Abstract
Purpose of Review
Neurogenic heterotopic ossification (NHO) is the abnormal formation of extra-skeletal bones in periarticular muscles after damage to the central nervous system (CNS) such as spinal cord injury (SCI), traumatic brain injury (TBI), stroke, or cerebral anoxia. The purpose of this review is to summarize recent developments in the understanding of NHO pathophysiology and pathogenesis. Recent animal models of NHO and recent findings investigating the communication between CNS injury, tissue inflammation, and upcoming NHO therapeutics are discussed.
Recent Findings
Animal models of NHO following TBI or SCI have shown that NHO requires the combined effects of a severe CNS injury and soft tissue damage, in particular muscular inflammation and the infiltration of macrophages into damaged muscles plays a key role. In the context of a CNS injury, the inflammatory response to soft tissue damage is exaggerated and persistent with excessive signaling via substance P-, oncostatin M-, and TGF-β1-mediated pathways.
Summary
This review provides an overview of the known animal models and mechanisms of NHO and current therapeutic interventions for NHO patients. While some of the inflammatory mechanisms leading to NHO are common with other forms of traumatic and genetic heterotopic ossifications (HO), NHOs uniquely involve systemic changes in response to CNS injury. Future research into these CNS-mediated mechanisms is likely to reveal new targetable pathways to prevent NHO development in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
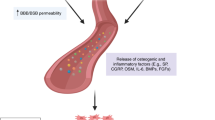
Neurogenic heterotopic ossifications (NHOs) are abnormal extra-skeletal bone formations mostly in periarticular muscles [1] after severe damage to the central nervous system (CNS) such as spinal cord injury (SCI), traumatic brain injury (TBI), stroke, or cerebral anoxia [2] and hence their name “neurogenic.” NHOs were first identified in spine-injured soldiers during World War I with the first use of radiography in battlefield injuries [3,4,5] and are still very prevalent in defense personnel with battlefield injuries, with up to 60% of blast and gunshot victims developing NHO when there is concomitant spinal damage [6,7,8]. NHO also occurs in up to 25% of civilians with severe SCI and 5–20% with TBI [9,10,11,12]. NHOs develop within a few months after CNS injury in periarticular muscles, with decreasing frequencies in the hip, elbow, knee, and shoulder (Fig. 1). NHO can be very incapacitating, mainly due to their large size (up to 2 kg), often causing significant pain and gradual reduction in the range of motion of affected limbs which often progresses to complete joint ankylosis. This exacerbates functional disabilities by increasing difficulty in sitting, eating, and dressing [13]. NHO growth can also cause nerve and blood vessel compression, further increasing patient morbidity [14, 15].
Treatment is still currently limited to surgical resection. Once NHOs have become troublesome, the orthopedic surgeon identifies a cleavage plan on the computed tomography (CT) scan, and patient’s comorbidities are under control [16]. Surgical resection remains challenging, particularly when ossifications entrap the whole joint and adjacent large blood vessels and nerves. Following surgical resection, NHO can re-occur in around 6% of patients [2]. Additional treatment modalities have been investigated, but most provided limited efficacy. Radiation therapy (RT) has been explored as a treatment for multiple forms of heterotopic ossification (HO) [17, 18] and shown to be beneficial in patients following total hip arthroplasty [19]. However, similar treatment regimens did not effectively prevent the recurrence of NHO [20], and there are concerns that RT could delay repair of skeletal fracture associated with the initial CNS injury. While some studies suggested RT limited the progression of NHO around the hip after SCI [21] and reduced pain and increased mobility in patients with established NHO [22], other studies have shown less favorable outcomes [23]. A recent retrospective study suggests that RT was associated with an increased risk of postoperative sepsis [24]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are also used to treat NHO [25, 26], and a recent retrospective study on 108 SCI patients has shown significant reduction of NHO diagnosis following prophylactic indomethacin or celecoxib for 2 weeks after CNS injury [27•]. The mechanism is believed to be through the systemic inhibition of prostaglandins, with subsequent changes in mesenchymal cell differentiation into osteoblasts [25, 26]. While studies have shown that NSAIDs can be beneficial for NHO after SCI [25], with a reduction in NHO diagnosis and volume as well as reduced inflammatory symptoms, there has been concerns of long-term NSAID use on gastrointestinal complications [28], and delayed fracture healing was also reported in other studies [29, 30]. Bisphosphonates have been investigated as preventative treatment of NHO in SCI and TBI patients [31,32,33,34] with varying results, and some studies suggested bisphosphonates potentially increase the risk of developing heterotopic ossification (HO). However, an additional risk of delayed fracture healing comes with the additional musculoskeletal injuries that often accompany SCI and TBI patients [35,36,37]. Overall the development of improved treatments for NHO has been slow, and trials of pharmacological interventions have continued to show limited effectiveness [10] reflecting the current limited knowledge on the etiology and pathophysiology of NHO. This is in part due to previous studies in SCI/TBI patients being retrospective with little insight into early initiating cellular and molecular events which drive NHO pathogenesis. Therefore, it is essential to establish animal models of NHO to delineate the mechanisms of NHO pathogenesis in order to identify new biomarkers to predict NHO development after SCI/TBI, and new therapeutic treatments that would prevent or reduce NHO development.
Animal Models of Heterotopic Ossification
Genetic Models
Beside NHO in patients with severe CNS injuries, rare genetically driven HOs are also well known in humans and have been at the forefront of animal model development in the last decade. This is particularly the case of fibrodysplasia ossificans progressiva (FOP, Stoneman syndrome). FOP is a very rare genetic disease (1 case in 2,000,000 humans) caused by dominant activating missense point mutations in the coding sequence of the ACVR1 gene. ACVR1 encodes the activin A receptor type I (ACVR1), also called activin receptor-like kinase-2 (ALK2), a bone morphogenetic protein (BMP) type I receptor [38,39,40,41]. Children with FOP develop a progressive ossification of an extensive portion of their body which is ultimately fatal. FOP animal models include the introduction (by means of mutant allele knock-in the ACVR1 gene or expression of a mutant transgene) of causal missense point mutations in ACVR1 such as ACVR1R206H which changes an arginine residue to a histidine residue [40, 42, 43]. In mice carrying the ACVR1R206H mutation, HOs develop locally in tissues following an injury or inflammation in a similar presentation to FOP patients with HO “flare-ups.” Additional mouse models were developed based on alternative ACVR1 mutations such as the constitutively active ALK2 model (caALK2) or ACVR1Q207D [44]. ACVR1R206H, which is the most frequent causal ACVR1 mutation in FOP patients [45], alters the signaling pathways that the ACVR1 receptor elicits upon binding of its physiological ligands. Specifically while wild-type ACVR1 inhibits bone morphogenetic protein (BMP) signaling in response to BMP-2 and BMP-4 binding to the BMP receptor 1A and BMP receptor 1B, the AVCR1R206H mutant loses this inhibitory function and gains BMP signaling function following direct binding of activin A [42, 46]. Mouse models in which recombinant BMPs are locally injected or surgically implanted or an inducible BMP4 transgene is expressed have also been used to induce HO formation [38, 47,48,49,50]. Together, these models have been invaluable to demonstrate the role of inflammation, mast cells and macrophages in triggering FOP “flare-ups” [51•], and the over-activation of SMAD1/5/8-mediated signaling downstream of causal ACVR1 mutations [52]. These models have been utilized for the development and pre-clinical testing of treatments for FOP and to identify cell types responsible for HO in FOP [43, 44, 53,54,55]. However, the relevance of these genetic models of FOP to NHO is questionable as pathological NHOs occur in genetically normal patients of a broad range of ethnicities, hence the necessity to develop animal models specific to NHO.
