Abstract
Accurate cardiovascular risk stratification forms the foundation to guide the allocation of preventive interventions in the primary prevention of atherosclerotic cardiovascular disease (ASCVD) events. The coronary artery calcium (CAC) score is a guideline-endorsed decision tool for further risk assessment and personalized management in asymptomatic individuals. Current United States guidelines recommend using the Pooled Cohort Equations to estimate 10-year risk of ASCVD, followed by a clinician–patient discussion that may be enriched using CAC for individuals at borderline or intermediate estimated risk who are uncertain about their risk management. CAC is a highly specific marker of coronary atherosclerosis, is strongly and independently associated with incident ASCVD events, and has emerged as a widely available tool that can inform a personalized allocation of preventive pharmacotherapies in a number of scenarios. Current guidelines focus on the role of CAC in the allocation of statin therapy, with CAC = 0 identifying patients who may safely refrain from therapy. In the near future, CAC may play a significant role in the allocation of multiple other preventive therapies in asymptomatic individuals. This chapter discusses the utility of CAC in cardiovascular risk assessment in primary prevention.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Atherosclerotic cardiovascular disease
- Coronary artery calcium
- Primary prevention
- Risk assessment
- Risk stratification
Introduction
Atherosclerotic cardiovascular disease (ASCVD), inclusive of coronary heart disease (CHD) and stroke events, remains a leading cause of morbidity and mortality in the United States and globally (Virani et al. 2021). Traditional risk scoring tools provide a quantitative estimation of the absolute risk of having an ASCVD event within a given timeframe. These risk estimators use demographic variables and other traditional risk factors to inform the allocation and intensity of primary prevention interventions and represent a guideline-endorsed first step for ASCVD risk assessment in the United States and Europe (Arnett et al. 2019; Grundy et al. 2019; Visseren et al. 2021). Clinical risk scores, however, use epidemiological data of ASCVD event rates observed in large populations to inform an individual’s risk of having an event over time, which results in important challenges when providing meaningful, personalized estimations of risk at the patient level. Moreover, these scores tend to underestimate risk among higher-risk younger individuals who would benefit from early preventive therapies; and to overestimate risk in many middle-aged and older individuals, allowing for little individualization of risk management in these groups and raising concerns of overtreatment (Lloyd-Jones et al. 2019). In this context, tools that can help further improve risk stratification and guide a more personalized allocation of preventive interventions can significantly enrich shared decision-making conversations with patients in primary prevention, resulting in a more “precise” allocation of lifelong preventive interventions.
Coronary artery calcium (CAC) scoring allows visualization of calcifications in the coronary walls, a finding that is a highly specific marker of coronary atherosclerosis. Coronary atherosclerotic plaque is the primary underlying substrate for coronary atherosclerotic events, and among individuals with no prior clinical ASCVD, multiple studies have consistently shown that the presence, extent, and severity of coronary atherosclerosis provide additional prognostic information and improve risk stratification beyond clinical risk scores (Nasir and Cainzos-Achirica 2021; Greenland et al. 2018). While the performance of the Pooled Cohort Equations (PCE) is reasonably good at the extremes of risk (<5% and >20% estimated 10-year ASCVD risk), there is significant room for nuance in the borderline (5–<7.5%) and intermediate (7.5–<20%) risk groups. In those individuals, current guidelines around the world acknowledge that other features, from race/ethnicity and advanced lipid measurements to the burden of coronary plaque, can enhance and further personalize risk assessment and management, matching the intensity of interventions to a most accurate estimation of absolute ASCVD risk. Among available tools, US and European guidelines acknowledge the CAC score as the best-established imaging technique to enhance risk assessment (Arnett et al. 2019; Grundy et al. 2019; Visseren et al. 2021).
This chapter focuses on the clinical role of CAC and its utility for shared decision-making, enhanced risk stratification, and guiding a more personalized management in the primary prevention of ASCVD. We also discuss implications for cost-effectiveness and long-term adherence to preventive interventions using CAC to inform the allocation of preventive therapies.
Pathophysiology of Coronary Artery Calcifications
Coronary artery calcification accompanies the development of atherosclerosis, and the extent of calcification reflects the progression of atherosclerosis. Coronary calcification occurs predominantly within the intima layer (intimal calcification) of the coronary artery as opposed to peripheral arteries, where calcification occurs mostly within the media layer (medial calcification). The process of coronary calcification appears to start within the atheromatous components of vascular plaque (lipid pools) and progresses with inflammatory and metabolic mediators, such as lipoproteins and cytokines, leading to the development of a necrotic core (Demer and Tintut 2008). Coronary artery calcification is initially seen within a thickened intima that contains microcalcifications with a size ranging from 0.5 to 15.0 μm. Early microcalcification is thought to originate from the apoptosis of smooth muscle cells resulting in fine microcalcification; this is followed by infiltration of macrophages into the lipid pool, which also undergoes apoptosis and release of matrix vesicles producing a relatively larger microcalcification appearance. These microcalcified deposits are commonly seen in the deeper areas of necrotic core close to the internal elastic lamina, which eventually coalesce to form more prominent speckles and fragments of calcifications, and further progression leads to a plaque with calcified sheet-like deposits more than 3 mm in size (Mori et al. 2018). Coronary artery calcification leads to arterial stiffness, decreased compliance, impaired vasomotor response, and compromised myocardial perfusion.
