Abstract
Purpose of Review
There has been great interest in understanding why T regulatory cells (Tregs) are reduced in number and/or in function in several autoimmune diseases including systemic lupus erythematosus (SLE). Although research has provided some answers, there is still much to learn.
Recent Findings
Recent investigations on the mechanisms responsible for the impairment of the Tregs in SLE have identified relevant abnormalities in cellular and molecular pathways that have been instrumental in the design of studies in animal models and in the development of pilot immunotherapeutic studies in lupus patients.
Summary
We review the progress made in the field in the last 5 years, discussing the mechanistic studies, together with the preclinical and clinical works that are moving forward the understanding of the physiopathology of Tregs in SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
T regulatory cells (Tregs) comprise different subpopulations of T lymphocytes that contribute to the maintenance of peripheral immune self-tolerance by suppressing the activation/expansion of effector cells, and their production of proinflammatory mediators. While acknowledging the existence of multiple types of Tregs (Tables 1 and 2), this review will only focus on CD4+ Tregs—the most studied type of Tregs.
General Considerations on Tregs
Tregs can suppress the activation, expansion, and differentiation of multiple types of cells including CD4+ T helper cells, CD8+ T cells, and B cells [1,2,3,4]. Tregs are schematically divided into thymus-derived (tTregs) and induced (iTregs), the latter differentiating in the periphery upon stimulation of naïve T cell precursors in the presence of TGF-β [5]. Interestingly, TGF-β is not only required for the induction of iTregs but is also involved in the mechanisms of Tregs suppression of CD4+ T effector cells and B cell production of antibodies [6], either through soluble TGF-β or via a direct contact with TGF-β on the cell surface of the Tregs [7, 8]. Importantly, the generation (but also the suppressive function) of tTregs and iTregs is highly dependent on IL-2 [9], where phosphorylated STAT5 represents a key intermediate between IL-2 signaling and FoxP3 gene transcription [10]. Of interest, the signaling lymphocytic activation molecule family 3 (SLAMF3) can improve sensitivity to IL-2 in SLE, since SLAMF3 costimulation promotes the differentiation of functional Tregs from naïve CD4+ T cells and increases lupus CD4+ T cell proliferation to IL-2 through activation of the IL-2/IL-2R/STAT5 pathway [11].
FOXP3 is the lineage-specific and most important transcription factor in the maintenance of phenotype and suppressive function of both tTregs and iTregs [12]. Yet human T cells, differently from mice, can transiently upregulate FoxP3 without necessarily acquiring a suppressive function [13]. For this reason, bona fide human Tregs are considered those Tregs that, in addition to FoxP3 and high surface levels of CD25, present hypomethylation of a conserved region within the FOXP3 gene called Treg-specific demethylated region (TSDR). This allows a good separation because CpG residues in this region are fully demethylated in Tregs but methylated in non-Tregs (both in humans and in mice), and the demethylation of this region associates with elevated and stable expression of FOXP3 [14].
Tregs and SLE
In murine SLE, the protective role of Tregs is supported by data from adoptive transfer experiments in lupus-prone mice in which the transfer of Tregs delayed disease progression, reduced renal pathology, and improved the survival of the mice [15]. Conversely, a reduction of Tregs following injection with depleting anti-CD25 antibody in young lupus-prone mice resulted in an accelerated development of lupus manifestations [16].
