Abstract
Purpose of Review
Recent advances in minimally invasive technology have compelled surgeons to perform nephrectomy with inferior vena cava thrombectomy using robotic assistance. Here, we aim to review the data comparing open versus robot-assisted nephrectomy with IVC thrombectomy, as well as review operative robotic techniques for nephrectomy with IVC thrombectomy.
Recent Findings
Over the last decade, there have been increasing reports of successful robotic-assisted IVC thrombectomy among skilled robotic surgeons, with case series detailing operative technique, as well as operative and oncologic outcomes for levels I-IV caval thrombus.
Summary
While there is immense promise in the future of robotic-assisted IVC thrombectomy, further studies with direct comparison to open surgical intervention will be needed to ensure the oncologic principles and outcomes are non-inferior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) is among the most common genitourinary malignancies, with an expected 76,080 new diagnoses in 2020 in the USA [1]. In recent history, RCC incidence has increased, as incidental renal mass diagnosis occurs with the increased use of abdominal imaging [2]. Despite the increased incidence over time, RCC exhibits the highest mortality of the genitourinary malignancies, with a mortality of 3.6 per 100,000 population [1]. RCC is unique in its proclivity for vascular invasion with potential for tumor to extend from the kidney itself along the path of venous drainage. Malignancy can extend from the renal vein into the inferior vena cava (IVC) as venous tumor thrombus (VTT). In fact, VTT exists at the time of diagnosis in approximately 8.8% of patients with RCC [3].
When untreated, VTT portends a poor outcome, with median survival of 5 months [4]. Since the landmark publication by Skinner et al. describing the first nephrectomy with IVC thrombectomy, the standard of care for RCC with IVC tumor thrombus has been open nephrectomy with IVC thrombectomy [5]. In the decades since this practice changing publication, nephrectomy with IVC thrombectomy has been found to provide a significant survival benefit, with median recurrence-free survival of 15 months [6]. Despite the potential curative surgical therapy of open nephrectomy with IVC thrombectomy, surgical resection via open approach has been shown to carry burdensome morbidity and mortality, with overall complication rate of over 30% and perioperative mortality of ~5–10% [7,8,9].
Recent advances in minimally invasive technology have compelled surgeons to perform VTT using robotic assistance. Over the last decade, there have been increasing reports of successful robotic-assisted IVC thrombectomy. Here, we aim to review the data comparing open versus robot-assisted nephrectomy with IVC thrombectomy, as well as review operative robotic techniques for nephrectomy with IVC thrombectomy.
History of Laparoscopic IVC Thrombectomy
Prior to the advent of robotic IVC thrombectomy, the roadmap for success was created in laparoscopic-assisted surgery. Although application of laparoscopy on initial reports was limited to the nephrectomy part of the procedure and subsequent open incision for VTT thrombectomy, Savage and Gill reported the fully laparoscopic level I VTT radical nephrectomy in 2000, confirming the feasibility of this procedure [10]. This experience was validated by Sundaram et al. in 2002, who reported on an experience with hand-assisted radical nephrectomy with IVC thrombectomy in a patient with level I thrombus [11]. Using hand-assisted laparoscopic technique, a vessel loop was used to obtain proximal and distal control of the inferior vena cava in combination with a Satinsky clamp to encompass the caval thrombus [11]. Subsequently, Hoang et al. reported pure laparoscopic radical nephrectomy with IVC thrombectomy for patients with right-sided tumors. The reported outcomes included patients with a median tumor size of 9.1 cm (range 5.7–12.8 cm). Overall, the authors reported a median operative time 240 min (range 159–330 min), EBL 600 cc (440–4850 mL), and open conversion in 1 out of seven patients (14.2%) due to direct caval wall invasion [12]. While most of these early reports involved levels I and II VTT, Shao et al. performed the first laparoscopic nephrectomy with thoracoscope-assisted open atriotomy for level IV VTT in 2015 [13]. Despite the growing excitement in minimally invasive management, significant barriers remained with laparoscopic utilization, including limited dexterity, two dimension visualization, and unstable visual picture.
History of Robotic-Assisted Nephrectomy with IVC Thrombectomy
With the verification of safety and feasibility of laparoscopic radical nephrectomy with IVC thrombectomy, the next frontier in technological advancement was the advent of the robot-assisted surgery. Herein, we review utilization of robotic assistance in the IVC thrombectomy space, assessing the state of the literature by thrombus level (Table 1).
