Abstract
Purpose of Review
The purpose of this review is to describe the current state of our thinking regarding bone-muscle interactions beyond the mechanical perspective.
Recent Findings
Recent and prior evidence has begun to dissect many of the molecular mechanisms that bone and muscle use to communicate with each other and to modify each other’s function. Several signaling factors produced by muscle and bone have emerged as potential mediators of these biochemical/molecular interactions. These include muscle factors such as myostatin, Irisin, BAIBA, IL-6, and the IGF family and the bone factors FGF-23, Wnt1 and Wnt3a, PGE2, FGF9, RANKL, osteocalcin, and sclerostin.
Summary
The identification of these signaling molecules and their underlying mechanisms offers the very real and exciting possibility that new pharmaceutical approaches can be developed that will permit the simultaneous treatments of diseases that often occur in combination, such as osteoporosis and sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Our understanding of the relationship between skeletal muscle and bone has undergone a major renaissance in the past decade or so with exponentially increasing numbers of published papers on the subject (Fig. 1). Skeletal muscle and bone are no longer viewed from the simple mechanical perspective; i.e., bone provides an attachment site for skeletal muscles and skeletal muscles apply load to bone. The musculoskeletal system is a complex organ system that involves multiple structural components (skeletal muscles, bones, ligaments, tendons, nervous system, vasculature, joints, and connective tissues). Both skeletal muscle and bone are also recognized as a source of a number of signaling molecules that can act through autocrine, paracrine, and endocrine mechanisms. Importantly, the functional activities of skeletal muscle and bone reciprocally affect each other (and other tissues/organs) through crosstalk mechanisms that play important roles during development and the aging spectrum. This crosstalk involves biochemical, molecular, and genetic coupling that we are only now beginning to understand. Several excellent reviews have been written in the past few years describing the emerging evidence of skeletal muscle and bone crosstalk [1,2,3,4,5,6,7,8,9]. In this review article, we will present some of the most current literature, in the context of prior literature that has advanced our understanding of these crosstalk mechanisms.
Published peer-reviewed papers in scientific journals: The search was conducted using PubMed with the search term “musculoskeletal interaction.” Search was conducted on March, 2020. This graph has been updated from Maurel et al., 2017 [142]
Evidence for Skeletal Muscle and Bone Interactions/Crosstalk
Some of the earliest evidence for muscle-bone crosstalk comes out of the orthopedic literature involving the treatment of complex fractures in which muscle flaps used to cover fracture sites showed accelerated healing and reduced infection compared with non-covered fractures [10] [11] [12] [13]. A study by Richards and colleagues [12] in canines demonstrated that the biomechanical properties at the site of an osteotomy were superior when a muscle flap covered the site versus a local skin flap. The potential contributors to improved healing include improved vascular supply, improved recruitment of mesenchymal stem cells/osteoprogenitor cells, providing a supply of cytokines and growth factors such as myokines and antimicrobial properties [14].
More recently, many studies in humans have shown an association between osteoporosis and sarcopenia in at least a subset of the population. Osteoporosis is defined by the World Health Organization as “… a bone mineral density (BMD) at the hip or lumbar spine that is less than or equal to 2.5 standard deviations below the mean BMD of a young-adult reference population” [15]. Sarcopenia was redefined by the European Working Group on Sarcopenia in Older People (2018 Group 2) in 2019 [16] with changes from its original 2010 definition [17], as “…low levels of measures for three parameters: (1) muscle strength, (2) muscle quantity/quality and (3) physical performance as an indicator of severity,” The new definition also recognizes that sarcopenia may be acute or chronic and may be primary or secondary (associated with another disease). While a precise cause-and-effect relationship has not been established, mounting evidence supports the hypothesis that there is molecular coupling and crosstalk between muscle and bone. Both conditions can occur coincidentally in the same individuals and have resulted in the use of the term sarco-osteopenia [18, 19] or osteosarcopenia [20,21,22,23]. Binkley and Buehring [19] have made the strong recommendation that muscle weakness needs to be recognized as a contributor to fracture risk, which subsequently has been supported in a number of studies. The evidence suggesting a relationship between osteoporosis and sarcopenia in a subset of the human aged population has led to great interest in molecular signaling between the two tissues, beyond the mechanical perspective.
There are several clinical studies that have examined parameters of muscle mass (lean mass), fat mass, and bone density. While many of the results offer disparate findings, factors such as age, gender, and ethnicity are possible confounders when examining multiple studies. In this regard, a meta-analysis performed by Ho-Pham and colleagues [24] found that lean mass was a more important determinant of bone mineral density in both sexes across all ages and ethnic groups versus fat mass. However, fat mass was equivalent to lean mass in postmenopausal women. They suggested that the importance of lean mass is reflected in physical activity, which is important during development and growth and maintenance of bone mass in the skeleton. An analysis by Huh and colleagues [25] studied an elderly cohort of participants in the Fourth Korea National Health and Nutrition Examination Surveys (KHANES IV). They found an association of muscle mass with bone density and femoral bone geometry in the elderly that was more prominent in men than in women. They also found a significant increase in the risk of osteoporosis in men and women with sarcopenia. Interestingly, the relationship between increased muscle mass and reduction in the occurrence of osteoporosis was strongest at the total hip and femoral neck in both sexes, but the reduction relationship at the lateral spine was only observed in men. Muscle mass is not the best indication of muscle function as it does not truly reflect muscle strength. Recently, Luo et al. [26•] published their results of the relationship between muscle strength (measured by grip strength) and bone mineral density (BMD). They used data from the National Health and Nutrition Examination Survey (NHANES 2013–2014), with a total of 1850 subjects aged from 40 to 80 years of age. Grip strength was measured using a handgrip dynamometer. BMD was measured on the femoral neck and total lumber spine (overall BMD from L1 to L4). After adjusting for several factors (i.e., age, ethnicity, social habits, genetics, nutrition, physical activity among others), they found that grip strength of the dominant hand is associated with increased femoral neck and total lumber spine BMD in men and premenopausal and postmenopausal women [26•].
Recently a meta-analysis by Locquet et al. [27] examined changes in bone mass and muscle parameters from childhood to adult life. Fifteen papers were included in their analysis, and despite heterogeneity between these studies, they did find a significant correlation between bone mineral density and markers of muscle function during aging. Some of these associations might be the consequence of muscle loading on bone, but diet and gene expression were also contributing to changes in muscle and bone during aging. In this regard, there is clear evidence for pleiotropic genes that regulate both muscle and bone [9] [28] (see below).