SCI-Induced NHO Models
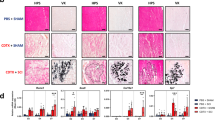
To fill this knowledge gap, we have developed the first clinically relevant animal model of NHO in genetically non-modified mice, without artificial implantation of osteogenic proteins such as BMPs [56•]. In our model, we perform a transection of the spinal cord between T11 and T13 rendering mice paraplegic. Without any additional intervention, NHO never develops in mice with SCI. The prevalence of NHO is significantly associated with (1) the severity of the CNS injury [57], (2) the presence of additional injuries or inflammation [12], and (3) is particularly high in combat-related casualties which are mostly multi-traumatic [6, 7]. For this reason, we hypothesized that an associated muscle injury was required for NHO to develop. To test this, we modeled muscle damage via an intramuscular injection of cardiotoxin (CDTX) purified from Naja snake venom, a well-accepted model of muscle damage and repair [58]. Likewise, CDTX-mediated muscle injury alone never caused HO with CDTX-injured muscles repairing within 1–3 weeks [56, 58]. However when combined with a SCI, NHOs develop within the CDTX-injured muscle over 1–3 weeks and recapitulate most clinical features of NHO in SCI patients [56•]. We also established that SCI with an intramuscular CDTX injection lead to NHO development in multiple mouse strains such as C57BL/6, C3H/He, and BALB/c (Fig. 2), confirming that this phenomenon is not restricted to the genetic make-up of any particular inbred strain. Therefore the development of NHO requires a dual insult of both the CNS and soft tissue [56•] (Fig. 3). Unlike genetically altered or BMP-implant models of FOP, our new model provides a powerful tool for determining the pathogenesis of NHO and assessing preventative therapies which is discussed further in sections below.
NHOs develop in multiple inbred mouse strains after combined SCI and muscle injury. C57BL/6, C3H/He, and BALB/c mice (n = 5/group) all underwent SCI between T12 and T13 together with an intramuscular injection of CDTX. NHO volume was quantified at 14 days post-surgery by micro-CT. Results show that NHOs develop in all mouse strains confirming that NHO development is not restricted to the genetic make-up of mouse strains. Data are presented as mean ± SD
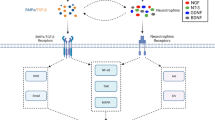
Schematic representation of SCI-NHO pathogenesis deduced from mouse models and retrospective studies in patients. Damage to the central nervous system (CNS) causes release of substance P. High-level SCI can also cause autonomic dysreflexia (AD). Systemic factors released after CNS injury and possibly AD causes abnormally high and prolonged activation of macrophages in injured muscles with persistent release and accumulation of OSM and TGF-β1. This may induce uncontrolled proliferation of fibro-adipogenic progenitors (FAPs) within injured muscles, FAP osteogenic differentiation, and formation of NHOs. Cyclooxygenase (COX) inhibitors such as indomethacin and celecoxib reduce NHO incidence in CNS-injured patients whereas the selective JAK1/2 tyrosine kinase inhibitor ruxolitinib reduces NHO volumes in mice with SCI
TBI-Induced NHO Models
More recently, small animal models of TBI-NHO have also been developed. A rat model using a polytrauma approach of TBI, femoral fracture, and muscle crush injury combined [59] has illustrated ectopic bone development in injured limbs. Other groups have utilized a combination of TBI with an Achilles tendon rupture (tenotomy) to establish NHO and illustrated changes in matrix metalloproteinase expression levels during TBI-NHO development [60]. Others used a combination of TBI and fracture, which increased serum calcitonin-related peptide release [61]. All these models have the potential to further identify mechanisms of TBI-NHO.
Trauma-Induced HO Models
As the development of HO is common in polytrauma patients [2, 62, 63], numerous models of trauma-associated HO have been developed, some of which do not have an accompanying CNS injury [64]. It is well established that HO develops after severe burns and other forms of soft tissue trauma [65]. A small animal model of burn-induced HO has been developed with a combination of burn and tenotomy with HO developing in the damaged limb [66]. This model has recently unraveled numerous pathways involved in burn-induced HO [67,68,69] and more recently excluded a role for activin A in burn-induced HO [70•]. Interestingly, these trauma models share some similarities with our SCI-NHO model and illustrate the importance of multiple traumas in the development of HO, as the incidence of HO was significantly higher with burn plus tenotomy compared with each insult alone [66]. Rat models of polytrauma have been developed using a combination of blast-related limb injury, bone fracture, quadriceps crush, amputation, and infection with methicillin-resistant Staphylococcus aureus (MRSA), reflective of combat-associated HO [71, 72]. Other models of trauma HO include mouse and rat tenotomy models [73,74,75] and a rabbit HO model where HO develops after hip surgery [76].
Overall, there has been substantial development of multiple small animal models for both neurogenic and non-neurogenic HO in the last decade [64]. These animal models will be vital to further understand HO pathogenesis and for development and pre-clinical testing of new therapeutics. Interestingly, multiple models demonstrate a commonality of multi-level trauma for the development of HO. The link between the dual injury process of CNS injury and muscle trauma is further discussed below.
The Nexus Between CNS Injury and Tissue Inflammation
We have previously established that a dual insult of SCI and muscle damage is required for SCI-NHO as NHO usually does not develop in mice with only SCI or only muscle injury [56•]. A dual-insult effect is also seen in models of TBI-NHO, where TBI alone does not induce NHO; however, the combination of TBI with multiple traumas (fracture or fracture + muscle crush) increases NHO prevalence accordingly [59]. Therefore, the development of pathological NHO following CNS injury requires a combined insult from both the CNS and tissue trauma (Fig. 3). These observations are consistent with patient data where there is a higher prevalence of NHO (up to 60%) in army personnel victims of combat inflicted blast and gunshot injuries with concomitant spinal damage [6,7,8]. Likewise, NHOs are more prevalent in SCI/TBI patients with concomitant bed sores, tracheostomy, pneumonia, smoking, systemic, or urinary tract infections, all signs of local or general inflammation [11, 12, 57].
