Abstract
Objective
A systematic review and meta-analysis was performed to determine the efficacy of non-invasive neuromodulation modalities for the treatment of acute migraine.
Background
Although pharmacological treatments are the gold standard for the management of acute migraine, some patients may require non-pharmacological treatment options. Non-invasive neuromodulation may provide an alternative, and techniques include transcranial magnetic stimulation (TMS), non-invasive vagal nerve stimulation (nVNS), non-painful remote electrical stimulation (NRES), and external trigeminal nerve stimulation (e-TNS).
Methods
This systematic review and meta-analysis was performed following PRISMA guidelines. We searched PUBMED, EMBASE, ClinicalTrials.gov, Cochrane Center Register of Controlled Trials, and LILACS databases. We included randomized controlled clinical trials studying patients with migraine treated with any form of non-invasive neuromodulation. Primary outcome was pain freedom within 2 h post-treatment. Secondary outcomes were pain relief within 2-h post-treatment and sustained pain freedom and sustained pain relief 48 h post-treatment.
Results
Pooled analysis demonstrated a significant effect of non-invasive neuromodulation on pain-free rates within 2 h (RR, 1.66; 95% CI, 1.35 to 2.05; P < 0.00001) and pain relief rates within 2 h (RR, 1.52; 95% CI, 1.13 to 2.05; P = 0.005) post-treatment. Non-invasive neuromodulation had no significant effect on sustained pain freedom at 48 h (RR, 1.56; 95% CI, 0.68 to 3.59; P = 0.29) or sustained pain relief at 48 h (RR, 1.47; 95% CI, 0.57 to 3.77; P = 0.43) after administration.
Conclusion
Neuromodulation has demonstrated some efficacy in acute migraine management and may be considered in the treatment paradigm of acute migraine in patients with contraindications to pharmacological therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Despite advances in the pharmacological treatment for migraine, there are numerous reasons that may lead patients to consider second- and third-line therapies. Regarding acute pharmacological management (i.e., agents that abort an individual migraine attack), first-line agents include acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), triptans, and ergots [1].
Regarding NSAIDs, the most common adverse effects (AEs) include gastrointestinal effects and risks of bleeding. Besides these, selective COX-2 inhibitors and traditional NSAIDs may increase risk of cardiovascular effects such as heart attack and stroke—AEs that may be reduced, but not entirely avoided by dose reduction [2]. In terms of ergot medications, adverse effects include nausea, vomiting, and paresthesia in > 5% [1], while systematic review of 2 observational studies noted a pooled odds ratio of 2.28 for serious ischemic events [3]. The same paper also evaluated 3 observational studies of triptans and did not demonstrate a negative cardiovascular effect of triptans. Instead, traditional triptans (5-HT1B/1D receptor agonists) have been associated with chest discomfort, throat discomfort, muscle pain, and paresthesia reported in up to 24%. While 5-HT1F receptor agonists such as lasmiditan do not demonstrate the usual adverse effect profile of traditional triptans, common AEs include CNS symptoms such as dizziness, paresthesia, nausea, somnolence, and fatigue [4]. Finally, frequent use of abortive medications may induce medication overuse headache (MOH). MOH is regarded as the most common type of chronic daily headache evolving from an episodic headache. It occurs in 1% of the general population and 11–70% among patients with chronic daily headache [5].
Based on the adverse events noted above and the high cost of novel pharmacological treatments such as small molecule CGRP receptor antagonists, patients may seek out alternatives for the acute management of migraine [6].
Non-invasive neuromodulation is an evolving group of therapies for migraine. These techniques work through non-invasive manipulation of neurotransmitter systems important to the sensation of pain [7]. Non-invasive neuromodulation can be broadly subdivided into modalities that target either the brain or peripheral nerves through transcranial or transcutaneous stimulation, respectively. These therapies are thought to work through the “gate control theory” developed by Melzack and Wall, which proposes that competing nociceptive and non-nociceptive signals regulate the sensation of pain. Stimulation of peripheral nerves in different locations through neuromodulation is thought to modulate afferent input of pain signals travelling to the brainstem and subsequent higher order processing [8] leading to reduction of pain sensation. Each neuromodulation subtype is described below.
Transcranial neuromodulation
Transcranial magnetic stimulation (TMS) involves an electromagnetic coil delivering single pulses (sTMS) or rapid successions (rTMS) of electrical current. This is thought to generate action potentials that inhibit cortical spreading depression and disrupt the cortico-thalamic circuits responsible for migraine [9].
