Abstract
Purpose of review
It is well established that symptomatic severe aortic stenosis (AS) carries a poor prognosis and requires valvular replacement for definitive treatment. While moderate AS has traditionally been thought of as a benign prelude to the aforementioned, recent data suggests that it is associated with increased morbidity and mortality. This article will consider treatment strategies for moderate AS, including early surgical and transcatheter aortic valve replacement and medical therapy.
Recent findings
There are few randomized controlled trials dedicated to medical and surgical therapies for moderate AS. Statins, antihypertensive agents and bisphosphonates have not consistently demonstrated an effect on AS progression, timing of aortic valve replacement, or improvement in patient outcomes. Early surgical intervention for patients with concomitant left ventricular dysfunction has been studied in a retrospective manner and appears promising.
Summary
Aside from the routine management of comorbidities (i.e., coronary artery disease, hypertension, hyperlipidemia), no specific treatment is recommended that is exclusively directed towards moderate AS. Clinicians should maintain a high vigilance for AS progression and the development of symptoms. Given the safety and efficacy of transcatheter aortic valve replacement, a randomized controlled trial is underway to evaluate its benefits in patients with moderate AS and left ventricular systolic dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past half-century, the landscape of aortic stenosis (AS) has changed tremendously. Once dominated by younger patients with rheumatic disease, AS now primarily affects older adults through calcific valvular degeneration [1]. Approximately 4% of patients over the age of 65 have moderate or severe AS, a number that is expected to more than double in both the United States and Europe by the year 2050 [2]. Moreover, as aortic valve (AV) calcification progresses with age, elderly patients (> 75 years old) are at greatest risk.
It is well known that untreated, symptomatic severe AS necessitates valvular intervention given its significant morbidity and poor survival [3, 4]. While surgical aortic valve replacement (SAVR) has been the mainstay of therapy for this patient population for years, transcatheter aortic valve replacement (TAVR) has demonstrated similar or improved outcomes since its initial approval by the Food and Drug Administration in 2011 [5,6,7,8,9]. Indeed, in 2017 over 50,000 TAVR procedures were performed in the United States [10].
The time honored “watchful waiting” strategy for moderate AS (defined as an aortic valve area [AVA] > 1.0 cm2 with a mean AV gradient of 20–39 mmHg or a peak velocity of 3.0–3.9 m/s) has recently come under scrutiny. Average rates of progression for moderate AS are a decrease in AVA by 0.1 cm2/year, an increase in mean gradient by 7 mmHg/year, and an increase of peak velocity by 0.3 m/s per year [11]. While these data suggest that careful monitoring with serial echocardiography should be satisfactory to avoid a transition to severe AS, these values vary widely among patients. Moreover, moderate AS itself has been associated with significant mortality, particularly in conjunction with a reduced left ventricular ejection fraction (LVEF) [12••, 13••]. Patients with moderate AS present with a high degree of cardiovascular comorbidities and exhibit reduced survival compared to age- and sex-matched patients without AS [14]. Given the potential clinical ramifications, a paradigm shift towards earlier treatment clearly needs to be investigated. As such, we aim to review the evidence in support of available medical, surgical, and interventional treatments for moderate AS.
Aortic stenosis and ventricular maladaptation
Much like severe AS, alterations in LVEF play an important role in prognostication for patients with moderate AS. Patients are typically grouped into those with preserved left ventricular function (LVEF ≥ 50%) and those with systolic dysfunction (LVEF < 50%).
Patients with moderate AS and left ventricular (LV) systolic dysfunction have worse survival compared to those with preserved LVEF [15]. It has been hypothesized that the failing LV is a “double-loaded ventricle” with significantly higher afterload due to increased arterial stiffness (in an elderly population) and a greater relative (and therefore more detrimental) effect of “only” moderate AS [16••]. The clinical implications are significant with one study demonstrating death or heart failure hospitalization in 48% of patients over 4 years [13••]. Moreover, 1-year aortic valve replacement (AVR) rates were higher than those historically reported for patients with asymptomatic severe AS or moderate AS with a preserved LVEF (13% vs. 5–9%, and 4–8%, respectively), suggestive of accelerated rates of clinical deterioration. Importantly, in the setting of a reduced LVEF, low aortic valve gradients and an AVA ≤ 1.0 cm2, low-flow low-gradient severe AS must be distinguished from moderate AS due to differences in current guideline recommendations for surgical or transcatheter valve replacement [11, 17]. Interestingly, the presence of a low-normal LVEF (50–60%) has also been demonstrated to predict further LVEF deterioration in patients with moderate AS, even after adjustment for confounders [18].