Coronary calcification is heavily influenced by demographic factors such as age, gender, and race/ethnicity. Age is a strong (albeit imperfect) predictor of CAC burden, and for a given age, men tend to have higher CAC scores than women, the development of coronary calcification in women is delayed by 10–15 years compared to men, which is likely due to the protective effects of estrogens in the premenopausal years. For racial/ethnic groups, coronary calcification is highest among non-Hispanic Whites and South Asians, followed by Chinese, Hispanics, and non-Hispanic Blacks (Mori et al. 2018; Kanaya et al. 2014; McClelland et al. 2006).
Measurement and Quantification of CAC and Clinically Relevant Cutpoints
The presence and extent of CAC can be seen using various imaging modalities, including radiography, computed tomography (CT), and intravascular imaging. Nevertheless, non-contrast-enhanced cardiac CT is the test of choice for the quantification of CAC scores in 2022. Specifically, multidetector CT (MDCT) has largely superseded the use of prior imaging modalities, such as electron-beam CT, and its clinical use is backed by a compelling body of international studies confirming the correlation between CAC scores and incident ASCVD outcomes across multiple cohorts. MDCT is safe, highly sensitive for detecting calcium-dense lesions, and an effective imaging tool producing 128–320 sections of the heart using a low radiation dose. Based on the method described by Dr. Arthur Agatston, each lesion detected with an area ≥1 mm2 and radiological attenuation >130 Hounsfield units is assigned a score that measures both the area and the radiological density of the lesion. Then, the overall score is calculated based on the sum of the individual lesions, and the final score ranges from zero (indicates no detectable calcified plaque) to thousands of Agatston units (higher score indicates higher calcified plaque burden).
CAC scores can be interpreted either as an absolute value with fixed cutoff points that are the same for all demographic groups or using age-, sex-, and race/ethnicity-specific thresholds. Absolute CAC scores are more commonly used in risk assessment, simpler, and easier to communicate. Absolute CAC scores are typically classified into four broad categories that signal increased risk of CHD/ASCVD event: 0, 1 to 99, 100 to 399, and ≥400 (Fig. 22.1). Of note, a CAC score ≥400 identifies individuals with event rates similar to those of secondary prevention populations, while CAC = 0 is associated with low event rates, particularly for CHD. In contrast, age-, sex-, and race/ethnicity-specific CAC cutpoints allow providers to examine whether an individual has a high CAC score relative to others with similar demographic characteristics, and may allow for more personalized risk management decisions in women, individuals at the extremes of age, and racial/ethnic minorities. These cutpoints should be derived based on diverse population-based cohort data obtained in the same country where the patient is being evaluated, and in the United States, data from the Multi-Ethnic Study of Atherosclerosis (MESA) is typically used for this purpose (https://www.mesa-nhlbi.org/Calcium/input.aspx).
CAC Burden as a Predictor of Future ASCVD Events
A wealth of epidemiological studies has demonstrated a strong association between baseline burden of CAC and the risk of incident ASCVD events, with studies such as MESA now confirming these associations at up to 18 years of follow-up (Al Rifai et al. 2021). CAC provides robust prognostic information in both men and women, across age strata, in multiple racial/ethnic groups, and in populations with varying burdens of traditional risk factors. Moreover, CAC provides prognostic information that is independent of and substantially incremental to traditional ASCVD risk factors, with several studies reporting statistically significant improvements in risk discrimination for the prediction of CHD/ASCVD events (Detrano et al. 2009; Erbel et al. 2010).
Compared with individuals with CAC = 0, individuals with CAC 1 to 99, CAC 100 to 399, and CAC ≥ 400 have 2- to 3-fold, 4- to 7-fold, and 9- to 16-fold higher risk of cardiovascular events and mortality, respectively (Detrano et al. 2009; Erbel et al. 2010; Hecht et al. 2017). Table 22.1 illustrates the strong correlation between CAC scores and 10-year ASCVD event rates in MESA. Of note, multiple studies have demonstrated that the absence of CAC is associated with a very low risk of CHD events, low risk of ASCVD events, and of cardiovascular death, in asymptomatic individuals from the general primary prevention population (Blaha et al. 2016; Nasir et al. 2015). Results of these studies have led to the concept of “power of zero, ” highlighting the fact that asymptomatic individuals with CAC = 0 have a low risk of cardiovascular events, or at least the lowest risk within populations at increased average risk (e.g., populations with diabetes or familial hypercholesterolemia). Moreover, studies have also shown that individuals without traditional ASCVD risk factors such as cigarette smoking, dyslipidemia, diabetes mellitus, hypertension, or family history of CHD, but who have elevated CAC have significantly higher cardiovascular events and mortality rates than individuals with multiple traditional ASCVD risk factors but CAC = 0 (Lakoski et al. 2007; Nasir et al. 2012).