Transcutaneous neuromodulation
Non-invasive vagal nerve stimulation (nVNS) involves a transcutaneous electrical current applied to the neck. In animal studies, this treatment has been shown to reduce extracellular glutamate levels, as well as decrease cortical spreading depression [10, 11]. External trigeminal nerve stimulation (e-TNS) implements an adhesive electrode on the forehead and transmits impulses to supraorbital and supratrochlear nerves. The mechanism of action is thought to act through modulation of pain sensing fibers in the trigeminal ganglion [12]. In non-painful remote electrical stimulation (NRES), an electrode applies an imperceptible current to the skin of the upper arm. This stimulation is thought to activate pain inhibitory centers through the conditioned pain modulation paradigm [13].
Previous literature
There have been several systematic reviews of the safety profile and efficacy of each of the above neuromodulation techniques for acute migraine; however, few of these have included a meta-analysis [14,15,16]. Lan et al. performed a meta-analysis of TMS on the acute and chronic management of migraine. Although analysis included 5 studies, only one of these studies evaluated TMS for acute symptoms. The study was performed by Lipton et al. and demonstrated a significant increase in pain-free rate at 2 h post-treatment [17, 18]. Lai et al. conducted a systematic review and meta-analysis on nVNS for the acute and chronic treatment of patients with migraine and cluster headache. Their analysis included 6 studies, of which 2 provided data for the acute treatment of cluster headache and 1 provided data for the acute treatment of migraine. Their evaluation showed a significant improvement in pain-free rates at 30 min, pain relief rates at 30 min, pain relief rates at 60 min, and abortive medication use [19]. Previous systematic reviews and meta-analyses often include both acute and chronic migraine and multiple types of migraine to increase statistical power. Furthermore, different neuromodulation techniques have not been directly compared.
Therefore, the aim of this systematic review and meta-analysis is to determine whether non-invasive neuromodulation is effective in the acute treatment of migraine. To our knowledge, this is the first meta-analysis to evaluate the effects of acute outcomes across all neuromodulation subtypes.
Methods
We followed the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines in reporting this systematic review and meta-analysis [20]. The study protocol was also registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42018094414. The following databases were searched: PUBMED, EMBASE, ClinicalTrials.gov, Cochrane Center Register of Controlled Trials, and LILACS with last search May 1, 2020.
Eligibility criteria
The research strategy was based on Population, Intervention, Comparator, Outcome, Timing, and Setting (PICOTS) framework. The population (P) in our study included adults with migraine headache. Intervention (I) included any type of non-invasive neuromodulation such as sTMS, rTMS, e-TNS, nVNS, NRES, or transcutaneous electrical nerve stimulation (TENS). Comparators (C) were either a sham group or pharmacological therapy. Outcomes (O) were pain-free rates, pain relief rates, change in pain intensity, and use of analgesia after migraine treatment. Timing (T) was within 2 h of treatment, based on the 2019 International Headache Society (IHS) guidelines for controlled trials of acute treatment of migraine attacks [21]. The timepoint of 48 h after migraine treatment was also used as a measure of relapse. In- or out-patient hospital settings (S) were included in our study.
We included studies that meet the following criteria: (1) randomized controlled clinical trials; (2) patients with episodic migraine without aura, with aura, or chronic migraine (International Classification of Headache Disorders [ICHD] 3rd edition, class 1.1, 1.2, and 1.3, respectively[22]); and (3) studies that used any type of non-invasive neuromodulation in one arm for the management of acute migraine. There was no language restriction applied to our review. We included studies in the gray literature such as conference and poster presentations to avoid publication bias. We excluded all studies with any of the following criteria: (1) non-randomized or observational studies, (2) uncontrolled trials, (3) studies that used invasive neuromodulation, and (4) studies with the aim of prophylactic treatment of migraine.
Outcome measurement
The primary outcome was pain freedom within 2 h of neuromodulation administration. Secondary outcomes were pain relief within 2 h of treatment, sustained pain freedom 48 h after treatment, and sustained pain relief 48 h after treatment. Pain relief was defined according to the IHS guidelines for controlled studies of migraine medications, which defines pain relief as a decrease in pain intensity from moderate (2) or severe (3) to mild (1) or no (0) pain on a 4-point scale [23]. For studies where this was not reported, pain relief was reported as a > 50% reduction in pain according to the visual analog scale (VAS) [24]. Change in pain intensity was measured according to the VAS or the numeric pain scale (NPS).
