Abstract
Purpose of Review
Bone marrow fat expresses mixed characteristics, which could correspond to white, brown, and beige types of fat. Marrow fat could act as either energy storing and adipokine secreting white fat or as a source of energy for hematopoiesis and bone metabolism, thus acting as brown fat. However, there is also a negative interaction between marrow fat and other elements of the bone marrow milieu, which is known as lipotoxicity. In this review, we will describe the good and bad roles of marrow fat in the bone, while focusing on the specific components of the negative effect of marrow fat on bone metabolism.
Recent Findings
Lipotoxicity in the bone is exerted by bone marrow fat through the secretion of adipokines and free fatty acids (FFA) (predominantly palmitate). High levels of FFA found in the bone marrow of aged and osteoporotic bone are associated with decreased osteoblastogenesis and bone formation, decreased hematopoiesis, and increased osteoclastogenesis. In addition, FFA such as palmitate and stearate induce apoptosis and dysfunctional autophagy in the osteoblasts, thus affecting their differentiation and function.
Summary
Regulation of marrow fat could become a therapeutic target for osteoporosis. Inhibition of the synthesis of FFA by marrow fat could facilitate osteoblastogenesis and bone formation while affecting osteoclastogenesis. However, further studies testing this hypothesis are still required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bone marrow microenvironment is a very active milieu, which involves multiple interactions between cells, hormones, growth factors, neural connections, and the vasculature [1,2,3]. These interactions are affected by the normal aging process and in higher proportion in conditions such as osteoporosis. The increasing volumes of marrow fat, which are observed in aging and osteoporosis, are associated with a set of local changes that are explained by the adipocytes and their secreted factors [4•, 5]. There is growing evidence that these factors regulate not only bone metabolism but also other functions of the bone marrow such as hematopoiesis [5, 6••].
There is still controversy whether high volumes of marrow fat play a negative, positive, or neutral role in the bone marrow [2, 7,8,9], which would depend on the physiological or pathological role of marrow fat and its secreted factors. Indeed, the understanding of the biological role of marrow adipocytes has gone from just replacing the vacuum left by other bone tissues to having very active thermogenic and metabolic roles [10, 11]. In contrast, other authors have proposed that marrow fat is a toxic presence within the marrow microenvironment [12, 13].
The hypothesis that the only biological role of marrow fat is to replace the vacuum left by decreased bone mass and lower hematopoiesis could be easily contradicted by evidence. The loss of bone mass observed in osteoporotic bone is not just replaced by marrow fat. In fact, marrow fat volumes gained in osteoporotic bones are much higher than the bone volumes lost in the same anatomical areas, thus suggesting that marrow fat is not there to replace the bone volumes, but that marrow fat could play a role in the pathophysiology of this disease [7, 10]. In this review, we will focus on both the positive and negative roles of marrow fat in bone biology. Recent evidence on the thermogenic and metabolic roles of marrow fat will be reviewed. In addition, the new evidence on the lipotoxic role of marrow fat will be summarized. Finally, the potential therapeutic applications of regulating lipotoxicity in the bone will also be discussed.
The Good Marrow Fat
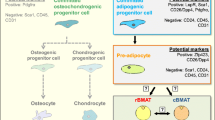
The question whether marrow fat has the characteristics attributed to white and/or brown fat would determine its predominant biological role (Fig. 1) [4•, 14]. Krings et al. [14] demonstrated that marrow fat has a very distinctive phenotype that resembles both white and brown adipose tissue (WAT and BAT, respectively), thus suggesting that marrow fat corresponds to a third type, which the authors described as “beige.” BAT is known to regulate thermogenesis and stimulate energy expenditure thus controlling body weight by promoting energy dissipation [15]. Beige fat contains multilocular droplets and is spread throughout WAT, albeit in low amounts during normal physiological conditions [16]. Earlier thought to exist only in small mammals and newborns, the presence of BAT in adult humans was established by fludeoxyglucose F 18 (18F-FDG)-PET/CT scans, and its location was established in the supraclavicular, axillary, and paravertebral regions [17]. Many studies have also established the thermo-regulatory role of BAT in response to cold [15, 18]. This effect is carried out by the sympathetic nervous system stimulation through norepinephrine by increasing the expression of uncoupling protein (UCP)-1, a protein that converts metabolic energy generated in the mitochondria to heat [19]. UCP-1 expression is induced by norepinephrine binding to the α-adrenergic receptors [20].