Level I VTT
The first described series of robotic nephrectomy with IVC tumor thrombectomy was published in 2010 by Abaza (Fig. 1) [14]. Their case series included five patients with level I right-sided VTT, with technique focused on identification of VTT extent via ultrasound probe and tangential IVC clamping. After incising the IVC along the Satinsky clamp, the tumor thrombi were delivered intact with the nephrectomy specimen. In this first report, excellent patient outcomes were reported with estimated blood loss (EBL) of 170 cc, operative time of 327 min, no transfusion requirements, and average length of stay (LOS) of 1.2 days. At 15 months follow-up, no patients had disease recurrence, and the one patient who had undergone cytoreductive nephrectomy had stable metastatic disease on systemic therapy. With these reassuring surgical and oncologic outcomes, the door was opened to usher in minimally invasive surgery for patients with RCC and caval thrombus, but data remained limited to results from high-volume, experienced surgeons.
Levels II–III VTT
Following the reports of level I VTT success, the first two series of robotic-assisted VTT surgical resection of levels II and III thrombi were reported in 2015 and 2016 by several groups (Table 1) [15,16,17]. Among these cohorts of 16 and 32 patients, there was increased surgical complexity as compared to prior reports. Within the case series reported by Abaza et al., 24 patients required IVC cross-clamping, one patient required synthetic patch cavoplasty, and one patient death was reported [16].
The majority of patients in this multicenter case series were level II thrombi (30/32), but this case series did include several level III (2/32) VTT. The nine surgeons that participated in this study were all highly experienced in robotics who were able to progress with this procedure with no conversions to open surgery, with a mean operative time of 292 min despite increased complexity of thrombi and caval reconstruction. The exact surgical technique varied by surgeon in this series; all procedures were performed transperitoneally, but the IVC was controlled with various methods (modified Rommel tourniquets vs bulldog clamp) and port placement and number (between 4 and 8) varied based on surgeon preference. The authors reported a mean EBL 399 cc, and only 3/32 patients required a blood transfusion with median LOS of 3.2 days. Given that this is among the first to describe level III intrahepatic, infradiaphragmatic thrombi, methodology was defined to manage the short hepatic veins by clipping and dividing prior to laparoscopic ultrasound to identify the most cranial aspect of the tumor for Rummel tourniquet placement.
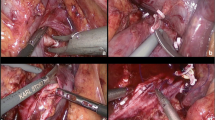
Subsequent robotic utilization of complex level III was further described in 2015 and 2016 by Bratslavsky and Cheng and Ramirez et al., respectively [17, 18]. Technical considerations were similar to the reports from lower grade thrombi, with additional steps largely focused on mobilization of the liver and division of the short hepatic veins. Consistent with the open approach, mobilization of the liver included incising the peritoneum below the liver, and the right triangular ligament, as well as division of hepatic veins to the posterior right and caudate lobes to facilitate exposure of the retrohepatic IVC (Fig. 2) [15]. It is imperative to allow for adequate exposure of the IVC prior to utilization of intraoperative ultrasound, to minimize manipulation and decrease likelihood of dislodging the tumor thrombus.
A Retrohepatic and B supradiaphragmatic robotic IVC thrombectomy. C Patient positioning and port placement for retrohepatic IVC thrombectomy, demonstrating a seven-port technique. Photos courtesy of Wang et al. [22]
Once the cranial extent of the tumor has been established, appropriate vascular control with combination of vascular loops and a bulldog clamp can be attempted. Unlike an open approach, cephalad and lateral nephropexy utilizing Weck clips have been described to suspend the kidney and adrenal upwards to allow for stable exposure during the cavotomy and repair. Operative time was reported at 353–366 min with 150–1200-cc blood loss and 0–4 units of red blood cell transfusion [17,18,19].
Subsequently, additional case series of levels II and III thrombi have been reported with good safety and oncologic outcomes [20,21,22]. While this experience showed widespread safety despite the variability among surgeons, it is not necessarily generalizable to all robotic surgeons. Similarly, careful patient selection for these case series enables successful robotic experience but is not representative of the population at large [16].