Search strategy
Our search process involved key terms and search modifiers specific to each database. The search plan involved a large list of neuromodulation subtypes. These key words were coupled with the Boolean OR operator, ending with the Boolean and operator and “headache”. A list of search terms used for each database is included in supplemental materials.
Study selection, data collection process, and data items
Screening, eligibility, and data extraction were completed by two independent reviewers using DistillerSR (Evidence Partners, Ottawa, Canada). Conflicts in screening and eligibility were flagged by the software and resolved through a discussion between researchers. None of the researchers were blinded during the study selection process.
We extracted data items from each study and where data was not measured, the data item was left blank. Data items were taken from ClinicalTrials.gov website in addition to the original manuscript. If the study provided evidence that data was measured, but did not provide the results, study investigators were contacted to retrieve these data items. To assess patient population, the country, migraine type, gender demographics, mean age, and age range of the patients were extracted. We evaluated the randomization process by noting treatment and control group ages, gender demographics, duration of migraine history, monthly migraines, duration of migraines, and monthly analgesic intake. Because our analysis included multiple types of neuromodulation, we summarized the treatment type, the type of sham stimulation, the device model, stimulation location, dose, frequency of use, and total number of treatments. In performing our meta-analysis, we extracted all primary and secondary outcomes. Intent-to-treat analysis (ITT) was preferred as the analysis cohort, and if this was unavailable, modified intent-to-treat analysis (mITT) or per-protocol (PP) analysis was selected. In the case that outcome was not measured at 2 h post-treatment, we used the same outcome if measured at < 2 h (e.g., 1 h post-treatment). This is in line with the 2019 IHS guidelines for controlled trials of acute treatment of migraine attacks, which allow consideration of pain outcomes before 2 h in non-oral treatments [21]. In the case of multiple treatment groups, we selected only the treatment group with the strongest form of stimulation. For studies in which patients were treated multiple times, and first-attack outcomes were available, first-attack data was preferred [25]. We evaluated safety of each neuromodulation by extracting the number of dropouts per treatment group, the number of patients experiencing ≥ 1 minor adverse event (AE) in each group, and specific symptoms that patients experienced. For one study [26] that did not provide the number of patients experiencing ≥ 1 minor AEs, we instead used the overall number of minor AEs.
Risk of bias
We evaluated the risk of bias in individual studies using the revised Cochrane Risk of Bias Assessment Tool [27]. The risk of bias was assessed by two independent reviewers and controversies were discussed and resolved with a third reviewer. The revised tool measures bias in five domains and provides an overall score classified as “low,” “some concern,” and “high.” The five domains are bias in the randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. In each of these domains, multiple questions are asked, with the option to answer “yes,” “probably yes,” “probably no,” “no,” or “no information”. To correctly follow the suggested judgment of bias according to the answers within each domain, we used the Excel tool available on the Cochrane Risk of Bias website. We evaluated the risk of bias across studies using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessment tool [28].
Summary measures and synthesis of results
In summarizing dichotomous and continuous data, we evaluated risk ratio (RR) and mean difference, respectively. In calculating RR, we used the Mantel–Haenszel statistical method with random effects and a 95% confidence interval. For mean difference in pain scores, we used the inverse variance method with random effects and a 95% confidence interval. Data was synthesized and plotted using Review Manager (RevMan) version 5.4 [29]. In creating forest plots, we conducted a pooled analysis, considering that each treatment subtype relies on the “gate control theory” of pain and involves a similar mechanism of action [8]. We also created subgroups based on neuromodulation type and calculated mean outcomes by subgroup. Study heterogeneity was calculated using the I2 statistic, where a value > 50% was considered to demonstrate a high degree of heterogeneity. We did not perform sensitivity analyses due to small sample size.
Results
Study selection
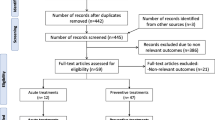
Figure 1 illustrates the flow diagram of the study selection process and the reasons for excluded studies. Following database research, a total of 2823 articles were retrieved. Removing duplicates, screening for title and abstract resulted in exclusion of 2077 unrelated articles. Full-text article assessment further excluded 202 studies for not meeting inclusion criteria, study completion, termination with no results, or for duplicate papers. One abstract met inclusion criteria, but was later excluded due to insufficient information for bias assessment or data analysis [30].
