both carry periprocedural risk. In a population with low surgical risk, SAVR has low periprocedural mortality risk (~ 1%), ~ 1–3% stroke risk, and 21–40% risk of atrial fibrillation [53, 55, 56]. Life-threatening or disabling bleeding risk is as high as 12% [56]. Conversely, TAVI has a periprocedural mortality ~ 0.4–0.8%, 0.6–3% stroke risk, ~ 2% risk of life-threatening or disabling bleed, and carries a risk for permanent pacemaker placement (6–17% depending on the type of valve deployed) [55, 56]. These data are predominantly from studies on symptomatic patients and thus may not reflect the risks for an asymptomatic patient population. With higher risk patients, the risks of intervention increase with both SAVR and TAVI. In asymptomatic populations, since the patient is not pursuing intervention for symptomatic relief, the onus of determining true benefit from early intervention falls heavily on the clinician.
Choice of valve type and valve durability is also important to evaluate when considering AVR. The main risks to balance include the risk of anticoagulation versus risk of valve degeneration when considering mechanical or bioprosthetic valve replacement. For patients under 60 years of age, mechanical aortic valve replacement is generally recommended over bioprosthetic valves [6,7,8]. Mechanical prostheses have lower rates of valve degeneration, lower rates of reoperation, and better rates of survival in patients < 60 years [57,58,59,60,61,62,63,64]. The risk of bioprosthetic valve degeneration is higher in patients who are implanted at a younger age [65]. However, mechanical prostheses carry the lifelong risk of anticoagulation and thus bleeding risk and contraindications to anticoagulation should be evaluated. In elderly patients, bioprosthetic SAVR or TAVI may be considered since valve degeneration requiring reintervention may not occur in the patient’s lifetime. Overall, national trends show an increase in bioprosthetic valve use, even in younger populations [66, 67]. Therefore, a careful discussion including risks, benefits, and patient preference is required.
Aortic valve replacement timing: ongoing trials
There are currently at least six trials underway to determine the benefit of early valve replacement for patients with asymptomatic severe AS [68,69,70,71,72,73] (Table 3). The EARLY TAVR trial will compare TAVR versus clinical surveillance, whereas AVATAR and ESTIMATE will evaluate SAVR compared with watchful waiting [69, 70, 73]. The remaining three trials (DANAVR, EVoLVeD, and EASY-AS) will compare both SAVR and TAVR versus watchful waiting [68, 71, 72]. Notably, the DANAVR trial will aim to recruit patients that have signs of elevated filling pressure or subclinical LV dysfunction including impaired GLS. This may shed light on whether GLS is an appropriate biomarker to use when considering early intervention. Similarly, the EVoLVeD trial will randomize patients after an initial CMR to assess the presence of mid wall fibrosis, and thus will clarify the prognostic value of CMR when determining appropriate timing of AVR.
Conclusions
Asymptomatic patients with severe AS require routine close follow-up to determine onset of signs or symptoms requiring valve replacement. If AVR is required, shared decision-making is critical for determining the choice of valve and implantation approach. There remains ongoing debate of whether early AVR in asymptomatic severe AS is appropriate, with ongoing trials dedicated to this question.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62(11):1002–1012. doi:https://doi.org/10.1016/j.jacc.2013.05.015.
Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30(9):962–70. https://doi.org/10.1016/j.cjca.2014.03.022.
Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol. 1990;15(5):1012–7. https://doi.org/10.1016/0735-1097(90)90234-g.
Kushiyama A, Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Inada T, Nagao K, Mabuchi H, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino-Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Jinnai T, Morikami Y, Saito N, Minatoya K, Kimura T, on behalf of the CURRENT AS Registry Investigators Age-related differences in the effects of initial aortic valve replacement vs. conservative strategy on long-term outcomes in asymptomatic patients with severe aortic stenosis. Circ J 2020;84(2):252–261. doi:https://doi.org/10.1253/circj.CJ-19-0431.
Otto CM. Calcific aortic valve disease: outflow obstruction is the end stage of a systemic disease process. Eur Heart J 2009;30(16):1940–1942. doi:https://doi.org/10.1093/eurheartj/ehp175.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):e57-185. doi:https://doi.org/10.1016/j.jacc.2014.02.536. This article contains American guidelines for evaluation and management of valvular heart disease including asymptomatic severe aortic stenosis.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2017;135(25):e1159-e95. doi:https://doi.org/10.1161/cir.0000000000000503. This article contains updates to American guidelines for evaluation and management of valvular heart disease including asymptomatic severe aortic stenosis.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739-2791. doi:https://doi.org/10.1093/eurheartj/ehx391. This article contains European guidelines for evaluation and management of valvular heart disease including asymptomatic severe aortic stenosis.
Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino-Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Sakata R, Kimura T, CURRENT AS Registry Investigators. Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2015;66(25):2827–2838. doi:https://doi.org/10.1016/j.jacc.2015.10.001.
Miyake M, Izumi C, Taniguchi T, Morimoto T, Amano M, Nishimura S, Kitai T, Kato T, Kadota K, Ando K, Furukawa Y, Inada T, Inoko M, Ishii K, Sakaguchi G, Yamazaki F, Koyama T, Komiya T, Yamanaka K, Nishiwaki N, Kanemitsu N, Saga T, Ogawa T, Nakayama S, Tsuneyoshi H, Iwakura A, Shiraga K, Hanyu M, Ohno N, Fukumoto A, Yamada T, Nishizawa J, Esaki J, Minatoya K, Nakagawa Y, Kimura T, on behalf of the CURRENT AS Registry Investigators Early surgery vs. surgery after watchful waiting for asymptomatic severe aortic stenosis. Circ J 2018;82(10):2663–2671. doi:https://doi.org/10.1253/circj.CJ-18-0416.
Campo J, Tsoris A, Kruse J, Karim A, Andrei AC, Liu M, Bonow RO, McCarthy P, Malaisrie SC Prognosis of severe asymptomatic aortic stenosis with and without surgery. Ann Thorac Surg 2019;108(1):74–79. doi:https://doi.org/10.1016/j.athoracsur.2019.01.031.
Kim HJ, Kim JB, Kim HR, Ju MH, Kang DY, Lee SA, Lee S, Ahn JM, Kim DH, Jung SH, Park DW, Song JM, Choo SJ, Chung CH, Song JK, Lee JW, Park SJ Impact of valve replacement on long-term survival in asymptomatic patients with severe aortic stenosis. Am J Cardiol 2019;123(8):1321–1328. doi:https://doi.org/10.1016/j.amjcard.2019.01.035.
Lee SA, Park SJ, Lee S, Kim DH, Song JM, Park SW, et al. Long-term survival of asymptomatic patients with very severe aortic stenosis: early surgery versus conventional treatment. J Am Coll Cardiol. 2020;75(18):2379–80. https://doi.org/10.1016/j.jacc.2020.03.036.
Frank S, Johnson A, Ross J Jr. Natural history of valvular aortic stenosis. Br Heart J. 1973;35(1):41–6. https://doi.org/10.1136/hrt.35.1.41.
Chizner MA, Pearle DL, de Leon AC Jr. The natural history of aortic stenosis in adults. Am Heart J. 1980;99(4):419–24. https://doi.org/10.1016/0002-8703(80)90375-0.
Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J. 1987;8(5):471–83. https://doi.org/10.1093/oxfordjournals.eurheartj.a062307.
Otto CM, Pearlman AS. Doppler echocardiography in adults with symptomatic aortic stenosis. Diagnostic utility and cost-effectiveness. Arch Intern Med. 1988;148(12):2553–60.
Oh JK, Taliercio CP, Holmes DR, Jr., Reeder GS, Bailey KR, Seward JB, Tajik AJ Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: prospective Doppler-catheterization correlation in 100 patients. J Am Coll Cardiol 1988;11(6):1227–1234. doi:https://doi.org/10.1016/0735-1097(88)90286-0.
Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115(22):2856–2864. doi:https://doi.org/10.1161/circulationaha.106.668681.
Zilberszac R, Gabriel H, Schemper M, Laufer G, Maurer G, Rosenhek R. Asymptomatic severe aortic stenosis in the elderly. JACC Cardiovasc Imaging 2017;10(1):43–50. doi:https://doi.org/10.1016/j.jcmg.2016.05.015.
Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart. 2001;86(4):381–386. doi:https://doi.org/10.1136/heart.86.4.381.
Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis 2002;11(2):204–209.
Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J 2005;26(13):1309–13. doi:https://doi.org/10.1093/eurheartj/ehi250.
Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95(9):2262–2270. doi:https://doi.org/10.1161/01.cir.95.9.2262.
Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Jr., Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes 2009;2(6):533–539. doi:https://doi.org/10.1161/circoutcomes.109.848259.
Lancellotti P, Magne J, Dulgheru R, Clavel MA, Donal E, Vannan MA, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. 2018;3(11):1060–8. https://doi.org/10.1001/jamacardio.2018.3152.
Calin A, Mateescu AD, Popescu AC, Bing R, Dweck MR, Popescu BA. Role of advanced left ventricular imaging in adults with aortic stenosis. Heart. 2020. doi:https://doi.org/10.1136/heartjnl-2019-315211, 106, 962, 969.
Everett RJ, Tastet L, Clavel MA, Chin CWL, Capoulade R, Vassiliou VS, Kwiecinski J, Gomez M, van Beek EJR, White AC, Prasad SK, Larose E, Tuck C, Semple S, Newby DE, Pibarot P, Dweck MR Progression of hypertrophy and myocardial fibrosis in aortic stenosis: a multicenter cardiac magnetic resonance study. Circ Cardiovasc Imaging 2018;11(6):e007451. doi:https://doi.org/10.1161/circimaging.117.007451.
Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, et al. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105(4):470–6. https://doi.org/10.1161/hc0402.102931.
Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. https://doi.org/10.1038/nrdp.2016.6.
Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109(19):2302–2308. doi:https://doi.org/10.1161/01.Cir.0000126825.50903.18.
Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, et al. B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol. 2014;63(19):2016–25. https://doi.org/10.1016/j.jacc.2014.02.581.
Capoulade R, Magne J, Dulgheru R, Hachicha Z, Dumesnil JG, O'Connor K, et al. Prognostic value of plasma B-type natriuretic peptide levels after exercise in patients with severe asymptomatic aortic stenosis. Heart. 2014;100(20):1606–12. https://doi.org/10.1136/heartjnl-2014-305729.
Nakatsuma K, Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, et al. B-type natriuretic peptide in patients with asymptomatic severe aortic stenosis. Heart. 2019;105(5):384–90. https://doi.org/10.1136/heartjnl-2018-313746.
Vollema EM, Sugimoto T, Shen M, Tastet L, Ng ACT, Abou R, Marsan NA, Mertens B, Dulgheru R, Lancellotti P, Clavel MA, Pibarot P, Genereux P, Leon MB, Delgado V, Bax JJ Association of left ventricular global longitudinal strain with asymptomatic severe aortic stenosis: natural course and prognostic value. JAMA Cardiol 2018;3(9):839–847. doi:https://doi.org/10.1001/jamacardio.2018.2288.
Ng ACT, Prihadi EA, Antoni ML, Bertini M, Ewe SH, Ajmone Marsan N, et al. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging. 2018;19(8):859–67. https://doi.org/10.1093/ehjci/jex189.
Magne J, Cosyns B, Popescu BA, Carstensen HG, Dahl J, Desai MY, et al. Distribution and prognostic significance of left ventricular global longitudinal strain in asymptomatic significant aortic stenosis: an individual participant data meta-analysis. JACC Cardiovasc Imaging. 2019;12(1):84–92. https://doi.org/10.1016/j.jcmg.2018.11.005.
Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, et al. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging. 2014;7(6):938–45. https://doi.org/10.1161/circimaging.114.002041.
Nagata Y, Takeuchi M, Wu VC, Izumo M, Suzuki K, Sato K et al. Prognostic value of LV deformation parameters using 2D and 3D speckle-tracking echocardiography in asymptomatic patients with severe aortic stenosis and preserved LV ejection fraction. JACC Cardiovasc Imaging 2015;8(3):235–245. doi:https://doi.org/10.1016/j.jcmg.2014.12.009.
Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56(4):278–87. https://doi.org/10.1016/j.jacc.2009.12.074.
Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, Japp AG, Prasad SK, Semple S, Newby DE, Dweck MR Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10(11):1320–1333. doi:https://doi.org/10.1016/j.jcmg.2016.10.007.
Dweck MR, Joshi S, Murigu T, Alpendurada F, Jabbour A, Melina G, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58(12):1271–9. https://doi.org/10.1016/j.jacc.2011.03.064.
Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin C, Dobson LE, Pica S, Loudon M, Malley T, Rigolli M, Foley JRJ, Bijsterveld P, Law GR, Dweck MR, Myerson SG, McCann GP, Prasad SK, Moon JC, Greenwood JP Myocardial scar and mortality in severe aortic stenosis. Circulation. 2018;138(18):1935–1947. doi:https://doi.org/10.1161/circulationaha.117.032839.
