Abstract
Purpose of Review
Historically, therapies for migraine have generally involved pharmacological treatments using non-selective or selective analgesics and preventive treatments. However, for many patients these treatments are not effective, while others prefer to use non-pharmacological-based therapies. To fill this need, over the last 15 years, neuromodulatory devices have entered the market for migraine treatment. Here, we will review the most recent findings for the use of these devices in the treatment of migraine.
Recent Findings
Non-invasive vagus nerve stimulation and spring-pulse transcranial magnetic stimulation are both cleared for the treatment of migraine, supported by preclinical studies that validate efficacy and mechanism of action, and complemented with clinical trial data. Other options also authorized for use include transcutaneous supraorbital nerve stimulation and remote electrical neuromodulation.
Summary
Various options are available to treat migraine using authorized neuromodulatory devices. These data support their efficacy in the treatment of episodic migraine, although further studies are necessary to elucidate their mechanism of action and to provide rigor to clinical trial data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine treatment has evolved dramatically over the last 50 years [1]. The acute treatment of migraine was based on traditional analgesics, namely acetaminophen (paracetamol) and non-steroidal anti-inflammatory drugs, and ergot derivatives as the first migraine-specific acute medications [2]. Overcoming some of the safety concerns of the latter, triptans (5HT1B/1D receptor agonists) showed efficacy and reasonably good tolerability with the main downside being their contraindication in patients with cardiovascular diseases, due to their vasoactive properties [3]. It also had not been long since amitriptyline was first described as a potentially efficacious preventive treatment for headache [4]. The therapeutic arsenal has grown exponentially over the years and various medications including other antidepressants, antihypertensives, calcium antagonists, and anticonvulsive drugs have all shown useful as migraine preventives [5]. With a better understanding of the pathophysiology of migraine, including the discovery of calcitonin gene-related peptide (CGRP) and understanding its role as a nociceptive neurotransmitter [6, 7], this has changed the traditional therapeutic approach towards more migraine-targeted drugs, such as preventive treatments based on monoclonal antibodies targeting the CGRP pathway [8], acute treatments with ditans (5HT1F agonists), and acute and/or preventive treatments with small-molecule CGRP antagonists (gepants) [5, 9].
Yet, despite the therapeutic advances of the last 50 years, for many migraine patients, the currently available preventive and acute medications are not efficacious. A good proportion do not tolerate these drugs, or cannot take them due to contraindications and drug-drug interactions, or they prefer to choose non-pharmaceutical options [10]. As our understanding of migraine pathophysiology and technology has evolved, so too has the opportunity to utilize new and novel neuromodulation approaches to treat migraine [11]. This review will cover the most promising devices showing evidence for the treatment of episodic migraine.
Non-invasive Vagal Nerve Stimulation
Historical Background
Stimulation of the vagus nerve was utilized initially for the treatment of epilepsy. In 1990, four patients were implanted with a device for intermittent vagal nerve stimulation [12]. Following positive multicenter randomized controlled trials, vagal nerve stimulation was FDA-cleared for this indication in 1997 [13]. Despite its benefits, side effects such as cough, dyspnea, and hoarseness were reported [14]. Non-invasive vagal nerve stimulation (nVNS) in the auricular area was trialed in 60 patients with pharmacoresistant epilepsy, leading not only to a reduction in seizure frequency but also improvement in quality of life and depression scales [15]. The use of VNS in depression was tested in a randomized sham-controlled trial and a subsequent open-label extension phase, with the latter showing improvement in depression scales [16, 17]. Consequently, VNS was also cleared by the FDA for the indication of depression in 2005. It was not until the beginning of the twenty-first century that the possibility of treating headache and pain with nVNS was addressed.