Study characteristics
Table 1 summarizes the date, location, and patient characteristics of the 6 included studies. The studies were conducted in the USA, Italy, and Israel between 2010 and 2019, and 5 studies were published in English language [18, 24,25,26, 31,32,33]. One of these studies was not published and only has results on the ClinicalTrials.gov website [26]. One study [31] was single centered and five [18, 24,25,26, 32] were multi-centered. The sample size of each study ranged from 86 to 446 and the mean age ranged from 39 to 43. Total patient population was 1297 with 1050 (81%) female patients. The most common ethnic background was Caucasian. Other ethnicities included Asian, Black, and Egyptian.
Table 2 summarizes patient characteristics after randomization. Five hundred eighty-nine (49%) patients were randomized to active therapy, and 622 (51%) were randomized to the control group. Four hundred eighty-one (82%) of patients receiving active therapy were female, whereas 500 (80%) were female in the control group. At baseline, the mean number of monthly migraine attacks were reported in 3 studies [18, 24, 25] and ranged from 4.2 to 5.4 in active group and 4.6 to 5.3 in control group. Two studies [25, 32] reported baseline monthly migraine days, which ranged 6.3–6.6 in the active group and 6.2–6.6 in the control group. Regarding patient demographics, one study noted significant baseline differences in migraine type, duration, and acute medication use [24].
There were 4 neuromodulation devices used including sTMS, nVNS, NRES, and e-TNS. The treatment used in each study is summarized in Supplemental Table 1. Duration of treatment ranged from 1 to 60 min per migraine episode. All treatments were used once per attack. Stimulation location was forehead, occiput, neck, or arm. Comparison was with sham devices.
Risk of bias in individual studies
Cochrane Bias Assessment was performed in 5 domains on 5 studies and revealed low bias in 2 studies [18, 25]. Assessment of bias could not be performed on one study that was unpublished and only had data on ClinicalTrials.gov [26]. Three studies demonstrated a degree of bias in at least 1 domain [24, 31, 32]. Yarnitsky et al. (2017) provided an unclear concealment process, which was assigned some concerns of bias in the “Randomization process” and “Overall bias” domain. In the study by Chou et al., there were baseline differences between treatment and control groups with respect to migraine type, duration, and medication use. This warranted some concerns of bias in the “Randomization process” and the “Overall bias” domain. Yarnitsky et al. (2019) was assigned some concerns of bias due to “Missing outcome data.” Of 252 randomized patients, 45 were not included in analysis due to insufficient migraine attacks, withdrawal of consent, or unknown reasons. All studies demonstrated low bias in the “Deviations from intended interventions,” “Measurement of the outcome,” and “Selection of the reported result” categories (Fig. 2).
Primary outcome—pain freedom within 2 h
Primary and secondary outcomes are presented in Supplemental Table 2. Figure 3 shows pooled analysis of the primary outcome. Neuromodulation demonstrated a significant increase in pain-free rates at ≤ 2 h (RR, 1.66; 95% confidence interval [CI], 1.35 to 2.05; P < 0.00001) and an I2 value of 0%, suggesting low heterogeneity.
Effect of neuromodulation on secondary outcomes
Regarding secondary outcomes, pain relief rates at ≤ 2 h post-treatment (Fig. 4) were significantly increased (RR, 1.52; 95% CI, 1.13 to 2.05; P = 0.005) with a high degree of heterogeneity (I2 = 86%). For outcomes at 48 h, neuromodulation had no significant effect on sustained pain freedom (RR, 1.56; 95% CI, 0.68 to 3.59; P = 0.29; Supplemental Fig. 1) or sustained pain relief (RR, 1.47; 95% CI, 0.57 to 3.77; P = 0.43; Supplemental Fig. 2). Outcomes of pain-free rates at 48 h were markedly inconsistent (I2 = 82%), as well as pain relief rates at 48 h (I2 = 91%).
Safety and adverse events (AEs)
All devices used were generally safe and resulted in no death or severe harm to patients. Supplemental Table 3 summarizes the number of patients with > 1 minor AE, number of dropouts, and notable symptoms. Studies reported common device-related AEs such as application site pain, discomfort, erythema, paresthesia, numbness, or burning sensation. Other AEs included headache, sinusitis, nasopharyngitis, and influenza. Supplemental Fig. 3 shows the forest plot of number of patients experiencing at least 1 minor AE during experimental or sham therapy. Pooled analysis demonstrated a non-significant effect of neuromodulation treatment on minor AEs (RR, 1.43; 95% CI, 0.99 to 2.08; P = 0.06) and a low degree of heterogeneity (I2 = 20%).