For human SLE, the data are less direct and the reports on numbers and function of Tregs have been at times contradictory. Although most studies reported reduced numbers or impaired function of circulating Tregs in SLE patients, some others found no apparent abnormalities and even increased levels of Tregs in SLE as compared with healthy controls [17]. These discrepancies may have arisen from differences in the use of selected phenotypic markers for the identification of the Tregs (an aspect that was frequent before the relative consensus reached among investigators in recent years), in addition to the protocol of isolation or stimulation of these cells prior to staining. Notwithstanding the recent improvements in characterization, there is still a lack of consensus about the optimal combination of markers to identify human Tregs unequivocally. Given that CD25 and FoxP3 can be expressed by activated T cells [13], CD45RA expression has been proposed as a marker to distinguish naïve and effector CD4+ cells from memory T cells (memory T cells lose CD45RA to become CD45RO+). Specifically, three phenotypically and functionally different subsets can be recognized on the basis of the expression of CD45RA and FoxP3 [18]. The subsets CD45RA+FoxP3low resting Tregs (group I) and CD45RA−FoxP3high activated Tregs (group II) exert suppressive activities in vitro. CD45RA−FoxP3low T cells (group III) are cytokine-producing, non-suppressive cells that probably correspond to effector cells where FoxP3 expression had been induced by cell activation. Based on this phenotypic characterization, active SLE patients’ blood appear to display decreased in percentages of group II Tregs (activated Tregs), increased group I Tregs (resting Tregs), and a significant increase in percentages of group III cells (cytokine-secreting, non-suppressive, effector-like cells) [17]. Of note, the increase in circulating group I Tregs positively correlated with disease activity and serum anti-DNA antibodies [19].
Another proposed phenotypic characterization of human Tregs considers that the transcription factor Helios is preferentially expressed (as mRNA and protein) in Tregs as compared with naïve T cells [20]. CD4+FoxP3+Helios+ Tregs have an elevated suppressive potential and display the characteristic of remaining fully demethylated at the TSDR [20, 21], in addition to expressing CXCR3 and CCR4—which allow them to migrate into inflamed tissues [22]. The finding that high percentages of FoxP3+Helios+ cells have been found in active SLE patients and that their numbers correlate with disease activity (despite a full functional suppressive capacity and migratory potential into inflamed tissues) [22] suggests that these functionally active Tregs may not be sufficient to effectively suppress the inflammatory responses in SLE.
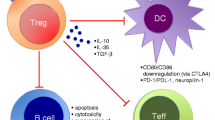
Other types of Tregs with the phenotype CD4+CD25−LAG3+ (LAG3+ Tregs) are regulated by Egr2, a zinc-finger transcription factor required for the induction of T cell anergy, and produce TGF-β3 in an Egr2- and Fas-dependent manner [23]. This cytokine is required for their suppression of lupus B cells, whose suppression requires PD-1 expression on the B cell. The frequency of LAG3+ Tregs that suppress antibody production is reduced in SLE patients [23].
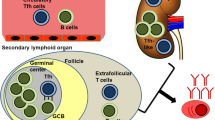
More recently, a population of Tregs called follicular Tregs (CD4+FoxP3+CXCR5+Bcl-6+)—which similarly to T follicular helper (Tfh) cells express CXCR5—has been shown to regulate humoral immune responses in the germinal centers, suppressing local B cell production of antibodies and thus limiting the germinal center response [24,25,26]. Other studies identified CD4+CD25low/-GITR+ cells as suppressive Tregs that expand in about half of SLE patients—all with inactive SLE—suggesting that these cells can contribute to the suppression of ongoing disease [27].
Finally, the screening of human T cells with a panel of monoclonal antibodies identified CD15s (sialyl Lewis x) as expressed by activated, terminally differentiated, and highly suppressive FOXP3high effector Tregs (eTregs), rather than FoxP3+CD4+ T cells in general [28], providing a new specificity in phenotyping of Tregs with a suppressive function. In this context, it has to be noted that Tregs in inflammatory settings adapt to the unfavorable surroundings by increasing the expression of effector markers such as inducible costimulator (ICOS) and glucocorticoid-induced tumor necrosis factor receptor (GITR), to become eTregs [29]. Those eTregs produce IL-10, whose expression is coordinated by the interferon regulatory factor 4 (IRF-4)/BLIMP-1 axis. IRF-4 is modulated by interactions with its partners DEF-6 and switch-associated protein 70 (SWAP-70) and has a central role in the functional program of lupus eTregs through a fine tuning of the function and survival of eTregs via mechanisms that include a modulation of the eTregs autophagy [30]. This allows eTregs to adapt to the unfavorable proinflammatory conditions of SLE, maintaining fitness and functional suppressive capability.