Below we summarize the evidence of CAC providing prognostic value across key groups.
Younger and Older Adults
Although CAC burden correlates with age, several other various factors also contribute to an individual’s CAC burden, including genetics, lifetime exposure to traditional and novel risk factors, individual susceptibility vs. resilience to atherosclerosis, and other factors. In this context, CAC accurately stratifies ASCVD risk across age strata, including among individuals at the extremes of the age continuum. This is important, as the PCE perform poorly in these groups, usually resulting in low-risk estimations in younger adults and high-risk estimations in the elderly. Specifically, young individuals with any CAC had a 4-fold higher risk of major ASCVD events, and a 10-fold higher risk when CAC > 100 compared with individuals of the same age with a CAC score of 0 (Miedema et al. 2019). For individuals over 75 years of age, CAC also independently predicts CHD/ASCVD events and mortality (Tota-Maharaj et al. 2014). Interestingly, young and elderly adults with CAC = 0 have a similar 5-year survival rate, and elderly adults with CAC = 0 have a lower mortality rate than younger adults with high CAC (Tota-Maharaj et al. 2012).
Men and Women
Biological sex affects the development of atherosclerosis. For the same age, women tend to have lower CAC scores, and CAC is detected on average 10 years after than in men. When sex-specific presence and pattern of CAC is examined, women and men with CAC = 0 have similar long-term CVD mortality, whereas if CAC > 0, women (in whom this finding is less frequent) have a 1.3-fold higher risk of CVD mortality when compared with men. Regarding calcification patterns, women tend to have more dense plaques but fewer calcified lesions and vessels and less volume of calcifications (Shaw et al. 2018). Using sex-specific CAC cutpoints can help improve ASCVD risk stratification and management in women.
Racial/Ethnic Groups
Given the fact that the PCE is limited to non-Hispanic White and non-Hispanic Black individuals, the ACC/AHA guideline recommended using the PCE version for non-Hispanic White individuals as an initial approximation to ASCVD risk in other racial/ethnic groups. However, the guidelines acknowledged that this approach could result in overestimating risk in certain racial/ethnic groups with lower ASCVD risk than their non-Hispanic White counterparts, such as East Asian individuals (e.g., Chinese, Koreans, or Japanese) (Lloyd-Jones et al. 2019), and underestimating risk in other racial/ethnic groups with higher ASCVD risk than their non-Hispanic White counterparts, such as South Asian individuals (e.g., Indian, Pakistani, or Bangladeshi) (Volgman et al. 2018). Therefore, measuring CAC score may be particularly helpful in refining initial risk estimates in these groups. In the CAC Consortium, a large prospective cohort of individuals undergoing self- or clinically referred CAC scores, CAC has shown predictive value independent of traditional CVD risk factors for both all-cause and CVD-specific mortality in non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, and Hispanic individuals (Orimoloye et al. 2018). The same is true in the population-based MESA cohort. Also, a recent study has suggested that CAC may also have value in personalizing risk management in US Asian Indian adults free of diabetes and at borderline estimated risk (Haque et al. 2021).
Individuals with a Family History of Premature CHD/ASCVD
Family history of premature CHD/ASCVD is an established risk factor of future ASCVD events independent of traditional risk factors (Patel et al. 2018). Physicians are constantly challenged with assessing ASCVD risk among individuals who report a family history but with no clearly abnormal traditional risk factors. Future risk among those individuals will not be captured using the PCE, and in this context, multiple studies have demonstrated that CAC testing is effective in stratifying ASCVD risk (Patel 2015; Cohen et al. 2014). In MESA, approximately half of individuals with family history of premature CHD/ASCVD had CAC = 0 and were at low absolute risk for events over a median follow-up of 10 years, whereas those with CAC ≥ 400 had a 4-fold future risk compared with CAC = 0 (Patel 2015). Several guidelines recommend a selective use of CAC in low estimated-risk individuals (<5% 10-year ASCVD risk with the PCE) with a family history of premature CHD/ASCVD. The absence of CAC (CAC = 0) would confirm their low-risk status, while the presence of CAC (CAC >0) would identify a group who might benefit from greater intensity of lifestyle modification and preventive therapies (Hecht et al. 2017).