An appropriate inflammatory response is also essential for muscle repair [58, 77, 78]. Macrophages are highly plastic and can direct the regeneration process toward either normal regeneration or pathological scar formation and fibrosis. In injured muscles without CNS injury, infiltration of monocytes/macrophages peaks 3–4 days post injury in mice and switches from a Ly6C+/CX3CR1low to a Ly6C−/CX3CR1hi phenotype with a change in expression of pro-inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin-1β (IL1β) to pro-regenerative transforming growth factor-β (TGF-β) [79,80,81]. Macrophages also produce various cytokines that enhance myogenesis such as interleukin-6 (IL6) and insulin-like growth factor 1 (IGF1) [82]. The recruitment of macrophages during muscle repair is mediated by CC chemokine ligand 2 (CCL2) and its receptor CC chemokine receptor 2 (CCR2). Inhibition of myeloid cell infiltration by depleting intramuscular CD11b+ cells or deletion of Ccl2 or Ccr2 genes significantly reduced intramuscular monocyte/macrophages and subsequently delayed clearance of necrotic muscle and muscle regeneration [83,84,85,86]. In addition, emerging evidence suggests macrophages are important in preventing fibrosis through inducing the apoptosis and clearance of muscle fibro-adipogenic progenitors (FAPs) via TNF [87]. Blocking TNF or myeloid-specific knockout of Tnf gene or Ccr2 gene deficiency all resulted in FAP accumulation and fibrosis in injured muscles [87].
Unlike dystrophic calcification post muscle injury, which is resolved over time via TNF-mediated mechanisms [88], SCI-NHOs at 21 days post-surgery contain osteocalcin-expressing osteoblasts and osterix-expressing osteocytes embedded within NHO foci in injured muscles [56•]. Importantly, we have illustrated that monocyte/macrophages infiltration into muscles is higher at 4 days post-surgery in mice with a SCI and CDTX muscle injury compared to mice without SCI, and this persists up to day 28 when NHO are mature [89•]. This is also observed in human NHO with CD68+ macrophages accumulating in the muscle at the periphery of the NHO [56•]. The key role of phagocytic macrophages in NHO, FOP, and traumatic HO pathogenesis is highlighted by the fact that their depletion by intravenous injection of clodronate-loaded liposomes significantly reduces HO formation in various animal models including NHO, FOP, burn and tenotomy HO model, and HO induced by exogenous BMP2 plus injury [51, 56, 68, 90]. Importantly we have found that neutrophils, unlike macrophages, do not play an important role in SCI-NHO pathogenesis. Mice with loss-of-function mutations in the gene encoding the granulocyte colony-stimulating factor (G-CSF) receptor are profoundly neutropenic yet still develop NHO in response to SCI and muscle injury similar to their wild-type controls with similar density of osterix-expressing osteoblasts [91•]. Likewise twice-daily administration of recombinant G-CSF, a treatment suggested to favor neuroregeneration after SCI or TBI, did not alter the course of NHO development [91•]. Therefore while G-CSF administration suppresses osteoblasts on endosteal surfaces of skeletal bones [92], it has no effect on osteoblasts forming NHOs in muscle [91•], similar to osteoblasts present on periosteal surfaces [92]. This further illustrates that the suppressive effect of G-CSF on endosteal osteoblasts is not direct but mediated by the adjacent bone marrow macrophages [92, 93].
The mechanism by which macrophages promote NHO development involves aberrant activation of the oncostatin M (OSM)/signal transducer and activator of transcription (STAT)-3 signaling pathway. SCI causes a persistent overexpression of OSM in injured muscles whereas in the absence of SCI, OSM expression normalizes over 3 weeks [94•]. Persistent OSM accumulation is an important driver of NHO pathogenesis as mice lacking the OSM receptor (OSMR) gene had a fourfold reduction in NHO volumes compared to wild-type controls. These results in mice were validated in humans as patients with NHO have higher OSM plasma concentration. OSM is also secreted by macrophages isolated from NHO biopsies, and this OSM promotes osteogenic differentiation of stromal cells derived from the muscles surrounding NHOs [94•]. OSM signals by binding to a cell surface receptor made of OSMR complexed to GP130. This complex subsequently signals by activating Janus tyrosine kinases JAK1 and JAK2, which then phosphorylate the transcription factor STAT3 enabling its translocation to the nucleus and activation. Indeed persistent OSM overexpression in injured muscles after SCI leads to persistent phosphorylation of STAT3. The functional relevance of STAT3 activation is highlighted by the fact that treatment with ruxolitinib, a selective JAK1/2 tyrosine-kinase inhibitor used to treat myeloproliferative neoplasms, significantly reduced NHO formation in mice [89•].
The Role of the CNS Injury
In mice, SCI exacerbates the inflammatory response to muscle injury ultimately leading to NHO formation instead of muscle repair [56, 89, 90, 94]. The question of which mechanisms initiated by the SCI lead to the sequence of pathological events driving NHO formation remains unresolved and a subject of intense investigation. Several risk factors have been identified in retrospective studies on patients with severe CNS injuries. The advantage of animal models is that these “risk factors” can be tested for their potential to promote NHO formation. For instance, muscle spasticity was identified as a significant risk factor of developing NHO in patients with TBI, stroke, or cerebral anoxia [95]. However, in our mouse model of SCI-NHO, injection of botulinum toxin A in the CDTX-injured muscle, to block the neuromuscular junctions and prevent muscular spasticity, did not reduce but instead increased NHO volumes. This suggests that muscle spasticity is not a trigger of NHO but rather the consequence of heterotopic bones growing in the muscle [96, 97].
Another possibility is that the SCI triggers the secretion of neuromediators at the site of the SCI, which excite neurons that synapse in the injured muscle, and ultimately modify the inflammatory response in the injured muscle. For instance, substance P has been described to be released in the dorsal horn of the spinal cord after SCI in humans [98] and rats [99]. However, in the mouse model of SCI-NHO, NHO volumes were increased in denervated limbs in which sciatic and femoral nerves were both excised [100]. This contradicts a direct role of the afferent nerves in promoting NHO in the injured muscle.
Retrospective case studies in SCI and TBI patients have also shown that autonomic dysreflexia (AD) is a significant risk factor associated with enhanced prevalence of NHO [62, 63, 101]. AD is frequent in patients with high-level SCI typically at and above vertebra T6. It is caused by a loss of the central control of post-ganglionic sympathetic nerve flow below the SCI. Typically, prolonged stimulation of sympathetic sensory nerves imposed by visceral stressors, such as overfull bladder or fecal compaction, stimulates the sympathetic nervous system. These impulses cannot be regulated by preganglionic nerves below the SCI because they have lost central control. This initiates an uncontrolled sympathetic reflex that causes very high norepinephrine release which constricts arteries with a sudden elevation of arterial pressure. A parasympathetic negative feedback reflex takes place by which baroreceptors in the carotid sinus sense the arterial hypertension and signal back to the brain which responds via the uninjured parasympathetic vagus nerve to decrease the heart rate. A parallel sympathetic reflex also takes place to relieve the vasoconstriction by reducing sympathetic release of norepinephrine. However, this sympathetic negative feedback via preganglionic sympathetic nerves is disconnected due to the SCI, and as a result, the hypertension and norepinephrine release remain unopposed while heart rate decreases [102, 103]. Hypertension combined with bradycardia represents the clinical signs of AD. If not managed rapidly, AD can lead to seizures, stroke, coma, cardiac arrest, and death. Although the mechanistic link between AD and immunodepression observed in severe CNS injuries has been explored [104, 105], the potential of AD and more generally systemic complications of SCI to trigger NHO development remains to be explored in animals. This is clearly an area of interest.