Level IV VTT
In VTT cases, operative complexity is determined by the cranial extent of the VTT. This is particularly true for level IV VTT which extends above suprahepatic IVC and into the right atrium (Fig. 1). Initial reports of robotic nephrectomy with level IV caval thrombus were published in 2019 and 2020 [19, 23]. This surgical technique mimics that of pure laparoscopy for level IV thrombus, with minimally invasive nephrectomy with mini thoracotomy for cardiac control (aortic cross clamping, cardiopulmonary bypass with hypothermic cardiac arrest and superior vena cava occlusion with transesophageal echocardiography). Simultaneous antegrade-retrograde thrombectomy was performed after transthoracic opening of the right atrium and transabdominal opening of the infrahepatic IVC. Complete thrombus removal was confirmed with intracaval balloon catheter and robot-controlled endoluminal venacavoscopy [13, 19]. In these scenarios, there is potential for complications including vascular injury, hemorrhage, and thrombus shedding. Despite the reported feasibility of robotic caval thrombectomy for levels III and IV thrombi, there has been limited adoption in the years thereafter, likely due to rarity of cases, the uncertain oncologic outcomes, and the time-consuming process of intraoperative patient repositioning and robot redocking. Additionally, caval thrombectomies are becoming increasingly rare surgical cases in the post-CARMENA era, with increased use of upfront systemic therapy in patients who present with metastatic disease [24].
Preoperative Considerations
Preoperative considerations in the management of VTT are largely the same between open and robotic approaches, with considerations of VTT level, grade, stage, nutritional status, and Charlson Comorbidity Index. High-quality preoperative imaging studies to delineate the extent of tumor thrombus and assess for direct caval wall invasion with associated intraoperative assessment of tumor thrombus are of utmost importance [14, 16]. Preoperative angioembolization remains controversial and is ultimately surgeon dependent, with some case series reporting strict recommendation for angioembolization preoperatively, while others recommend never performing angioembolization in part due to concerns regarding necrosis and embolization of tumor thrombus [20, 22, 25, 26].
Operative Techniques
Abaza et al. first defined the technique of robotic nephrectomy with IVC thrombectomy in level II VTT in 2011 among five patients with right-sided tumors [14]. In brief, these are performed transperitoneally in lateral decubitus position. The colon is reflected medially and the duodenum is kocherized. Early control of the artery is performed at either the hilum or in the interaortocaval space. Once vascular control has been established, gentle circumferential IVC dissection above and below the renal vein is performed, taking care not to dislodge the thrombus. To identify the cranial extent of the VTT, laparoscopic ultrasound delineates the extent of the tumor thrombus to identify the location for IVC control. Lumbar veins are identified and controlled with clips or bipolar cautery depending on the caliber of the collaterals. Nephropexy with the robotic arm or with Weck clips can retract the kidney to both shorten the tumor in the IVC lumen and ensure identification of vessels and venous collaterals [14, 17, 18]. As described by Abaza et al., tangential IVC clamping with Satinsky clamp can then be performed [16]. This involves incision of the IVC on the inner curvature of the Satinsky, and delivery of the tumor thrombus with subsequent IVC reconstruction with 4–0 polypropylene suture [14].
When cross-clamping of the IVC is necessary, most commonly, a modified Rommel tourniquet is created with vessel loops placed twice around the IVC above and below the thrombus and around the contralateral renal vein. Alternatively, IVC control can be achieved with bulldogs, laparoscopic Satinsky clamps, or an intra-caval Fogarty catheter [16, 21]. A small incision is made in the IVC to ensure that all lumbar veins have been appropriately controlled. Once the IVC is opened and the thrombus extracted, the lumen is irrigated with heparinized saline before closing [14].
Retrohepatic IVC thrombectomy most often requires a seven-port technique (Fig. 2) [27]. For a proximal thrombus inferior to the first porta hepatis, some (usually 1–3) short hepatic veins are ligated but the liver does not require mobilization (similarly to level II thrombus). For thrombus between the first and second porta hepatis, the right lobe of the liver is mobilized from the IVC by ligating additional short hepatic veins (usually 3–5). If the thrombus is above the second porta hepatis but below the diaphragm, both the left and right lobes of the liver are mobilized to obtain high proximal control of the suprahepatic and infradiaphragmatic IVC as well as clamping the first porta hepatis.
Technique by Side
In comparing left- vs right-sided IVC thrombectomy, specific techniques differentiate the two [20, 22, 25]. Unlike the right-sided approach, the left-sided approach starts with caval thrombectomy and ends with radical nephrectomy. Due to these differences in the approach, some emphasize the importance of preoperative embolization of the left main and segmental arteries, as the renal vein may be disconnected well before control of the renal artery. Furthermore, as the approach involves caval thrombus management first, the patient is first secured with the right side up to perform the caval thrombectomy, followed by re-docking and re-positioning the patients with the left side up for the kidney portion of the procedure.