Everett RJ, Treibel TA, Fukui M, Lee H, Rigolli M, Singh A, Bijsterveld P, Tastet L, Musa TA, Dobson L, Chin C, Captur G, Om SY, Wiesemann S, Ferreira VM, Piechnik SK, Schulz-Menger J, Schelbert EB, Clavel MA, Newby DE, Myerson SG, Pibarot P, Lee S, Cavalcante JL, Lee SP, McCann GP, Greenwood JP, Moon JC, Dweck MR Extracellular myocardial volume in patients with aortic stenosis. J Am Coll Cardiol 2020;75(3):304–316. doi:https://doi.org/10.1016/j.jacc.2019.11.032.
Bing R, Cavalcante JL, Everett RJ, Clavel MA, Newby DE, Dweck MR. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging. 2019;12(2):283–96. https://doi.org/10.1016/j.jcmg.2018.11.026.
Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71(8):860–71. https://doi.org/10.1016/j.jacc.2017.12.035.
Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–56. https://doi.org/10.1056/NEJMoa0804602.
Monin JL, Lancellotti P, Monchi M, Lim P, Weiss E, Piérard L, et al. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120(1):69–75. https://doi.org/10.1161/circulationaha.108.808857.
Lancellotti P, Donal E, Magne J, Moonen M, O'Connor K, Daubert JC, et al. Risk stratification in asymptomatic moderate to severe aortic stenosis: the importance of the valvular, arterial and ventricular interplay. Heart. 2010;96(17):1364–71. https://doi.org/10.1136/hrt.2009.190942.
Kang DH, Park SJ, Rim JH, Yun SC, Kim DH, Song JM, et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation. 2010;121(13):1502–9. https://doi.org/10.1161/circulationaha.109.909903.
Maréchaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31(11):1390–7. https://doi.org/10.1093/eurheartj/ehq076.
Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, et al. Natural history of very severe aortic stenosis. Circulation. 2010;121(1):151–6. https://doi.org/10.1161/circulationaha.109.894170.
Kang DH, Park SJ, Lee SA, Lee S, Kim DH, Kim HK, et al. Early surgery or conservative care for asymptomatic aortic stenosis. N Engl J Med. 2020;382(2):111–9. https://doi.org/10.1056/NEJMoa1912846. This randomized controlled trial evaluated early surgery compared with conservative management in patients with very severe asymptomatic aortic stenosis and found a mortality benefit favoring surgery.
Nishimura RA, O'Gara PT, Bavaria JE, Brindis RG, Carroll JD, Kavinsky CJ, et al. AATS/ACC/ASE/SCAI/STS expert consensus systems of care document: a proposal to optimize care for patients with valvular heart disease: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Catheter Cardiovasc Interv. 2019;94(1):3–26. https://doi.org/10.1002/ccd.28196.
Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, Askew J, Sorajja P, Rovin J, Chetcuti SJ, Adams DH, Teirstein PS, Zorn GL 3rd, Forrest JK, Tchétché D, Resar J, Walton A, Piazza N, Ramlawi B, Robinson N, Petrossian G, Gleason TG, Oh JK, Boulware MJ, Qiao H, Mugglin AS, Reardon MJ, Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380(18):1706–1715. doi:https://doi.org/10.1056/NEJMoa1816885.
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, Leipsic J, Hahn RT, Blanke P, Williams MR, McCabe J, Brown DL, Babaliaros V, Goldman S, Szeto WY, Genereux P, Pershad A, Pocock SJ, Alu MC, Webb JG, Smith CR, PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380(18):1695–1705. doi:https://doi.org/10.1056/NEJMoa1814052.
Khan SS, Trento A, DeRobertis M, Kass RM, Sandhu M, Czer LS, et al. Twenty-year comparison of tissue and mechanical valve replacement. J Thorac Cardiovasc Surg. 2001;122(2):257–69. https://doi.org/10.1067/mtc.2001.115238.
Chan V, Jamieson WR, Germann E, Chan F, Miyagishima RT, Burr LH et al. Performance of bioprostheses and mechanical prostheses assessed by composites of valve-related complications to 15 years after aortic valve replacement. J Thorac Cardiovasc Surg 2006;131(6):1267–1273. doi:https://doi.org/10.1016/j.jtcvs.2005.11.052.