Quality assessment
Assessment of quality of evidence was performed by using the GRADE assessment tool. Results are shown in Fig. 5. The highest rating of quality of evidence was measurement of adverse outcomes, which was graded as “high.”
Discussion
To our knowledge, this is the first systematic review and meta-analysis to evaluate the efficacy and safety across all non-invasive neuromodulation treatment modalities for the management of acute migraine. Our analysis shows that these treatments provide significant benefit to patients through cessation and relief of pain within 2 h of administration, as illustrated in Fig. 3 and Fig. 4, respectively. The anticipated absolute effect of non-invasive neuromodulation on pain freedom within 2 h of treatment is 302 per 1000 patients, or 30.2%, as shown in Fig. 5. Similarly, the absolute effect of treatment on pain relief within 2 h is 700 per 1000 patients, or 70%. Notably, this effect of treatment on pain freedom and pain relief is not sustained at 24 or 48 h. Lastly, as illustrated in Supplemental Fig. 3, non-invasive neuromodulation treatments are not significantly associated with minor adverse events.
Effective management strategies for migraine include education, lifestyle modification strategies, acute treatment, and preventive approaches. For acute migraine treatment, attacks should be managed rapidly and consistently to restore patient function and minimize the use of abortive therapy [34]. Treatment includes the use of drug therapy, nerve blocks, and other behavioral strategies, and choice of intervention depends on patient medical history, severity and frequency of attacks, and associated symptoms [35]. In 2013, the Canadian Headache Society published guidelines on acute drug therapy for migraine headache [36]. Acetaminophen was recommended for mild-moderate attacks; ibuprofen, naproxen, and diclofenac for all attack severity; triptans and dihydroergotamine for moderate or severe attacks. In addition, antiemetics were recommended to reduce or resolve nausea. Considering non-pharmacological approaches, a large retrospective study concluded effectiveness of occipital nerve block in reducing pain scores for acute migraine [37].
Our results provide an alternative analysis, consistent with previous research, which suggests non-invasive neuromodulation for adjunctive or second-line therapy for acute migraine. Triptans, which represent first-line management for the abortion of acute migraine, have thus far demonstrated greater efficacy. A study conducted by Ng-Mak et al. in 2007 evaluated the efficacy of rizatriptan, sumatriptan, almotriptan, eletriptan, zolmitriptan, frovatriptan, and naratriptan in 673 patients with a total of 1346 migraine attacks. Of patients taking rizatriptan, 60.9% achieved pain freedom within 2 h of administration, while patients treated with other triptans achieved pain freedom in 49.9% of cases. Pain relief within 2 h was achieved in 88.1% of patients taking rizatriptan and 81.% of patients taking other triptans [38]. While our analysis shows statistically significant outcomes for pain freedom and pain relief at the same time points, the results are less impressive than the clinical efficacy demonstrated by the majority of published triptan studies.
Another clinical marker of efficacy in treating acute migraine is pain freedom at 2 h with no recurrence of headache at 24 h or 48 h. Headache recurrence can occur in 15–40% of patients taking a triptan, and is much higher in those taking simple, combined analgesics and opioids [39]. The neuromodulation studies analyzed in this paper did not demonstrate sustained efficacy when used as monotherapy. These findings in a clinical trial setting of initial efficacy but lack of sustained efficacy most likely correlate closely to what is seen in clinical practice by many physicians, and thus may contribute to the lower adoption of neuromodulation as an acute treatment for migraine headache.
Despite the efficacy of triptans, these groups of medications can carry intolerable side effects and limited use in certain patients due to contraindications. In these circumstances, less invasive, low-risk treatment modalities should be considered.
One population that may benefit from alternative migraine treatments is women in pregnancy, although evidence for treatment in this population is currently lacking. The hormonal changes of pregnancy can drastically worsen migraines, particularly in the first trimester [40]. Many women prior to conception may wish to discontinue migraine prophylaxis and abortive agents such as triptans, despite recent studies supporting the safety of Sumatriptan during pregnancy [41]. Neuromodulation may be an option for these patients; however, there are currently no randomized controlled trials that evaluate the safety and efficacy of neuromodulation within a pregnant patient population. Additionally, neuromodulation may be an option for migraine headache patients with poor renal function, gastrointestinal disease, hepatic dysfunction, or cardiovascular disease, who cannot tolerate therapies such as triptans or NSAIDs. Finally, patients with chronic migraine complicated by medication overuse headache (MOH) may benefit from non-invasive neuromodulation. These patients are difficult to treat due to limitations on the type and quantity of medication that can be prescribed. Neuromodulation may be an option to help alleviate moderate migraine attacks that the patient would otherwise treat with over the counter analgesics, triptans, or opioids.