Rationale
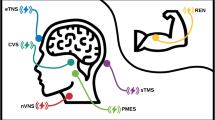
The rationale behind its use in headache is based on the anatomy and physiology of the vagus nerve. Unlike other cranial nerves, the vagus nerve is both motor (efferent) and sensory (afferent), with afferent being the majority of the nerve fibers by ratio of 80:20. At the same time, these afferent fibers are divided in different types. Large A-fibers carry somatic information, small A-fibers carry visceral information, B-fibers carry autonomic information, and unmyelinated C-fibers, which are the most common, are responsible for the transmission of pain as well as sensory visceral, stretching, temperature, and chemical information [18, 19]. At the level of the vagal ganglia, the most relevant neurotransmitters include substance P, glutamate, as well as CGRP [20]. Indeed, the vagus nerve acts as a pain mediator. Its afferent fibers synapse in the area postrema and the spinal nucleus of the trigeminal nerve as well as the nucleus tractus solitarius (NTS) (Fig. 1). From the NTS, there are projections to the locus coeruleus (LC) and the periaqueductal gray (PAG). Some of the major projections of the vagus nerve connect to the thalamus, the parabrachial and ventral posteromedial (VPM) nuclei [18, 21]. The thalamus and PAG have been described as paramount in migraine pathophysiology [11].
Schematic representation of afferent projections of the vagus nerve. AP, area postrema; PAG, periaqueductal gray; PB, parabrachial nucleus; DRN, dorsal raphe nucleus; LC, locus coeruleus; NTS, nucleus tractus solitaries; SuS, superior salivatory nucleus (preganglionic parasympathetic neurons); TCC, trigeminocervical complex (trigeminal nucleus caudalis and its cervical extension to C1 and C2)
Preclinical and Clinical Studies
Preclinical experiments have shown the potential of VNS in pain modulation, but particularly, intracranial migraine-like nociception. Studies demonstrate invasive and non-invasive VNS inhibits responses in rodent models of migraine-like dural-trigeminovascular nociception [22], migraine aura [23], and migraine-like facial allodynia [24]. Studies in models of cortical spreading depression (CSD), the experimental correlate of migraine aura, confirm that central afferents that relay through the NTS are involved in this inhibitory mechanism of action [25]. Furthermore, both serotonin and norepinephrine (noradrenaline) are strongly implicated, supporting a likely role of both LC and raphe nuclei, which receive direct and indirect projections from the NTS. Non-invasive VNS in healthy humans was studied with functional MRI showing deactivation of the spinal trigeminal nucleus [26], as well as a complex network of functional connectivity that also includes the hypothalamus, pontine nucleus, and parahippocampal gyrus [27].
Clinical Trial Evidence
Of note, while studies observing the effects of implanted (invasive) VNS devices for treatment in epilepsy and depression have reported significant improvements in migraine [28,29,30,31], there are no controlled clinical trials of invasive VNS indicated for migraine prevention or acute treatment. Two modalities of nVNS have been tested for migraine treatment: transcutaneous cervical (gammaCore®) and auricular (VITOS®) devices [32].
The gammaCore device stimulates the vagus nerve in the cervical region, where it is flanked by the carotid artery and jugular vein. This device uses fixed stimulus parameters (1 ms pulses of 5 kHz sine waves repeated at 25 Hz for 2 min) with current applied determined by the patient to a comfortable level (24 V peak voltage and 60 mA peak output current). The efficacy of the gammaCore device as a headache abortive was studied in one randomized, sham-controlled study, the PRESTO trial. This study involved 243 migraine patients, where nVNS was applied bilaterally (1 × 2 min for each side) with the option to repeat stimulation if no improvement occurred after 15 min. The primary endpoint, pain freedom at 2 h, was achieved by 30.4% of patients in the active group compared to 19.7% in the sham group. However, the difference was not statistically significant (P = 0.067) [33]. Despite this, for secondary endpoints of pain freedom at both 30 and 60 min, nVNS was statistically superior to sham. Preventive use of this device was tested in the PREMIUM trial, a multicenter, randomized, double-blind sham-controlled study that involved 332 patients with episodic migraine. Here, the preventive treatment protocol included bilateral VNS (1 × 2 min each side) administered three times daily. The primary endpoint, the mean reduction in migraine days per month was not met; after a 12-week follow-up period, this was 2.26 reduction for active and 1.80 for sham (P = 0.15). However, a post hoc analysis involving patients whose therapeutic adherence was high, namely more than 67% per month, did show a statistically significant difference favoring the active group [34, 35]. Additionally, there is evidence from another clinical investigation that the sham treatment may influence the trigemino-autonomic reflex [36]. It may be speculated that the sham device could have had a small effect reducing the difference between the active and sham groups and thereby influencing the study outcome. Therefore, further studies in migraine would be warranted to confirm the findings.