Kulik A, Bédard P, Lam BK, Rubens FD, Hendry PJ, Masters RG, et al. Mechanical versus bioprosthetic valve replacement in middle-aged patients. Eur J Cardiothorac Surg. 2006;30(3):485–91. https://doi.org/10.1016/j.ejcts.2006.06.013.
Ruel M, Chan V, Bédard P, Kulik A, Ressler L, Lam BK et al. Very long-term survival implications of heart valve replacement with tissue versus mechanical prostheses in adults <60 years of age. Circulation. 2007;116(11 Suppl):I294–I300. doi:https://doi.org/10.1161/circulationaha.106.681429.
van Geldorp MW, Eric Jamieson WR, Kappetein AP, Ye J, Fradet GJ, Eijkemans MJ et al. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg 2009;137(4):881–886, 6e1-5. doi:https://doi.org/10.1016/j.jtcvs.2008.09.028.
Badhwar V, Ofenloch JC, Rovin JD, van Gelder HM, Jacobs JP. Noninferiority of closely monitored mechanical valves to bioprostheses overshadowed by early mortality benefit in younger patients. Ann Thorac Surg. 2012;93(3):748–53. https://doi.org/10.1016/j.athoracsur.2011.12.032.
Weber A, Noureddine H, Englberger L, Dick F, Gahl B, Aymard T, et al. Ten-year comparison of pericardial tissue valves versus mechanical prostheses for aortic valve replacement in patients younger than 60 years of age. J Thorac Cardiovasc Surg. 2012;144(5):1075–83. https://doi.org/10.1016/j.jtcvs.2012.01.024.
Glaser N, Jackson V, Holzmann MJ, Franco-Cereceda A, Sartipy U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur Heart J. 2015;37(34):2658–67. https://doi.org/10.1093/eurheartj/ehv580.
Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000;36(4):1152–1158. doi:https://doi.org/10.1016/s0735-1097(00)00834-2.
Dunning J, Gao H, Chambers J, Moat N, Murphy G, Pagano D et al. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use--an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database. J Thorac Cardiovasc Surg. 2011;142(4):776–782.e3. doi:https://doi.org/10.1016/j.jtcvs.2011.04.048.
Isaacs AJ, Shuhaiber J, Salemi A, Isom OW, Sedrakyan A. National trends in utilization and in-hospital outcomes of mechanical versus bioprosthetic aortic valve replacements. J Thorac Cardiovasc Surg. 2015;149(5):1262–1269.e3. doi:https://doi.org/10.1016/j.jtcvs.2015.01.052.
Leicester Uo, Unit LCT, University Hospitals L, Australia TUoW, University of Auckland NZ. The early valve replacement in severe asymptomatic aortic stenosis study. https://ClinicalTrials.gov/show/NCT04204915; 2020.
Serbia CCo. Aortic valve replacement versus conservative treatment in asymptomatic severe aortic stenosis. https://ClinicalTrials.gov/show/NCT02436655; 2015.
Lifesciences E. Evaluation of transcatheter aortic valve replacement compared to surveillance for patients with asymptomatic severe aortic stenosis. https://ClinicalTrials.gov/show/NCT03042104; 2017.
Edinburgh Uo, Trust SJTC. Early valve replacement guided by biomarkers of LV decompensation in asymptomatic patients with severe AS. https://ClinicalTrials.gov/show/NCT03094143; 2017.
Hospital OU, Rigshospitalet D, Hospital AU, Hospital AU, Hospital ZU. Danish national randomized study on early aortic valve replacement in patients with asymptomatic severe aortic stenosis. https://ClinicalTrials.gov/show/NCT03972644; 2019.
Paris AP-Hd. Early surgery for patients with asymptomatic aortic stenosis. https://ClinicalTrials.gov/show/NCT02627391; 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jasleen K Tiwana and Catherine M Otto declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Approval
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Valvular Heart Disease
Rights and permissions
About this article
Cite this article
Tiwana, J.K., Otto, C.M. Contemporary Workup and Management of Asymptomatic Patients with Severe Aortic Stenosis. Curr Treat Options Cardio Med 22, 47 (2020). https://doi.org/10.1007/s11936-020-00837-7
Published:
DOI: https://doi.org/10.1007/s11936-020-00837-7