Genome-wide association studies (GWAS) have provided another source of evidence for bone-muscle crosstalk. Several pleiotropic genes that affect bone and muscle have been detected [2•]. One of these, METTL21C, has been shown in vitro to alter both muscle function and osteocyte viability and resistance to undergo apoptosis through modulation of the NF-κB signaling pathway [29]. The identity of the muscle/bone factor(s) that might regulate the pleiotropic effects of METTL21C gene expression is not known. In a study of four pediatric cohorts using a bivariate GWAS approach, Medina-Gomez et al. [30] identified pleiotropic effects for total body lean mass and total body BMD (less head) in eight loci, seven of which had already been identified as BMD loci. These included WNT4, GALNT3, MEPE, CPED/WNT16, TNFSF11, RIN3, PPP6R3/LRP5, and SREBF1/TOM1L22. The SREBF1 gene is important for differentiation in both osteoblasts and myoblasts, but has opposite roles in those two cell types. A mechanism by which the SREBF1 gene could be involved in the regulation of muscle and bone mass might be through its active gene product, SREBP-1 [31]. Gorski et al. [32] reported that mice with a targeted deletion of the proprotein convertase Mbtps1 in osteocytes (using Dmp1-Cre) had an age-related increase in Soleus muscle mass and contractile force. Based on these observations, Gorski and Price [33] have proposed a model for bone-muscle crosstalk that involves a complex pathway centered on the regulation of core circadian clock genes, Dec1 and Dec2, by SREBP-1 and the subsequent downstream targets. Other clock genes have also been implicated in the control of bone mineral density and architectural properties. For example, deletion of the transcription factor brain and muscle ARNT-like protein 1 (BMAL1) results in a rapid aging phenotype with decreased BMD and reductions in trabecular and cortical bone parameters [34]. This is in contrast to deletion of the clock genes Per and Cry, which result in a high bone mass phenotype [35]. BMAL1 has also been shown to be important in maintenance of skeletal muscle, as mice lacking BMAL1 have significantly reduced normalized maximal force [36]. This has led to its implication in bone-muscle crosstalk through control of several myokines with known effects on bone [37]. Finally, in addition to these pleiotropic genes identified by multivariate GWAS, there are a number of examples of animal models with targeted deletion in muscle or bone that display changes in the reciprocal tissue. Some of these will be discussed in the subsequent sections.
The existence of pleiotropic genes, while perhaps not surprising from a development perspective, suggests that share genetics could lead to the coincident occurrence of osteoporosis and sarcopenia in some individuals. This highlights the need to consider the advantages of therapeutic approaches that simultaneously target all elements of the musculoskeletal system as has been suggested [28] [9] [2•].
Mechanisms of Skeletal Muscle and Bone Crosstalk
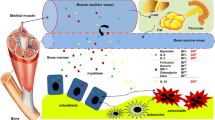
Figure 2 illustrates possible mechanisms of communication between bone and muscle. The release of key proteins/small molecules into the bloodstream would seem to be the most obvious means of crosstalk communication between tissues. Every cell in our body depends on obtaining their nutrients from the bloodstream and removing metabolic waste into the circulation. The fact that certain cytokines specifically produced in muscle can reach high concentration levels in the bloodstream after exercise is strong evidence that myokines are being released directly into circulation. Likewise, several bone-derived factors are known to circulate.
Muscle-bone possible way of interaction: Possible routes that myokines can reach bone and how osteokines can reach muscle. Both tissues are highly vascularized. (1) In skeletal muscle, secreted molecules can get into circulation through the extracellular fluid present in the endomysium. (2) In bone, osteocytes through the lacunar-canalicular system are in close proximity to blood vessels. It has been observed that osteocytes can extend their dendrites into blood vessels. (3) Due to the close proximity of bone and muscle, molecules (smaller than 40 kDa) can crossed the periosteum by diffusion (figure was created using BioRender.com)
Both muscle and bone are highly vascularized tissues. Each group of myofibers is supplied by an artery and two veins and innervated by a nerve. Muscle fibers are formed into bundles called fascicles and are surrounded by a connective tissue called the perimysium. Inside each perimysium are muscle fibers individually surrounded by a thin connective tissue composed mainly of type I and type III collagen known as the endomysium. Inside the endomysium, the muscle fiber is bathed in a nutrient-rich fluid. In human bone, at the center of each osteon runs a nerve, an artery, and a vein. Each osteocyte inside exists within a lacunae or “cave” in the bone. Osteocytes are connected to each other through a large number of dendrites that extend through canaliculi (or “tunnels”). This lacunar-canalicular system forms a network that facilitates transporting small proteins (< 70 kDa) and molecules to and from the bloodstream (Fig. 3).
Osteocyte arrangement in murine bones. Scanning electron microscopy images of resin-embedded, acid-etched murine cortical bone. a Inset shows how osteocytes interact with blood vessels. b Osteocytes are connected with each other via a dense lacunar-canalicular network. Arrows point to a blood vessel running through the cortical bone. Note the osteocytes closed to blood vessels extending their dendrites around it
Another mechanism of cell-to-cell communication is the shedding of cellular components in the form of microvesicles, exosomes, or extracellular vesicles (EVs). This concept was first embraced in the cancer field. Cancer cells are known for uncontrolled proliferation and excessive release of EVs into the environment to attract and develop new, but leaky, blood vessels (angiogenesis) to support the cancerous cell growth and metastasis. EVs are membrane vesicles that contain a wide spectrum of molecules (proteins, mRNAs, and microRNAs) that reflect the state of the cell. The shed EVs can act locally (autocrine effect) or be delivered into the bloodstream and act on distant organs (paracrine effect). This uptake can happen through at least three known mechanisms: (1) a ligand-receptor mechanism involving molecules expressed on the EV membrane, (2) fusing with the membrane of the targeted cells and releasing its content inside the cell, or (3) EV uptake into the cell and subsequent processing that releases its cargo intracellularly.
There is mounting evidence that supports the idea that nearly every cell is able to shed various types of microvesicles (extracellular vesicles, EVs) including bone cells. Qin and Dallas extensively reviewed the literature on EVs shed from different bone cells as well as from bone marrow stromal cells and muscle cells (see [38•] for more details). In 2015, Ge et al. [39] reported more than 1000 proteins inside microvesicles obtained from osteoblastic cells MC3T3. Besides proteins involved in biogenesis, processing, and uptake of EVs, other proteins involved in osteogenesis were also reported. Cui et al. [40] reported the presence of 457 microRNAs (miRNA), out of which 43 were highly expressed including miR-1192, miR-680, and miR302a that are known to be expressed in osteoblasts. Other miRNAs found in the exosomes of this cell line are known to regulate the Wnt/β-catenin pathway, an important pathway in the bone development, growth, and homeostasis [40]. Deng et al. [41] showed that osteoblast microvesicles contain RANKL and that treatment of RAW264.7 cells with these EVs promoted TRAP-positive multinucleated cells, suggesting that osteoblasts can support osteoclastogenesis. Osteoclast cells are also capable of producing microvesicles. Huynh et al. [42] reported EVs from osteoclast precursors enhanced osteoclastogenesis, but EVs from mature osteoclasts inhibited further osteoclast formation, suggesting an autocrine effect of the EVs produced by osteoclasts. This is due to the presence of RANKL receptor, RANK, in the EVs therefore working as a decoy for RANKL in the microenvironment [42]. Li et al. [43] reported that osteoclastic miR-214-3p in human biopsies correlate with the high levels of the same miRNA (in serum exosomes) in elderly women with fracture compared with age-matched controls. Osteoclast-derived exosomes containing miRNA-214 transferred to osteoblastic cells have been shown to inhibit bone formation [44].
A less explored field is the production/role of EVs by osteocytes. Only a handful of papers had been reported on the microvesicles released by osteocytes that can have an effect on other cells [45] [46]. In 2013, Veno et al. tested microvesicles isolated from late differentiated IDG-SW3 cells (a cell line that can differentiate from osteoblast to osteocyte) onto undifferentiated IDG-SW3 cells or the Dmp1-mem-GFP calvaria cells (from a transgenic mouse expressing a membrane-targeted GFP in osteocytes). They observed a strong induction of alkaline phosphatase, increased expression of Dmp1-GFP within 48 h, and E11/gp38. They concluded that microvesicles from osteocytes contain cargo that may induce differentiation of osteoblasts [46]. Qin et al. [45] reported that exosomes produced by osteocytes (using the Ocy454 cell line) that had been pretreated with myostatin can be taken up by osteoblasts (MC3T3 cell line); this uptake caused reduced levels of Runx2 and reduced osteoblastic differentiation by downregulating the Wnt signaling pathway (see more details in the “Skeletal Muscle as an Endocrine Organ—Myokines” section). Sato et al. [47] have studied the expression of miRNAs present in MLO-Y4 cells (osteocyte-like cell line). This group also analyzed the circulating exosomes from an osteocyte-less (OL) mouse model. They concluded that the observed decrease in the number of circulating miRNAs in the serum of the OL mice may be caused due to the lack of osteocytes [47].