The concept that systemic complications of CNS injury could trigger NHO is supported by a remarkable feature of our SCI-NHO mouse model in which NHOs develop in the CDTX-injected muscle regardless of whether CDTX is injected in the mobile non-paralyzed front limb or the paralyzed hind limb [56•]. NHO in non-paralyzed limbs are frequent following stroke and TBI but also observed (albeit quite rarely) in SCI patients with NHO in the non-paralyzed shoulder/elbow particularly if fractured [2]. These findings suggest that systemic factors promoting NHO are released in the circulation in response to SCI [2, 56]. In support of this, we have shown that plasma from mice that underwent both SCI and CDTX-mediated muscle injury had a greater osteogenic potential in vitro when cultured with mesenchymal progenitor cells and satellite cells isolated from mouse muscles, compared to plasma from mice that had no SCI [56•]. This is also consistent with the observation that TBI combined with bone fracture in rats increased calcitonin gene-related peptide (CGRP) plasma concentration, as well as CGRP expression in muscles, which correlated with accelerated fracture repair [61].
Several osteogenic peptides have been found to be increased in the plasma of patients developing NHO such as substance P [56•] and OSM [94•]. Substance P increases mineralization of sorted muscle mesenchymal progenitor cells and satellite cells in vitro [56•]. The fact that administration of a selective inhibitor of the substance P receptor NK1R reduced NHO volumes following SCI in mice suggests that substance P plays an important role in NHO pathogenesis [56•]. In support of this, implantation of a bio-scaffold containing substance P against the Achilles tendon in mice was sufficient to induce HO formation whereas scaffolds containing CGRP had no such effect and even inhibited the promoting effect of substance P [106•]. Of note, implantation of the scaffold required drilling of the calcaneus bone to anchor the implant where the HO subsequently developed [106•]. Therefore the inflammatory component necessary for HO formation could have been elicited from the inflamed calcaneus.
OSM is also increased in the plasma of NHO patients and stimulates mineralization of sorted muscle mesenchymal progenitor cells and satellite cells in vitro [94•]. Deletion of the OSM receptor gene significantly reduced NHO volumes after SCI in mice suggesting an important role in NHO pathogenesis [94•]. However OSM is abundantly produced locally by activated myeloid cells in the injured muscle [89, 94]; thus, its increase in plasma could be a consequence of exacerbated unresolved muscular inflammation rather than a direct consequence of the SCI.
Likewise, serum concentrations of TGF-β1 are increased in NHO patients particularly in the early osteogenesis phase [107•], and TGF-β1 is well known as an important regulator of bone formation and coupling with bone resorption [108]. TGF-β1 expression was also increased in percutaneously injured Achilles tendons forming HO in mice despite the absence of a CNS injury. Administration of a neutralizing anti-TGF-β1 antibody or conditional deletion of the Tgfbr2 gene (encoding the main TGF-β receptor) in mesenchymal cells prevented HO development in this model [107•]. However, the fact that HOs were prevented in mice with conditional deletion of the Tgfb1 gene specifically in myeloid cells [107•] suggests that TGF-β1 is not released in the circulation as a consequence of the SCI but rather locally by inflammatory myeloid cells infiltrating the damaged tissue developing HO.
While there is a plethora of reports on the role of BMPs and activin A in FOP pathogenesis, there is a very few in respect to their potential role in NHO pathogenesis. There is evidence that some BMPs such as BMP-2 and BMP-9 can be induced in non-neurogenic trauma-induced HO particularly when endochondral bone formation is involved [109]. Many BMPs (e.g., BMP-2, BMP-4, BMP-6, BMP-7, and BMP-9) are osteoinductive when administered in one form or another in muscles or tendons in vivo whereas others, such as BMP-3, inhibit HO induced by osteoinductive BMPs [110]. However, whether BMPs have any role in NHO pathogenesis remains to be demonstrated. We could find only one article in which BMP concentration was measured in the plasma of CNS-injured patients with and without NHO, however, which BMP was measured that was not specified [111]. It has been recently shown that SP7/osterix-expressing cells present in the endoneurium that surrounds myelinated and non-myelinated axons can exit the nerve and migrate to the site of HO formation induced by recombinant adenovirus producing BMP-2 [112]. However, the fact that HOs were induced by adenoviral BMP-2 questions the relevance of these findings to NHO. In the same study, osterix-expressing cells were also found in nerves entrapped in human NHO biopsies together with cells in which Smad1/5/8 was phosphorylated. This study however did not assess whether BMPs were produced in the surrounding muscle to induce osteogenic differentiation of these cells. We measured mRNA expression for BMP-2, BMP-4, and BMP-7 in mouse muscles in our NHO model 4 days after injury. The mRNA for all these BMPs was downregulated in CDTX-injured muscles in the presence of a SCI. Furthermore, daily injection of LDN-193189, a selective inhibitor of BMPR1a, BMPR1b, and ACVR1 kinase activity that blocks Smad1/5/8 activation downstream of BMP binding, had surprisingly no effect of SCI-NHO development in our mouse model (Tseng HW et al., submitted), whereas LDN-193189 treatment inhibits HO in mouse models of FOP driven by ACVR1Q207D [44]. Overall, a potential of BMPs and their receptors in NHO pathogenesis is possible but remains to be established unlike FOP which is clearly caused by modulating point mutations in the AVCR1 gene, and BMP-type receptor kinase inhibitors may not be as effective at preventing NHO development as they are with FOP.
Conclusion
The recent development of animal model of NHO in the past 5 years has enabled the identification of some mechanisms involved in its pathogenesis. The nexus between the CNS injury and tissue trauma/inflammation is key in the development of NHO and explains why NSAIDs given early following CNS trauma have shown some success at reducing NHO in patients [27•]. Experiments in mice have shown that JAK1/2 inhibitors may also have this potential in patients by targeting the GP130/JAK pathway instead of the cyclooxygenase pathway. A better understanding of how systemic and autonomic deregulations following CNS injury cooperate with muscle inflammation to promote NHO development will no doubt provide novel targets to prevent NHO development in patients. These will also be likely to lead to the identification of novel biomarkers to predict the onset of NHO and to develop treatments for patients to prevent this debilitating condition. Finally this research area has revealed intriguing interactions between the nervous system and innate immunity that enables muscle regeneration while when deregulated leads to bone formation instead.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ohlmeier M, Suero EM, Aach M, Meindl R, Schildhauer TA, Citak M. Muscle localization of heterotopic ossification following spinal cord injury. Spine J. 2017;17(10):1519–22. https://doi.org/10.1016/j.spinee.2017.04.021.
Genet F, Jourdan C, Schnitzler A, Lautridou C, Guillemot D, Judet T, et al. Troublesome heterotopic ossification after central nervous system damage: a survey of 570 surgeries. PLoS One. 2011;6(1):e16632. https://doi.org/10.1371/journal.pone.0016632.