The Evolving Role of CAC for Statin Therapy Allocation in Primary Prevention
In the late 1990s and early 2000s, CAC was seen as a tool that could help identify apparently healthy individuals with subclinical atherosclerosis who could benefit from more aggressive preventive interventions, typically statins. However, in the last decade, with the broadening of eligibility of statins to reduce ASCVD risk and background of risk overestimation with the PCE (Karmali et al. 2014; DeFilippis et al. 2015), after 2013, a substantial proportion of adult individuals in the United States became potential candidates for statin therapy (Nasir et al. 2015). In this context, the CAC score gained popularity in identifying statin-eligible individuals expected to derive small absolute benefit from treatment (Nasir and Cainzos-Achirica 2021; Greenland et al. 2018; Nasir et al. 2015). As discussed above, many studies have shown that the absence of CAC (CAC = 0) indicates low risk for future ASCVD events, particularly CHD events (Nasir et al. 2015; Blaha et al. 2009; Sarwar et al. 2009). Analyses in MESA showed that among statin-eligible candidates according to the AHA/ACC guidelines for cholesterol management, approximately one-half had CAC = 0 and had a lower 10-year ASCVD risk than the threshold recommended for treatment. Specifically, the absence of CAC reclassified risk below the threshold for statin consideration in almost 50% of those with borderline to intermediate 10-year ASCVD risk (5–20%; Fig. 22.2). Moreover, over a median follow-up of about 10 years, most ASCVD events occurred among those with detectable CAC, consistent with 10-year risk levels suggested by guidelines for statin therapy (Nasir et al. 2015). These findings have been replicated in other prospective cohorts from the United States and elsewhere.
Utility of the absence of CAC in reclassifying 10-year ASCVD risk below the risk threshold for statin consideration in the borderline- and intermediate-risk groups. (Reprinted from Nasir et al. 2015 with permission)
Large observational studies with baseline CAC data and including users and nonusers of statins also suggest that the presence and severity of CAC is associated with the benefit that can be derived from statin therapy for the primary prevention of CHD/ASCVD, at least on a 10-year timeframe (Mitchell et al. 2018). Specifically, among individuals with detected CAC (CAC > 0), statin therapy is associated with significant reductions in CHD/ASCVD events, and these are larger the higher the baseline CAC score. Conversely, in patients with baseline CAC = 0, these observational analyses suggest a very modest benefit, if any at all. Analysis from the Walter Reed Cohort Study showed that the number of individuals needed to be treated with statin to prevent one initial CHD/ASCVD event over 10 years ranged from 100 for CAC 1–100 to 12 for CAC >100 (Fig. 22.3).
Cumulative incidence of major adverse cardiovascular events stratified by statin treatment and CAC severity. Blue line; no statin; dashed red line, statin therapy. (Reprinted from Mitchell et al. 2018 with permission)
CAC Compared to Other Biomarkers
Several studies have compared the value of CAC and of other various markers, such as serum biomarkers, carotid plaque, carotid intima-media thickness, and ankle-brachial index, among others, for ASCVD risk assessment and prediction of ASCVD events. Of note, studies have consistently shown minimal to no improvement in risk reclassification beyond traditional risk factors with those other markers, as opposed to CAC (Blaha et al. 2016). Importantly, the reliability of absence of CAC score as a marker of low ASCVD risk is superior to absence of risk-enhancing factors, low levels of biomarkers, or absence of carotid plaque (Peters et al. 2012).
CAC for Shared Decision-Making in the Allocation of Statin Therapy and Implications for Adherence
Shared decision-making refers to the process by which clinicians learn about, consider, and incorporate patients’ values, goals, and preferences and jointly discuss risk stratification and therapeutic options and potential benefits/harms of available therapeutic options. Shared decision-making means involving the patient at the core of the risk assessment and therapeutic process, engaging them in selecting meaningful risk assessment strategies and interventions. This can enhance adherence to recommendations, as patients have a better understanding of the rationale of the decisions being made and are directly involved in those, actively engaging in such decisions (Montori et al. 2013). In addition, patients who have higher insight into their disease and risk factors are regularly engaged in self-monitoring and are more motivated to control their risk factors more than their peers who do not (Bodenheimer et al. 2002).
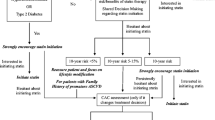
In this context, CAC testing can provide additional relevant information among patients who are uncertain about their management after clinical risk scoring and are willing to use the burden of coronary plaque to inform their decisions about preventive statin therapy. On the contrary, for patients unlikely to change their management based on this information, CAC scoring would be of low value and is not recommended. This represents an important conceptual departure from the notion of CAC as a “screening” tool, rather, it serves as a decision aid in specific patients willing to consider additional information to make a final decision. Figure 22.4 describes the proposed role of CAC scoring in shared decision-making in the allocation of statin therapy in primary prevention.
CAC score as a decision aid in shared decision-making in risk assessment and management for primary ASCVD prevention. (Reprinted from Nasir and Cainzos-Achirica 2021 with permission)
Long-term adherence to preventive interventions is a critical unmet need in the primary prevention of ASCVD, and this is an area where CAC can have a very important impact on improving preventive care and potential outcomes. Indeed, several studies suggest improved adherence to preventive care (lifestyle modifications and medications) following CAC scoring (Mamudu et al. 2014). Interestingly, a randomized trial (CorCal) was conducted and compared CAC-based vs. PCE risk score-based strategy for initiation of statin therapy for primary ASCVD prevention. After 1 year, CAC-based strategy resulted in superior statin adherence rate, lower low-density lipoprotein cholesterol (LDL-C) levels, similar or reduced estimated costs, and fewer ASCVD events occurred compared to PCE risk score-based strategy (Muhlestein et al. 2021). In order to communicate with patients even more effectively, physicians may consider providing visual graphics and resources to patients to help them understand their risk, as visualization of CAC images may improve patient understanding and compliance (Kalia et al. 2006).