Another potential form of bone-muscle crosstalk is by diffusion. This is possible due to the close proximity of both tissues. The periosteum is a cell layer (~ 60 microns thick) that surrounds bone and muscle. Lai et al. [48] showed that molecules < 40 kDa can easily diffuse through the semi-permeable periosteum. This implies that small molecules like PGE2, NO, BAIBA, and Irisin can easily reach the adjacent tissue by passive diffusion, whereas osteokines or myokines > 40 kDa most likely are being transported via the circulation or as EV cargo.
Skeletal Muscle as an Endocrine Organ—Myokines
It is now well recognized that muscle can produce cytokines or “myokines” that have an effect on distant organs. The concept of the myokine was first introduced by Pedersen and colleagues (reviewed in [49] [50] [51]) based on their studies of interleukin (IL)-6, which has shown to be released from contracting skeletal muscle. In this section, we will highlight the recent literature on the most prominent myokines.
Myostatin
In 1997, McPherron et al. first reported myostatin, also known as growth differentiation factor-8 (GDF-8), is a muscle-secreted cytokine [52]. Myostatin belongs to the TGF superfamily and shares homology with other members of this family, e.g., GDF11, BMP9, BMP10, TGFβ1. Unlike other members of the TGF family, myostatin is expressed exclusively in the myotome of the somite during embryogenesis and in skeletal muscle during growth. In mice, global deletion of myostatin results in a 2–3-fold increase in muscle mass compared with controls. Concomitantly, there are changes in site-specific trabecular and cortical bone mineral content and bone volume [53]. Exercise has been shown to also increase bone properties in these mice [54]. Myostatin deficiency has also been reported in cattle where mutations in the MSTN gene give rise to what is known as the double-muscled phenomenon, which is characterized by a 20% increase in muscle mass (mainly hyperplasia—increase in fiber number rather than fiber size) [55,56,57]. In humans, mutations in the MSTN gene produce myostatin-related muscle hypertrophy that can be inherited [58].
In addition to its inhibitory effect on muscle, myostatin also affects bone cells. Myostatin binds to the type IIB activin receptor (AcvrIIB), which is highly expressed in bone marrow–derived stem cells (BMSC) [59]. Recombinant myostatin injection represses bone formation and increases osteoclastogenesis. Qin et al. [45] reported that myostatin treatment of osteocytes increased the expression of the bone inhibitors, sclerostin and Dkk1. EVs released from the myostatin-treated osteocyte (Ocy454 cell line) can be taken up by osteoblasts (MC3T3 cell line) resulting in the downregulation of Runx2, a key player in osteoblast differentiation. This inhibition was completely reversed by the expression of exogenous miR-218 through inhibition of Sost, a Wnt signaling inhibitor [45]. Other in vitro studies have also shown that myostatin increased RANKL-induced osteoclastogenesis [60, 61].
Inhibition of myostatin in order to improve muscle and bone disorders such as Duchenne muscular dystrophy (DMD) and rheumatoid arthritis (RA) has yielded intriguing results, but there are potential concerns. A humanized neutralizing antibody to mysostatin, Domagrozumab, was developed by Pfizer and went into clinical trials to treat juvenile patients with DMD. However, the trial was stopped in phase II since patients did not show improvement in the endpoint tested after 1 year of treatment. Interestingly, synovial tissues from patients with RA, as well as of a mouse model of RA, have elevated levels of myostatin. In a mouse model of RA that carries the human TNF-α transgenic mouse, treatment with an anti-myostatin antibody ameliorated the joint erosion [60]. Myostatin inhibitors such as ACE-031 have also been developed and clinical trials to treat DMD patients had been designed. However, the clinical trial on this drug was stopped due to safety concerns (epistaxis (nasal bleeding) and telangiectasias (spider veins)) [62]. As explained by Long et al. [63], some of the concerns with inhibition of myostatin as have been developed thus far is the high degree of homology between myostatin and other growth factors, such as GDF11 causing undesirable side effects. Myostatin, similar to other members of the TGF family, is produced as a pro-form that must be enzymatically processed into the mature active form. Long and colleagues reported favorable results using muSRK-015P, a monoclonal antibody that binds exclusively to pro- and latent myostatin. Using a mouse model of spinal muscular atrophy, the authors reported that muSRK-015P was not only effective in increasing muscle mass and function but also effective in increasing cortical thickness and trabecular bone properties [63]. SRK-015, an optimized version of SRK-015P, is currently in phase 2 of clinical trials to treat patients with later-onset spinal muscular atrophy. The study is set to end in April 2021.
Irisin
In 2012, Boström et al. [64] elegantly reported the discovery of a novel myokine, which they named Irisin, after the Greek messenger goddess Iris. Irisin is the cleavage product of a type I membrane protein encoded by the FNDC5 gene which is regulated by peroxisome proliferator–activated receptor γ (PPARγ) coactivator 1α (PGC1-α). Levels of Irisin in plasma were elevated in mice subjected to 3 weeks of voluntary wheel running and in humans after 10 weeks of supervised endurance exercise training. Overexpression of Fndc5 (using an adenoviral vector that expressed full-length of FNDC5) resulted in increased levels of Irisin in plasma and significant increases of brown fat and the thermogenesis genes Upc-1 and Cidea mRNA in subcutaneous fat (inguinal) as well as changes in mitochondria genes and oxygen consumption in adipocytes, but not skeletal muscles or cultured myocytes. In this original paper, Irisin also improved glucose tolerance in mice fed with high-fat diet [64].
In 2014, Colaianni et al. [65] showed that Irisin was able to induce the differentiation of bone marrow stromal cells into osteoblasts. Although these were in vitro experiments using primary cells, these findings implied a potential muscle-fat-bone axis. The bone-fat relationship had been previously suggested by studies on leptin acting at the level of the brain to control bone formation (reviewed in [66,67,68,69]). Colaianni and colleagues [70] subsequently showed that low doses of Irisin (100 μg/kg body weight) were enough to increase cortical bone mass and strength (trabecular bone changes were almost significant). This low dose was not enough to turn white adipose to brown adipose tissue that was observed with higher doses (3500 μg/kg of body weight). The increase in bone mass was due to increase osteoblast number and decreased osteoclast numbers. The authors also showed that in bone marrow stromal cells, Irisin treatment increased ERK phosphorylation and increased expression of osteogenesis-related genes [70]. These findings have been confirmed recently using MC3T3 cells [71], and in mice subjected to a volunteer wheel running regimen [72]. Irisin is also capable of blocking the effects of hindlimb unloading, namely restoring bone mass and blocking muscle atrophy [73].
Recently Kim et al. [74] identified the Irisin receptor as a subset of integrin complexes. The αV/β5 integrin complex receptor had the highest affinity for Irisin. They showed that binding to its receptor causes the phosphorylation of focal adhesion kinase (FAK) at tyrosine 397, which in turns phosphorylated Akt at threonine 308 (but not at serine 473) and phosphorylation of the cyclic AMP response element-binding protein (CREB) after 5 min of Irisin treatment [74]. Similar to PTH treatment, the dose and type of administration (continuously versus intermittent) appear to be of critical importance in producing Irisin effects on bone. For the anabolic effect on mouse bones, intermittent treatment with 100 μg/kg once a week is more effective in decreasing Sost and Dkk1 mRNA expression [70] [73] [75]. On the other hand, researchers have reported a catabolic effect when using daily Irisin injections for 6 days. This regime seems to increase bone remodeling by increasing Sost mRNA expression in osteocyte-enriched bone [74].