Recent Studies on the Suppression by Tregs in SLE
In addition to T cells [8], tTregs and iTregs can directly suppress activation and proliferation of B cells. While nTregs suppress B cell responses through cytotoxic mechanisms that involve the expression of granzymes and perforin [4], iTregs appear to suppress B cells directly in a non-cytotoxic fashion that depends on TGF-β signaling [31]. Tregs can also inhibit production of autoantibodies in lupus mouse B cells by promoting B cell anergy, both in vitro and in vivo [32]. This phenomenon associates with a reduction in Ca++ flux in B cells, and CTLA-4 blockade inhibits the effects of Tregs on the anergic lupus B cells [32].
The suppressive capacity of Tregs also depends on programmed death-1 (PD-1) signaling, which induces resistance to apoptosis and prolongs the survival and suppression of Tregs in lupus mice [33]. Moreover, ex vivo-generated iTregs suppress upon transfer a lupus-like chronic graft-versus-host disease by preventing the expansion of immunogenic dendritic cells (DCs) and inducing tolerogenic DCs through TGF-β-dependent mechanisms [34].
At a molecular level, the activity of calcium/calmodulin-dependent protein kinase IV (CaMK4) is increased in lupus patients’ T cells, where it reduces IL-2 production by promoting the effects of the transcriptional repressor cAMP-responsive element modulator-α (CREMα) on the IL-2 promoter. T cells from MRL/lpr lupus mice had increased levels of CaMK4, and the genetic deletion of Camk4 improved mice survival by restoring IL-2 production, reducing T cell activation, and favoring Tregs activity and number. In SLE patients, the silencing of CaMK4 in T cells increased the expression of FoxP3 upon cell stimulation [35].
Tregs also require protein phosphatase 2A (PP2A) to maintain their suppressive capacity in vivo [36••]. PP2A is elevated in patients with SLE, where it contributes to reduced production of IL-2 and increased IL-17, decreased CD3ζ and increased FcRγ expression on T cells, hypomethylated lupus-related genes, and increased expression of CREMα. The deficiency of PP2A specifically in Tregs resulted in a severe lymphoproliferative and autoimmune disease with autoantibodies and clinical similarities to the phenotype of scurfy mice secondary to the loss of restraint on the mTORC1 pathway. As a result, mice with PP2A-deficient Tregs had a greater activation of both CD4+ and CD8+ T cells and produced larger amounts of proinflammatory cytokines [36••, 37].
Some studies have also pointed to the contribution of the adipokine leptin in the pathogenesis of SLE. Leptin increase in SLE patients seems to correlate directly with disease activity and inversely with Tregs frequency [38]. In (NZB × NZW)F1 (BW) lupus mice, elevated leptin levels correlated with disease manifestations, and the administration of leptin accelerated development of autoantibodies and renal disease through mechanisms that involved an inhibition of Tregs [39]. Conversely, leptin antagonism delayed disease progression and increased survival of severely nephritic mice [39]. Since during fasting there is a reduction of the levels of circulating leptin, fasting-induced hypoleptinemia in BW lupus mice induced an expansion of functional regulatory T cells that was reversed by leptin replacement, explaining in part the known beneficial effects of fasting in SLE patients [40].
In any case, it must be taken into account that sometimes lupus Tregs might not have functional defects. Their impaired responses could simply be secondary to an acquired resistance of the lupus effector T cells to suppression by Tregs [41].