Although some methodologies encourage preoperative angioembolization prior to all left-sided thrombectomies (e.g., Wang et al.), some surgeons do not perform angioembolization prior to any cases (e.g., Rose et al.), while others perform angioembolization on a case-by-case basis (e.g., Chopra et al.) [20, 22, 26]. While preoperative angioembolization does not improve morbidity or mortality for open VTT, it is reasonable to use preoperative angioembolization for left-sided robot-assisted VTT due to surgical complexity, with decision based on surgeon preference and comfort.
Comparing Robotic-Assisted vs Open Approach
At present, few studies directly compare operative and oncologic outcomes between robotic-assisted and open nephrectomy with IVC thrombectomy. While the blueprints for open IVC VTT surgery were established decades ago with well-studied perioperative and oncologic outcomes, there are few direct comparisons between robotic-assisted and open approaches to VTT have limited comparable evaluation [28]. Whether the robotic approach is generalizable to more than a handful of select centers and can safely replace open surgery without significantly compromising oncologic and perioperative safety is largely unknown due to lack of prospective, long-term data. The opposite problem may become true in the future, as comfort with open surgery among younger surgeons is diminishing. Among retrospective reviews, after controlling for selection bias, the benefits of robotic nephrectomy with caval thrombectomy become noticeable given that robotic approach can quicken perioperative recovery without apparent compromise of oncologic outcomes.
Comparing Open vs Robotic Peri-Operative Outcomes
Retrospective evaluations of robotic VTT surgery with immediate peri-operative outcomes exist but are limited to a small cohort of patients. Among a retrospective, matched comparison of 68 patients undergoing levels I–II IVC thrombectomy compared 31 robotic and 37 open IVC thrombectomies [29]. Prior to matching, there were significant differences between the two cohorts, but they were evenly balanced in terms of tumor size, age, gender, BMI, ASA, CCI, tumor side/size, level and length of thrombus, and preoperative renal function. Among this cohort, the robotic group had significantly lower EBL (250 ml vs 1000 ml, p < 0.001), fewer blood transfusions (6.5% vs 54.8%, p < 0.001), shorter LOS (5 days vs 9 days, p < 0.001), and fewer complications (9.7% vs 29.0%, p = 0.7) [29]. The differences in the cohorts prior to matching confirm that well-selected patients are more likely to be offered robotic thrombectomies. Similarly, Rose et al. report that robotic-assisted cases experienced fewer blood transfusions (21% vs 82% p < 0.01) and shorter LOS (3 days vs 7 days, p < 0.01), as compared to open [26]. Of note, this large series does note that open thrombectomies do report fewer complications among robotic vs open thrombectomies (17% vs 43%, p < 0.01), including fewer Clavien IV and V complications (0% vs 7.1%) [26]. While this may provide reassurance about the safety and feasibility of the robotic approach, it may also be reflective of a selective cohort of favorable patients that are amenable to the robotic approach [26]. Despite shorter length of hospitalization, the robotic group had higher mean direct cost than the open group ($12,987 vs $7337, p < 0.001) [29].
Conflicting reports of the difference in operative time are noted when comparing open vs robotic approach—likely based on surgeon variability. Gu et al. report that robotic surgery had shorter overall median operating time (150 min vs 230 min, p < 0.001), though left-sided tumors required significantly more time with the robotic approach (260 min vs 249 min, p = 0.662) [29]. Whereas Rose et al. report that the robot-assisted group had overall longer operative times (242 vs 284 min, p = 0.03) [26].
Comparing Open vs Robotic Oncologic Outcomes
In comparing open vs robotic oncologic outcomes for VTT, no significant differences are reported in the literature. With a median follow-up of 27 months for the robotic group and 45 months for the open group, Gu et al. report no significant difference in progression free-survival (PFS) (p = 0.71) or overall survival (OS) (p = 0.18) between the two groups [29]. Similarly, Rose et al. reported no difference in OS (51 vs 49 months, p = 0.16) or RFS (33.2 vs 36.5 months, p = 0.68) [26]. Despite efforts to match cohorts between open and robotic cases in these series, there is certainly provider bias in choosing patients eligible for robotic caval thrombectomy. To date, there is no level I data comparing survival outcomes between open and robotic IVC thrombectomy; thus, our understanding of these outcomes is limited to retrospective analysis and institutional data.