β-Aminoisobutyric Acid
The effect of β-aminoisobutyric acid (BAIBA) on fat was first reported in 2004 in an attempt to elucidate the mechanism by which certain antiretroviral nucleoside reverse-transcriptase inhibitors (mainly stavudine (d4T) and zidovudine (AZT) which are analogs of thymine) reduce body fat [76]. BAIBA is one of the metabolites produced by the degradation of d4T and AZT to thymine. They observed that BAIBA treatment mimics the effects of d4T and AZT and is more potent in reducing body fat content in RjOrl Swiss mice [76]. This is achieved by increasing fatty acid oxidation (FAO) [77]. This effect on FAO was also reported by Igoudjil et al. [78]. BAIBA appears to prevent diet-induced obesity in mice with partial leptin deficiency [79], through a leptin-dependent stimulation of mitochondrial FAO [80].
In 2008, Calvo et al. described the creation of a muscle-specific PGC-1α transgenic mouse [81]. This mutant mouse model has enhanced ability to perform endurance exercise with increased ability for oxygen uptake. In 2014, Roberts used liquid chromatography mass spectrophotometry (LC-MS) to identify small molecules expressed by myocytes with PGC-1α overexpression. They found 4 small molecules: BAIBA, GABA, cytosine, and 2′-deoxycitosine. Out of the four, only BAIBA increased the expression of brown adipocyte-specific genes (UCP-1 and Cidea). BAIBA was capable of inducing brown adipocyte-like phenotype in human induced pluripotent stem cells through a PPARα-dependent mechanism [82]. Interestingly, the authors also demonstrated that forced overexpression of PGC-1α in primary myocytes altered the transcription of several genes identified in the Framingham Heart Study genome-wide association analysis that are genetic determinants of BAIBA levels in humans.
Kitase et al. [83] reported administration of L-BAIBA in drinking water protects bone loss due to disuse by preventing ROS-induced cell death and protecting mitochondrial integrity. Zhu et al. [84] showed that BAIBA stimulates proliferation and differentiation of osteoprogenitor cells (MC3T3-E1 cells) by activating the NAD(P)H/ROS signaling. Recent reports have also demonstrated the effects of BAIBA on other tissues such as liver [85] and kidney [86].
Most recently, Wang et al. [87] developed an easy method to detect aminobutyric acid isomers and found correlations between bone mineral density and osteoporotic fractures. They reported lower expression of GABA in non-osteoporotic as well as in osteoporotic women between 60 and 80 years of age. D-BAIBA levels in older non-osteoporotic (control) females ranged from 0.71 to 2.24 μM and osteoporotic women ranged from 0.38 to 1.97 μM. D-BAIBA serum concentration was positively correlated with T score (hip) in older women without any fracture, but not correlated in older women with fractures. D-BAIBA and GABA showed significant positive association with physical activity in the different populations. L-AABA was strongly associated with alcohol intake. D-BAIBA was not associated with hip BMD in 85 young Caucasian women without osteoporosis/osteopenia, but there was a positive correlation in 38 lean women and a negative correlation in 47 obese individuals. Interestingly it appears that humans and mice differ in terms of which enantiomer of BAIBA is biologically important.
Interleukin-6 Family
The interleukin-6 (IL-6) superfamily has several members, i.e., IL-6, LIF, and CNTF. A main characteristic of this family is the use of the co-receptor glycoprotein-130 (gp130). Binding to this co-receptor leads to downstream activation of the JAK/STAT or the ERK signaling pathway. It is well known that exercise releases several cytokines such as tumor necrosis factor (TNF-α) and interleukins (IL-1, IL-6, IL-10). IL-6 plasma concentration increases by 10-fold after 30 min of exercise and up to 100-fold after 2 h of eccentric exercise. IL-6 increases are directly related to the duration, intensity, and amount of muscles involved in exercise [88]. Previously it was hypothesized that IL-6 production was due to an inflammatory response after exercise [89]. Steensberg et al. showed that the increased IL-6 plasma concentration after exercise was consequence of muscle contraction [90] and more specifically by type 2 muscle fibers [91]. We now understand that levels of IL-6 in monocytes (immune cells responsible for IL-6 production during sepsis) do not change during exercise [92] [93].
Besides its action on glucose uptake and fatty acid oxidation, IL-6 increases both osteoclast formation [94] and osteoblast differentiation [91]. Bakker et al. [95] showed that IL-6 had no effect on the osteocyte response to mechanical loading in an in vitro model. However, IL-6 treatment of osteocytes results in decreased alkaline phosphatase activity and Runx2 expression in osteoblasts, and increased expression of the proliferation marker Ki67 and osteocalcin [95].
IL-6 has two basic mechanisms of action: cis and trans. Classic (Cis) happens when the cells express the IL-6 receptor (IL-6R) bound to the cell membrane. Upon binding, a homodimer of the co-receptor gp-130 is recruited and a series of downstream signaling mechanisms are activated. Trans-signaling occurs when IL-6 binds to a soluble receptor (sIL-6R) before binding to a homodimer of gp130 on the cell surface. McGregor et al. [96] reported that it was the trans-signaling, and not cis-signaling, that promoted bone formation and osteoclastogenesis in a calvaria mouse model.
Bone as an Endocrine Organ—Osteokines
Bone is a dynamic tissue undergoing modeling during growth and development to achieve proper shape and remodeling throughout a person’s lifetime as a means of renewing bone’s structure and integrity. Our current understanding suggests that the activities of the bone forming osteoblasts and bone resorbing osteoclasts are largely orchestrated by the osteocyte, the most abundant cell in bone. Together these three cells utilize highly conserved mechanisms throughout the vertebrate animal kingdom to create a skeleton that is capable of providing six major functions: structural support for the body, facilitation of movement, protection for the internal organs, a site for hematopoiesis, a reservoir of calcium and phosphate and fat storage, and a more recently recognized endocrine function.
The concept of bone as an endocrine organ took off in 2006 and since then several reviews in the past decade have discussed the importance of bone as an endocrine tissue and more specifically the important role of the osteocyte and osteoblast in this function (see reviews by [97,97,98,99,100,101,102,104]). There is now a growing list of bone-produced molecules that have effects on other organs. This list includes a wide range of molecules from proteins like FGF23 and osteocalcin to small molecules like PGE2.
Fibroblast Growth Factor 23
Fibroblast growth factor 23 (FGF23) was first identified in 2000 [105]. In 2006, it was reported that patients with hypophosphatemia rickets have high levels of circulating FGF23 [106]. It was later determined that bone-secreted FGF23 targets kidney proximal tubules where it regulates the expression of the type II sodium/phosphate co-transporters Na-Pi 2a and Na-Pi 2c that are responsible for absorption and reabsorption of phosphate [107]. This leads to a decrease in phosphate absorption and hypophosphatemia. FGF23 also regulates vitamin D metabolism by inhibiting 1-α-hydroxylase, which is responsible for the conversion of 1,25 (OH)2 D to its active form: 1,25 (OH)2D3 [108]. These studies clearly established a bone-kidney axis.
FGF23 binds to the FGF receptor (FGFR) in the presence of the co-receptor Klotho [31, 109]. FGF23 can also activate FGFR4 independently of Klotho [110]. Expression of FGF23 is regulated by PHEX and Dmp1, additional products of the osteocyte, which inhibit the expression of FGF23. Mutations in PHEX lead to increased levels of FGF23.