Recent Progress in Understanding the Role of Epigenetics in Lupus Tregs
Epigenetic modifications include stable, reversible changes in gene expression that result from DNA or chromatin modification or post-transcriptional mechanisms (not associated with changes in DNA coding sequences) that can be passed through cell divisions to cell progenies for multiple generations or indefinitely. Therefore, the failure to maintain epigenetic homeostasis can result in altered cell nuclear activity, changed transcriptome, or aberrant gene expression. Epigenetic changes typically include nucleic acid methylation and histone post-translational modifications, in addition to a modulation of microRNA (miRNA) expression [42]. Considering that epigenetic modifications impart critical changes on cell development and function, as well as direct pathogenic changes, they have been object of multiple studies in SLE, e.g., how miRNAs can act as post-transcriptional regulators of specific aspects of the disease process [43]. A miRNA profile studying abnormal expression of circulating miRNAs in SLE patients as compared with patients with rheumatoid arthritis (RA) and healthy controls identified miR-126 as specifically enriched only in the blood of the SLE patients and other miRNAs (miR-21, miR-451, miR-223, and miR-16) as upregulated in both SLE and RA patients. In contrast, miR-125a-3p, miR-155, and miR-146a appeared to be reduced levels in SLE patients [44]. While preliminary and in need of further analyses, these findings may be of interest for a better understanding of the immune regulation in SLE, also considering the recent findings that miR-125a can stabilize both the commitment and immunoregulatory capacity of Tregs [45•]. In this sense, in the bm12 → B6 cGVHD model of SLE, the deficiency in miR-21 associated with a reduction in autoantibody titers and splenomegaly and an expansion of Tregs [46].
Recent Developments in Tregs-Based Therapies in SLE
Efforts to correct the impairment of Tregs in SLE have been undertaken in multiple experimental models and preclinical settings [17]. Considering that Treg deficits in SLE patients associate with IL-2 deficiency, attempts have been made for the restoration of Tregs activity through IL-2 therapy. The treatment of MRL/lpr lupus mice after onset of disease with an IL-2-recombinant adeno-associated virus resulted in an expansion of Tregs and decreased IL-17-producing CD3+CD4−CD8− double-negative (DN) T cells that associated with significantly reduced organ damage in the skin, lungs, and kidneys [47]. In humans, the findings in vitro that low-dose IL-2 stimulation resulted in an increase of CD25 and anti-apoptotic Bcl-2 expression in lupus Tregs prompted a small study in five patients with refractory SLE. After giving those patients a low-dose IL-2 regimen consisting of daily subcutaneous injections of 1.5 million IU of IL-2 on five consecutive days (in analogy to the in vitro studies), an increased proliferation of Tregs was observed in all patients [48•]. A larger study investigating the effects of low-dose IL-2 subcutaneous treatment on Tregs from patients with active SLE consisted of three cycles of administration at a dose of 1 million IU every other day for 2 weeks, followed by a 2-week break in treatment [49•]. Over the course of rhIL-2 administration, a significant increase in the relative number of Tregs accompanied an improved function of these cells in ex vivo suppression assays. This associated with reduced relative number of Tfh cells, Th17 cells, and DN T cells. All 38 patients who completed therapy showed decreased disease activity at the end of study, as compared to baseline disease activity, without no serious adverse events, and lupus manifestations improved in most patients as compared to controls that had been recruited subsequently [49•]. Thus, the expansion of Tregs in patients with active SLE through low-dose IL-2 associated with quantifiable clinical changes, although randomized trials are required to validate the potential therapeutic effects of this therapy.
SLE patients’ T cell dysfunction is also regulated through mitochondrial transmembrane potential and mechanistic target of rapamycin (mTOR) by glutathione (GSH). In a randomized, double-blind, placebo-controlled study with the GSH precursor N-acetylcysteine, the reduced activity of mTOR associated with an increased FoxP3 expression in Tregs and a reduction of DN T cells and anti-DNA antibody production [50]. Indeed, blockade of mTOR has therapeutic benefits in SLE patients, where mTOR complex 1 (mTORC1) activity was increased and rapamycin inhibited mTORC1 in Tregs, promoting their expansion [51]. Thus, in SLE, mTORC1 expands DN T and Th17 cells, and contracts Tregs [51, 52].