Although there are no direct, prospective comparisons between open and robotic techniques, limited oncologic data have previously been published in the literature. Chopra et al. report that among their cohort of robotic caval thrombectomies, 46% developed new-onset metastatic disease, and 42% received adjuvant therapy within 12 months, with median follow-up of 16 months [20]. For Wang et al., only 7.6% developed metastatic disease, and 15.2% died during the median 18-month follow-up [19]. Gill et al. report 11.1% metastatic disease and 0% mortality in their initial series with median 7-month follow-up [15]. The incredible heterogeneity of oncologic outcomes among these studies is likely attributable to patient selection among the robotic thrombectomy cohorts that has created variable risk of metastasis and death among studies. Although there are no direct comparisons for cancer recurrence between open and robotic surgery, the aforementioned data are comparable to reports of 30–40% 5-year relapse rates after open nephrectomy for stages II and III RCC [30].
Conclusion
While there is immense promise in the future of robotic-assisted IVC thrombectomy, further studies with direct comparison to open surgical intervention will be needed to ensure the oncologic principles and outcomes are non-inferior.
Abbreviations
- cm:
-
Centimeter
- EBL:
-
Estimated blood loss
- IVC:
-
Inferior vena cava
- LOS:
-
Length of stay
- min:
-
Minute
- mL:
-
Milliliter
- RCC:
-
Renal cell carcinoma
- VTT:
-
Venous tumor thrombus
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020;70(2020):7–30.
King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high-grade disease: United States 2001 to 2010. J Urol. 2014;191:1665–70.
Whitson JM, Reese AC, Meng MV. Population based-analysis of survival in patients with renal cell carcinoma and venous tumor thrombus. Urol Oncol. 2013;31:259–63.
Reese AC, Whitson JM, Meng MV. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol Oncol. 2013;31:1305–9.
Skinner DG, Pfister RF, Colvin R. Extension of renal cell carcinoma into the vena cava: the rationale for aggressive surgical management. J Urol. 1972;107:711–6.
Haddad AQ, Wood CG, Abel EJ, Krabbe LM, Darwish OM, Thompson RH, Heckman JE, Merril MM, Gayed BA, Sagalowsky AI, Boorjian SA, Margulis V, Leibovich BC. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: a contemporary multicenter cohort. J Urol. 2014;192:1050–6.
Boorjian SA, Sengupta S, Blute ML. Renal cell carcinoma: vena caval involvement. BJU Int. 2007;99:1239–44.
Blute ML, Leibovich BC, Lohse CM, Cheville JC, Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004;94:33–41.
Boorjian SA, Blute ML. Surgery for vena caval tumor extension in renal cancer. Curr Opin Urol. 2009;19:473–7.
Savage SJ, Gill IS. Laparoscopic radical nephrectomy for renal cell carcinoma in a patient with level I renal vein tumor thrombus. J Urol. 2000;163:1243–4.
Sundaram CP, Rehman J, Landman J, Joseph OH. Hand-assisted laparoscopic radical nephrectomy for renal cell carcinoma with inferior vena caval thrombus. J Urol. 2002;168:176–9.
Hoang AN, Vaporcyian AA, Matin SF. Laparoscopy-assisted radical nephrectomy with inferior vena caval thrombectomy for level II to III tumor thrombus: a single-institution experience and review of the literature. J Endourol. 2010;24:1005–12.
Shao P, Li J, Qin C, Lv Q, Ju X, Li P, Shao Y, Ni B, Yin C. Laparoscopic radical nephrectomy and inferior vena cava thrombectomy in the treatment of renal cell carcinoma. Eur Urol. 2015;68:115–22.
Abaza R. Initial series of robotic radical nephrectomy with vena caval tumor thrombectomy. Eur Urol. 2011;59:652–6.
Gill IS, Metcalfe C, Abreu A, Duddalwar V, Chopra S, Cunningham M, Thangathurai D, Ukimura O, Satkunasivam R, Hung A, Papalia R, Aron M, Desai M, Gallucci M. Robotic level III inferior vena cava tumor thrombectomy: initial series. J Urol. 2015;194:929–38.
Abaza R, Shabsigh A, Castle E, Allaf M, Hu JC, Rogers C, Menon M, Aron M, Sundaram CP, Eun D. Multi-institutional experience with robotic nephrectomy with inferior vena cava tumor thrombectomy. J Urol. 2016;195:865–71.
Bratslavsky G, Cheng JS. Robotic-assisted radical nephrectomy with retrohepatic vena caval tumor thrombectomy (level III) combined with extended retroperitoneal lymph node dissection. Urology. 2015;86:1235–40.