Biological roles of bone marrow fat. Marrow fat acting as a brown-like type stimulates bone formation and osteoblastogenesis. In contrast, when acting a yellow-like type, marrow fat secretes multiple factors which have been associated with a deleterious effect on hematopoiesis, bone metabolism, and bone quality
In contrast, WAT is composed of monolocular lipid droplets that function as a storage compartment for the release of lipids, when required. WAT is found in the viscerae and subcutaneously and displays several important physiological functions, including the storage of postprandial glucose as triglyceride, and the secretion of signaling factors that regulate appetite and energy homeostasis [21•,22]. Interestingly, contrary to its effect on WAT, caloric restriction has been associated with a significant increase in marrow fat, which may indicate that increasing volumes of this fat are induced as a potential metabolic supplier [23]. This could be explained by the capacity of marrow fat to store triglycerides similarly as WAT [24].
The beige characteristics of marrow fat suggest that marrow adipocytes constitute a particular adipose depot, which could have some beneficial roles associated with their brown component. For instance, marrow adipocytes store significant quantities of fat and produce adipokines, leptin, and adiponectin, which are known for their role in the regulation of energy metabolism [25]. In addition, marrow fat is found in areas of high bone turnover, thus suggesting that it could play a role as an energy provider in this process [26]. Scheller et al. [27••] highlighted the differences in marrow fat that occur due to spatial orientation and named distally located fat as constitutive marrow adipose tissue (cMAT) and the proximally located fat as regulated marrow adipose tissue (rMAT). cMAT is expressed earlier in life and is less affected by physiological changes, whereas rMAT is affected by physiological changes like temperature. Levels of marrow fat are higher in the axial skeleton where the temperature is higher. Also, higher marrow fat volumes occur in animals exposed to low temperatures [28••].
Beige fat secretes endocrine/paracrine factors that are beneficial for the skeleton. An interesting study by Rahman et al. [29], using FoxC2(AD)(+/Tg) mice, a well-established model for inducible beige fat, showed a beneficial effect on the bone. FoxC2(AD)(+/Tg) mice showed high bone mass due to increased bone formation associated with high bone turnover. Inducible BAT activated endosteal osteoblasts whereas osteocytes had decreased sclerostin expression and elevated levels of receptor activator of nuclear factor kappa-β ligand (RANKL). Also, conditioned media collected from beige adipocytes derived from FoxC2(AD)(+/Tg) mice activated osteoblast phenotype and increased phospho-AKT and β-catenin expression in recipient cells. This effect was reversed by using anti-wnt 10b and anti-I insulin-like growth factor binding protein 2 antibodies, confirming the involvement of endocrine/paracrine secretions in the observed anabolic effect of beige fat.
The beneficial role of marrow fat via their brown and white characteristics could be attenuated by conditions such as aging and diabetes [14]. In both conditions, the systemic energy metabolism and thermogenic response are significantly decreased. Marrow fat seems to play a role in this phenomenon by losing its capacity to assume a brown-like phenotype. This finding has been associated with alterations in the levels of several hormones (i.e., growth hormone and sex steroids), increasing levels of adipokines, and lower expression of brown fat gene markers (i.e., UCP1, Dio2, PGC1α, and β3 adrenergic receptor) [14].
The Bad Marrow Fat
It would be expected that when the phenotype of the bone marrow fat closely corresponds to WAT, the accumulation and concurrent secretion of fatty acids and adipokines could play a deleterious effect on the bone marrow microenvironment, a phenomenon known as lipotoxicity [30]. Lipotoxicity is defined as the effect of fat on nonadipose tissues [31]. These effects include generalized steatosis, lipotoxicity, and lipoapoptosis [31, 32]. Abnormal fat infiltration and lipotoxicity are observed in several organs and is associated with a variety of diseases [31]. Lipotoxicity of pancreatic beta-cells, myocardium, and skeletal muscle leads, respectively, to type 2 diabetes, cardiomyopathy, and insulin resistance [31, 33]. Sarcopenia, a disease that has very similar risk factors than osteoporosis, is also associated with fat infiltration and lipotoxicity [34, 35••]. Considering that fat infiltration of the marrow space is a regular finding in aged and osteoporotic bone, it has been proposed that lipotoxicity could explain some of the changes observed in bone metabolism and tissue quality usually associated with osteoporosis and fractures [8, 13].