To improve current therapies, and with the knowledge that SLE patients taking oral prednisone display a modest increase in the proportion of circulating Tregs, a study in 17 SLE patients investigated the effects of intravenous high-dose methylprednisolone (MP) on Tregs. MP infusions associated with active proliferation and expansion of eTregs in the first 3 days, to decline to baseline values at day 8. Of interest, the absence of flare after 1 year of follow-up was associated with a higher frequency of eTregs at day 2, suggesting that this increase may contribute to the preventive effect of MP on subsequent flares in SLE [53].
Other authors suggested that Treg-based immunotherapy could help maintain disease remission in SLE [54], and experimental testing in mice employed new approaches to possibly enhance Tregs activities. A77 1726, the active metabolite of leflunomide, attenuated the manifestations of lupus nephritis and increased the frequency of Tregs, possibly because of an ability of this molecule to potentiate the conversion of naïve conventional T cells into iTregs through the inhibition of Akt [55], also suppressing the expansion of DN T cells and inhibiting T and B cell activation.
In MRL/lpr lupus mice, treatment with the CaMK4 inhibitor KN-93 favored the generation of Tregs in vitro and in vivo, and this was accompanied by a reduced accumulation of inflammatory cells in tissue, together with decreased skin and kidney damage [56].
Tregs could also be induced by histone-derived peptides in inactive SLE patients, and in active SLE, Tregs induction by the peptides was unmasked by dexamethasone or hydroxychloroquine [57]. The histone peptide-induced Tregs depended on TGFβ/ALK-5/pSmad 2/3 signaling and suppressed type I IFN-related gene expression in SLE patients. Regarding the IFN signature, mice with established SLE treated with the anti-DNA peptide hCDR1 downregulated IFN-α gene expression significantly and had improved clinical manifestations. In human SLE, hCDR1 reduced IFN-α gene expression in vitro and in vivo in patients treated for 24 weeks by weekly subcutaneous injections, with resulting decrease of disease activity [58].
Conclusions
The last 5 years has seen significant progress in understanding the role of Tregs in SLE. Many investigations on the mechanisms that are responsible for the impairment of Tregs in SLE have led to significant progress in defining new cellular and molecular events that lead and/or sustain the dysfunction of these cells in the disease. Improved phenotypic characterization and unveiling of cellular mechanisms have resulted in new approaches that are now translating into strategies of restoration of the impaired Tregs function in SLE. There is hope that the near future will see implementation of some of these strategies into clinical settings.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–96. https://doi.org/10.1084/jem.188.2.287.
McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A. 2011;108(18):7529–34. https://doi.org/10.1073/pnas.1103782108.
Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+CD25+ regulatory T cells. J Immunol. 2005;175(7):4180–3. https://doi.org/10.4049/jimmunol.175.7.4180.
Iikuni N, Lourenço EV, Hahn BH, La Cava A. Cutting edge: regulatory T cells directly suppress B cells in systemic lupus erythematosus. J Immunol. 2009;183(3):1518–22. https://doi.org/10.4049/jimmunol.0901163.
Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–7. https://doi.org/10.1038/nri1032.
Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, et al. TGF-β1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172(2):834–42. https://doi.org/10.4049/jimmunol.172.2.834.
Oida T, Xu L, Weiner HL, Kitani A, Strober W. TGF-β-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J Immunol. 2006;177(4):2331–9. https://doi.org/10.4049/jimmunol.177.4.2331.
La Cava A, Ebling FM, Hahn BH. Ig-reactive CD4+CD25+ T cells from tolerized (New Zealand Black x New Zealand White)F1 mice suppress in vitro production of antibodies to DNA. J Immunol. 2004;173(5):3542–8. https://doi.org/10.4049/jimmunol.173.5.3542.
Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+CD25+CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201(5):723–35. https://doi.org/10.1084/jem.20041982.
Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571–9. https://doi.org/10.1182/blood-2006-02-004747.
Comte D, Karampetsou MP, Kis-Toth K, Yoshida N, Bradley SJ, Mizui M, et al. Engagement of SLAMF3 enhances CD4+ T-cell sensitivity to IL-2 and favors regulatory T-cell polarization in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2016;113(33):9321–6. https://doi.org/10.1073/pnas.1605081113.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. https://doi.org/10.1126/science.1079490.
Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-β dependent but does not confer a regulatory phenotype. Blood. 2007;110(8):2983–90. https://doi.org/10.1182/blood-2007-06-094656.
Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur J Immunol. 2007;37(9):2378–89. https://doi.org/10.1002/eji.200737594.
Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177(3):1451–9. https://doi.org/10.4049/jimmunol.177.3.1451.
Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A. 2010;107(1):204–9. https://doi.org/10.1073/pnas.0903158107.
Giang S, La Cava A. Regulatory T cells in SLE: biology and use in treatment. Curr Rheumatol Rep. 2016;18(11):67. https://doi.org/10.1007/s11926-016-0616-6.
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. https://doi.org/10.1016/j.immuni.2009.03.019.
Pan X, Yuan X, Zheng Y, Wang W, Shan J, Lin F, et al. Increased CD45RA+FoxP3low regulatory T cells with impaired suppressive function in patients with systemic lupus erythematosus. PLoS One. 2012;7(4):e34662. https://doi.org/10.1371/journal.pone.0034662.
Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–41. https://doi.org/10.4049/jimmunol.0904028.
Kim YC, Bhairavabhotla R, Yoon J, Golding A, Thornton AM, Tran DQ, et al. Oligodeoxynucleotides stabilize Helios expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119(12):2810–8. https://doi.org/10.1182/blood-2011-09-377895.
Alexander T, Sattler A, Templin L, Kohler S, Gross C, Meisel A, et al. Foxp3+Helios+ regulatory T cells are expanded in active systemic lupus erythematosus. Ann Rheum Dis. 2013;72(9):1549–58. https://doi.org/10.1136/annrheumdis-2012-202216.
Okamura T, Sumitomo S, Morita K, Iwasaki Y, Inoue M, Nakachi S, et al. TGF-β3-expressing CD4+CD25−LAG3+ regulatory T cells control humoral immune responses. Nat Commun. 2015;6:6329.
Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–82. https://doi.org/10.1038/nm.2425.
Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–8. https://doi.org/10.1038/nm.2426.
Fujio K, Okamura T, Sumitomo S, Yamamoto K. Regulatory cell subsets in the control of autoantibody production related to systemic autoimmunity. Ann Rheum Dis. 2013;72(Suppl 2):ii85–9. https://doi.org/10.1136/annrheumdis-2012-202341.
Nocentini G, Alunno A, Petrillo MG, Bistoni O, Bartoloni E, Caterbi S, et al. Expansion of regulatory GITR+CD25low/-CD4+ T cells in systemic lupus erythematosus patients. Arthritis Res Ther. 2014;16(5):444. https://doi.org/10.1186/s13075-014-0444-x.
Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci U S A. 2015;112(23):7225–30. https://doi.org/10.1073/pnas.1508224112.
La Cava A. Survive to fight: effector Treg cells in systemic lupus erythematosus. Arthritis Rheumatol. 2016;68(6):1327–9. https://doi.org/10.1002/art.39616.
Chandrasekaran U, Yi W, Gupta S, Weng CH, Giannopoulou E, Chinenov Y, et al. Regulation of effector Treg cells in murine lupus. Arthritis Rheumatol. 2016;68(6):1454–66. https://doi.org/10.1002/art.39599.