Ramirez D, Maurice MJ, Cohen B, Krishnamurthi V, Haber GP. Robotic level III IVC tumor thrombectomy: duplicating the open approach. Urology. 2016;90:204–7.
Wang B, Huang Q, Liu K, Fan Y, Peng C, Gu L, Shi T, Zhang P, Chen W, Du S, Niu S, Liu R, Zhao G, Li Q, Xiao C, Wang R, Li S, Wang M, Liu F, Wang H, Li H, Ma X, Zhang X. Robot-assisted level III-IV inferior vena cava thrombectomy: initial series with step-by-step procedures and 1-yr outcomes. Eur Urol. 2020;78:77–86.
Chopra S, Simone G, Metcalfe C, de Castro Abreu AL, Nabhani J, Ferriero M, Bove AM, Sotelo R, Aron M, Desai MM, Gallucci M, Gill IS. Robot-assisted level II-III inferior vena cava tumor thrombectomy: step-by-step technique and 1-year outcomes. Eur Urol. 2017;72:267–74.
Kundavaram C, Abreu AL, Chopra S, Simone G, Sotelo R, Aron M, Desai MM, Gallucci M, Gill IS. Advances in robotic vena cava tumor thrombectomy: intracaval balloon occlusion, patch grafting, and vena cavoscopy. Eur Urol. 2016;70:884–90.
Wang B, Li H, Ma X, Zhang X, Gu L, Li X, Fan Y, Gao Y, Liu K, Zhu J. Robot-assisted laparoscopic inferior vena cava thrombectomy: different sides require different techniques. Eur Urol. 2016;69:1112–9.
Gill IS, Cacciamani GE, Duddalwar V, Thangathurai D, Cunningham M. Renal cancer with extensive level IV intracardiac tumour thrombus removed by robot. Lancet. 2020;396:e88.
Mejean A, Ravaud A, Thezenas S, Colas S, Beauval JB, Bensalah K, Geoffrois L, Thiery-Vuillemin A, Cormier L, Lang H, Guy L, Gravis G, Rolland F, Linassier C, Lechevallier E, Beisland C, Aitchison M, Oudard S, Patard JJ, Theodore C, Chevreau C, Laguerre B, Hubert J, Gross-Goupil M, Bernhard JC, Albiges L, Timsit MO, Lebret T, Escudier B. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379:417–27.
Rose KM, Navaratnam AK, Abdul-Muhsin HM, Faraj KS, Eversman SA, Moss AA, Eversman WG, Stone WM, Money SR, Davila VJ, Castle EP. Robot-assisted surgery of the vena cava: perioperative outcomes, technique, and lessons learned at the Mayo Clinic. J Endourol. 2019;33:1009–16.
Rose KM, Navaratnam AK, Faraj KS, Abdul-Muhsin HM, Syal A, Elias L, Moss AA, Eversman WG, Stone WM, Money SR, Davila VJ, Tyson MD, Castle EP. Comparison of open and robot-assisted radical nephrectomy with level I and II inferior vena cava tumor thrombus: the Mayo Clinic experience. Urology. 2020;136:152–7.
Wang B, Li H, Huang Q, Liu K, Fan Y, Peng C, Gu L, Li X, Guo G, Liu R, Hu M, Zhao G, Wang H, Liu F, Xiong J, Zhang X, Ma X. Robot-assisted retrohepatic inferior vena cava thrombectomy: first or second porta hepatis as an important boundary landmark. Eur Urol. 2018;74:512–20.
Masic S, Kutikov A. Robotic inferior vena cava thrombectomy: are we entering the house through an attic window? Eur Urol Focus. 2018;4:641–2.
Gu L, Ma X, Gao Y, Li H, Li X, Chen L, Wang B, Xie Y, Fan Y, Zhang X. Robotic versus open level I-II inferior vena cava thrombectomy: a matched group comparative analysis. J Urol. 2017;198:1241–6.
Sharma T, Tajzler C, Kapoor A. Is there a role for adjuvant therapy after surgery in “high risk for recurrence” kidney cancer? An update on current concepts. Curr Oncol. 2018;25:e444–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have existing conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Kidney Diseases
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prunty, M., Bell, S., Kutikov, A. et al. Review of Robotic-Assisted Radical Nephrectomy with Inferior Vena Cava Thrombectomy in Renal Cell Carcinoma. Curr Urol Rep 23, 363–370 (2022). https://doi.org/10.1007/s11934-022-01120-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11934-022-01120-x