Mechanisms and Consequences of Lipotoxicity in the Bone
Bone tissue homeostasis is largely regulated and maintained by the bone marrow microenvironment. Increasing levels of marrow fat would affect bone metabolism through a variety of mechanisms. This has been demonstrated by studies showing that osteoporosis can be introduced in normal mice by injection of total bone marrow cells from old mice directly into the bone marrow cavity of normal recipients [36,37,38,39]. However, the specific mechanisms involved in this lipotoxic effect have been partially explored.
Based on the observation that increasing levels of marrow fat are associated with low bone mass [40, 41], we initially explored the possibility that marrow fat behaves differently in young than older bone. To test this hypothesis, we characterized the age-related changes in cytokine expression in bone marrow flush as compared to subcutaneous fat isolated from young (4-month-old) and old (24-month-old) male C57BL/6J mice, using proteomic analysis. Proteins showing a significant change were grouped according to their known function in the bone. We found a significant age-induced difference in the expression of 53 cytokines. As compared with subcutaneous fat, aging bone marrow showed a more pro-adipogenic, anti-osteoblastogenic, and pro-apoptotic phenotype [42]. Our data suggested that, with aging, the bone marrow microenvironment becomes significantly more toxic than subcutaneous adipocytes. We concluded that these adipokines, if secreted, could play a role in the pathogenesis of age-related bone loss by affecting other cells within the marrow milieu.
The next question was whether the increasingly toxic profile of aging adipocytes could have an impact on other cells in their vicinity. MSC exposed to adipocyte-secreted factors lose their capacity to differentiate into bone cells while favoring adipogenesis [43, 44], an effect that could be explained by several mechanisms including oxidative stress, which favors adipogenesis, and secretion of inflammatory cytokines (i.e., IL-6 and TNFα) and adipokines.
To test whether adipokines are involved in osteoblast differentiation and function, we mimicked the bone marrow microenvironment in vitro using a two-chamber system co-culturing normal human osteoblast with differentiating pre-adipocytes in the absence or presence of the inhibitor of fatty acid synthase (FAS) cerulenin [45]. After 3 weeks in co-culture, osteoblasts showed significantly lower levels of differentiation and function as indicated by lower mineralization and expression of alkaline phosphatase, osterix, osteocalcin, and Runx2. In addition, osteoblast survival was decreased while also showing higher levels of apoptosis, which was associated with higher activation of caspases 3/7. Additionally, we analyzed the composition of the supernatants obtained from the osteoblast side of the chamber. We found that two free fatty acids (FFA)—palmitate and stearate—were the most prevalent FFA observed in these supernatants. Interestingly, a previous study in humans identified palmitate as the most prevalent FFA in the bone marrow of osteoporotic women [46], thus suggesting a potential role of palmitate in the pathogenesis of lipotoxicity in the bone.
Subsequently, we characterized the lipotoxic effect of palmitate on human osteoblasts [47•, 48]. Initially, we tested for changes in palmitoylation in this model. Palmitoylation is associated with the activation/inhibition of the aspartate-histidine-histidine-cysteine (DHHC) palmitoyl transferases genes that have a specific response to palmitate [49].
Initially, we found negative changes in the expression of palmitoyl transferase genes in human osteoblasts exposed to palmitate in vitro, thus confirming that palmitate is internalized and has a biological effect on osteoblasts. We found that palmitate negatively affected differentiation and bone nodule formation and mineralization by osteoblasts. Furthermore, from a mechanistic approach, we assessed changes in the nuclear activity of β-catenin and runt-related transcription factor 2 (Runx2)/phosphorylated mothers against decapentaplegic (Smad) complexes. Although the expression of β-catenin in palmitate-treated cells was not affected, there was a significant reduction in the transcriptional activities of both β-catenin and Runx2. A more recent study by Yeh et al. [50], confirmed these findings using fetal rat calvarial cells.