CAC in Current Primary Prevention Guidelines
Current guidelines around the world recommend considering CAC as part of the evaluation among individuals with borderline and intermediate-risk for ASCVD in case of uncertainty regarding decisions for initiation of preventive therapies. Table 22.2 summarizes major guidelines and expert consensus on use of CAC for risk assessment in primary prevention.
In the United States, the 2018 American College of Cardiology/American Heart Association (ACC/AHA) / Multisociety guideline authors concluded that it is appropriate to consider CAC testing in the context of shared decision-making for asymptomatic individuals without underling clinical ASCVD who are 40–75 years of age, have a 10-year ASCVD risk between 5% and 20%, and are uncertain about their risk management after clinical risk estimation (class of recommendation IIa) (Grundy et al. 2019). Also, the guideline endorsed the selective consideration of CAC testing among individuals with estimated risk <5% with a family history of premature CHD/ASCVD (class of recommendation IIa). Similar recommendations were included in the 2019 ACC/AHA Primary Prevention Guideline. Both guidelines emphasized the ability of CAC = 0 to de-risk individuals at borderline/intermediate risk who are not active smokers and do not have diabetes as a group where statins can be avoided given an expected small absolute risk reduction and the interventions focus on lifestyle modification. Similarly, for adults 75 years of age or older, guidelines highlight the role of measuring CAC to reclassify those with CAC = 0 to avoid statin therapy (class of recommendation IIa) (Arnett et al. 2019).
In contrast, the ACC/AHA guideline authors concluded that CAC testing has limited impact on decisions regarding preventive therapy utilization among individuals with low (<5%) and no family history, as well as in those with high (>20%) 10-year calculated ASCVD risk. Among the low-risk group, the majority of individuals have CAC = 0 and have an extremely low 10-year ASCVD event rate of 1.6%, and <5% of individuals have CAC > 100 (Nasir et al. 2015). At the other end of the risk spectrum, the majority of high-risk individuals (estimated risk >20%) have detectable CAC, and despite the fact that high-risk individuals with CAC = 0 have a lower observed event rate than the calculated risk (<20%), CAC is unlikely to have an impact on the decision to initiate preventive statin therapy, as the risk remains above the >7.5% threshold suggested for treatment (Nasir et al. 2015).
In Europe, the 2021 European Society of Cardiology (ESC) guidelines recommended the use of CAC as a risk modifier that can reclassify CVD risk upward and downward in addition to conventional risk factors, and may thus be considered in men and women with calculated risks around decision thresholds and uncertain about their management (Visseren et al. 2021).
CAC for the Allocation of Other Preventive Pharmacotherapies Beyond Statins
The primary aim of ASCVD risk assessment is to identify individuals who would benefit the most (i.e., largest absolute risk reduction) from available preventive pharmacotherapies that are proven to reduce risk. Similarly, accurate risk stratification can help identify individuals expected to derive the smallest benefit from an intervention, which is an important consideration when therapeutic decisions involve treatments that are expensive or have potential side effects. In this context, using CAC to inform not only statin allocation but the use of multiple other preventive treatments is an active area of research. Below we discuss studies on the prognostic value of CAC in several special populations where despite increased average ASCVD risk, CAC can help further stratify risk and thereby inform a more personalized use of specific add-on therapies. Some of these uses of CAC are now discussed in recent expert consensus documents by the National Lipid Association and the Endocrine Society (Orringer et al. 2021; Newman et al. 2020), but they have not yet been incorporated into ACC/AHA or the ESC guidelines. Based on those novel studies, in Table 22.3, we present a summary of the proposed role of CAC in guiding treatment decisions for multiple preventive therapies in primary prevention.
Aspirin
ACC/AHA guidelines recommend considering low-dose aspirin therapy for adults at very high ASCVD risk and not at high bleeding risk (class of recommendation IIa). However, the optimal approach for identifying appropriate, very high-risk candidates for therapy is unclear. Analyses from two cohorts (MESA and the Dallas Heart Study) suggest that among individuals at low bleeding risk, CAC ≥ 100, and particularly a CAC score ≥ 400, identifies individuals who would likely derive net benefit from aspirin. In contrast, CAC = 0 identifies individuals who would likely derive net harm from aspirin, even among those at low bleeding risk. Conversely, the PCE failed to identify subgroups expected to derive net benefit, not even among those at estimated ASCVD risk >20%. CAC can thus provide a valuable tool for a selective, most personalized allocation of low-dose aspirin in primary prevention (Ajufo et al. 2021; Cainzos-Achirica et al. 2020).