The reason why some patients experience ventricular maladaptation in the presence of moderate AS and others do not remains unclear. While prior studies have suggested that women are more likely to respond to the pressure load imposed by valvular stenosis with concentric hypertrophy, clinical predictors of systolic dysfunction have been challenging to identify. Pathology studies have implicated two distinct types of LV fibrosis in response to the increased afterload from valvular stenosis—reactive interstitial fibrosis and replacement fibrosis [19•, 20, 21]. The former, characterized by myofibroblast activity and collagen deposition, occurs in the early stages of AS and has been demonstrated to regress following AVR [22]. Conversely, replacement fibrosis occurs later and is permanent. The extent of both forms of maladaptive left ventricular fibrosis appears to worsen as AS progresses and is emblematic of the detrimental effects of even early stages of AS [23].
Unsurprisingly, greater degrees of replacement fibrosis have been associated with impaired LV recovery after AVR and poorer outcomes [19•]. In a longitudinal observational study of 674 patients with severe AS, the presence of late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (a marker of replacement fibrosis) was associated with higher all-cause mortality (26.4% vs. 12.9%, p < 0.001) and cardiovascular mortality (15.0% vs. 4.8%, p < 0.001) regardless of surgical or transcatheter intervention [24]. Moreover, greater degrees of LGE correlated with higher rates of all-cause and cardiovascular mortality. In a prospective study of 203 patients (of which 166 had at least mild AS), the extent of myocardial fibrosis was observed to gradually worsen across the progression of AS from mild to moderate to severe [23]. Thus, the obstructive aortic valve lesion may adversely impact left ventricular remodeling, even before symptoms or severe AS develop, thereby providing an important pathologic basis for the need for early intervention. The prospective EVoLVeD trial (NCT03094143) will seek to answer this question for patients with asymptomatic severe AS, randomizing 400 patients with midwall LGE to early AVR as compared to medical therapy [25]. If in fact the presence of LGE identifies patients that benefit from early AVR for asymptomatic severe AS, future studies will be needed to investigate whether this strategy should be expanded to moderate AS.
Surgical and transcatheter aortic valve replacement
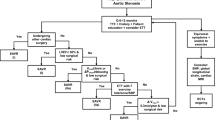
In light of the aforementioned data regarding the adverse consequences of moderate AS, the question of whether these patients should undergo valve replacement gains relevance (see Fig. 1). While moderate AS imposes a significant hemodynamic load on the left ventricle ( with increased wall stress and myocyte fibrosis), this load is manageable for the normal heart. Moreover, for asymptomatic patients with moderate AS and a normal LVEF, the finite durability and life span of bioprosthetic valves in addition to the periprocedural risks of AVR are important considerations. Given our inability to accurately predict the progression of AS, it remains inappropriate and unnecessary to globally recommend surgical intervention for all patients with moderate AS in the absence of some evidence of cardiac or clinical decompensation.
In patients with existing LV dysfunction, however, relief of fixed valvular stenosis may improve left ventricular hemodynamics and limit (or allow reversal of) myocyte fibrosis, thereby theoretically precluding the worsening (or onset) of LV dysfunction [26]. In heart failure with reduced LVEF, the mainstay of therapy is afterload reduction—alleviation of the fixed afterload imposed by moderate AS may therefore provide therapeutic benefit. Similarly, the morbidity and mortality conferred by progressive AS should be alleviated with valve replacement. Unfortunately, no prospective randomized controlled trials have evaluated the role of AVR (surgical or transcatheter) in patients with moderate AS (with a preserved or reduced LVEF). In a retrospective study of 263 patients ≥ 70 years old with moderate AS who were referred for coronary artery bypass grafting (CABG), performance of a concomitant SAVR compared to CABG alone conferred no additional in-hospital mortality (4.2% vs. 6.0%, p = 0.8), change in 5-year survival (62.3 ± 5.5% vs. 64.2 ± 4.3%), or freedom from AVR (98.9 ± 1.1% vs. 97.8 ± 1.2%, p = 0.13) [27]. In a more recent study of 1634 patients with moderate or severe AS and an LVEF ≤ 50%, the effect of SAVR with or without CABG was compared to medical therapy [28]. In the subgroup of patients with moderate AS (classified by either mean gradient or AVA), SAVR was associated with a lower mortality over a 5-year follow-up period (hazard ratio [HR] 0.58, 95% confidence interval [CI] 0.39–0.89, p < 0.001).
With the advent of TAVR, the morbidity, mortality, and recovery times associated with AVR have markedly improved, making early utilization of AVR a potentially advantageous therapeutic strategy. Given the poor prognostic implications of moderate AS with a reduced LVEF, the prospective TAVR-UNLOAD trial (NCT02661451) is ongoing and seeks to randomize 300 patients (≥ 18 years old) with heart failure (defined as heart failure hospitalizations or an elevated serum BNP/NT-proBNP), moderate AS, and a reduced LVEF (20–50%) to either TAVR plus optimal medical therapy or optimal medical therapy alone [29]. The primary endpoint of the study is a composite outcome of all-cause mortality, disabling stroke, cardiovascular hospitalization (due to heart failure, stroke or symptomatic aortic valve disease), and change in Kansas City Cardiomyopathy Questionnaire relative to baseline. With TAVR-UNLOAD on the horizon, we will soon learn whether early valve replacement for moderate AS will change the trajectory of heart failure progression.