In addition to MSC and osteoblasts, osteoclasts could also be targeted by marrow fat thus increasing bone resorption and decreasing bone mass. Age-related marrow adipogenesis is linked to increased expression of RANKL [51•], which is a strong inducer of osteoclast differentiation and activity. Adipogenic transcription factors C/EBPβ and C/EBPδ, but not peroxisome proliferator-activated receptor γ, bind to the RANKL promoter and stimulate RANKL gene transcription with concomitant downregulation of osteoprotegerin. A similar study also reported that palmitate enhances RANKL-stimulated osteoclastogenesis and is sufficient to induce osteoclast differentiation even in the absence of RANKL [52]. Interestingly, in this study, adenovirus-mediated expression of diacylglycerol acyl transferase 1 (DGAT1), a gene involved in triglyceride synthesis, inhibits palmitate-induced osteoclastogenesis, suggesting a protective role of DGAT1 for bone health. Overall, by increasing osteoclastic activity and thus bone resorption while decreasing osteoblast differentiation and function, FFA (and predominantly palmitate) affect bone metabolism, which could add a lipotoxic component to the pathogenesis of osteoporosis.
Additionally to its deleterious effect on bone metabolism, marrow fat also affects hematopoiesis. Whereas a recent study reports that bone marrow adipocytes support hematopoietic stem cell survival [53], most of the studies have demonstrated that increasing levels of marrow fat have a negative effect on hematopoiesis. Bone-resident cells committed to the adipocytic lineage inhibit hematopoiesis and bone healing, potentially by producing excessive amounts of dipeptidyl peptidase-4, a protease that is a target of diabetes therapies [6••]. In addition, adipocytes of both rabbit and human secrete a soluble factor(s) that inhibits B lymphopoiesis. Pretreatment of BM mononuclear cells with adipocyte-conditioned medium dramatically inhibited their differentiation into proB cells in cocultures with OP9 stromal cells [54].
In summary, increasing levels of marrow fat are associated with global changes in the bone marrow microenvironment which includes low levels of osteoblastogenesis, decreased osteoblast function, reduced mineralization, high levels of bone resorption, and alterations in hematopoiesis. These effects are explained by both direct and indirect interaction between adipocytes and their secreted factors (predominantly FFA) and the cells in their vicinity.
Lipoapoptosis
Apoptosis plays an important role in bone metabolism. Whereas osteoclasts undergo apoptosis after a cycle of bone resorption, a percentage of osteoblasts undergo apoptosis under physiological conditions. However, the number of apoptotic osteoblasts is dramatically increased in aged and osteoporotic bone [55].
The intrinsic mechanisms explaining these high levels of osteoblast apoptosis remain unknown. Nevertheless, there are several identified triggers such as IL-6, TNFα, and corticosteroids, which activate a variety of apoptotic pathways in bone cells [56]. Although there is no evidence that high levels of marrow fat are also associated with osteoblast apoptosis in vivo, some in vitro studies suggest that adipocyte-secreted products could trigger osteoblast apoptosis, a process known as lipoapoptosis. Unger and Orci [57] defined lipoapoptosis as “a metabolic cause of programmed cell death, which has been identified to occur in obesity and aging”. Lipoapoptosis, which is usually mediated by palmitate [58], has been associated with the mechanisms of diabetes, cardiomyopathy, and fatty liver disease [57]. Considering that the fat infiltration observed in aged and osteoporotic bone is similar to the one observed in these diseases, it has been proposed that the apoptosis observed in the osteoblasts in aged and osteoporotic bone could be associated with the secretion of high levels of FFA by marrow fat into the bone marrow milieu.