Xu A, Liu Y, Chen W, Wang J, Xue Y, Huang F, et al. TGF-β-induced regulatory T cells directly suppress B cell responses through a noncytotoxic mechanism. J Immunol. 2016;196(9):3631–41. https://doi.org/10.4049/jimmunol.1501740.
Liu Y, Liu A, Iikuni N, Xu H, Shi FD, La Cava A. Regulatory CD4+ T cells promote B cell anergy in murine lupus. J Immunol. 2014;192(9):4069–73. https://doi.org/10.4049/jimmunol.1302897.
Wong M, La Cava A, Hahn BH. Blockade of programmed death-1 in young (New Zealand Black x New Zealand White)F1 mice promotes the suppressive capacity of CD4+ regulatory T cells protecting from lupus-like disease. J Immunol. 2013;190(11):5402–10. https://doi.org/10.4049/jimmunol.1202382.
Lan Q, Zhou X, Fan H, Chen M, Wang J, Ryffel B, et al. Polyclonal CD4+Foxp3+ Treg cells induce TGFβ-dependent tolerogenic dendritic cells that suppress the murine lupus-like syndrome. J Mol Cell Biol. 2012;4(6):409–19. https://doi.org/10.1093/jmcb/mjs040.
Koga T, Ichinose K, Mizui M, Crispín JC, Tsokos GC. Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. J Immunol. 2012;189:3490–6.
•• Apostolidis SA, Rodríguez-Rodríguez N, Suárez-Fueyo A, Dioufa N, Ozcan E, Crispín JC, et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat Immunol. 2016;17(5):556–64. This study revealed the importance of the serine-threonine phosphatase PP2A in the function of Tregs and in the prevention of autoimmunity. Tregs were found to have high PP2A activity, and the ablation of the PP2A complex in Tregs led to development of severe autoimmune manifestations. https://doi.org/10.1038/ni.3390.
Sharabi A, Kasper IR, Tsokos GC. The serine/threonine protein phosphatase 2A controls autoimmunity. Clin Immunol. 2018.
Wang X, Qiao Y, Yang L, Song S, Han Y, Tian Y, Ding M, Jin H, Shao F, Liu A. Leptin levels in patients with systemic lupus erythematosus inversely correlate with regulatory T cell frequency. Lupus. 2017;26:1401–6. https://doi.org/10.1177/0961203317703497.
Lourenço EV, Liu A, Matarese G, La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci U S A. 2016;113(38):10637–42. https://doi.org/10.1073/pnas.1607101113.
Liu Y, Yu Y, Matarese G, La Cava A. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J Immunol. 2012;188(5):2070–3. https://doi.org/10.4049/jimmunol.1102835.
Yu Y, Liu Y, Shi FD, Zou H, Hahn BH, La Cava A. Tolerance induced by anti-DNA Ig peptide in (NZB × NZW)F1 lupus mice impinges on the resistance of effector T cells to suppression by regulatory T cells. Clin Immunol. 2012;142(3):291–5. https://doi.org/10.1016/j.clim.2011.11.004.
Liu A, La Cava A. Epigenetic dysregulation in systemic lupus erythematosus. Autoimmunity. 2014;47(4):215–9. https://doi.org/10.3109/08916934.2013.844794.
Amarilyo G, La Cava A. miRNA in systemic lupus erythematosus. Clin Immunol. 2012;144(1):26–31. https://doi.org/10.1016/j.clim.2012.04.005.
Wang H, Peng W, Ouyang X, Li W, Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl Res. 2012;160(3):198–206. https://doi.org/10.1016/j.trsl.2012.04.002.
• Pan W, Zhu S, Dai D, Liu Z, Li D, Li B, et al. MiR-125a targets effector programs to stabilize Treg-mediated immune homeostasis. Nat Commun. 2015;6:7096. This study identified miR-125a as a critical factor in the stabilization of Tregs and the control of autoimmune disease. https://doi.org/10.1038/ncomms8096.