The auricular VNS, the VITOS® (or NEMOS®, indicated for treatment of epilepsy) device has been tested for preventive treatment in chronic migraine in a double-blind, monocentric, parallel group, controlled trial. The outcome of the treatment groups differed based on frequency of stimulation, with the 1 Hz group showing a significantly (P = 0.035) greater reduction of headache days per month (−7.0 ± 4.6 days) compared to the 25 Hz group (−3.3 ± 5.4 days). Patient compliance was high during this trial, which could also account for its positive results as compared to the cervical VNS studies. Again, despite the efficacy and overall good tolerability, only two patients discontinued the 1 Hz treatment due to local ulcer, no more studies have been published on this modality of stimulation [37].
Single Pulse Transcranial Magnetic Stimulation
Historical Background
Transcranial magnetic stimulation (TMS) of the brain became available in 1985. Prior to this, direct electric brain stimulation was only possible during neurosurgical operations [38]. TMS has become a useful tool, utilizing a copper electromagnetic coil, which generates a perpendicular electromagnetic pulse that is applied to the cortex rather than to the scalp and skull. Stimulation of the motor cortex on one side was able to produce movement on the contralateral side and has since been used as a tool to examine motor pathways. Stimulation of non-motor areas was tested for the first time in the early 90s [39]. Of note, the electrical field that is generated on the surface of the cortex can be of up to 150 V/m and evidence shows that it reaches depths of between 1.5 and 3 cm [40]. Following initial single pulse stimulations, stimulation in pairs and repeated pulse stimulation were developed. In the latter, trains of up to 1000s of stimuli can be applied to the cortex. Studies of this technique have shown that low frequency stimulation of less than 1 Hz produces an inhibitory effect, whereas high frequency, namely more than 1 Hz, activates the cortex [41].
Rationale
In approximately 30% of patients with migraine, attacks are preceded by a migraine aura [42]. The most frequent type of aura is visual aura. Following its description by Leao [43], it was accepted that the physiological mechanism behind the aura phenomenon is very likely cortical spreading depression (CSD) (now also termed cortical spreading depolarization). This is a wave of near complete neuronal and glial depolarization that propagates slowly across the cortex and leads to a suppression/depression of neural activity, whereby for a short period of time it is not possible to mediate further depolarizations. Therefore, CSD of the occipital area is thought to account for the symptoms of visual aura [11]. Additionally, considerable evidence suggests that migraine is characterized by neuronal hyperexcitability. Indeed, levels of the main excitatory neurotransmitter, glutamate, have been found raised in migraine patients [44]. This hyperexcitability was shown in the occipital cortex of migraineurs using functional MRI. Visually triggered attacks were followed by cortical suppression of the occipital area regardless of the presence of aura, pointing to this phenomenon in the activation of migraine with and without aura [45].
Preclinical Evidence
In a preclinical model using both rats and cats sTMS has shown the capacity to block mechanically and chemically triggered CSD. It also inhibited dural-responsive trigemino-thalamic, but not trigeminovascular, neurons. This suggests that the action of sTMS is local, at the level of thalamocortical responses, and it does not affect descending pathways that modulate migraine-like trigeminal neurons. The evidence also suggests that sTMS acts on ubiquitous excitatory and inhibitory mechanisms, inhibiting glutamate activity and enhancing GABAergic neurons [46,47,48].