Our group tested the effect of palmitate on osteoblast survival [48]. Lipoapoptosis was observed when osteoblasts were exposed to palmitate in the media, which induced apoptosis though the activation of the Fas/Jun kinase (JNK) apoptotic pathway. In addition, osteoblasts were rescued from palmitate-induced apoptosis adding the JNK inhibitor SP600125 to the media. Furthermore, other studies have identified additional mechanisms of lipoapoptosis in the bone. Kim et al. [59] reported that palmitate induces osteoblast apoptosis through the impaired activation of ERK. Dong et al. [60], used a co-culture system, to demonstrate that FFA-generated reactive oxygen species responsible for adipocytes-induced activation of ERK/P38 signaling thus of osteoblast lipoapoptosis. In addition, the phosphorylation of extracellular signal-regulated kinase (ERK)/P38 was increased, and inhibition of ERK/P38 significantly suppressed lipotoxicity.
Fat-Induced Autophagy
Autophagy has been identified as an important regulator of bone metabolism [61, 62]. Autophagy is an endocytic process that is mediated by lysosomes and is critical for an efficient cell defense to stressful stimuli [63]. Autophagy is responsible for the recycling of cellular materials, such as unwanted or damaged proteins, and the generation of amino acids during periods of starvation, as well as adaptive immunity.
The process of autophagy involves interaction among several key proteins. It starts with activation of UNC-51-like kinase (ULK), phosphorylation of autophagy protein (ATG) 13 and FIP 200 [64,65,66]. ULK1 phosphorylation leads to translocation of class III PI3K complex I, containing atg6 (also known as beclin), beclin 1-regulated autophagy (AMBRA). ATG14L, Vps15, and class III phosphoinositide 3-kinase (PI3K CIII) then generate phosphatidylinositol 3-phosphate (PI3P), leading to the formation of the autophagosome [67], which is then fused with a lysosome resulting in digestion of their contents.
Autophagy affects various cells types. In MSC derived from umbilical cord, autophagy leads to increased stemness [68]. In bone marrow-derived MSC, autophagy leads to increased survival under oxidative stress conditions [69]. Accumulation of large quantities of autophagic vacuoles have been reported in undifferentiated MSC [70]. These vacuoles are consumed during early osteogenic differentiation of MSC, suggesting that they are used as energy sources [71]. Autophagy is also important during differentiation in MSC and autophagy inhibitors like bafilomycin, NH4Cl, and shRNA-mediated knockdown of LC3 are reported to impair differentiation of dental pulp-derived MSC into osteoblasts [72].
Autophagy increases osteoblast differentiation and mineralization [71, 73]. Autophagic vesicles containing apatite needles are found in osteoblasts, suggesting their role in intracellular mineralization, a phenomenon that has been reported by several groups [74,75,76]. Autophagy inhibition in osteoblasts using 3-MA and chloroquine leads to impairment in osteoblast differentiation [73], as does knocking down of key genes in autophagy like atg7 and beclin 1 [71].
Autophagy failure has been linked not only to degenerative diseases of the nervous system, such as Alzheimer’s and Parkinson’s disease [77], but also to osteoporosis [78•]. In our study [48], palmitate induced dysfunctional autophagy in cultured osteoblasts, which was preceded by the activation of autophagosomes that surround palmitate droplets. In addition, osteoblasts were protected from lipotoxicity by inhibiting autophagy with the phosphoinositide kinase inhibitor 3-methyladenine.
In addition, autophagy is also reported in osteocytes [79]. Autophagy is induced in this cell type under stressful conditions like starvation, hypoxia [80], and oxidative stress [81]. However, whether FFA induce dysfunctional autophagy in osteocytes remains unknown.
Therapeutic Implications
Regarding reversibility of marrow fat-induced lipotoxicity, several potential therapeutic approaches have been recently tested. In our in vitro model, inhibition of FAS and thus of FFA production rescued osteoblasts from lipoapoptosis and lipotoxicity [45]. Another study has reported that bezafibrate, a fibrate drug used as a lipid-lowering agent to treat hyperlipidemia, inhibits palmitate-induced apoptosis via the NF-κB signaling pathway [82]. Gillet et al. [83] reported that oleate abrogates palmitate-induced lipotoxicity and proinflammatory response in human bone marrow-derived MSC and osteoblasts.