FGF23 has specific effects on cardiac muscle [111, 112] and one study has raised the possibility that it may have direct effects on skeletal muscle. Kido et al. [113] have implicated FGF23 in muscle atrophy associated with chronic kidney disease by inhibition of insulin/IGF signaling in skeletal muscle. However, another study by Avin et al. [114] found that despite expressing FGFR (1–4) and α-Klotho, in vitro treatments with FGF23 did not alter skeletal muscle C2C12 myotube function and in vivo acute treatment with FGF23 did not alter Soleus and EDL muscle contractility measured ex vivo. These authors speculated that FGF23 might be working indirectly or in concert with another co-factor. Thus, while FGF23 is a putative factor involved in bone-muscle crosstalk, its exact role is an open question.
Fibroblast Growth Factor 9
Fibroblast growth factor 9 (FGF9) belongs to the FGF superfamily. Using the newly developed calvaria-derived osteocyte-like cell lines, OmGPF10 and OmGFP66, Wang et al. reported that when osteoblasts differentiate into osteocytes, the production of FGF23 is increased [115]. McCormick et al. [116] reported that FGF9 was moderately expressed in osteoblast and strongly expressed in the osteoid osteocyte cells, and also showed moderate expression in osteocytes isolated from 2- and 4-week-old mouse femurs Treatment of OmGFP66 with FGF9 induced FGF23 mRNA expression significantly (~ 1200-fold) within 24 h and FGF23 protein secretion 90-fold. Recently Huang et al. reported the effect of FGF9 on muscle cells. Using C2C12 cell line and human cells, they observed an inhibition in myogenic differentiation, decreasing expression of MyoG and Mhc, and increasing expression of myostatin.
Osteocalcin
Osteocalcin is a secreted protein produced by late osteoblast and osteocytes and it is stored locally in the bone matrix due to its high affinity for hydroxyapatite. It is also known as γ-carboxyglutamic acid or BGLAP. It is a relatively small molecule with a molecular weight of 5.6 kDa. Upon decarboxylation (due to low pH), it is released into the circulation. Osteocalcin binds to its receptor, Gpcr6a, located in the plasma membrane of a wide variety of cells. It has been reported that osteocalcin acts on pancreatic cells to increase β-cell proliferation and insulin secretion. It also acts on the testis (Leydig cells) to promote testosterone production [117]. In muscle, osteocalcin increases insulin sensitivity.
The health benefits of exercise are well known, which has effects on the regulation of glucose metabolism, increases lipolysis, and increases muscle and bone mass. The mechanism of action of the effect of exercise on IL6 and osteocalcin was recently published by [118]. The authors reported that exercise-induced IL6 acts on osteoblasts (and most likely osteocytes), leading to an increase in the production of RANKL that in turn activates osteoclasts to acidify and remodel bone matrix. This leads to the release of osteocalcin from the matrix into circulation where it can go to the muscle and bind to its receptor Gpcr6 [118] and thereby regulate muscle function.
Transforming Growth Factor β
Transforming growth factor β (TGFβ) is mainly produced by osteoblasts and is also stored in the bone matrix in its latent form. To become active, TGF β has to be released from the extracellular matrix, which is achieved by low pH (osteoclast acidification of local matrix). Bone destruction induced by cancer metastasis leads to increase levels of TGFβ in the circulation. Deletion of TGFβ in osteocytes leads to decreases in osteocyte RANKL production and perilacunar/canalicular remodeling, which results in decreased bone quality due to decreased canalicular length (hence osteocyte connectivity) [119].
Pathological release of TGFβ from bone matrix induces muscle weakness by decreasing Ca2+-induced muscle force production. This is achieved by increased NADPH oxidase (Nox4), which leads to increased oxidation of skeletal muscle proteins including the ryanodine receptor/calcium release channel, leading to a leakage of Ca2+ needed for muscle contraction [120].
Regulators of the Wnt/β-Catenin Pathway
The Wnt/β-catenin pathway is an important signaling pathway in regulating bone growth and bone mass accrual. It plays roles in the differentiation of bone cells during growth and development, bone homeostasis during adulthood including bone regeneration during fracture healing, and bone accrual in response to mechanical loading. This is a multifactorial pathway with several key molecules involved in the regulation of bone formation. Positive regulators produced by bone cells include Wnt-1, Wnt3a, and Wnt10. Regulation of the pathway is also achieved by several inhibitors including sclerostin and Dkk-1. Although these secreted factors are produced in bone and their concentration can be high locally, some can also be detected in serum (e.g., sclerostin), which makes them candidates for mediators of bone-muscle crosstalk. Serum sclerostin levels have been shown to negatively correlate with skeletal muscle mass in hemodialysis patients with diabetes [121] and in a non-diabetic, Korean cohort [122]. Interestingly, the use of an anti-sclerostin antibody in a breast cancer mouse model system was protective against bone destruction and also improved muscle function [123]. These studies imply a role for sclerostin in the regulation of muscle function, but the exact mechanism is unclear at this time.
The canonical Wnt/β-catenin pathway and non-canonical Wnt pathways also play important roles in skeletal muscle development [124, 125]. Huang et al. [126] demonstrated using the C2C12 myoblast/myotube cell line treated with conditioned media from MLO-Y4 osteocyte-like cells that Wnt3a produced by the MLO-Y4 cells could stimulate myogenesis and enhance myotube contractile properties. They further showed that Wnt3a was more potent than Wnt1 in inducing myogenesis. However, in vivo evidence for a role of Wnt proteins in muscle-bone crosstalk is lacking. One of the critical questions, especially for the Wnts, is can they act at a distance, via an endocrine type mechanism in the circulation or do they perhaps use an extracellular vesicle type of transport to signal from bone to muscle?
Prostaglandin E2
Prostaglandin E2 (PGE2) is released by many cells in response to mechanical stimulation including osteocytes and skeletal muscle cells. In response to fluid flow shear stress, MLO-Y4 osteocyte-like cells (and 2T3 osteoblast-like cells) rapidly release significant amounts of PGE2 [127]. Brotto and colleagues have shown that PGE2 modulates Ca+2 homeostasis [128] and stimulates C2C12 myoblasts to differentiate into myotubes [129]. How PGE2 produced by osteocytes/osteoblasts might signal to skeletal muscle is a key question, especially given the short half-life and rapid metabolism of PGE2 in circulation [130]. Because skeletal muscle and bone are intimately associated tissues, a diffusion type of mechanism is entirely possible (see the “Mechanisms of Skeletal Muscle and Bone Crosstalk” section, above).
Receptor Activator of Nuclear Factor Kappa β Ligand
Receptor activator of nuclear factor kappa β ligand (RANKL) is produced by immune cells as well as osteocytes; however, osteocytes are the major source for RANKL [131,131,133]. Mice with deletion of RANKL show severe osteopetrosis, are toothless, and lack osteoclasts [134]. Osteoprotegerin (OPG) is a decoy receptor for RANKL. Two studies using the Opg−/− mouse have implicated a role for RANKL in bone-muscle crosstalk and suggest it may be a therapeutic target for treating sarcopenia. Bonnet et al. [135] showed that in women taking the anti-RANKL antibody denosumab, for 3 years, there were improvements in lean muscle mass and strength. Using the huRANKLTg+ mouse model, they demonstrated that denosumab and the RANKL inhibitor OPG-Fc produced improvements in muscle function. Hamoudi et al. [136] observed similar results using the Opg−/− mouse model, which displays the expected weaker bones due to elevated RANKL levels and atrophy of the fast twitch type IIb muscle fibers. Treatment of these mice with an anti-RANKL antibody partially alleviated these deficits. These studies suggest that RANKL plays a role in bone-muscle crosstalk and, in addition to being a pharmaceutical target for treating osteoporosis, could well be a target for treating sarcopenia.