Clinical Evidence
Currently, the device spring (s)TMS by eNeura is cleared by the FDA for both the acute and preventive treatment of migraine since 2019. The efficacy of sTMS as an acute therapy was addressed in three randomized controlled trials [49,50,51]. The largest of these involved 164 migraine with aura patients that treated one migraine attack either with sTMS or sham. The primary endpoint, pain freedom at 2 h, was met by 39% of patients on active and 22% on sham stimulation (P = 0.0179). Sustained response at 24 and 48 h was also statistically significant in the sTMS group compared to sham, with no difference in the rate of adverse events [49]. The efficacy of sTMS as a migraine preventive has not been studied in large randomized controlled trials and these are warranted. The largest open-label study to date, the ESPOUSE study, was a multicenter trial conducted in the USA that included 217 patients with episodic migraine who were followed up for 3 months. The study showed that compared to a baseline of 9 headache days per month, frequency was reduced by 2.75 ± 0.40 headache days. Interestingly, other efficacy variables were positive such as the 50% responder rate which was 46%. There was also a reduction in the scores of the headache impact test (HIT-6) and the use of analgesics [52].
Other Neurostimulation Devices
Other neurostimulation devices based on different mechanisms of action are also available, supported by single, smaller trials. Transcutaneous supraorbital nerve stimulation involves stimulation of branches of the ophthalmic division of the trigeminal nerve, and it is hypothesized that nociceptive firing would be inhibited following the gate control theory [53]. Here, the Cefaly® device was developed as a stimulator of the supraorbital and supratrochlear branches of V1. Some neuromodulation involving important structures of the pain matrix, such as the anterior cingulate cortex has been shown using 18-F-FDG-PET after 3 months of preventive treatment with Cefaly® [54]. Indeed, a small, randomized, sham-controlled, clinical trial was conducted in 67 patients with episodic migraine who were followed up for 3 months. The primary endpoint of change in monthly migraine days was met for active (6.94 to 4.88; P = 0.023) and not for sham (6.54 to 6.22: P = 0.608) [55]. This neuromodulation device has also been studied as a potential migraine acute therapy in a multi-center, randomized, sham-controlled, clinical trial. Fifty-four patients were assigned to verum and 52 to sham and the device was used for 1 h to treat 1 migraine attack. The primary endpoint, reduction in the visual analogue scale compared to baseline was met (−3.46 ± 2.32 vs. −1.78 ± 1.89; P < 0.0001) [56]. A transcutaneous mastoid stimulator showed efficacy in migraine prevention when tested against sham in a randomized clinical trial. Each treatment arm included 40 participants. After a 3-month period, there was a reduction of 1.13 headache days in the sham group against 3.99 in the active. However, it should be noted that the baseline number of headache days was imbalanced, with 5.6 in the active group and 7.85 in the sham [57]. A supraorbital stimulator was tested in a similar study showing analogous results. Both devices have been recently compared against each other with similar values in efficacy measured as reduction in migraine days (60.5% vs. 53.8%, P = 0.88) [55]. Of note, the reduction in the HIT-6 assessment was higher in the supraorbital stimulator group (36.5% vs. 25.6%, P = 0.041) and so was the presence of paresthesia (13.3% vs. 0%, P = 0.026) [58]. Another transcutaneous device is Relivion®. This device has been designed to electrically stimulate up to 6 branches of the trigeminal and occipital nerves and is self-administered. Two studies, one in episodic and one in chronic migraine have shown efficacy when compared to sham [59]. Consistent long-term data is warranted. It was FDA-cleared in 2021 for the acute treatment of migraine.
Lastly, perhaps the most recent approach to be cleared for the treatment of migraine, is remote electrical neuromodulation (REN). This treatment works on the principle of conditioned pain modulation [60]. The upper arm nerves are stimulated to engage endogenous descending analgesic mechanisms using a subthreshold conditioning stimulus, inhibiting pain in remote body regions (such as the head). In a randomized, double-blind, sham-controlled, multi-center study, the Nerivio™ device, cleared by the FDA in 2020, was demonstrated to provide clinically meaningful relief from migraine pain (66.7% vs. 38.8%, p < 0.001) and complete pain freedom (37.4% vs. 18.4%, p < 0.05) at 2 h compared to sham [61, 62]. These outcomes were sustained through 48 h.