Overall, therapeutic approaches to lipotoxicity would involve one or some of the following approaches: (1) affecting the capacity of marrow adipocytes to produce lipotoxic products; (2) inhibiting lipoapoptosis and promoting autophagy of fat products; or (3) changing the type of marrow fat from a toxic (WAT-like) to a bone-forming phenotype.
Conclusion
Cell interactions within the bone marrow milieu are complex. Whereas brown-like and beige marrow fat seem to act similarly to other types of fat in the body, white-like marrow fat has a toxic effect on most of the cells in its vicinity. High levels of marrow fat observed in aged and osteoporotic bone have been associated with low bone mass. Although this finding could be explained by the release of several adipocyte-secreted factors, the current evidence indicates that palmitate is the predominant factor that is not only highly prevalent in the bone marrow but also has the strongest toxic effect on osteoblasts and hematopoietic cells while it also induces osteoclastic activity and bone resorption.
Understanding the role of marrow fat in regulating bone metabolism is pivotal. Marrow adipocytes have moved from being peripheral components of the pathogenesis of osteoporosis into major regulators of the bone marrow microenvironment and potential therapeutic targets. Therefore, lipotoxicity is a promissory target that deserves further exploration.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tamma R, Ribatti D. Bone niches, hematopoietic stem cells, and vessel formation. Int J Mol Sci. 2017;18(1):151.
Craft CS, Scheller EL. Evolution of the marrow adipose tissue microenvironment. Calcif Tissue Int. 2017;100:461–75.
Smith JNP, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31:1044–50.
• Hardouin P, Rharass T, Lucas S. Bone marrow adipose tissue: to be or not to be a typical adipose tissue? Front Endocrinol (Lausanne). 2016;7:85. Interesting and relevant review on the biology of marrow fat.
Paccou J, Hardouin P, Cotten A, Penel G, Cortet B. The role of bone marrow fat in skeletal health: usefulness and perspectives for clinicians. J Clin Endocrinol Metab. 2015;100:3613–21.
•• Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20:771–84. Excellent report describing the effect of marrow fat on hematopoiesis and bone metabolism.
Kawai M, de Paula FJA, Rosen CJ. New insights into osteoporosis: the bone–fat connection. J Int Med. 2012;272:317–29.
Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol. 2008;20:429–34.
Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteoporos Rep. 2011;9:67–75.
Hardouin P, Marie PJ, Rosen CJ. New insights into bone marrow adipocytes: report from the First European Meeting on Bone Marrow Adiposity (BMA 2015). Bone. 2016;93:212–5.
Hardouin P, Pansini V, Cortet B. Bone marrow fat. Joint Bone Spine. 2014;81:313–9.
Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors—a mini-review. Gerontology. 2011;57:66–75.
Ng A, Duque G. Osteoporosis as a lipotoxic disease. IBMS BoneKEy. 2010;7:108–23.
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–52.
Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63.
Park A, Kim WK, Bae KH. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells. 2014;6:33–42.
Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–8.
Rogers NH. Brown adipose tissue during puberty and with aging. Ann Med. 2015;47:142–9.
Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510:76–83.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
• Sulston RJ, Cawthorn WP. Bone marrow adipose tissue as an endocrine organ: close to the bone? Horm Mol Biol Clin Investig. 2016;28:21–38. Good review on fat and bone interactions.
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30.
Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CMH, et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology. 2016;157:508–21.
Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–24.
Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50:534–9.
Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30.
•• Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. Excellent report on regional distribution of marrow fat.
•• Devlin MJ, Rosen CJ. The bone–fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3:141–7. Comprehensive review on the role of marrow fat in health and disease.
Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013;154:2687–701.
Carobbio S, Pellegrinelli V, Vidal-Puig A. Adipose tissue function and expandability as determinants of lipotoxicity and the metabolic syndrome. Adv Exp Med Biol. 2017;960:161–96.
Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–36.
Mittendorfer B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr Opin Clin Nutr Metab Care. 2011;14:535–41.
Zlobine I, Gopal K, Ussher JR. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim Biophys Acta. 2016;1861:1555–68.
Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. 2015;12:22–6.