Dejerine M, Ceillier A, Dejerine Y. Para-osteo-arthropathies des paraplegiques par lesion medullaire: Etude anatomique et histologique. Rev Neurol. 1919;26:399–407.
Dejerine M, Ceillier MA. Trois cas d'ostéomes – Ossifications périostés juxta-musculaires chez les paraplegiques par lesion traumatique de la moelle épinière. Rev Neurol. 1918;1:159–74.
Schurch B, Dollfus P. The ‘Dejerines’: an historical review and homage to two pioneers in the field of neurology and their contribution to the understanding of spinal cord pathology. Spinal Cord. 1998;36(2):78–86. https://doi.org/10.1038/sj.sc.3100561.
Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91(5):1084–91. https://doi.org/10.2106/jbjs.h.00792.
Bevevino AJ, Lehman RA Jr, Tintle SM, Kang DG, Dworak TC, Potter BK. Incidence and morbidity of concomitant spine fractures in combat-related amputees. Spine J. 2014;14(4):646–50. https://doi.org/10.1016/j.spinee.2013.06.098.
Hoyt BW, Pavey GJ, Potter BK, Forsberg JA. Heterotopic ossification and lessons learned from fifteen years at war: a review of therapy, novel research, and future directions for military and civilian orthopaedic trauma. Bone. 2018;109:3–11. https://doi.org/10.1016/j.bone.2018.02.009.
Aubut JA, Mehta S, Cullen N, Teasell RW. A comparison of heterotopic ossification treatment within the traumatic brain and spinal cord injured population: an evidence based systematic review. NeuroRehabilitation. 2011;28(2):151–60. https://doi.org/10.3233/NRE-2011-0643.
Haran M, Bhuta T, Lee B. Pharmacological interventions for treating acute heterotopic ossification. Cochrane Database Syst Rev. 2004(4):Cd003321. https://doi.org/10.1002/14651858.CD003321.pub3.
Dizdar D, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S. Risk factors for developing heterotopic ossification in patients with traumatic brain injury. Brain Inj. 2013;27(7–8):807–11. https://doi.org/10.3109/02699052.2013.775490.
Reznik JE, Biros E, Marshall R, Jelbart M, Milanese S, Gordon S, et al. Prevalence and risk-factors of neurogenic heterotopic ossification in traumatic spinal cord and traumatic brain injured patients admitted to specialised units in Australia. J Musculoskelet Neuronal Interact. 2014;14(1):19–28.
Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37(3):129–36. https://doi.org/10.1080/16501970510027628.
Bradleigh LH, Perkash A, Linder SH, Sullivan GH, Bhatt HK, Perkash I. Deep venous thrombosis associated with heterotopic ossification. Arch Phys Med Rehabil. 1992;73(3):293–4.
Salga M, Jourdan C, Durand MC, Hangard C, Denormandie P, Carlier RY, et al. Sciatic nerve compression by neurogenic heterotopic ossification: use of CT to determine surgical indications. Skelet Radiol. 2014;44:233–40. https://doi.org/10.1007/s00256-014-2003-6.
Genet F, Chehensse C, Jourdan C, Lautridou C, Denormandie P, Schnitzler A. Impact of the operative delay and the degree of neurologic sequelae on recurrence of excised heterotopic ossification in patients with traumatic brain injury. J Head Trauma Rehabil. 2012;27(6):443–8. https://doi.org/10.1097/HTR.0b013e31822b54ba.
Popovic M, Agarwal A, Zhang L, Yip C, Kreder HJ, Nousiainen MT, et al. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta-analysis of published data. Radiother Oncol. 2014;113(1):10–7. https://doi.org/10.1016/j.radonc.2014.08.025.
Seegenschmiedt MH, Makoski HB, Micke O. German cooperative group on radiotherapy for benign D. Radiation prophylaxis for heterotopic ossification about the hip joint--a multicenter study. Int J Radiat Oncol Biol Phys. 2001;51(3):756–65. https://doi.org/10.1016/s0360-3016(01)01640-6.
Liu JZ, Frisch NB, Barden RM, Rosenberg AG, Silverton CD, Galante JO. Heterotopic ossification prophylaxis after total hip arthroplasty: randomized trial of 400 vs 700 cGy. J Arthroplast. 2017;32(4):1328–34. https://doi.org/10.1016/j.arth.2016.10.030.
Cipriano C, Pill SG, Rosenstock J, Keenan MA. Radiation therapy for preventing recurrence of neurogenic heterotopic ossification. Orthopedics. 2009;32(9). https://doi.org/10.3928/01477447-20090728-33
Museler AC, Grasmucke D, Jansen O, Aach M, Meindl R, Schildhauer TA, et al. In-hospital outcomes following single-dose radiation therapy in the treatment of heterotopic ossification of the hip following spinal cord injury-an analysis of 444 cases. Spinal Cord. 2017;55(3):244–6. https://doi.org/10.1038/sc.2016.112.
Lee CH, Shim SJ, Kim HJ, Yang H, Kang YJ. Effects of radiation therapy on established neurogenic heterotopic ossification. Ann Rehabil Med. 2016;40(6):1135–9. https://doi.org/10.5535/arm.2016.40.6.1135.
Ploumis A, Belbasis L, Ntzani E, Tsekeris P, Xenakis T. Radiotherapy for prevention of heterotopic ossification of the elbow: a systematic review of the literature. J Shoulder Elb Surg. 2013;22(11):1580–8. https://doi.org/10.1016/j.jse.2013.07.045.
Honore T, Bonan I, Salga M, Denormandie P, Labib A, Genet G, et al. Effectiveness of radiotherapy to prevent recurrence of heterotopic ossification in patients with spinal cord injury and traumatic head injury: a retrospective case-controlled study. J Rehabil Med. 2020. https://doi.org/10.2340/16501977-2692.
Banovac K, Williams JM, Patrick LD, Haniff YM. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal Cord. 2001;39(7):370–4. https://doi.org/10.1038/sj.sc.3101166.
Banovac K, Williams JM, Patrick LD, Levi A. Prevention of heterotopic ossification after spinal cord injury with COX-2 selective inhibitor (rofecoxib). Spinal Cord. 2004;42(12):707–10. https://doi.org/10.1038/sj.sc.3101628.
• Zakrasek EC, Yurkiewicz SM, Dirlikov B, Pence BT, Crew JD. Use of nonsteroidal anti-inflammatory drugs to prevent heterotopic ossification after spinal cord injury: a retrospective chart review. Spinal Cord. 2019;57(3):214–20. https://doi.org/10.1038/s41393-018-0199-3 A retrospective study confirming NSAID prophylaxis reduces prevalence and extent of NHO.
Oni JK, Pinero JR, Saltzman BM, Jaffe FF. Effect of a selective COX-2 inhibitor, celecoxib, on heterotopic ossification after total hip arthroplasty: a case-controlled study. Hip Int. 2014;24(3):256–62. https://doi.org/10.5301/hipint.5000109.