Further evidence for a role of RANKL in bone-muscle crosstalk comes from recent studies by Frenette and colleagues in their studies of Duchenne muscular dystrophy (see review [137]). Their recent work has shown that treatment of the mdx/utrn+/− mouse with anti-RANKL antibody improved EDL muscle maximum specific force and increased the stiffness of the tibia measured by 3-point mechanical testing [138]. In a more recent study with the osteoprotegerin (OPG)-deficient mouse model (OPG is a decoy receptor for RANKL), they observed a similar improvement in EDL and bone biomechanical properties after treatment with an anti-RANKL antibody [136]. It is interesting that the effect of anti-RANKL therapy appears to be targeted to fast twitch muscle fibers. Given the use of anti-RANKL therapies to treat osteoporosis, baseline and post-treatment assessment of muscle function in those patients (perhaps grip strength testing) should reveal whether this therapy can improve sarcopenia in those patients.
Unanswered Questions and Future Directions
The last two decades have witnessed an exponential increase in published literature (Fig. 1) describing potential bone-muscle crosstalk or interactions. Still there remain many unanswered questions. One of the most important of these is what is the mechanism by which biochemical /molecular coupling of these two tissues occurs? As described in the sections above, there is evidence for the classical mechanisms of endocrine and paracrine signaling and newer, evolving understandings of mechanisms such as extracellular vesicles that may play important roles. Understanding how or if osteokines and myokines crosstalk to the other tissue, and crosstalk specificity, is critical if we are to use these molecules therapeutically. The list of myokines and osteokines continues to grow. Critical to determining which of these are mediators of bone-muscle crosstalk will be studies in animal model systems in which targeted deletion of these molecules in one tissue is coupled with determining the consequences on both bone and muscle (and other tissues). Another important question is does crosstalk signaling change across the aging spectrum? It is entirely conceivable and likely that crosstalk mechanisms in play during development might differ from those during pubertal growth versus adulthood versus the aged musculoskeletal system. Understanding which molecules and mechanisms are active during these different periods of like will be necessary if we are going to manipulate these mechanisms to treat various diseases that occur at different ages. All of these mechanisms occur on the landscape of varying genetic backgrounds between individuals and understanding the influence of modifying genetics that may control crosstalk will also be essential. Investigators have long appreciated the mechanical coupling of bone and muscle, and so teasing out mechanisms that may underlie mechanical versus biochemical interactions represents a challenge. Another important area for future consideration is the role of the central nervous system or other organ systems that may be involved such as adipose tissue and the pancreas. Bone-muscle crosstalk need not necessary be direct, and there is already evidence that supports bone-derived factors that target other organ systems, which could then have effects on muscle function [139, 140] [4]. Another important aspect of bone-muscle crosstalk that has only been superficially explored relates to gender differences and how changes in key hormones such as estrogen in females during menopause might alter these mechanisms. Thus, despite a literal explosion in our understanding and appreciation of the importance of bone-muscle crosstalk, there are still several important questions that need to be addressed. This is especially critical when it comes to the design of new therapeutic agents for the treatment of various human diseases that involve the musculoskeletal system (see reviews [22, 137, 141,141,142,144]).
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. 2020;94:155–218.
• Trajanoska K, et al. Genetics of bone and muscle interactions in humans. Curr Osteoporos Rep. 2019;17(2):86–95 High Importance: This manuscript describes key pleiotropic loci that have been identified by multivariate GWAS studies.
Bonewald L. Use it or lose it to age: a review of bone and muscle communication. Bone. 2019;120:212–8.
Karsenty G, Mera P. Molecular bases of the crosstalk between bone and muscle. Bone. 2018;115:43–9.
Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70.
Brotto M, Bonewald L. Bone and muscle: interactions beyond mechanical. Bone. 2015;80:109–14.
Cianferotti L, Brandi ML. Muscle-bone interactions: basic and clinical aspects. Endocrine. 2014;45(2):165–77.
Brotto M, Johnson ML. Endocrine crosstalk between muscle and bone. Curr Osteoporos Rep. 2014;12(2):135–41.
Karasik D, Kiel DP. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone. 2010;46(5):1226–37.
Byrd HS, Spicer TE, Cierney G 3rd. Management of open tibial fractures. Plast Reconstr Surg. 1985;76(5):719–30.
Cierny G 3rd, Byrd HS, Jones RE. Primary versus delayed soft tissue coverage for severe open tibial fractures. A comparison of results. Clin Orthop Relat Res. 1983;178:54–63.
Richards RR, Mahoney JL, Minas T. Influence of soft tissue coverage on the healing of cortical defects in canine diaphyseal bone. Ann Plast Surg. 1986;16(4):296–304.
Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285–92.
Chan JK, et al. Soft-tissue reconstruction of open fractures of the lower limb: muscle versus fasciocutaneous flaps. Plast Reconstr Surg. 2012;130(2):284e–95e.
Cosman F, de Beur SJ, LeBoff M, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–81.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Kull M, Kallikorm R, Lember M. Impact of a new sarco-osteopenia definition on health-related quality of life in a population-based cohort in Northern Europe. J Clin Densitom. 2012;15(1):32–8.
Binkley N, Buehring B. Beyond FRAX: it’s time to consider “sarco-osteopenia”. J Clin Densitom. 2009;12(4):413–6.
Sepulveda-Loyola W, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21(2):220–5.
Kirk B, Al Saedi A, Duque G. Osteosarcopenia: a case of geroscience. Aging Med (Milton). 2019;2(3):147–56.
Fatima M, Brennan-Olsen SL, Duque G. Therapeutic approaches to osteosarcopenia: insights for the clinician. Ther Adv Musculoskelet Dis. 2019;11:1759720x19867009.
Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 2015;16(4):290–5.
Ho-Pham LT, Nguyen UD, Nguyen TV. Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab. 2014;99(1):30–8.
Huh JH, Song MK, Park KH, Kim KJ, Kim JE, Rhee YM, et al. Gender-specific pleiotropic bone-muscle relationship in the elderly from a nationwide survey (KNHANES IV). Osteoporos Int. 2014;25(3):1053–61.
. Luo Y, Jiang K, He M. Association between grip strength and bone mineral density in general US population of NHANES 2013–2014. Arch Osteoporos. 2020;15(1):47 High Importance: This manuscript describes a US population study of hand grip strength and bone mineral density of the femoral neck and total lumbar spine. They found that grip strength can be associated with nonadjacent bones and grip strength of the dominant arm was highly correlated with BMD.
Locquet M, Beaudart C, Durieux N, Reginster JY, Bruyère O. Relationship between the changes over time of bone mass and muscle health in children and adults: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2019;20(1):429.
Karasik D, Kiel DP. Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res. 2008;23(6):788–802.
Huang J, Hsu YH, Mo C, Abreu E, Kiel DP, Bonewald LF, et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-kappaB signaling pathway. J Bone Miner Res. 2014;29(7):1531–40.
Medina-Gomez C, Kemp JP, Dimou NL, Kreiner E, Chesi A, Zemel BS, et al. Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus. Nat Commun. 2017;8(1):121.
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–3.
Gorski JP, Huffman NT, Vallejo J, Brotto L, Chittur SV, Breggia A, et al. Deletion of Mbtps1 (Pcsk8, S1p, Ski-1) gene in osteocytes stimulates soleus muscle regeneration and increased size and contractile force with age. J Biol Chem. 2016;291(9):4308–22.