Conclusion
Several neuromodulation techniques and devices were discussed in this review. Because of the overall good tolerability of this approach, the lack of interactions or contraindications and some patients’ preference to avoid pharmacological-based therapies for their migraine treatment, more studies on the efficacy and long-term utility of these devices are necessary. Vagus nerve stimulation has proven to be an effective therapy in patients with unilateral migraine that do not respond to standard migraine preventives. Also, its utility in trigeminal autonomic cephalalgias [63, 64] and indomethacin-sensitive headaches [65, 66] make it a useful additional to the headache treatment armory. Nevertheless, additional randomized controlled trials of nVNS for migraine prevention, utilizing a different sham, is essential to clarify its broad utility [34, 35]. Similarly, based on the supporting preclinical and clinical data for sTMS, patients with migraine with aura could be good candidates for this treatment if other treatments specific for migraine aura prevention, such as flunarizine [67], are not tolerated or not efficacious. Therapeutic use of other neuromodulatory devices is also supported by clinical trials, although larger controlled clinical studies would further confirm and validate their efficacy for the treatment of migraine, coupled with preclinical studies to help describe their mechanism of action.
References
Chan C, Goadsby PJ. Recent advances in pharmacotherapy for episodic migraine. CNS Drugs. 2019;33(11):1053–71.
Schiff PL. Ergot and its alkaloids. Am J Pharm Educ. 2006;70(5):98.
Cameron C, Kelly S, Hsieh SC, Murphy M, Chen L, Kotb A, et al. Triptans in the acute treatment of migraine: a systematic review and network meta-analysis. Headache. 2015;55(Suppl 4):221–35.
Lance JW, Curran DA. Treatment of chronic tension headache. Lancet. 1964;1(7345):1236–9.
Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ. Targets for migraine treatment: beyond calcitonin gene-related peptide. Curr Opin Neurol. 2021.
Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61.
Goadsby PJ, Edvinsson L. Joint 1994 Wolff Award Presentation. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache. 1994;34(7):394–9.
Edvinsson L. CGRP Antibodies as Prophylaxis in Migraine. Cell. 2018;175(7):1719.
Moreno-Ajona D, Perez-Rodriguez A, Goadsby PJ. Gepants, calcitonin-gene-related peptide receptor antagonists: what could be their role in migraine treatment? Curr Opin Neurol. 2020;33(3):309–15.
Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81.
Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97(2):553–622.
Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(Suppl 2):S40–3.
Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51(1):48–55.
Ryvlin P, Gilliam FG, Nguyen DK, Colicchio G, Iudice A, Tinuper P, et al. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia. 2014;55(6):893–900.
Aihua L, Lu S, Liping L, Xiuru W, Hua L, Yuping W. A controlled trial of transcutaneous vagus nerve stimulation for the treatment of pharmacoresistant epilepsy. Epilepsy Behav. 2014;39:105–10.
Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol Psychiatry. 2005;58(5):347–54.
Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol Psychiatry. 2005;58(5):355–63.
Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: part I. Headache. 2016;56(1):71–8.
Krahl SE, Senanayake SS, Handforth A. Destruction of peripheral C-fibers does not alter subsequent vagus nerve stimulation-induced seizure suppression in rats. Epilepsia. 2001;42(5):586–9.
Lyubashina OA, Sokolov AY, Panteleev SS. Vagal afferent modulation of spinal trigeminal neuronal responses to dural electrical stimulation in rats. Neuroscience. 2012;222:29–37.
Krahl SE, Clark KB. Vagus nerve stimulation for epilepsy: a review of central mechanisms. Surg Neurol Int. 2012;3(Suppl 4):S255–9.
Akerman S, Simon B, Romero-Reyes M. Vagus nerve stimulation suppresses acute noxious activation of trigeminocervical neurons in animal models of primary headache. Neurobiol Dis. 2017;102:96–104.
Chen SP, Ay I, Lopes de Morais A, Qin T, Zheng Y, Sadeghian H, et al. Vagus nerve stimulation inhibits cortical spreading depression. Pain. 2016;157(4):797–805.
Oshinsky ML, Murphy AL, Hekierski H Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155(5):1037–42.