Kjaersgaard-Andersen P, Schmidt SA. Indomethacin for prevention of ectopic ossification after hip arthroplasty. Acta Orthop Scand. 1986;57(1):12–4. https://doi.org/10.3109/17453678608993206.
Kjaersgaard-Andersen P, Schmidt SA. Total hip arthroplasty. The role of antiinflammatory medications in the prevention of heterotopic ossification. Clin Orthop Relat Res. 1991;263:78–86.
Finerman GA, Stover SL. Heterotopic ossification following hip replacement or spinal cord injury. Two clinical studies with EHDP. Metab Bone Dis Relat Res. 1981;3(4–5):337–42. https://doi.org/10.1016/0221-8747(81)90050-3.
Stover SL, Hahn HR, Miller JM 3rd. Disodium etidronate in the prevention of heterotopic ossification following spinal cord injury (preliminary report). Paraplegia. 1976;14(2):146–56. https://doi.org/10.1038/sc.1976.25.
Banovac K. The effect of etidronate on late development of heterotopic ossification after spinal cord injury. J Spinal Cord Med. 2000;23(1):40–4. https://doi.org/10.1080/10790268.2000.11753507.
Banovac K, Gonzalez F, Wade N, Bowker JJ. Intravenous disodium etidronate therapy in spinal cord injury patients with heterotopic ossification. Paraplegia. 1993;31(10):660–6. https://doi.org/10.1038/sc.1993.106.
Kates SL, Ackert-Bicknell CL. How do bisphosphonates affect fracture healing? Injury. 2016;47(Suppl 1(0 1)):S65–8. https://doi.org/10.1016/s0020-1383(16)30015-8.
Kidd LJ, Cowling NR, Wu AC, Kelly WL, Forwood MR. Bisphosphonate treatment delays stress fracture remodeling in the rat ulna. J Orthop Res. 2011;29(12):1827–33. https://doi.org/10.1002/jor.21464.
Sloan AV, Martin JR, Li S, Li J. Parathyroid hormone and bisphosphonate have opposite effects on stress fracture repair. Bone. 2010;47(2):235–40. https://doi.org/10.1016/j.bone.2010.05.015.
Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;165(4):1107–15. https://doi.org/10.1016/S0002-9440(10)63372-X.
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–7. https://doi.org/10.1038/ng1783.
Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, et al. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27(8):1746–56. https://doi.org/10.1002/jbmr.1637.
Kaplan FS, Fiori J, De La Peña LS, Ahn J, Billings PC, Shore EM. Dysregulation of the BMP-4 signaling pathway in fibrodysplasia ossificans progressiva. Ann N Y Acad Sci. 2006;1068:54–65. https://doi.org/10.1196/annals.1346.008.
Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. 2015;7(303):303ra137. https://doi.org/10.1126/scitranslmed.aac4358.
Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA, et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018;9(1):471. https://doi.org/10.1038/s41467-018-02872-2.
Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14(12):1363–9. https://doi.org/10.1038/nm.1888.
Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, et al. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30(3):379–90. https://doi.org/10.1002/humu.20868.
• Hildebrand L, Stange K, Deichsel A, Gossen M, Seemann P. The fibrodysplasia ossificans progressiva (FOP) mutation p.R206H in ACVR1 confers an altered ligand response. Cell Signal. 2017;29:23–30. https://doi.org/10.1016/j.cellsig.2016.10.001This papers shows the switch in function of BMP ligands signaling occurring as a consequence of the ACVR1R206H mutation which is the most common in FOP patients.
Yano M, Kawao N, Okumoto K, Tamura Y, Okada K, Kaji H. Fibrodysplasia ossificans progressiva-related activated activin-like kinase signaling enhances osteoclast formation during heterotopic ossification in muscle tissues. J Biol Chem. 2014;289(24):16966–77. https://doi.org/10.1074/jbc.M113.526038.
Hamilton PT, Jansen MS, Ganesan S, Benson RE, Hyde-Deruyscher R, Beyer WF, et al. Improved bone morphogenetic protein-2 retention in an injectable collagen matrix using bifunctional peptides. PLoS One. 2013;8(8):e70715. https://doi.org/10.1371/journal.pone.0070715.
Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM, et al. BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res. 2011;26(6):1166–77. https://doi.org/10.1002/jbmr.311.
Li L, Jiang Y, Lin H, Shen H, Sohn J, Alexander PG, et al. Muscle injury promotes heterotopic ossification by stimulating local bone morphogenetic protein-7 production. J Orthop Translat. 2019;18:142–53. https://doi.org/10.1016/j.jot.2019.06.001.
• Convente MR, Chakkalakal SA, Yang E, Caron RJ, Zhang D, Kambayashi T, et al. Depletion of mast cells and macrophages impairs heterotopic ossification in an Acvr1(R206H) mouse model of fibrodysplasia ossificans progressiva. J Bone Miner Res. 2018;33(2):269–82. https://doi.org/10.1002/jbmr.3304This study established that depletion of macrophages or mast cells impaired injury-induced HO in the ACVR1R206H mouse FOP model demonstrating that the innate immune system contributes to HO in FOP.
Haupt J, Xu M, Shore EM. Variable signaling activity by FOP ACVR1 mutations. Bone. 2017;109:232–40. https://doi.org/10.1016/j.bone.2017.10.027.
Cappato S, Giacopelli F, Ravazzolo R, Bocciardi R. The horizon of a therapy for rare genetic diseases: a “druggable” future for fibrodysplasia ossificans progressiva. Int J Mol Sci. 2018;19(4):989. https://doi.org/10.3390/ijms19040989
Maekawa H, Kawai S, Nishio M, Nagata S, Jin Y, Yoshitomi H, et al. Prophylactic treatment of rapamycin ameliorates naturally developing and episode -induced heterotopic ossification in mice expressing human mutant ACVR1. Orphanet J Rare Dis. 2020;15(1):122. https://doi.org/10.1186/s13023-020-01406-8.
Pulik L, Mierzejewski B, Ciemerych MA, Brzoska E, Legosz P. The survey of cells responsible for heterotopic ossification development in skeletal muscles-human and mouse models. Cells. 2020;9(6):1324. https://doi.org/10.3390/cells9061324
• Genêt F, Kulina I, Vaquette C, Torossian F, Millard S, Pettit AR, et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J Pathol. 2015;236(2):229–40. https://doi.org/10.1002/path.4519Description of the first clinically relevant mouse model of SCI-NHO that does not involve genetic manipulation or implants containing BMPs.
Citak M, Suero EM, Backhaus M, Aach M, Godry H, Meindl R, et al. Risk factors for heterotopic ossification in patients with spinal cord injury: a case-control study of 264 patients. Spine. 2012;37(23):1953–7. https://doi.org/10.1097/BRS.0b013e31825ee81b.
Mounier R, Théret M, Arnold L, Cuvellier S, Bultot L, Göransson O, et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18(2):251–64. https://doi.org/10.1016/j.cmet.2013.06.017.