Gorski JP, Price JL. Bone muscle crosstalk targets muscle regeneration pathway regulated by core circadian transcriptional repressors DEC1 and DEC2. Bonekey Rep. 2016;5:850.
Samsa WE, Vasanji A, Midura RJ, Kondratov RV. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone. 2016;84:194–203.
Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122(5):803–15.
Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci U S A. 2010;107(44):19090–5.
Riley LA, Esser KA. The role of the molecular clock in skeletal muscle and what it is teaching us about muscle-bone crosstalk. Curr Osteoporos Rep. 2017;15(3):222–30.
. Qin W, Dallas SL. Exosomes and extracellular RNA in muscle and bone aging and crosstalk. Curr Osteoporos Rep. 2019; High Importance: This review discusses the role of extracellular vesicles in bone-muscle crosstalk.
Ge M, Ke R, Cai T, Yang J, Mu X. Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun. 2015;467(1):27–32.
Cui Y, Luan J, Li H, Zhou X, Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–92.
Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, et al. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37–42.
Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J, et al. Characterization of regulatory extracellular vesicles from osteoclasts. J Dent Res. 2016;95(6):673–9.
Li D, Liu J, Guo B, Liang C, Dang L, Lu C, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7(1):10872.
Sun W, Zhao C, Li Y, Wang L, Nie G, Peng J, et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2:16015.
Qin Y, Peng Y, Zhao W, Pan J, Ksiezak-Reding H, Cardozo C, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J Biol Chem. 2017;292(26):11021–33.
Veno PPM, Dusevich V, Bonewald L, Dallas S. Osteocytes release microvesicles that regulate osteoblast function. J Bone Miner Res. 2013;28(Suppl 1):S253.
Sato M, Suzuki T, Kawano M, Tamura M. Circulating osteocyte-derived exosomes contain miRNAs which are enriched in exosomes from MLO-Y4 cells. Biomed Rep. 2017;6(2):223–31.
Lai X, Price C, Lu X(L), Wang L. Imaging and quantifying solute transport across periosteum: implications for muscle-bone crosstalk. Bone. 2014;66:82–9.
Pedersen BK, Febbraio M. Muscle-derived interleukin-6--a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun. 2005;19(5):371–6.
Pedersen BK, et al. Role of myokines in exercise and metabolism. J Appl Physiol (1985). 2007;103(3):1093–8.
Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–406.
McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90.
Hamrick MW, McPherron AC, Lovejoy CO. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif Tissue Int. 2002;71(1):63–8.
Hamrick MW, Samaddar T, Pennington C, McCormick J. Increased muscle mass with myostatin deficiency improves gains in bone strength with exercise. J Bone Miner Res. 2006;21(3):477–83.
Grobet L, Royo Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17(1):71–4.
Kambadur R, Sharma M, Smith TPL, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7(9):910–6.
Smith TP, et al. Myostatin maps to the interval containing the bovine mh locus. Mamm Genome. 1997;8(10):742–4.
Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350(26):2682–8.
Hamrick MW, Shi X, Zhang W, Pennington C, Thakore H, Haque M, et al. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone. 2007;40(6):1544–53.
Dankbar B, Fennen M, Brunert D, Hayer S, Frank S, Wehmeyer C, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. 2015;21(9):1085–90.
Chen Y-S, Guo Q, Guo LJ, Liu T, Wu XP, Lin ZY, et al. GDF8 inhibits bone formation and promotes bone resorption in mice. Clin Exp Pharmacol Physiol. 2017;44(4):500–8.
Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55(4):458–64.
Long KK, O’Shea KM, Khairallah RJ, Howell K, Paushkin S, Chen KS, et al. Specific inhibition of myostatin activation is beneficial in mouse models of SMA therapy. Hum Mol Genet. 2019;28(7):1076–89.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8.
Colaianni G, et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol. 2014;2014:–902186.
Huang L, Li C. Leptin: a multifunctional hormone. Cell Res. 2000;10(2):81–92.
Thomas T, Burguera B. Is leptin the link between fat and bone mass? J Bone Miner Res. 2002;17(9):1563–9.
Gimble JM, Nuttall ME. Bone and fat: old questions, new insights. Endocrine. 2004;23(2–3):183–8.
Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310(1–2):21–9.
Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, et al. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112(39):12157–62.
Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, et al. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep. 2016;6:18732.
Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone research. 2017;5:–16056.
Colaianni G, Mongelli T, Cuscito C, Pignataro P, Lippo L, Spiro G, et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci Rep. 2017;7(1):2811.
Kim H, et al. Irisin mediates effects on bone and fat via αV integrin receptors, 1756. Cell. 2018;175(7):–1768.e17.
Storlino G, et al. Irisin prevents disuse-induced osteocyte apoptosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2019. https://doi.org/10.1002/jbmr.3944.
Maisonneuve C, Igoudjil A, Begriche K, Lettéron P, Guimont MC, Bastin J, et al. Effects of zidovudine, stavudine and beta-aminoisobutyric acid on lipid homeostasis in mice: possible role in human fat wasting. Antivir Ther. 2004;9(5):801–10.
Note R, Maisonneuve C, Lettéron P, Peytavin G, Djouadi F, Igoudjil A, et al. Mitochondrial and metabolic effects of nucleoside reverse transcriptase inhibitors (NRTIs) in mice receiving one of five single- and three dual-NRTI treatments. Antimicrob Agents Chemother. 2003;47(11):3384–92.
Igoudjil A, Abbey-Toby A, Begriche K, Grodet A, Chataigner K, Peytavin G, et al. High doses of stavudine induce fat wasting and mild liver damage without impairing mitochondrial respiration in mice. Antivir Ther. 2007;12(3):389–400.
Begriche K, et al. Beta-aminoisobutyric acid prevents diet-induced obesity in mice with partial leptin deficiency. Obesity (Silver Spring, Md). 2008, 2053-2067;(16):9.
Begriche K, Massart J, Fromenty B. Effects of β-aminoisobutyric acid on leptin production and lipid homeostasis: mechanisms and possible relevance for the prevention of obesity. Fundam Clin Pharmacol. 2010;24(3):269–82.
Calvo JA, et al. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol (1985). 2008;104(5):1304–12.
Roberts LD, Boström P, O’Sullivan JF, Schinzel RT, Lewis GD, Dejam A, et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014;19(1):96–108.
Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jähn K, Yi J, et al. beta-Aminoisobutyric acid, l-BAIBA, is a muscle-derived osteocyte survival factor. Cell Rep. 2018;22(6):1531–44.
Zhu XW, Ding K, Dai XY, Ling WQ. β-aminoisobutyric acid accelerates the proliferation and differentiation of MC3T3-E1 cells via moderate activation of ROS signaling. J Chin Med Assoc. 2018;81(7):611–618. https://doi.org/10.1016/j.jcma.2017.12.005.
Shi CX, Zhao MX, Shu XD, Xiong XQ, Wang JJ, Gao XY, et al. beta-Aminoisobutyric acid attenuates hepatic endoplasmic reticulum stress and glucose/lipid metabolic disturbance in mice with type 2 diabetes. Sci Rep. 2016;6:21924.
Wang H, Qian J, Zhao X, Xing C, Sun B. beta-Aminoisobutyric acid ameliorates the renal fibrosis in mouse obstructed kidneys via inhibition of renal fibroblast activation and fibrosis. J Pharmacol Sci. 2017;133(4):203–13.