Morais A, Liu TT, Qin T, Sadhegian H, Ay I, Yagmur D, et al. Vagus nerve stimulation inhibits cortical spreading depression exclusively through central mechanisms. Pain. 2020;161(7):1661–9.
Frangos E, Komisaruk BR. Access to Vagal Projections via Cutaneous Electrical Stimulation of the Neck: fMRI Evidence in Healthy Humans. Brain Stimul. 2017;10(1):19–27.
Moller M, Mehnert J, Schroeder CF, May A. Noninvasive vagus nerve stimulation and the trigeminal autonomic reflex: an fMRI study. Neurology. 2020;94(10):e1085–93.
Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25(2):82–6.
Cecchini AP, Mea E, Tullo V, Curone M, Franzini A, Broggi G, et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci. 2009;30(Suppl 1):S101–4.
Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain. 2003;4(9):530–4.
Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia. 2002;22(6):482–4.
Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22(9):1260–8.
Tassorelli C, Grazzi L, de Tommaso M, Pierangeli G, Martelletti P, Rainero I, et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology. 2018;91(4):e364–73.
Diener HC, Goadsby PJ, Ashina M, Al-Karagholi MA, Sinclair A, Mitsikostas D, et al. Non-invasive vagus nerve stimulation (nVNS) for the preventive treatment of episodic migraine: the multicentre, double-blind, randomised, sham-controlled PREMIUM trial. Cephalalgia. 2019;39(12):1475–87.
Silberstein SD, Mechtler LL, Kudrow DB, Calhoun AH, McClure C, Saper JR, et al. Non-invasive vagus nerve stimulation for the acute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 Study. Headache. 2016;56(8):1317–32.
Schroeder CF, Möller M, May A. nVNS sham significantly affects the trigeminal-autonomic reflex. A randomized controlled study. 2019;93(5):e518–21.
Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain. 2015;16:543.
Dressler D, Schönle P, Conrad B. [Transcranial brain stimulation. Methods and results]. Nervenarzt. 1988;59(9):504–13.
Rossini PM, Caramia MD. Central conduction studies and magnetic stimulation. Curr Opin Neurol Neurosurg. 1992;5(5):697–703.
Lipton RB, Pearlman SH. Transcranial magnetic simulation in the treatment of migraine. Neurotherapeutics. 2010;7(2):204–12.
Lan L, Zhang X, Li X, Rong X, Peng Y. The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails. J Headache Pain. 2017;18(1):86.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211.
Marshall WH. Spreading cortical depression of Leao. Physiol Rev. 1959;39(2):239–79.
Tripathi GM, Kalita J, Misra UK. Role of glutamate and its receptors in migraine with reference to amitriptyline and transcranial magnetic stimulation therapy. Brain Res. 2018;1696:31–7.
Cao Y, Welch KM, Aurora S, Vikingstad EM. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol. 1999;56(5):548–54.
Lloyd JO, Chisholm KI, Oehle B, Jones MG, Okine BN, Al-Kaisy A, et al. Cortical mechanisms of single-pulse transcranial magnetic stimulation in migraine. Neurotherapeutics. 2020;17(4):1973–87.
Barker AT, Shields K. Transcranial magnetic stimulation: basic principles and clinical applications in migraine. Headache. 2017;57(3):517–24.
Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain. 2016;139(Pt 7):2002–14.
Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9(4):373–80.
Clarke BM, Upton AR, Kamath MV, Al-Harbi T, Castellanos CM. Transcranial magnetic stimulation for migraine: clinical effects. J Headache Pain. 2006;7(5):341–6.
Mohammad TM, Hughes G, Nkrumah M et al (2006) Self-administered transcranial magnetic stimulation (TMS) during the aura phase improved and aborts headache. Headache 46:857 (abstract).
Starling AJ, Tepper SJ, Marmura MJ, Shamim EA, Robbins MS, Hindiyeh N, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia. 2018;38(6):1038–48.
Wall PD. The gate control theory of pain mechanisms. A re-examination and re-statement Brain. 1978;101(1):1–18.