Brady RD, Zhao MZ, Wong KR, Casilla-Espinosa PM, Yamakawa GR, Wortman RC, et al. A novel rat model of heterotopic ossification after polytrauma with traumatic brain injury. Bone. 2020;133:115263. https://doi.org/10.1016/j.bone.2020.115263.
Shi WZ, Ju JY, Xiao HJ, Xue F, Wu J, Pan MM, et al. Dynamics of MMP9, MMP2 and TIMP1 in a rat model of brain injury combined with traumatic heterotopic ossification. Mol Med Rep. 2017;15(4):2129–35. https://doi.org/10.3892/mmr.2017.6275.
Song Y, Bi L, Zhang Z, Huang Z, Hou W, Lu X, et al. Increased levels of calcitonin gene-related peptide in serum accelerate fracture healing following traumatic brain injury. Mol Med Rep. 2012;5(2):432–8. https://doi.org/10.3892/mmr.2011.645.
van Kampen PJ, Martina JD, Vos PE, Hoedemaekers CW, Hendricks HT. Potential risk factors for developing heterotopic ossification in patients with severe traumatic brain injury. J Head Trauma Rehabil. 2011;26(5):384–91. https://doi.org/10.1097/HTR.0b013e3181f78a59.
Hendricks HT, Geurts AC, van Ginneken BC, Heeren AJ, Vos PE. Brain injury severity and autonomic dysregulation accurately predict heterotopic ossification in patients with traumatic brain injury. Clin Rehabil. 2007;21(6):545–53. https://doi.org/10.1177/0269215507075260.
Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, et al. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3(4):e10172. https://doi.org/10.1002/jbm4.10172.
Medina A, Shankowsky H, Savaryn B, Shukalak B, Tredget EE. Characterization of heterotopic ossification in burn patients. J Burn Care Res. 2014;35(3):251–6. https://doi.org/10.1097/BCR.0b013e3182957768.
Peterson JR, De La Rosa S, Sun H, Eboda O, Cilwa KE, Donneys A, et al. Burn injury enhances bone formation in heterotopic ossification model. Ann Surg. 2014;259(5):993–8. https://doi.org/10.1097/SLA.0b013e318291da85.
Hsu GC, Marini S, Negri S, Wang Y, Xu J, Pagani CA, et al. Endogenous CCN family member WISP-1 inhibits trauma-induced heterotopic ossification. JCI Insight. 2020;5. https://doi.org/10.1172/jci.insight.135432.
• Sorkin M, Huber AK, Hwang C, Carson WF, Menon R, Li J, et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat Commun. 2020;11(1):722. https://doi.org/10.1038/s41467-019-14172-4This paper characterized the inflammatory response, in particular the monocyte/macrophage subpopulations present in injured tissue using a burn and tenotomy-induced HO mouse model confirming the importance of monocyte/macrophages and TGF-β1 in burn-induced HO.
Loder SJ, Agarwal S, Chung MT, Cholok D, Hwang C, Visser N, et al. Characterizing the circulating cell populations in traumatic heterotopic ossification. Am J Pathol. 2018;188(11):2464–73. https://doi.org/10.1016/j.ajpath.2018.07.014.
• Hwang C, Pagani CA, Das N, Marini S, Huber AK, Xie L et al. Activin A does not drive post-traumatic heterotopic ossification. Bone. 2020;138:115473. https://doi.org/10.1016/j.bone.2020.115473. The results of this study establishes that anti-activin A neutralizing antibodies have no effect on burn / tenotomy-induced HO.
Qureshi AT, Dey D, Sanders EM, Seavey JG, Tomasino AM, Moss K, et al. Inhibition of mammalian target of rapamycin signaling with rapamycin prevents trauma-induced heterotopic ossification. Am J Pathol. 2017;187(11):2536–45. https://doi.org/10.1016/j.ajpath.2017.07.010.
Qureshi AT, Crump EK, Pavey GJ, Hope DN, Forsberg JA, Davis TA. Early characterization of blast-related heterotopic ossification in a rat model. Clin Orthop Relat Res. 2015;473(9):2831–9. https://doi.org/10.1007/s11999-015-4240-y.
Kim JM, Yang YS, Park KH, Ge X, Xu R, Li N, et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation. Nat Commun. 2020;11(1):2289. https://doi.org/10.1038/s41467-020-16038-6.
Zhang Q, Zhang Y, Yan M, Zhu K, Su Q, Pan J, et al. βig-h3 enhances chondrogenesis via promoting mesenchymal condensation in rat Achilles tendon heterotopic ossification model. Aging. 2020;12(8):7030–41. https://doi.org/10.18632/aging.103060.
Kusano T, Nakatani M, Ishiguro N, Ohno K, Yamamoto N, Morita M, et al. Desloratadine inhibits heterotopic ossification by suppression of BMP2-Smad1/5/8 signaling. J Orthop Res. 2020. https://doi.org/10.1002/jor.24625.
Rumi MN, Deol GS, Singapuri KP, Pellegrini VD Jr. The origin of osteoprogenitor cells responsible for heterotopic ossification following hip surgery: an animal model in the rabbit. J Orthop Res. 2005;23(1):34–40. https://doi.org/10.1016/j.orthres.2004.05.003.
Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31(2):384–96. https://doi.org/10.1002/stem.1288.
Varga T, Mounier R, Horvath A, Cuvellier S, Dumont F, Poliska S, et al. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J Immunol. 2016;196(11):4771–82. https://doi.org/10.4049/jimmunol.1502490.
Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, et al. Comparative study of injury models for studying muscle regeneration in mice. PLoS One. 2016;11(1):e0147198. https://doi.org/10.1371/journal.pone.0147198.
Novak ML, Weinheimer-Haus EM, Koh TJ. Macrophage activation and skeletal muscle healing following traumatic injury. J Pathol. 2014;232(3):344–55. https://doi.org/10.1002/path.4301.
Wang X, Zhao W, Ransohoff RM, Zhou L. Infiltrating macrophages are broadly activated at the early stage to support acute skeletal muscle injury repair. J Neuroimmunol. 2018;317:55–66. https://doi.org/10.1016/j.jneuroim.2018.01.004.
Blomster LV, Brennan FH, Lao HW, Harle DW, Harvey AR, Ruitenberg MJ. Mobilisation of the splenic monocyte reservoir and peripheral CX3CR1 deficiency adversely affects recovery from spinal cord injury. Exp Neurol. 2013;247(0):226–40. https://doi.org/10.1016/j.expneurol.2013.05.002.
Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204(5):1057–69. https://doi.org/10.1084/jem.20070075.
Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25(1):358–69. https://doi.org/10.1096/fj.10-171579.
Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–25. https://doi.org/10.1182/blood-2012-01-403386.
Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011;25(10):3344–55. https://doi.org/10.1096/fj.10-178939.
Lemos DR, Babaeijandaghi F, Low M, Chang C-K, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21(7):786–94. https://doi.org/10.1038/nm.3869.
Zhao Y, Urganus AL, Spevak L, Shrestha S, Doty SB, Boskey AL, et al. Characterization of dystrophic calcification induced in mice by cardiotoxin. Calcif Tissue Int. 2009;85(3):267–75. https://doi.org/10.1007/s00223-009-9271-5.