The socioeconomic burden of treatment for chronic diseases such as migraine may affect access to treatment options. Symptomatic management may require lifelong commitment of financial resources. The devices may be cost prohibitive for patients and many private insurance companies may not offer the same degree of coverage as offered for more conventional pharmacological interventions such as triptans. These factors may play a role in the limited adoption of neuromodulation for acute migraine attacks, despite the efficacy and safety that is shown in the limited number of randomized controlled trials included in this systematic review.
Given the specific situations in which non-invasive neuromodulation may prove clinically relevant, there have been numerous brands developed and approved for use in patients with migraine. Here, we review most common brands approved by the FDA including Cerena TMS in 2013, Cefaly e-TNS in 2014 for chronic migraine and in 2017 for acute migraine, and gammaCore nVNS in 2018 for acute migraine. In 2019, eNeura sTMS mini received FDA clearance for acute and preventive treatment of migraine in children 12 years of age and older. They received FDA clearance for adults in 2017. Nerivio Migra NRES was approved for treatment of acute treatment of migraine headache in 2018[42].
Limitations
By including all types of non-invasive neuromodulation, our analysis has greater statistical power compared to studies that pinpoint a single neuromodulation technique. However, the sample size presented here is very limited. Only 6 studies met inclusion criteria and only 5 provided the primary outcome for meta-analysis. In addition, heterogeneity of outcomes across studies resulted in small sample sizes for secondary outcomes, such as sustained pain freedom at 24 or 48 h, in which only 3 studies could be included. Lastly, we combine different neuromodulation techniques into a single pooled analysis, which provides an alternative perspective in comparison to other studies, which often include multiple types of headache or merge acute and chronic migraine outcomes [17, 19]. However, the combination of these different techniques can give rise to heterogeneous results, which may be interpreted as a product of the different treatment modalities or other experimental factors. This challenge in interpretation is an inherent limitation in our study.
It is therefore important to have additional, well-designed clinical trials that include patient-centered outcomes as recommended by the IHS [21], which can contribute to future meta-analysis and increase sample size. Furthermore, these studies should include pregnant patients, those with contraindication to other acute therapies and also patients with MOH. The data derived from these studies may be helpful in optimizing patient selection for neuromodulation in a clinical setting, determining the safety and efficacy of the devices in these unique populations, and perhaps even improving patient access to these treatment options.
Conclusion
This systematic review and meta-analysis offers evidence that non-invasive neuromodulation may provide significant benefit to patients through relief of pain within 2 h of administration. Although triptans remain the gold standard for acute migraine management, these neuromodulation techniques may be considered in some patients requiring low-risk non-invasive treatment modalities for acute migraine.
Abbreviations
- TMS:
-
Transcranial magnetic stimulation
- STMS:
-
Single-pulse transcranial magnetic stimulation
- rTMS:
-
Rapid succession transcranial magnetic stimulation
- nVNS:
-
Non-invasive vagal nerve stimulation
- e-TNS:
-
External trigeminal nerve stimulation
- NRES:
-
Non-painful remote electrical stimulation
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
Prospective Register of Systematic Reviews
- PICOTS:
-
Population, Intervention, Comparator, Outcome, Timing, Setting
- TENS:
-
Transcutaneous electrical nerve stimulation
- IHS:
-
International Headache Society
- ICHD:
-
International Classification of Headache Disorders
- VAS:
-
Visual analog scale
- NPS:
-
Numeric pain scale
- ITT:
-
Intent-to-treat analysis
- mITT:
-
Modified intent-to-treat analysis
- PP:
-
Per protocol
- AE:
-
Adverse event
- RR:
-
Risk ratio
- RCT:
-
Randomized controlled trial
- EG:
-
Experimental group
- CG:
-
Control group
- CI:
-
Confidence interval