Wang Z, Bian L, Mo C, Shen H, Zhao LJ, Su KJ, et al. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun Biol. 2020;3(1):39.
Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508(Pt 3):949–53.
Nehlsen-Cannarella SL, et al. Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol (1985). 1997;82(5):1662–7.
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529(Pt 1):237–42.
Hiscock N, Chan MHS, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18(9):992–4.
Starkie RL, Angus DJ, Rolland J, Hargreaves M, Febbraio MA. Effect of prolonged, submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. J Physiol. 2000;528(Pt 3):647–55.
Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am J Phys Cell Phys. 2001;280(4):C769–74.
Juffer P, Jaspers RT, Klein-Nulend J, Bakker AD. Mechanically loaded myotubes affect osteoclast formation. Calcif Tissue Int. 2014;94(3):319–26.
Bakker AD, Kulkarni RN, Klein-Nulend J, Lems WF. IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J Dent Res. 2014;93(4):394–9.
McGregor NE, Murat M, Elango J, Poulton IJ, Walker EC, Crimeen-Irwin B, et al. IL-6 exhibits both cis- and trans-signaling in osteocytes and osteoblasts, but only trans-signaling promotes bone formation and osteoclastogenesis. J Biol Chem. 2019;294(19):7850–63.
Tresguerres FGF, Torres J, López-Quiles J, Hernández G, Vega JA, Tresguerres IF. The osteocyte: a multifunctional cell within the bone. Ann Anat. 2020;227:151422.
Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16.
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Rev Endocr Metab Disord. 2015;16(2):93–8.
Schaffler MB, Cheung WY, Majeska R, Kennedy O. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2014;94(1):5–24.
Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell ... and more. Endocr Rev. 2013;34(5):658–90.
Pi M, Quarles LD. Novel bone endocrine networks integrating mineral and energy metabolism. Curr Osteoporos Rep. 2013;11(4):391–9.
DiGirolamo DJ, Clemens TL, Kousteni S. The skeleton as an endocrine organ. Nat Rev Rheumatol. 2012;8(11):674–83.
Schaffler MB, Kennedy OD. Osteocyte signaling in bone. Curr Osteoporos Rep. 2012;10(2):118–25.
Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277(2):494–8.
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–5.
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Ren Physiol. 2009;297(2):F282–91.
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8.
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4.
Richter B, Faul C. FGF23 actions on target tissues-with and without Klotho. Front Endocrinol (Lausanne). 2018;9:189.
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408.
Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, et al. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304(8):E863–73.
Kido S, Hashimoto Y, Segawa H, Tatsumi S, Miyamoto KI. Muscle atrophy in patients wirh ckd results from fgf23/klotho-mediated supression of insulin/igf-i signaling. Kidney Research and Clinical Practice. 2012;31(2):A44.
Avin KG, Vallejo JA, Chen NX, Wang K, Touchberry CD, Brotto M, et al. Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility. Am J Physiol Endocrinol Metab. 2018;315(4):E594–e604.
Wang K, le L, Chun BM, Tiede-Lewis LAM, Shiflett LA, Prideaux M, et al. A novel osteogenic cell line that differentiates into GFP-tagged osteocytes and forms mineral with a bone-like lacunocanalicular structure. J Bone Miner Res. 2019;34(6):979–95.
McCormick, L.A., et al., Role of FGF9 in promotion of early osteocyte differentiation and as a potent inducer of FGF23 expression in osteocytes. J Bone Mineral Res, 2016. American Society of Bone and Mineral Research (ASBMR Annual Meeting – Atlanta, GA 2016): p. Abstract 1122.
Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144(5):796–809.
Chowdhury S, Schulz L, Palmisano B, Singh P, Berger JM, Yadav VK, et al. Muscle derived interleukin-6 increases exercise capacity by signaling in osteoblasts. J Clin Invest. 2020.
Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, et al. Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21(9):2585–96.
Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21(11):1262–71.
Medeiros MC, et al. Serum sclerostin, body composition, and sarcopenia in hemodialysis patients with diabetes. Int J Nephrol. 2020;2020:4596920.
Kim JA, Roh E, Hong SH, Lee YB, Kim NH, Yoo HJ, et al. Association of serum sclerostin levels with low skeletal muscle mass: the Korean Sarcopenic Obesity Study (KSOS). Bone. 2019;128:115053.
Hesse E, et al. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;5.
Girardi F, Le Grand F. Wnt signaling in skeletal muscle development and regeneration. Prog Mol Biol Transl Sci. 2018;153:157–79.
Rudnicki MA, Williams BO. Wnt signaling in bone and muscle. Bone. 2015;80:60–6.
Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway. JBMR Plus. 2017;1(2):86–100.
Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone. 2010;47(5):872–81.
Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M. Prostaglandin E2: from clinical applications to its potential role in bone- muscle crosstalk and myogenic differentiation. Recent Pat Biotechnol. 2012;6(3):223–9.
Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L, et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle. 2015;14(10):1507–16.
Bothwell W, Verburg M, Wynalda M, Daniels EG, Fitzpatrick FA. A radioimmunoassay for the unstable pulmonary metabolites of prostaglandin E1 and E2: an indirect index of their in vivo disposition and pharmacokinetics. J Pharmacol Exp Ther. 1982;220(2):229–35.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4.
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900.
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–23.
Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019;129(8):3214–23.
Hamoudi D, Bouredji Z, Marcadet L, Yagita H, Landry LB, Argaw A, et al. Muscle weakness and selective muscle atrophy in osteoprotegerin-deficient mice. Hum Mol Genet. 2020;29(3):483–94.
Boulanger Piette A, Hamoudi D, Marcadet L, Morin F, Argaw A, Ward L, et al. Targeting the muscle-bone unit: filling two needs with one deed in the treatment of Duchenne muscular dystrophy. Curr Osteoporos Rep. 2018;16(5):541–53.
Hamoudi D, Marcadet L, Piette Boulanger A, Yagita H, Bouredji Z, Argaw A, et al. An anti-RANKL treatment reduces muscle inflammation and dysfunction and strengthens bone in dystrophic mice. Hum Mol Genet. 2019;28(18):3101–12.
Brun J, Berthou F, Trajkovski M, Maechler P, Foti M, Bonnet N. Bone regulates browning and energy metabolism through mature osteoblast/osteocyte PPARgamma expression. Diabetes. 2017;66(10):2541–54.
Mera P, Ferron M, Mosialou I. Regulation of energy metabolism by bone-derived hormones. Cold Spring Harb Perspect Med. 2018:8(6).
Picca A, Calvani R, Manes-Gravina E, Spaziani L, Landi F, Bernabei R, et al. Bone-muscle crosstalk: unraveling new therapeutic targets for osteoporosis. Curr Pharm Des. 2017;23(41):6256–63.
Maurel DB, Jahn K, Lara-Castillo N. Muscle-bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines. 2017:5(4).
Compston J. Emerging therapeutic concepts for muscle and bone preservation/building. Bone. 2015;80:150–6.
Girgis CM. Integrated therapies for osteoporosis and sarcopenia: from signaling pathways to clinical trials. Calcif Tissue Int. 2015;96(3):243–55.
Funding
N.L-C. and MLJ are funded by a grant from the NIH NIA 2P01 AG039355 (Dr. Lynda F. Bonewald, Indiana University School of Medicine, PI)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nuria Lara-Castillo and Mark L. Johnson declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Muscle and Bone
Rights and permissions
About this article
Cite this article
Lara-Castillo, N., Johnson, M.L. Bone-Muscle Mutual Interactions. Curr Osteoporos Rep 18, 408–421 (2020). https://doi.org/10.1007/s11914-020-00602-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00602-6