• Alexander KA, Tseng H-W, Fleming W, Jose B, Salga M, Kulina I et al. Inhibition of JAK1/2 tyrosine kinases reduces neurogenic heterotopic ossification after spinal cord injury. Front Immunol. 2019;10:377. https://doi.org/10.3389/fimmu.2019.00377. This study established that persistent STAT3 phosphorylation was present in muscles developing NHO using a mouse model of NHO after SCI and that treatment with ruxolitinib, a selective JAK1/2 tyrosine-kinase inhibitor, significantly reduced NHO formation in mice.
Levesque J-P, Sims NA, Pettit AR, Alexander KA, Tseng H-W, Torossian F, et al. Macrophages driving heterotopic ossification: convergence of genetically-driven and trauma-driven mechanisms. J Bone Miner Res. 2018;33(2):365–6. https://doi.org/10.1002/jbmr.3346.
• Tseng H-W, Kulina I, Salga M, Fleming W, Vaquette C, Genêt F et al. Neurogenic heterotopic ossifications develop independently of granulocyte-colony stimulating factor and neutrophils. J Bone Miner Res. 2020;in press. https://doi.org/10.1002/jbmr.4118. This research establishes that neutrophils do not play an important role in the pathogenesis of NHO in a mouse model of NHO after SCI.
Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116(23):4815–28. https://doi.org/10.1182/blood-2009-11-253534.
Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208(2):251–60. https://doi.org/10.1084/jem.20101700.
• Torossian F, Guerton B, Anginot A, Alexander KA, Desterke C, Soave S, et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight. 2017;2(21):e96034. https://doi.org/10.1172/jci.insight.96034This study established the role of oncostatin M in human NHO and a mouse model of NHO after SCI.
Bargellesi S, Cavasin L, Scarponi F, De Tanti A, Bonaiuti D, Bartolo M, et al. Occurrence and predictive factors of heterotopic ossification in severe acquired brain injured patients during rehabilitation stay: cross-sectional survey. Clin Rehabil. 2017;32(2):255–62. https://doi.org/10.1177/0269215517723161.
Wharton GW, Morgan TH. Ankylosis in the paralyzed patient. J Bone Joint Surg Am. 1970;52(1):105–12.
Salga M, Tseng H-W, Alexander KA, Jose B, Vaquette C, Debaud C, et al. Blocking neuromuscular junctions with botulinum toxin A injection enhances neurological heterotopic ossification development after spinal cord injury in mice. Ann Phys Rehabil Med. 2019 in press. https://doi.org/10.1016/j.rehab.2019.01.005.
Leonard AV, Manavis J, Blumbergs PC, Vink R. Changes in substance P and NK1 receptor immunohistochemistry following human spinal cord injury. Spinal Cord. 2014;52(1):17–23. https://doi.org/10.1038/sc.2013.136.
Kim MS, Yan J, Wu W, Zhang G, Zhang Y, Cai D. Rapid linkage of innate immunological signals to adaptive immunity by the brain-fat axis. Nat Immunol. 2015;16(5):525–33. https://doi.org/10.1038/ni.3133.
Debaud C, Salga M, Begot L, Holy X, Chedik M, de l’Escalopier N, et al. Peripheral denervation participates in heterotopic ossification in a spinal cord injury model. PLoS One. 2017;12(8):e0182454. https://doi.org/10.1371/journal.pone.0182454.
Putz C, Helbig L, Gerner HJ, Zimmermann-Stenzel M, Akbar M. Autonomic dysreflexia: a possible trigger for the development of heterotopic ossifications after traumatic spinal cord injury? Eur J Trauma Emerg Surg. 2014;40(6):721–6. https://doi.org/10.1007/s00068-013-0353-8.
Weaver LC, Marsh DR, Gris D, Brown A, Dekaban GA. Autonomic dysreflexia after spinal cord injury: central mechanisms and strategies for prevention. Prog Brain Res. 2006;152:245–63. https://doi.org/10.1016/S0079-6123(05)52016-8.
Sweis R, Biller J. Systemic complications of spinal cord injury. Curr Neurol Neurosci Rep. 2017;17(1):8. https://doi.org/10.1007/s11910-017-0715-4.
Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–86. https://doi.org/10.1038/nrn1765.
Zhang Y, Guan Z, Reader B, Shawler T, Mandrekar-Colucci S, Huang K, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci. 2013;33(32):12970–81. https://doi.org/10.1523/jneurosci.1974-13.2013.
• Tuzmen C, Verdelis K, Weiss L, Campbell P. Crosstalk between substance P and calcitonin gene-related peptide during heterotopic ossification in murine Achilles tendon. J Orthop Res. 2018;36(5):1444–55. https://doi.org/10.1002/jor.23833In a mouse model of tenotomy-induced HO, this research established that substance P delivered to the injury site promoted HO development whereas when substance P was delivered along with calcitonin gene-related peptide HO development was no longer promoted.
• Wang X, Li F, Xie L, Crane J, Zhen G, Mishina Y, et al. Inhibition of overactive TGF-β attenuates progression of heterotopic ossification in mice. Nat Commun. 2018;9(1):551. https://doi.org/10.1038/s41467-018-02988-5Using Acilles tendon puncture-induced and BMP-2-induced mouse models of HO, this research shows that TGF-β1 promotes HO formation.
Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-β1 to the bone. Endocr Rev. 2005;26(6):743–74. https://doi.org/10.1210/er.2004-0001.
Grenier G, Leblanc É, Faucheux N, Lauzier D, Kloen P, Hamdy RC. BMP-9 expression in human traumatic heterotopic ossification: a case report. Skelet Muscle. 2013;3(1):29. https://doi.org/10.1186/2044-5040-3-29.
Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11(17):1312–20. https://doi.org/10.1038/sj.gt.3302298.
Dong L, Dong G, Cao J, Zhang J. Association of α2-HS glycoprotein with neurogenic heterotopic ossification in patients with spinal cord injury. Med Sci Monit. 2017;23:5382–8. https://doi.org/10.12659/msm.904626.
Olmsted-Davis EA, Salisbury EA, Hoang D, Davis EL, Lazard Z, Sonnet C, et al. Progenitors in peripheral nerves launch heterotopic ossification. Stem Cells Transl Med. 2017;6(4):1109–19. https://doi.org/10.1002/sctm.16-0347.
Funding
JPL is supported by Research Fellowship 1136130 from the National Health and Medical Research Council of Australia (NHMRC). KAA, HWT, FG, and JPL research are supported by NHMRC Ideas Grant 1181053.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skeletal Biology and Regulation
Rights and permissions
About this article
Cite this article
Alexander, K.A., Tseng, HW., Salga, M. et al. When the Nervous System Turns Skeletal Muscles into Bones: How to Solve the Conundrum of Neurogenic Heterotopic Ossification. Curr Osteoporos Rep 18, 666–676 (2020). https://doi.org/10.1007/s11914-020-00636-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00636-w