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- MOH:
-
Medication overuse headache
References
González-Hernández A, Marichal-Cancino BA, MaassenVanDenBrink A, Villalón CM (2018) Side effects associated with current and prospective antimigraine pharmacotherapies, (in eng). Expert Opin Drug Metab Toxicol 14(1):25–41. https://doi.org/10.1080/17425255.2018.1416097
Tacconelli S, Bruno A, Grande R, Ballerini P, Patrignani P (2017) Nonsteroidal anti-inflammatory drugs and cardiovascular safety - translating pharmacological data into clinical readouts, (in eng). Expert Opin Drug Saf 16(7):791–807. https://doi.org/10.1080/14740338.2017.1338272
Roberto G et al (2015) Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies, (in eng). Cephalalgia 35(2):118–131. https://doi.org/10.1177/0333102414550416
Negro A, Koverech A, Martelletti P (2018) Serotonin receptor agonists in the acute treatment of migraine: a review on their therapeutic potential, (in eng). J Pain Res 11:515–526. https://doi.org/10.2147/jpr.s132833
Diener HC, Holle D, Solbach K, Gaul C (2016) Medication-overuse headache: risk factors, pathophysiology and management, (in eng). Nat Rev Neurol 12(10):575–583. https://doi.org/10.1038/nrneurol.2016.124
Burch R, Rayhill M (2018) New preventive treatments for migraine," (in eng), Bmj 361 k2507. https://doi.org/10.1136/bmj.k2507
Miller S, Matharu M (2017) Non-invasive neuromodulation in primary headaches, (in eng). Curr Pain Headache Rep 21(3):14. https://doi.org/10.1007/s11916-017-0608-x
Jenkins B, Tepper SJ (2011) Neurostimulation for primary headache disorders, part 1: pathophysiology and anatomy, history of neuromodulation in headache treatment, and review of peripheral neuromodulation in primary headaches, (in eng). Headache 51(8):1254–1266. https://doi.org/10.1111/j.1526-4610.2011.01966.x
Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ (2016) Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine, (in eng). Brain 139(Pt 7):2002–2014. https://doi.org/10.1093/brain/aww118
Oshinsky ML, Murphy AL, Hekierski H Jr, Cooper M, Simon BJ (2014) Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia, (in eng). Pain 155(5):1037–1042. https://doi.org/10.1016/j.pain.2014.02.009
Chen SP et al (2016) Vagus nerve stimulation inhibits cortical spreading depression, (in eng). Pain 157(4):797–805. https://doi.org/10.1097/j.pain.0000000000000437
Schwedt TJ, Vargas B (2015) Neurostimulation for treatment of migraine and cluster headache, (in eng). Pain Med 16(9):1827–1834. https://doi.org/10.1111/pme.12792
Grimsrud KW, Halker Singh RB (2018) Emerging treatments in episodic migraine, (in eng). Curr Pain Headache Rep 22(9):61. https://doi.org/10.1007/s11916-018-0716-2
Redgrave J et al (2018) Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review, (in eng). Brain Stimul 11(6):1225–1238. https://doi.org/10.1016/j.brs.2018.08.010
Reuter U, McClure C, Liebler E, Pozo-Rosich P (2019) Non-invasive neuromodulation for migraine and cluster headache: a systematic review of clinical trials, (in eng). J Neurol Neurosurg Psychiatry 90(7):796–804. https://doi.org/10.1136/jnnp-2018-320113
Stilling JM, Monchi O, Amoozegar F, Debert CT (2019) Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review, (in eng). Headache 59(3):339–357. https://doi.org/10.1111/head.13479
Lan L, Zhang X, Li X, Rong X, Peng Y (2017) The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails, (in eng). J Headache Pain 18(1):86. https://doi.org/10.1186/s10194-017-0792-4
Lipton RB et al (2010) Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial, (in eng). Lancet Neurol 9(4):373–380. https://doi.org/10.1016/s1474-4422(10)70054-5
Lai YH, Huang YC, Huang LT, Chen RM, Chen C (2020) Cervical noninvasive vagus nerve stimulation for migraine and cluster headache: a systematic review and Meta-Analysis, (in eng). Neuromodulation. https://doi.org/10.1111/ner.13122
Liberati A et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration, (in eng). Bmj 339 b2700. https://doi.org/10.1136/bmj.b2700
Diener HC et al (2019) Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: Fourth edition, (in eng). Cephalalgia 39(6) 687–710. https://doi.org/10.1177/0333102419828967
Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition, (in eng). Cephalalgia 38(1) 1–211. https://doi.org/10.1177/0333102417738202