Medical therapy
Beyond valve replacement, several pharmaceutical agents have been evaluated for their potential role in slowing the progression of moderate AS. It should be noted that the majority of trials included patients with varying degrees of stenosis rather than moderate AS exclusively. Further, while there are numerous retrospective and observational studies, randomized, prospective trials are few and far between (see Table 1).
Diet and lifestyle
AS has been identified as an active process of lipid retention, chronic inflammation, and osteoblast activation within the aortic valve. Similarly, metabolic syndrome is characterized by proinflammatory and prothrombotic abnormalities secondary to insulin resistance, often in the setting of abdominal obesity and increased visceral adiposity. It was hypothesized that both obesity and metabolic syndrome could therefore be linked to worsening progression and mortality in patients with AS. In a retrospective study of 105 patients with at least moderate AS, metabolic syndrome was associated with more rapid progression of valvular stenosis (− 0.14 ± 0.13 cm2/year vs. – 0.08 ± 0.08 cm2/year, p = 0.008) and decreased 3-year event-free survival (44 ± 8% vs. 69 ± 6%, p = 0.002) [38]. In a substudy of the prospective ASTRONOMER (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin) trial, 243 patients with mild to moderate AS were evaluated for the effects of metabolic syndrome on AS progression [39]. Metabolic syndrome was an independent predictor of AS progression, particularly in patients younger than 57 years old. This effect of metabolic syndrome has been similarly identified in patients with bioprosthetic aortic valves, with increased rates of deterioration [40].
There is debate, however, on the role of obesity as a stand-alone risk factor for the progression of AS. Earlier studies suggested an inverse relationship of body mass index (BMI) and aortic valve calcification (i.e., an “obesity paradox”), whereas others have implied either a neutral or negative effect [41,42,43]. In a substudy of the randomized controlled SEAS (Simvastatin Ezetimibe in Aortic Stenosis) trial, neither being overweight nor obese was associated with AS progression in patients with asymptomatic mild to moderate AS [44].
Despite the aforementioned, there is no available data on the role of diet and/or exercise in prevention or treatment of moderate AS. For competitive athletes with mild to moderate AS, the ACC/AHA recommend continued participation in low and moderate static or dynamic sports if exercise testing to at least the level of activity achieved in competition and training demonstrates satisfactory exercise capacity without symptoms, ST segment depression, ventricular arrhythmias, or abnormal blood pressure response (class IIa, level of evidence C) [45]. For non-athletes, however, there are no recommendations with regard to exercise. In terms of diet, we recommend following the 2019 ACC/AHA guidelines on the primary prevention of cardiovascular disease given the frequency with which cardiovascular comorbidities occur in conjunction with moderate AS [46].
Statin therapy
The pathogenesis of valvular calcification in AS is similar to that of atherosclerosis; with damage to the valvular endothelium, there is lipid deposition (e.g., lipoprotein(a) [Lp(a)] and low-density lipoprotein [LDL]) resulting in inflammation, macrophage infiltration, and ultimately calcification [47]. Indeed, AS and atherosclerosis share many of the same risk factors for progression, including long-term elevations in cholesterol levels [43, 48]. As such, it is reasonable to postulate that statins may have a beneficial effect in the treatment of AS.
The SEAS trial was the largest trial to evaluate this question. 1873 patients with asymptomatic mild to moderate AS and no other indication for lipid-lowering therapy were randomized to simvastatin plus ezetimibe versus placebo [30]. After a median of 52 months, there was no difference in major adverse cardiac events, rates of AVR, or AS progression. Similarly, the ASTRONOMER trial was a randomized, double-blind, placebo-controlled trial examining 269 patients with mild to moderate AS and no indication for lipid-lowering therapy [31]. After a median follow-up of 3.5 years, no change in rates of AS progression were noted. These findings have been replicated in other smaller randomized controlled trials using different statin agents [32,33,34]. A meta-analysis of 4 major prospective randomized controlled trials showed no differences in mean or peak aortic valve gradients, AVA, freedom from AVR, or death from cardiovascular causes if a statin was used compared to placebo [49].
As such, current US and European guidelines do not recommend statin therapy to slow progression or treat moderate AS [11, 17].
Alternative lipid-lowering therapies
Like LDL, high levels of Lp(a) have been associated with aortic valve calcification [48, 50, 51]. In one prospective study of 145 patients with AS (peak velocity > 2.0 m/s), patients in the top Lp(a) tertile had increased progression of valvular calcium score, faster hemodynamic progression on echocardiography, and higher risk of death and AVR compared to those in the lower tertile [52]. While a central role of Lp(a) in the pathogenesis of AS could explain the lack of benefit seen with statins, we await the results of ongoing randomized controlled trials evaluating therapies that target Lp(a), including PCSK9 inhibitors, niacin, and antisense oligonucleotides against apo(a) mRNA [50, 53, 54].