•• Ilich JZ, Kelly OJ, Inglis JE, Panton LB, Duque G, Ormsbee MJ. Interrelationship among muscle, fat, and bone: connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 2014;15:51–60. Excellent review on the interrelationship between bone, muscle, and fat.
Li J, Liu X, Zuo B, Zhang L. The role of bone marrow microenvironment in governing the balance between osteoblastogenesis and adipogenesis. Aging Dis. 2016;7:514–25.
Ueda Y, Inaba M, Takada K, Fukui J, Sakaguchi Y, Tsuda M, et al. Induction of senile osteoporosis in normal mice by intra-bone marrow-bone marrow transplantation from osteoporosis-prone mice. Stem Cell. 2007;25:1356–63.
Takada K, Inaba M, Ichioka N, Ueda Y, Taira M, Baba S, et al. Treatment of senile osteoporosis in SAMP6 mice by intra-bone marrow injection of allogeneic bone marrow cells. Stem Cells. 2006;24:399–405.
Ichioka N, Inaba M, Kushida T, Esumi T, Takahara K, Inaba K, et al. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells. 2002;20:542–51.
Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55:693–8.
Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2012;97:2782–91.
Gasparrini M, Rivas D, Elbaz A, Duque G. Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Exp Gerontol. 2009;44:613–8.
Casado-Díaz A, Santiago-Mora R, Dorado G, Quesada-Gómez JM. The omega-6 arachidonic fatty acid, but not the omega-3 fatty acids, inhibits osteoblastogenesis and induces adipogenesis of human mesenchymal stem cells: potential implication in osteoporosis. Osteoporos Int. 2013;24:1647–61.
Kruger MC, Coetzee M, Haag M, Weiler H. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res. 2010;49:438–49.
Elbaz A, Wu X, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med. 2010;14:982–91.
Griffith JF, Yeung DK, Ahuja AT, et al. A study of bone marrow and subcutaneous fatty acid composition in subjects of varying bone mineral density. Bone. 2009;44(6):1092–6.
• Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155:108–16. First report on the mechanisms of lipotoxicity in osteoblasts in vitro.
Gunaratnam K, Vidal C, Boadle R, Thekkedam C, Duque G. Mechanisms of palmitate-induced cell death in human osteoblasts. Biol Open. 2013;2:1382–9.
Greaves J, Chamberlain LH. DHHC palmitoyl transferases: substrate interactions and (patho)physiology. Trends Biochem Sci. 2011;36:245–53.
Yeh L-CC, Ford JJ, Lee JC, Adamo ML. Palmitate attenuates osteoblast differentiation of fetal rat calvarial cells. Biochem Biophys Res Commun. 2014;450:777–81.
• Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014;289:16699–710. This paper reports the connection between marrow adipogenesis and increased bone resorption.
Drosatos-Tampakaki Z, Drosatos K, Siegelin Y, Gong S, Khan S, van Dyke T, et al. Palmitic acid and DGAT1 deficiency enhance osteoclastogenesis, while oleic acid-induced triglyceride formation prevents it. J Bone Miner Res. 2014;29:1183–95.
Mattiucci D, Maurizi G, Izzi V, Cenci L, Ciarlantini M, Mancini S, et al. Bone marrow adipocytes support hematopoietic stem cell survival. J Cell Physiol. 2018;233(2):1500–11.
Bilwani FA, Knight KL. Adipocyte-derived soluble factor(s) inhibits early stages of B lymphopoiesis. J Immunology. 2012;189:4379–86.
Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am J Med. 2000;108:153–64.
Mollazadeh S, Fazly Bazzaz BS, Kerachian MA. Role of apoptosis in pathogenesis and treatment of bone-related diseases. J Orthop Surg Res. 2015;10:15.
Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–12.
Seeßle J, Liebisch G, Schmitz G, Stremmel W, Chamulitrat W. Palmitate activation by fatty acid transport protein 4 as a model system for hepatocellular apoptosis and steatosis. Biochim Biophys Acta. 2015;1851:549–65.
Kim JE, Ahn MW, Baek SH, Lee IK, Kim YW, Kim JY, et al. AMPK activator, AICAR, inhibits palmitate-induced apoptosis in osteoblast. Bone. 2008;43:394–404.