Tfelt-Hansen P et al (2012) Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators, (in eng). Cephalalgia 32(1) 6–38. https://doi.org/10.1177/0333102411417901
Chou DE, ShnaydermanYugrakh M, Winegarner D, Rowe V, Kuruvilla D, Schoenen J (2019) Acute migraine therapy with external trigeminal neurostimulation (ACME): a randomized controlled trial, (in eng). Cephalalgia 39(1):3–14. https://doi.org/10.1177/0333102418811573
Martelletti P et al (2018) Consistent effects of non-invasive vagus nerve stimulation (nVNS) for the acute treatment of migraine: additional findings from the randomized, sham-controlled, double-blind PRESTO trial, (in eng), J Headache Pain 19(1) 101. https://doi.org/10.1186/s10194-018-0929-0
Kuruvilla D. A Phase III Trial of e-TNS for the Acute Treatment of Migraine (TEAM) [Online] Available: https://clinicaltrials.gov/ct2/show/NCT03465904
Sterne JAC et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials, (in eng). Bmj 366 l4898. https://doi.org/10.1136/bmj.l4898
GradePro. GDT: GRADEpro Guideline Development Tool. Evidence Prime, Inc. gradepro.org (accessed)
Review Manager (RevMan) (2014) The Cochrane Collaboration, Copenhagen: the Nordic Cochrane Centre
Hering-Hanit R (2017) A prospective, randomized, single blind, parallel-group, placebo controlled clinical study to evaluate the short-term effectiveness of combined occipital and supraorbital transcutaneous nerve stimulation (OS-TNS) in treating migraine, in IHC Posters - Thursday and Friday. vol. 37, ed: Cephalalgia, p. 73
Yarnitsky D et al (2017) Nonpainful remote electrical stimulation alleviates episodic migraine pain, (in eng). Neurology 88(13):1250–1255. https://doi.org/10.1212/wnl.0000000000003760
Yarnitsky D et al (2019) Remote Electrical Neuromodulation (REN) Relieves acute migraine: a randomized, double-blind, placebo-controlled, multicenter trial, (in eng). Headache 59(8):1240–1252. https://doi.org/10.1111/head.13551
Tassorelli C et al (2018) Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study, (in eng). Neurology 91(4):e364–e373. https://doi.org/10.1212/wnl.0000000000005857
Silberstein SD (2000) Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology, (in eng). Neurology 55(6):754–762. https://doi.org/10.1212/wnl.55.6.754
Ong JJY, De Felice M (2018) Migraine treatment: current acute medications and their potential mechanisms of action, (in eng). Neurotherapeutics 15(2):274–290. https://doi.org/10.1007/s13311-017-0592-1
Worthington I et al (2013) Canadian Headache Society Guideline: acute drug therapy for migraine headache, (in eng). Can J Neurol Sci 40(5 Suppl 3):S1-s80
Allen SM, Mookadam F, Cha SS, Freeman JA, Starling AJ, Mookadam M (2018) Greater occipital nerve block for acute treatment of migraine headache: a large retrospective cohort study, (in eng). J Am Board Fam Med 31(2):211–218. https://doi.org/10.3122/jabfm.2018.02.170188
Ng-Mak DS, Hu XH, Chen Y, Ma L, Solomon G (2007) Times to pain relief and pain freedom with rizatriptan 10 mg and other oral triptans, (in eng). Int J Clin Pract 61(7):1091–1111. https://doi.org/10.1111/j.1742-1241.2007.01400.x
Antonaci F, Ghiotto N, Wu S, Pucci E, Costa A (2016) Recent advances in migraine therapy, (in eng). Springerplus 5:637. https://doi.org/10.1186/s40064-016-2211-8
Goadsby PJ, Goldberg J, Silberstein SD (2008) Migraine in pregnancy, (in eng). BMJ 336(7659):1502–1504. https://doi.org/10.1136/bmj.39559.675891.AD
Duong S, Bozzo P, Nordeng H, Einarson A (2010) Safety of triptans for migraine headaches during pregnancy and breastfeeding, (in eng). Can Fam Physician 56(6):537–539
FDA. 510(k) Clearances. US Food & Drug Administration. https://www.fda.gov/medical-devices/device-approvals-denials-and-clearances/510k-clearances (accessed October 24th, 2020)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Ana Marissa Lagman-Bartolome reports advisory board honorarium fees from Allergan, Novartis, TEVA, Eli Lilly, Lundbeck, and Aralez, outside the submitted work.
All the other authors declare no competing interests.
Ethical approval
None.
Registration
PROSPERO registration number CRD42018094414.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clark, O., Mahjoub, A., Osman, N. et al. Non-invasive neuromodulation in the acute treatment of migraine: a systematic review and meta-analysis of randomized controlled trials. Neurol Sci 43, 153–165 (2022). https://doi.org/10.1007/s10072-021-05664-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05664-7