Garchow B, Kiriakidou M. MicroRNA-21 deficiency protects from lupus-like autoimmunity in the chronic graft-versus-host disease model of systemic lupus erythematosus. Clin Immunol. 2016;162:100–6. https://doi.org/10.1016/j.clim.2015.11.010.
Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, et al. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4−CD8− IL-17-producing T cells. J Immunol. 2014;193(5):2168–77. https://doi.org/10.4049/jimmunol.1400977.
• von Spee-Mayer C, Siegert E, Abdirama D, Rose A, Klaus A, Alexander T, et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis. 2016;75(7):1407–15. This study showed that treatment of a small number of SLE patients with a low-dose IL-2 regimen expanded functional Tregs in vivo . https://doi.org/10.1136/annrheumdis-2015-207776.
• He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22(9):991–3. This study showed that treatment of 38 lupus patients with low-dose IL-2 resulted in the reductions of disease activity in all patients. The findings associated with increased Tregs frequency and reduced Tfh and Th17 cell responses. However, the results were not conclusive due to limitations in the study including the recruitment of the control group after end of treatment in the experimental group. https://doi.org/10.1038/nm.4148.
Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–46. https://doi.org/10.1002/art.34502.
Kato H, Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and CD4−CD8− double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol. 2014;192(9):4134–44. https://doi.org/10.4049/jimmunol.1301859.
Lai ZW, Borsuk R, Shadakshari A, Yu J, Dawood M, Garcia R, et al. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol. 2013;191(5):2236–46. https://doi.org/10.4049/jimmunol.1301005.
Mathian A, Jouenne R, Chader D, Cohen-Aubart F, Haroche J, Fadlallah J, et al. Regulatory T cell responses to high-dose methylprednisolone in active systemic lupus erythematosus. PLoS One. 2015;10(12):e0143689. https://doi.org/10.1371/journal.pone.0143689.
Weigert O, von Spee C, Undeutsch R, Kloke L, Humrich JY, Riemekasten G. CD4+Foxp3+ regulatory T cells prolong drug-induced disease remission in (NZB x NZW)F1 lupus mice. Arthritis Res Ther. 2013;15(1):R35. https://doi.org/10.1186/ar4188.
Qiao G, Yang L, Li Z, Williams JW, Zhang J. A77 1726, the active metabolite of leflunomide, attenuates lupus nephritis by promoting the development of regulatory T cells and inhibiting IL-17-producing double negative T cells. Clin Immunol. 2015;157(2):166–74. https://doi.org/10.1016/j.clim.2015.01.006.
Koga T, Mizui M, Yoshida N, Otomo K, Lieberman LA, Crispín JC, et al. KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3+ regulatory T cells in MRL/lpr mice. Autoimmunity. 2014;47(7):445–50. https://doi.org/10.3109/08916934.2014.915954.
Zhang L, Bertucci AM, Ramsey-Goldman R, Harsha-Strong ER, Burt RK, Datta SK. Major pathogenic steps in human lupus can be effectively suppressed by nucleosomal histone peptide epitope-induced regulatory immunity. Clin Immunol. 2013;149(3):365–78. https://doi.org/10.1016/j.clim.2013.08.008.
Sthoeger Z, Zinger H, Sharabi A, Asher I, Mozes E. The tolerogenic peptide, hCDR1, down-regulates the expression of interferon-α in murine and human systemic lupus erythematosus. PLoS One. 2013;8(3):e60394. https://doi.org/10.1371/journal.pone.0060394.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Systemic Lupus Erythematosus
Rights and permissions
About this article
Cite this article
La Cava, A. Tregs in SLE: an Update. Curr Rheumatol Rep 20, 6 (2018). https://doi.org/10.1007/s11926-018-0714-8
Published:
DOI: https://doi.org/10.1007/s11926-018-0714-8