Antihypertensive agents
Hypertension is a major risk factor for the development and progression of AS and has been estimated to account for up to one quarter of incident cases [55,56,57]. Pathologically, hypertension results in concentric left ventricular remodeling and diastolic dysfunction. In patients with mild to moderate AS, the presence of a higher left ventricular mass index has been shown to increase rates of cardiovascular morbidity and mortality [58, 59]. Moreover, the synergistic load imposed on the left ventricle by reduced arterial compliance and an increased transvalvular gradient, quantified by the valvuloarterial impedance index (Zva), have also been shown to correlate with an increased risk of left ventricular dysfunction and increased mortality in patients with at least moderate AS [60, 61].
In this section, we will briefly review the available evidence in support of different classes of antihypertensive therapies for patients with moderate AS.
β-Blockers
While there is an abundance of data in support of β-blockers for use in coronary artery disease, arrhythmia, and heart failure with a reduced ejection fraction (HFrEF), there are a dearth of studies evaluating their efficacy and safety in patients with AS. Mechanistically, β-blockers decrease afterload, heart rate and myocardial oxygen demand. As one retrospective study has demonstrated higher resting heart rates in patients with mild valvular stenosis, β-blockers can theoretically hit multiple targets associated with the development and progression of AS [62].
In a post hoc analysis of the SEAS trial, the use of β-blockers demonstrated lower all-cause mortality (HR 0.5, 95% CI 0.3–0.7, p < 0.001), sudden cardiac death (HR 0.2, 95% CI 0.1–0.6, p = 0.004), and cardiovascular death (HR 0.4, 95% CI 0.2–0.7, p < 0.001) in patients with mild to moderate AS compared to placebo [63]. In another study, 40 patients with asymptomatic moderate to severe AS were randomized to metoprolol or placebo for 22 weeks [35]. Patients who took metoprolol were noted to have lower peak and mean aortic valve gradients, lower myocardial consumption, and lower valvuloarterial impedance with no change in stroke volume. While these data are promising (both in terms of efficacy and safety), the confounding beneficial effects of β-blockade on other common comorbidities (including atherosclerosis, hypertension and arrhythmias) necessitate further dedicated studies for patients with moderate AS.
RAAS inhibitors
The renin-angiotensin-aldosterone system (RAAS) plays an important role in ventricular adaptation in the setting of AS. While myocytes initially hypertrophy to counteract the effects of prolonged increases in afterload and left ventricular wall stress, eventually the left ventricle will decompensate. Angiotensin II and aldosterone signaling have both been implicated in maladaptive ventricular behavior by driving myocyte apoptosis, activating cardiac myofibroblasts and promoting myocardial fibrosis [37, 64, 65]. Indeed, inhibition of these pathways by angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs) has well-known benefits on ventricular remodeling and clinical outcomes in patients with HFrEF. The aortic valve also expresses prorenin, renin, ACE, and angiotensin II receptors and they are thereby integrally linked to the RAAS system [53].
Given the connection of the RAAS system to the aortic valve and adverse ventricular remodeling, it is reasonable to suspect that RAAS antagonism could improve clinical outcomes or slow progression of AS. While several randomized controlled trials have investigated patients with severe AS, fewer have included the cohort of patients with moderate or less disease. The RIAS (Ramipril In Aortic Stenosis) trial was a randomized controlled trial of ramipril versus placebo in 100 patients with moderate to severe asymptomatic AS. Compared to placebo, ramipril resulted in statistically significant reductions in left ventricular mass and a trend towards a slower rate of AS progression after 1 year of follow-up (0.0cm2 vs. – 0.2cm2, p = 0.067) [36]. Like the RIAS trial, a substudy of the randomized controlled SEAS trial also demonstrated reductions in left ventricular mass in patients using ACE inhibitor or ARB therapy [66]. However, after a median follow-up of 4.3 years, there were no differences in AS progression or cardiovascular outcomes, including cardiovascular death and all-cause mortality.
The only randomized controlled trial to date to investigate MRA therapy in AS has been the ZEST trial [37]. Sixty-five patients with asymptomatic moderate to severe AS were randomized to placebo versus eplerenone. After a median follow-up of 19 months, there were no statistically significant differences in the onset of left ventricular dysfunction (systolic or diastolic), left ventricular mass, or progression of AS.
Calcium channel blockers
Like β-blockers, calcium channel blockers (CCBs) have multiple beneficial cardiovascular effects, including a decrease in afterload and myocardial oxygen demand. Neither dihydropyridine (DHP) nor non-DHP CCBs have been studied in a randomized controlled trial for patients with any degree of AS. A retrospective analysis of 314 patients examined the effects of CCBs (DHP and non-DHP) on patients with asymptomatic moderate or severe AS [67]. In this cohort, all-cause mortality was greater in the group of patients taking CCBs, independent of age, hypertension, diabetes, LVEF, and baseline AVA (HR 7.09, 95% CI 2.15–23.38, p = 0.001). Importantly, however, the type of CCB was not delineated and the prevalence of coronary artery disease was significantly higher in the group taking CCBs. It is therefore unclear whether CCB therapy poses safety concerns to patients with moderate AS without further randomized control trials.