Dong X, Bi L, He S, Meng G, Wei B, Jia S, et al. FFAs-ROS-ERK/P38 pathway plays a key role in adipocyte lipotoxicity on osteoblasts in co-culture. Biochimie. 2014;101:123–31.
Veldhuis-Vlug AG, Rosen CJ. Mechanisms of marrow adiposity and its implications for skeletal health. Metabolism. 2017;67:106–14.
Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21:369–74.
Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16:461–72.
Ganley IG, Lam du H, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284:12297–305.
Hosokawa N, Sasaki T, Lemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5:973–9.
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, et al. ULK–Atg13–FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003.
Polson HE, de Lartigue J, Rigden DJ, Reedijk M, Urbe S, Clague MJ, et al. Mammalian Atg18 (WIPI2) localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Autophagy. 2010;6:506–22.
Hou J, Han ZP, Jing YY, Yang X, Zhang SS, Sun K, et al. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013;4:e844.
Song C, Song C, Tong F. Autophagy induction is a survival response against oxidative stress in bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2014;16:1361–70.
Nuschke A, Rodrigues M, Stolz DB, Chu CT, Griffith L, Wells A. Human mesenchymal stem cells/multipotent stromal cells consume accumulated autophagosomes early in differentiation. Stem Cell Res Ther. 2014;5:140.
Nollet M, Santucci-Darmanin S, Breuil V, Al-Sahlanee R, Cros C, Topi M, et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10:1965–77.
Pantovic A, Krstic A, Janjetovic K, Kocic J, Harhaji-Trajkovic L, Bugarski D, et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52:524–31.
Liu F, Fang F, Yuan H, Yang D, Chen Y, Williams L, et al. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res. 2014;28:2414–30.
Rohde M, Mayer H. Exocytotic process as a novel model for mineralization by osteoblasts in vitro and in vivo determined by electron microscopic analysis. Calcif Tissue Int. 2007;80:323–36.
Boonrungsiman S, Gentleman E, Carzaniga R, Evans ND, McComb DW, Porter AE, et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci U S A. 2012;109:14170–5.
Mahamid J, Sharir A, Gur D, Zelzer E, Addadi L, Weiner S. Bone mineralization proceeds through intracellular calcium phosphate loaded vesicles: a cryo-electron microscopy study. J Struct Biol. 2011;174:527–35.
Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–11.
• Hocking LJ, Whitehouse C, Helfrich MH. Autophagy: a new player in skeletal maintenance? J Bone Miner Res. 2012;27:1439–47. A good review on the role of autophagy in bone metabolism.
Jilka RL, O'Brien CA. The role of osteocytes in age-related bone loss. Curr Osteoporos Rep. 2016;14:16–25.
Zahm AM, Bohensky J, Adams CS, Shapiro IM, Srinivas V. Bone cell autophagy is regulated by environmental factors. Cell Tissues Organs. 2011;194:274–8.
Yang Y, Zheng X, Li B, Jiang S, Jiang L. Increased activity of osteocyte autophagy in ovariectomized rats and its correlation with oxidative stress status and bone loss. Biochem Biophys Res Commun. 2014;451:86–92.
Zhong X, Xiu L, Wei G, Pan T, Liu Y, Su L, et al. Bezafibrate prevents palmitate-induced apoptosis in osteoblastic MC3T3-E1 cells through the NF-κB signaling pathway. Int J Mol Med. 2011;28:535–42.
Gillet C, Spruyt D, Rigutto S, Dalla Valle A, Berlier J, Louis C, et al. Oleate abrogates palmitate-induced lipotoxicity and proinflammatory response in human bone marrow-derived mesenchymal stem cells and osteoblastic cells. Endocrinology. 2015;156:4081–93.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lackshman Singh, Sonia Tyagi, Damian Myers, and Gustavo Duque declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Bone Marrow and Adipose Tissue
Rights and permissions
About this article
Cite this article
Singh, L., Tyagi, S., Myers, D. et al. Good, Bad, or Ugly: the Biological Roles of Bone Marrow Fat. Curr Osteoporos Rep 16, 130–137 (2018). https://doi.org/10.1007/s11914-018-0427-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0427-y