Blood Pressure (BP) Goals
The ACC/AHA guidelines for the management of high BP recommend using the PCE to estimate 10-year ASCVD risk to establish BP treatment goals. Analysis from multiple prospective cohorts showed that among individuals with elevated BP as well as among strata defined by increasing hypertension severity, those with CAC > 0 had a significantly higher incidence of CVD events as opposed to those with CAC = 0, and the number needed to be treated to prevent one future CVD event was lower if CAC > 0 than CAC = 0, in all groups. These results were consistent across racial/ethnic subgroups (Parcha et al. 2021). Furthermore, among individuals with systolic BP <160 mm Hg and 10-year ASCVD risk estimates between 5% and 15%, CAC > 100 can identify those who would likely benefit from initiation or intensification of systolic BP goal compared with CAC = 0 (McEvoy et al. 2017).
Diabetes
Individuals with diabetes are more likely to have ASCVD events, and guidelines recommend at least a moderate-intensity statin in all adults 40–75 years of age with diabetes; and high-intensity statin in those at higher ASCVD risk. Studies have shown that CAC can be useful in stratifying risk among individuals with diabetes (Jensen et al. 2020; Malik et al. 2017), as the risk for CHD and ASCVD events increases progressively with higher CAC scores. Moreover, CAC augments the prognostic information provided by diabetes duration, glycemic control, and insulin use. Thus, CAC may be used to personalize the intensity of statin therapy in patients with diabetes, and may help inform the allocation of novel, costly ASCVD risk-reduction pharmacotherapies in diabetes such as glucagon-like peptide-1 receptor agonists (GLP-1RAs) (Cainzos-Achirica et al. 2021a).
Hypertriglyceridemia
With a prevalence of 25% of hypertriglyceridemia in the general United States population, and evidence of an independent association between levels of triglyceride-rich particles and risk of ASCVD events, there is great interest in the identification of specific pharmacological interventions that can help further reduce ASCVD risk in these individuals. Icosapent ethyl (IPE) is currently the only omega-3-based therapy approved by the Food and Drug Administration (FDA) for ASCVD risk reduction in primary prevention patients with hypertriglyceridemia, and other fatty acids have and are being evaluated for this purpose. In this contest, a recent analysis pooling MESA and three other population-based cohorts suggested that CAC can have a role in identifying high-risk candidates for IPE in primary prevention, and this was true among individuals with and without diabetes (Cainzos-Achirica et al. 2021b).
Severe Hypercholesterolemia and Genetically Confirmed Familial Hypercholesterolemia
There is substantial heterogeneity in long-term ASCVD risk among individuals with severe hypercholesterolemia. Although individuals with high LDL-C (>190 mg/dL) and individuals with familial hypercholesterolemia (FH) are at increased risk for ASCVD compared to the general population, a considerable proportion of these individuals do not experience ASCVD events despite having lifelong elevated LDL-C levels. In this context, multiple studies have shown that CAC has the ability to accurately stratify ASCVD risk in these individuals. For instance, in MESA, among those with LDL-C ≥ 190 mg/dL and CAC = 0, 10-year ASCVD event rates were 3.7%, compared with 20% in individuals with LDL-C ≥ 190 mg/dL and CAC > 0 (Sandesara et al. 2020).
Similarly, among individuals with genetically confirmed FH, CAC has the ability to shed light on ASCVD risk heterogeneity and inform a more personalized management. During a 10-year follow-up of patients with heterozygous FH from Brazil, higher CAC scores were strongly associated with ASCVD, while events were remarkably lower among those with CAC = 0 (Miname et al. 2019). Of note, according to a meta-analysis of 9 small FH clinical cohorts, the prevalence of CAC = 0 in individuals with FH is 45% (Mszar et al. 2020). While guidelines are consistent in their recommendation of statin therapy in all individuals with genetically confirmed FH as well as in those with LDL-C > 190 mg/dL, these studies suggest that CAC may help individualize the allocation of more costly add-on interventions, such as PCSK9 inhibitors or inclisiran. Risk stratification using CAC tailored to patients with FH may further enhance the cost-effectiveness and resource utilization of these novel lipid-lowering treatments.
Follow-Up on Initial CAC Scan
Given the predictive and prognostic power of CAC, particularly the power of CAC = 0, there is an overall interest in knowing the “warranty period” during which individuals with CAC = 0 remain at low or lower risk of events, and when a repeat scan will most likely detect conversion to CAC > 0. The time for conversion to CAC > 0 varies according to baseline estimated ASCVD risk, age, sex, race/ethnicity, and diabetes status. Studies have shown that for individuals with CAC = 0 undergoing yearly CAC scans, conversion to CAC > 0 occurred in 15% of individuals between 3 and 7 years after the initial scan. Repeat CAC scanning can be considered in 5–7 years for patients at low 10-year ASCVD risk (<5%), 3–5 years in those at intermediate risk for ASCVD (5%–10%), and three years in those with diabetes (Dzaye et al. 2021).