Antihypertensive agents—a summary
Given the aforementioned, current ACC/AHA guidelines recommend the initiation of antihypertensive therapy in all patients with asymptomatic AS, started at a low dose and gradually uptitrated with frequent clinical monitoring (class I, level of evidence B) [11]. Given a lack of evidence in support of any one class of medication, recommendations suggest initiation of therapies “according to standard guideline directed medical therapy.”
Calcium and phosphorus metabolism
After the initial phases of inflammation and lipid deposition in aortic valve tissue, valvular calcification is dominated by the activation of osteoblast-like cells that develop an osteogenic phenotype [47]. There is upregulation and deposition of extracellular matrix proteins and dysregulation of RANKL/OPG signaling (receptor activator of nuclear factor-κB ligand and osteoprotegerin, respectively), which normally governs bone demineralization [47]. Similarly, there have been numerous associations established between patients with low bone density and aortic valve calcification [68, 69]. It has therefore been hypothesized that medications designed to prevent and treat osteoporosis may have a role in the management of AS.
Calcium and vitamin D supplementation have not been studied prospectively to determine their effects on progression or outcomes for patients with moderate AS. In a community-based observational study examining 144 women over a 4-year period, calcium supplementation resulted in no differences in aortic valve calcification [70]. However, a recent retrospective study of 2660 patients with mild to moderate AS found that calcium and/or vitamin D supplementation was associated with faster rates of progression of AS and the need for AVR (44.5% vs. 20.9%, p < 0.001) but no differences in overall mortality in a 6-year follow-up period [71].
Bisphosphonates represent one of the mainstays in therapy for osteoporosis. However, they have also been noted to have beneficial effects on vascular and aortic valve calcification through decreased bone resorption (and therefore, decreased release of calcium and phosphate), in addition to pleiotropic effects on inflammation and the reduction of inflammatory cytokines [47]. While no prospective trials have been performed to date, other studies have had mixed results. In an analysis of 3710 women enrolled in the MESA (Multi-Ethnic Study of Atherosclerosis) trial, the use of bisphosphonates was associated with lower degrees of aortic valve calcification in patients ≥ 65 years old [72]. In a retrospective study of 801 female patients with mild to moderate AS, the use of bisphosphonates resulted in no differences in AVA, peak and mean aortic valve gradients, survival, or freedom from AVR over an average 5.1 year follow-up [73]. Conversely, some smaller echocardiographic studies across a range of stenosis severity (i.e., mild to severe) have shown slower rates of progression of valve calcification for patients on bisphosphonates [74,75,76]. The beneficial effects of bisphosphonates should be interpreted with caution, as the aforementioned trials evaluated patients who were already taking these medications for osteoporosis and therefore have dysregulated calcium and phosphate metabolism.
Denosumab, a monoclonal antibody to RANKL and alternative therapy for osteoporosis, has also been proposed as a treatment for AS. While in vitro studies have demonstrated reductions in calcification by valvular interstitial cells, no studies have evaluated this therapy in patients with AS [77].
Clearly, prospective randomized controlled trials are needed to evaluate the efficacy of bisphosphonates and denosumab in patients with AS (and without osteoporosis). The SALTIRE II trial (NCT02132026) will hopefully shed some light on this question, comparing the use of alendronic acid, denosumab, and placebo to determine changes in aortic valve calcium score in patients with at least mild AS. At present, however, there are no guideline recommendations for the use of these therapies in patients with moderate AS [11, 17].
Warfarin
While there is an increasing shift towards the use of direct oral anticoagulants (DOAC) for cardiovascular diseases that require anticoagulation, warfarin is still a cornerstone therapy given its widespread availability, affordability, and familiarity. Recently, a retrospective analysis of 303 patients with at least mild AS and a preserved LVEF compared rates of AS progression between patients on warfarin, DOAC, or no anticoagulant therapy [78]. Annualized increases in aortic valve calcium scores and peak aortic valve gradients were higher in the warfarin group compared to the DOAC and no therapy groups. While prospective studies should be performed to confirm the aforementioned, for patients with moderate AS who have the option of either a DOAC or warfarin, we favor the use of the former (if feasible).
Future directions/emerging therapies
Recently, targets of phosphodiesterase type 5 (PDE5) have garnered interest as a therapy for AS. Murine and human hearts have shown increased expression of myocardial PDE5 in response to transverse aortic constriction and severe valvular AS, respectively [79]. Given the known benefits of PDE5 inhibitors and soluble guanylate cyclase (sGC) inhibitors in patients with pulmonary hypertension, it has been proposed that these therapies may offer benefit in patients with AS [80,81,82,83,84]. While multiple randomized controlled trials were initially underway that included patients with moderate AS, difficulty in enrollment has limited their progress.