Magis D, D’Ostilio K, Thibaut A, De Pasqua V, Gerard P, Hustinx R, et al. Cerebral metabolism before and after external trigeminal nerve stimulation in episodic migraine. Cephalalgia. 2017;37(9):881–91.
Schoenen J, Vandersmissen B, Jeangette S, Herroelen L, Vandenheede M, Gerard P, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80(8):697–704.
Chou DE, Shnayderman Yugrakh M, Winegarner D, Rowe V, Kuruvilla D, Schoenen J. Acute migraine therapy with external trigeminal neurostimulation (ACME): A randomized controlled trial. Cephalalgia. 2019;39(1):3–14.
Juan Y, Shu O, Jinhe L, Na Y, Yushuang D, Weiwei D, et al. Migraine prevention with percutaneous mastoid electrical stimulator: a randomized double-blind controlled trial. Cephalalgia. 2017;37(13):1248–56.
Deng Y, Zheng M, He L, Yang J, Yu G, Wang J. A head-to-head comparison of percutaneous mastoid electrical stimulator and supraorbital transcutaneous stimulator in the prevention of migraine: a prospective, randomized controlled study. Neuromodulation. 2020;23(6):770–7.
Daniel O, Sharon R, Tepper SJ. A device review of Relivion(R): an external combined occipital and trigeminal neurostimulation (eCOT-NS) system for self-administered treatment of migraine and major depressive disorder. Expert Rev Med Devices. 2021;18(4):333–42.
Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9(2):131–7.
Yarnitsky D, Dodick DW, Grosberg BM, Burstein R, Ironi A, Harris D, et al. Remote electrical neuromodulation (REN) relieves acute migraine: a randomized, double-blind, placebo-controlled, multicenter trial. Headache. 2019;59(8):1240–52.
Yarnitsky D, Volokh L, Ironi A, Weller B, Shor M, Shifrin A, et al. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology. 2017;88(13):1250–5.
Nesbitt AD, Marin JC, Tompkins E, Ruttledge MH, Goadsby PJ. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84(12):1249–53.
Gaul C, Magis D, Liebler E, Straube A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post hoc analysis of the randomised, controlled PREVA study. J Headache Pain. 2017;18(1):22.
Tso AR, Marin J, Goadsby PJ. Noninvasive vagus nerve stimulation for treatment of indomethacin-sensitive headaches. JAMA Neurol. 2017;74(10):1266–7.
Moreno-Ajona D, Villar-Martínez MD, Goadsby PJ, Hoffmann J. Primary cough headache treated with non-invasive vagal nerve stimulation. Neurology. 2020;95(13):593–4.
Karsan N, Palethorpe D, Rattanawong W, Marin JC, Bhola R, Goadsby PJ. Flunarizine in migraine-related headache prevention: results from 200 patients treated in the UK. Eur J Neurol. 2018;25(6):811–7.
Funding
Simon Akerman is supported by a National Institute of Neurological Disorders and Stroke (NINDS) grant, NS120930.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David Moreno Ajona reports no conflict of interest. Jan Hoffmann received personal fees for consulting, serving on advisory fees and/or speaking for Allergan, Autonomic Technologies Inc., Cannovex BV, Chordate Medical AB, Eli Lilly, Hormosan Pharma, Lundbeck, Novartis Pharma, Sanofi and Teva. He also received personal fees for Medico-Legal work as well as from Oxford University Press, Quintessence Publishing, Sage Publishing and Springer Healthcare. He receives research support from Bristol Myers Squibb. All these activities are unrelated to the submitted work. Simon Akerman reports personal fees from Amgen, Allergan, and Kallyope, and for Patent/Legal work in headache and orofacial pain, unrelated to this work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Episodic Migraine
Rights and permissions
About this article
Cite this article
Moreno-Ajona, D., Hoffmann, J. & Akerman, S. Devices for Episodic Migraine: Past, Present, and Future. Curr Pain Headache Rep 26, 259–265 (2022). https://doi.org/10.1007/s11916-022-01024-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-022-01024-y