The value of repeating the scan in individuals with CAC = 0 relies on the potential for changing preventive treatment recommendations, which will be more intensive if the CAC score increases, and therefore, absolute risk increases. On the other hand, in patients with CAC > 0, particularly those with CAC > 100, repeating the scan will unlikely change established management. In addition, serial CAC testing to assess the efficacy of preventive therapies is not recommended.
Conclusions
The CAC score is a marker of coronary atherosclerosis, is strongly and independently associated with incident CHD/ASCVD events, and is a powerful tool for risk assessment in primary prevention. Among patients uncertain about their risk management after initial clinical risk assessment, CAC can be used to reclassify risk by identifying individuals at higher risk (CAC > 0, and particularly CAC > 100), and de-risk individuals who are expected to derive modest absolute benefit from certain pharmacological interventions (CAC = 0). This is true across age groups, in both men and women, and across a wide range of clinical risk management scenarios. CAC helps further personalize the allocation of statins in primary prevention, a role that is currently endorsed across international cardiovascular prevention guidelines, with studies suggesting that this use of CAC may enhance physician prescription of statins and long-term adherence by patients. In the near future, CAC may also help personalize the allocation of multiple other preventive interventions among individuals free of clinical ASCVD, a very active area of research and innovation in this space.
References
Ajufo E, Ayers CR, Vigen R, Joshi PH, Rohatgi A, de Lemos JA, Khera A. Value of coronary artery calcium scanning in association with the net benefit of aspirin in primary prevention of atherosclerotic cardiovascular disease. JAMA Cardiol. 2021;6:179–87.
Al Rifai M, Blaha MJ, Nambi V, et al. Determinants of incident atherosclerotic cardiovascular disease events among those with absent coronary artery calcium: multi-ethnic study of atherosclerosis. Circulation. 2021. https://doi.org/10.1161/CIRCULATIONAHA.121.056705.
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596–646.
Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700.
Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease : the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133:849–58.
Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–75.
Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39:2401–8.
Cainzos-Achirica M, Miedema MD, McEvoy JW, et al. Coronary artery calcium for personalized allocation of aspirin in primary prevention of cardiovascular disease in 2019: the MESA study (Multi-Ethnic Study of Atherosclerosis). Circulation. 2020;141:1541–53.
Cainzos-Achirica M, Patel KV, Quispe R, et al. Coronary artery calcium for the allocation of GLP-1RA for primary prevention of atherosclerotic cardiovascular disease. JACC Cardiovasc Imaging. 2021a;14:1470–2.
Cainzos-Achirica M, Quispe R, Dudum R, et al. CAC for risk stratification among individuals with hypertriglyceridemia free of clinical atherosclerotic cardiovascular disease. JACC Cardiovasc Imaging. 2021b. https://doi.org/10.1016/J.JCMG.2021.10.017.
Cohen R, Budoff M, McClelland RL, Sillau S, Burke G, Blaha M, Szklo M, Uretsky S, Rozanski A, Shea S. Significance of a positive family history for coronary heart disease in patients with a zero coronary artery calcium score (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol. 2014;114:1210–4.
DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–75.
Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–48.
Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2009;358:1336–45.
Dzaye O, Dardari ZA, Cainzos-Achirica M, et al. Warranty period of a calcium score of zero: comprehensive analysis from MESA. JACC Cardiovasc Imaging. 2021;14:990–1002.
Erbel R, Mhlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–406.
Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–47.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:E1082–143.
Haque W, Grandhi GR, Kanaya AM, et al. Implications of the 2019 American College of Cardiology/American Heart Association primary prevention guidelines and potential value of the coronary artery calcium score among south Asians in the US: the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study. Atherosclerosis. 2021;334:48–56.
Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, Blankstein R, Narula J, Rumberger J, Shaw LJ. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11:157–68.
Jensen JC, Dardari ZA, Blaha MJ, et al. Association of body mass index with coronary artery calcium and subsequent cardiovascular mortality: the coronary artery calcium consortium. Circ Cardiovasc Imaging. 2020;13:9495.
Kalia NK, Miller LG, Nasir K, Blumenthal RS, Agrawal N, Budoff MJ. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis. 2006;185:394–9.
Kanaya AM, Kandula NR, Ewing SK, Herrington D, Liu K, Blaha MJ, Srivastava S, Dave SS, Budoff MJ. Comparing coronary artery calcium among US South Asians with four racial/ethnic groups: the MASALA and MESA studies. Atherosclerosis. 2014;234:102–7.
Karmali KN, Goff DC, Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:959–68.
Lakoski SG, Greenland P, Wong ND, Schreiner PJ, Herrington DM, Kronmal RA, Liu K, Blumenthal RS. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2007;167:2437–42.
Lin JS, Evans CV, Johnson E, Redmond N, Coppola EL, Smith N. Nontraditional risk factors in cardiovascular disease risk assessment: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;320:281–97.
Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC, Sperling LS, Virani SS, Blumenthal RS. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation. 2019;139:E1162–77.