A variety of other therapies have been proposed to slow the progression of AS due to their effects on cellular mechanisms and inflammation. These include endocannabinoids, ectonucleotidase inhibitors, disease modifying antirheumatic drugs (DMARDs), and dipeptidyl peptidase-4 (DPP-4) inhibitors [50, 53].
Conclusion
Moderate AS represents more than just a “latent period” before the development of severe AS. Rather, it is an active disease state characterized by inflammation and LV fibrosis and is associated with increased rates of morbidity and mortality when left ventricular function is decreased. While no medical therapies have emerged to slow its progression, there is a signal that early intervention may preclude or reverse maladaptive ventricular remodeling. Randomized controlled trials are underway to evaluate the benefits of early transcatheter valve replacement considering their efficacy and safety profile.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Braunwald E. Aortic stenosis: then and now. Circulation. 2018;137:2099–100.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528.
Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–12.
Ross J Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–7.
Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–8.
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607.
Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20.
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705.
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98.
Vemulapalli S, Carroll JD, Mack MJ, Li Z, Dai D, Kosinski AS, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380:2541–50.
Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–185.
Strange G, Stewart S, Celermajer D, Prior D, Scalia GM, Marwick T, et al. Poor long-term survival in patients with moderate aortic stenosis. J Am Coll Cardiol. 2019;74:1851–63 An observational registry examining the natural history and prognosis of aortic stenosis. Notably, patients with moderate aortic stenosis had a similar risk profile of cardiovascular morbidity and mortality to those with severe aortic stenosis.
van Gils L, Clavel MA, Vollema EM, Hahn RT, Spitzer E, Delgado V, et al. Prognostic implications of moderate aortic stenosis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2017;69:2383–92 This retrospective analysis demonstrated that patients with moderate aortic stenosis and left ventricular systolic dysfunction are at high risk for all-cause death, aortic valve replacement and heart failure hospitalizations.
Delesalle G, Bohbot Y, Rusinaru D, Delpierre Q, Marechaux S, Tribouilloy C. Characteristics and prognosis of patients with moderate aortic stenosis and preserved left ventricular ejection fraction. J Am Heart Assoc. 2019;8:e011036.
Kennedy KD, Nishimura RA, Holmes DR Jr, Bailey KR. Natural history of moderate aortic stenosis. J Am Coll Cardiol. 1991;17:313–9.
Pibarot P, Messika-Zeitoun D, Ben-Yehuda O, Hahn RT, Burwash IG, Van Mieghem NM, et al. Moderate aortic stenosis and heart failure with reduced ejection fraction: can imaging guide us to therapy? JACC Cardiovasc Imaging. 2019;12:172–84 A review of moderate aortic stenosis and heart failure with reduced left ventricular ejection fraction. The article examines the use of diagnostic modalities for evaluation of the degree of aortic stenosis, current recommendations for management of this patient population and the potential role of transcatheter aortic valve replacement.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91.
Ito S, Miranda WR, Nkomo VT, Connolly HM, Pislaru SV, Greason KL, et al. Reduced left ventricular ejection fraction in patients with aortic stenosis. J Am Coll Cardiol. 2018;71:1313–21.
Bing R, Cavalcante JL, Everett RJ, Clavel MA, Newby DE, Dweck MR. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging. 2019;12:283–96 This review describes the pathophysiology and prognostic implications of left ventricular remodeling and myocardial fibrosis in patients with aortic stenosis. Subsequently, imaging modalities for assessment of the aforementioned are discussed in detail.
Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–91.
Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, et al. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477–84.
Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–55.
Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10:1320–33.
Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin C, et al. Myocardial scar and mortality in severe aortic stenosis. Circulation. 2018;138:1935–47.
Bing R, Everett RJ, Tuck C, Semple S, Lewis S, Harkess R, et al. Rationale and design of the randomized, controlled Early Valve Replacement Guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis (EVOLVED) trial. Am Heart J. 2019;212:91–100.
Bastos MB, Schreuder JJ, Daemen J, Van Mieghem NM. Hemodynamic effects of transcatheter aortic valve replacement for moderate aortic stenosis with reduced left ventricular ejection fraction. JACC Cardiovasc Interv. 2019;12:684–6.
Dagenais F, Mathieu P, Doyle D, Dumont E, Voisine P. Moderate aortic stenosis in coronary artery bypass grafting patients more than 70 years of age: to replace or not to replace? Ann Thorac Surg. 2010;90:1495–9 discussion 9–500.
Samad Z, Vora AN, Dunning A, Schulte PJ, Shaw LK, Al-Enezi F, et al. Aortic valve surgery and survival in patients with moderate or severe aortic stenosis and left ventricular dysfunction. Eur Heart J. 2016;37:2276–86.