Malik S, Zhao Y, Budoff M, Nasir K, Blumenthal RS, Bertoni AG, Wong ND. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the multi-ethnic study of atherosclerosis. JAMA Cardiol. 2017;2:1332–40.
Mamudu HM, Paul TK, Veeranki SP, Budoff M. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults: a systematic review. Atherosclerosis. 2014;236:338–50.
McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–7.
McEvoy JW, Martin SS, Dardari ZA, et al. Coronary artery calcium to guide a personalized risk-based approach to initiation and intensification of antihypertensive therapy. Circulation. 2017;135:153–65.
Miedema MD, Dardari ZA, Nasir K, et al. Association of coronary artery calcium with long-term, cause-specific mortality among young adults. JAMA Netw Open. 2019;2:e197440.
Miname MH, Bittencourt MS, Moraes SR, Alves RIM, Silva PRS, Jannes CE, Pereira AC, Krieger JE, Nasir K, Santos RD. Coronary artery calcium and cardiovascular events in patients with familial hypercholesterolemia receiving standard lipid-lowering therapy. JACC Cardiovasc Imaging. 2019;12:1797–804.
Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, Cheezum M, Shaw LJ, Villines TC. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72:3233–42.
Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2503–4.
Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11:127–42.
Mszar R, Grandhi GR, Valero-Elizondo J, et al. Absence of coronary artery calcification in middle-aged familial hypercholesterolemia patients without atherosclerotic cardiovascular disease. JACC Cardiovasc Imaging. 2020;13:1090–2.
Muhlestein JB, Knowlton KU, Le VT, et al. Coronary artery calcium versus pooled cohort equations score for primary prevention guidance. JACC Cardiovasc Imaging. 2021. https://doi.org/10.1016/J.JCMG.2021.11.006.
Nasir K, Cainzos-Achirica M. Role of coronary artery calcium score in the primary prevention of cardiovascular disease. BMJ. 2021. https://doi.org/10.1136/BMJ.N776.
Nasir K, Rubin J, Blaha MJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–73.
Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66:1657–68.
Newman CB, Blaha MJ, Boord JB, et al. Lipid management in patients with endocrine disorders: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2020;105:3613–82.
Orimoloye OA, Budoff MJ, Dardari ZA, et al. Race/ethnicity and the prognostic implications of coronary artery calcium for all-cause and cardiovascular disease mortality: the coronary artery calcium consortium. 2018. J Am Heart Assoc. https://doi.org/10.1161/JAHA.118.010471.
Orringer CE, Blaha MJ, Blankstein R, Budoff MJ, Goldberg RB, Gill EA, Maki KC, Mehta L, Jacobson TA. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol. 2021;15:33–60.
Parcha V, Malla G, Kalra R, Li P, Pandey A, Nasir K, Arora G, Arora P. Coronary artery calcium score for personalization of antihypertensive therapy: a pooled cohort analysis. Hypertension. 2021;77:1106–18.
Patel J, Al Rifai M, Blaha MJ, et al. Coronary artery calcium improves risk assessment in adults with a family history of premature coronary heart disease: results from multiethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2015. https://doi.org/10.1161/CIRCIMAGING.115.003186.
Patel J, Al Rifai M, Scheuner MT, Shea S, Blumenthal RS, Nasir K, Blaha MJ, McEvoy JW. Basic vs more complex definitions of family history in the prediction of coronary heart disease: the multi-ethnic study of atherosclerosis. Mayo Clin Proc. 2018;93:1213–23.
Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37:1129–50.
Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98:177–84.
Sandesara PB, Mehta A, O’Neal WT, Kelli HM, Sathiyakumar V, Martin SS, Blaha MJ, Blumenthal RS, Sperling LS. Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol ≥190 mg/dL: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2020;292:224–9.
Sarwar A, Shaw LJ, Shapiro MD, et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–88.
Shaw LJ, Min JK, Nasir K, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC consortium. Eur Heart J. 2018;39:3727–35.
Tota-Maharaj R, Blaha MJ, McEvoy JW, et al. Coronary artery calcium for the prediction of mortality in young adults <45 years old and elderly adults >75 years old. Eur Heart J. 2012;33:2955–62.
Tota-Maharaj R, Blaha MJ, Blankstein R, Silverman MG, Eng J, Shaw LJ, Blumenthal RS, Budoff MJ, Nasir K. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective, population-based cohort. Mayo Clin Proc. 2014;89:1350–9.
Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics—2021 update. Circulation. 2021;143:E254–743.
Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2021;42:3227–337.
Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018;138:e1–e34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Taha, M.B., Ahuja, D., Patel, K.V., Cainzos-Achirica, M., Nasir, K. (2022). Coronary Artery Calcium. In: Shapiro, M.D. (eds) Cardiovascular Risk Assessment in Primary Prevention. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-98824-1_22
Download citation
DOI: https://doi.org/10.1007/978-3-030-98824-1_22
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-98823-4
Online ISBN: 978-3-030-98824-1
eBook Packages: MedicineMedicine (R0)