Spitzer E, Van Mieghem NM, Pibarot P, Hahn RT, Kodali S, Maurer MS, et al. Rationale and design of the Transcatheter Aortic Valve Replacement to UNload the Left ventricle in patients with ADvanced heart failure (TAVR UNLOAD) trial. Am Heart J. 2016;182:80–8.
Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–56.
Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, Investigators A. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–14.
Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–97.
van der Linde D, Yap SC, van Dijk AP, Budts W, Pieper PG, van der Burgh PH, et al. Effects of rosuvastatin on progression of stenosis in adult patients with congenital aortic stenosis (PROCAS Trial). Am J Cardiol. 2011;108:265–71.
Dichtl W, Alber HF, Feuchtner GM, Hintringer F, Reinthaler M, Bartel T, et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am J Cardiol. 2008;102:743–8.
Hansson NH, Sörensen J, Harms HJ, Kim WY, Nielsen R, Tolbod LP, et al. Metoprolol Reduces Hemodynamic and Metabolic Overload in Asymptomatic Aortic Valve Stenosis Patients: A Randomized Trial. Circ Cardiovasc Imaging. 2017 Oct;10(10):e006557. https://doi.org/10.1161/CIRCIMAGING.
Bull S, Loudon M, Francis JM, Joseph J, Gerry S, Karamitsos TD, et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur Heart J Cardiovasc Imaging. 2015;16:834–41.
Stewart RA, Kerr AJ, Cowan BR, Young AA, Occleshaw C, Richards AM, et al. A randomized trial of the aldosterone-receptor antagonist eplerenone in asymptomatic moderate-severe aortic stenosis. Am Heart J. 2008;156:348–55.
Briand M, Lemieux I, Dumesnil JG, Mathieu P, Cartier A, Despres JP, et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47:2229–36.
Capoulade R, Clavel MA, Dumesnil JG, Chan KL, Teo KK, Tam JW, et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol. 2012;60:216–23.
Briand M, Pibarot P, Despres JP, Voisine P, Dumesnil JG, Dagenais F, et al. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation. 2006;114:I512–7.
Larsson SC, Wolk A, Hakansson N, Back M. Overall and abdominal obesity and incident aortic valve stenosis: two prospective cohort studies. Eur Heart J. 2017;38:2192–7.
Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkila J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15:865–70.
Thanassoulis G, Massaro JM, Cury R, Manders E, Benjamin EJ, Vasan RS, et al. Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol. 2010;55:2491–8.
Rogge BP, Cramariuc D, Lonnebakken MT, Gohlke-Barwolf C, Chambers JB, Boman K, et al. Effect of overweight and obesity on cardiovascular events in asymptomatic aortic stenosis: a SEAS substudy (Simvastatin Ezetimibe in Aortic Stenosis). J Am Coll Cardiol. 2013;62:1683–90.
Bonow RO, Nishimura RA, Thompson PD, Udelson JE, American Heart Association E, Arrhythmias Committee of Council on Clinical Cardiology CoCDiYCoC, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 5: valvular heart disease: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e292–7.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:e177–232.
Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. 2015;66:561–77.
Smith JG, Luk K, Schulz CA, Engert JC, Do R, Hindy G, et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312:1764–71.
Thiago L, Tsuji SR, Nyong J, Puga ME, Gois AF, Macedo CR, et al. Statins for aortic valve stenosis. Cochrane Database Syst Rev. 2016;9:CD009571.
Goody PR, Hosen MR, Christmann D, Niepmann ST, Zietzer A, Adam M, et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol. 2020;40:885–900.
Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–12.
Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73:2150–62.
Marquis-Gravel G, Redfors B, Leon MB, Genereux P. Medical treatment of aortic stenosis. Circulation. 2016;134:1766–84.
Viney NJ, van Capelleveen JC, Geary RS, Xia S, Tami JA, Yu RZ, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomized, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388:2239–53.
Manolis AJ, Kallistratos MS, Poulimenos LE. Hypertension and aortic stenosis: no strangers, not anymore! J Hypertens. 2019;37:2156–8.
Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, Canoy D, Raimondi F, Ayala Solares JR, et al. Systolic blood pressure and risk of valvular heart disease: a Mendelian randomization study. JAMA Cardiol. 2019;4:788–95.
Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, et al. Association between cardiovascular risk factors and aortic stenosis: the CANHEART aortic stenosis study. J Am Coll Cardiol. 2017;69:1523–32.
Cioffi G, de Simone G, Cramariuc D, Mureddu GF, Gerdts E. Inappropriately high left-ventricular mass in asymptomatic mild-moderate aortic stenosis. J Hypertens. 2012;30:421–8.
Gerdts E, Rossebo AB, Pedersen TR, Cioffi G, Lonnebakken MT, Cramariuc D, et al. Relation of left ventricular mass to prognosis in initially asymptomatic mild to moderate aortic valve stenosis. Circ Cardiovasc Imaging. 2015;8:e003644 discussion e.
Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–8.
Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003–11.
de Oliveira Moraes AB, Stahli BE, Arsenault BJ, Busseuil D, Merlet N, Gebhard C, et al. Resting heart rate as a predictor of aortic valve stenosis progression. Int J Cardiol. 2016;204:149–51.
Bang CN, Greve AM, Rossebø AB, Ray S, Egstrup K, Boman K, et al. Antihypertensive Treatment With β-Blockade in Patients With Asymptomatic Aortic Stenosis and Association With Cardiovascular Events. J Am Heart Assoc. 2017 Nov 27;6(12):e006709. https://doi.org/10.1161/JAHA.117.006709.
Everett RJ, Dweck MR. Renin-angiotensin system inhibition in aortic stenosis: thinking beyond transcatheter aortic valve replacement. J Am Coll Cardiol. 2019;74:642–4.
Hale TM. Persistent phenotypic shift in cardiac fibroblasts: impact of transient renin angiotensin system inhibition. J Mol Cell Cardiol. 2016;93:125–32.
Bang CN, Greve AM, Kober L, Rossebo AB, Ray S, Boman K, et al. Renin-angiotensin system inhibition is not associated with increased sudden cardiac death, cardiovascular mortality or all-cause mortality in patients with aortic stenosis. Int J Cardiol. 2014;175:492–8.
Saeed S, Mancia G, Rajani R, Parkin D, Chambers JB. Antihypertensive treatment with calcium channel blockers in patients with moderate or severe aortic stenosis: relationship with all-cause mortality. Int J Cardiol. 2020;298:122–5.
Aksoy Y, Yagmur C, Tekin GO, Yagmur J, Topal E, Kekilli E, et al. Aortic valve calcification: association with bone mineral density and cardiovascular risk factors. Coron Artery Dis. 2005;16:379–83.
Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, et al. Association of coronary artery and aortic calcium with lumbar bone density: the MESA abdominal aortic calcium study. Am J Epidemiol. 2009;169:186–94.
Bhakta M, Bruce C, Messika-Zeitoun D, Bielak L, Sheedy PF, Peyser P, et al. Oral calcium supplements do not affect the progression of aortic valve calcification or coronary artery calcification. J Am Board Fam Med. 2009;22:610–6.
Karrthik A, Ahuja KR, Gad MM, Bazarbashi N, Kaur M, Collier P, et al. Abstract 15768: Does Vitamin D/Calcium Supplement Intake Worsen Aortic Stenosis Progression? Circulation. 2019;140:A15768.
Elmariah S, Delaney JA, O’Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, et al. Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (The Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56:1752–9.
Aksoy O, Cam A, Goel SS, Houghtaling PL, Williams S, Ruiz-Rodriguez E, et al. Do bisphosphonates slow the progression of aortic stenosis? J Am Coll Cardiol. 2012;59:1452–9.
Innasimuthu AL, Katz WE. Effect of bisphosphonates on the progression of degenerative aortic stenosis. Echocardiography. 2011;28:1–7.
Skolnick AH, Osranek M, Formica P, Kronzon I. Osteoporosis treatment and progression of aortic stenosis. Am J Cardiol. 2009;104:122–4.
Sterbakova G, Vyskocil V, Linhartova K. Bisphosphonates in calcific aortic stenosis: association with slower progression in mild disease--a pilot retrospective study. Cardiology. 2010;117:184–9.
Lerman DA, Prasad S, Alotti N. Denosumab could be a Potential Inhibitor of Valvular Interstitial Cells Calcification in vitro. Int J Cardiovasc Res. 2016 Jan 3;5(1):10.4172/2324-8602.1000249. https://doi.org/10.4172/2324-8602.1000249.
Tastet L, Pibarot P, Shen M, Clisson M, Cote N, Salaun E, et al. Oral anticoagulation therapy and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2019;73:1869–71.
Vandenwijngaert S, Pokreisz P, Hermans H, Gillijns H, Pellens M, Bax NA, et al. Increased cardiac myocyte PDE5 levels in human and murine pressure overload hypertrophy contribute to adverse LV remodeling. PLoS One. 2013;8:e58841.
Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–903.
Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–57.
Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–29.
Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369:330–40.
Rubin LJ, Badesch DB, Fleming TR, Galie N, Simonneau G, Ghofrani HA, et al. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER-2 study. Chest. 2011;140:1274–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Elmariah receives research grants from American Heart Association (19TPA34910170), National Institutes of Health (R01 HL151838), Edwards Lifesciences, Svelte Medical, and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Valvular Heart Disease
Rights and permissions
About this article
Cite this article
Bernard, S., Elmariah, S. Treating Moderate Aortic Stenosis: Too Early or Too Late?. Curr Treat Options Cardio Med 23, 6 (2021). https://doi.org/10.1007/s11936-020-00884-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-020-00884-0