Abstract
Purpose of Review
Craniopharyngiomas represent one of the most challenging diseases to treat. Despite their benign histology, and after many decades of surgical experience and technological advancements, there is still no clear consensus regarding the most effective management for this tumor. Due to their location and aggressive local characteristics, purely surgical approaches all too often result in unacceptable morbidity.
Recent Findings
Partial resection combined with radiation therapy results in similar control rates when compared to aggressive surgery, while also minimalizing the neuro-endocrinological morbidity.
Summary
In this manuscript, we describe the historical progression of the shifting strategies in the management of pediatric craniopharyngioma. Time has also altered our expectations for outcomes, evolving from purely morbidity and mortality to simple Glasgow Outcomes Scales, now to formal neuro-psychometric and quality of life data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas are uncommon, histologically benign, extra-axial, central nervous system (CNS) tumors, which arise from squamous epithelial cell remnants of Rathke’s pouch, along the path of the craniopharyngeal duct, an embryological tract from the nasopharynx to the diencephalon [1,2,3]. These tumors have a bimodal age distribution, with one peak at ages 5–14, and the second peak later in adulthood at ages 50–74, without a clear gender preference [1]. In the pediatric population, they are the most common suprasellar tumors and constitute about 5–15% of intracranial neoplasms [1, 3]. According to the new World Health Organization (WHO) classification of CNS tumors, craniopharyngiomas are classified as grade 1 tumors, with two distinct tumor types: adamantinomatous (aCP) and papillary (pCP) [4]. Due to their distinct clinical, demographic, molecular, and radiologic features, aCP and pCP are no longer considered to be subtypes of craniopharyngioma, but rather distinct tumor types [4]. Craniopharyngiomas most commonly arise in the suprasellar compartment, with occasional intrasellar extension [5]. Purely intrasellar location is possible. Extensions along the optic system, the ventricles, and the subarachnoid spaces and occasionally into the posterior fossa have been described [5,6,7,8,9,10]. Although they are considered benign, slowly growing tumors, they have “locally malignant” characteristics, mostly because they arise in a location crowded with vascular, visual, and endo-metabolic related structures. They can therefore result in a wide range of clinical manifestations [3, 11, 12]. Only rarely have cases of truly secondary malignant transformation, possibly triggered by irradiation, been reported [3, 13]. Likewise, CNS metastases may also be seen; however, these are more commonly iatrogenic following a previous intervention [14].
Despite their benign histology, craniopharyngiomas have troubled neurosurgeons over the years, ever since the time of Harvey Cushing, who has said that this tumor is “one of the most baffling problems that confront the neurosurgeon” [15,16,17]. In the current era of neurosurgery, craniopharyngiomas remain a challenge to even the most experienced neurosurgeon [18, 19]. Fierce debates are common during national and international meetings, as each neurosurgeon has his/her own theory and approach, leading to inconsistent treatment protocols, sometimes within the same neurosurgical department [18]. One certainty is that no single approach can be applied to all craniopharyngiomas, given the variability in tumor consistency (cystic, solid, calcified), location, and clinical presentation. A second certainty is, because they are neoplastic, they will inevitably enlarge over time; hence, they should generally be approached with curative intent.
Adult and pediatric craniopharyngiomas differ in the predominant histological type, macroscopic appearance, radiological characteristics, clinical presentation, genetics, recurrence rate, and mortality rates [12, 20•]. aCP are seen almost exclusively in children, whereas pCP are found almost exclusively in adults [12, 21]. Therefore, it now seems only logical that their treatment paradigms should be different and distinct. However, in the earliest days of neurosurgical treatment for these tumors, as experience with pediatric craniopharyngiomas was just beginning to accrue, the treatment approach used for adult craniopharyngiomas was replicated with children. There was, in the beginning, no real understanding or acknowledgment that these were actually different diseases with different implications and prognoses. Today, despite our improved understanding of the tumor types and their various manifestations and effects, treatment of craniopharyngioma in general, and pediatric craniopharyngioma, in particular, continues to be challenging; optimal management remains controversial [21,22,23].
This review describes the shifting trends in pediatric craniopharyngioma management over the last few decades. We begin with a review of the history of craniopharyngioma treatment, and then follow the different treatment approaches as understanding has increased over time, until we reach the currently accepted standards of treatment.
The First Descriptions
In 1838, Martin Rathke, following his mentor Karl Ernst von Baer, described for the first time the embryological origin of the pituitary gland [24]. A few years later, in 1857, Zenker first described a suprasellar mass of squamous epithelium cells containing cholesterol crystals [25,26,27]. Subsequently, in 1860, Luschka further studied the occurrence of squamous cells in the adenohypophysis area [28, 29]. The first hints of craniopharyngiomas date back to 1861, when Wagner described a tumor situated behind the optic chiasm, displacing the optic tracts, that was composed of small cysts with layers of epithelium, resembling in part the oral epithelium. Wagner noted that the ventricles were normal and that the anterior lobe of the hypophysis was intact [30]. In 1875, Von Mihalkovitcs demonstrated the embryonical stages in the formation of the pituitary gland. He also showed that the cells forming Rathke’s vesicle form a cavity structure known as Rathke’s cleft [24, 31]. Mihalkovitcs proposed a theory that the developing adenohypophysis undergoes a rotation upward and forward, causing the cells that were originally at the insertion of the craniopharyngeal duct to be drawn away and acquire a new position above the gland [24, 29, 31]. This theory might explain the predilection of craniopharyngiomas to originate in the suprasellar region [5, 24]. For many decades to come, although the occurrence of these cells in this area was clear, their clinical significance was not.
It was only in 1902, over 25 years later, that Saxer reported on an epithelial tumor, consisting of a mixture of epithelial cells that morphologically resembled the cells of the pars intermedia, compressing and distending the surrounding structures [24, 30, 32]. Notably, the anterior lobe of the hypophysis was normal; the tumor was found to be unconnected to the choroid plexus [30, 32]. In the same year, Cushing unsuccessfully operated on a 16-year-old obese and sexually undeveloped woman [16]. Although accurate pathological diagnosis could not be confirmed, there are multiple sources offering evidence that seems to indicate that this tumor was actually a craniopharyngioma [16]. In 1904, Erdheim thought that these epithelial cells can only be found in the glands of adult patients [33]. Based on anatomical and autopsy studies, Erdheim believed that these unknown tumors originated from the same nest of cells, and named them “hypophyseal duct neoplasms” [24, 33]. The first successful report of surgical resection of a tumor resembling this type (“Erdheim tumor”), through a transsphenoidal approach, was performed on a 39-year-old male with bi-temporal hemianopsia and polyuria. This operation was completed by Halstead at St. Luke’s Hospital in Chicago in 1910 [25, 27, 29, 30]. It was not until 1932 that Susman described similar squamous cells in the pituitary gland of children [24, 34]. In the same year, Cushing coined the name craniopharyngioma and believed these tumors originated from epithelial cells of the hypophyseal duct or craniopharyngeal duct [15, 16, 27].
The Radical Resection Era
Since the first successful removal of a craniopharyngioma in 1910, the technology has evolved [27]. During the 1950s, the operating microscope and micro-neurosurgery were introduced. Surgical options were expanded, including advances in the transsphenoidal approach, revived by Guiot and Hardy [27, 35,36,37]. In the 1960s, with the advent of oral glucocorticoids, Matson et al. published their experience with 57 children, advocating that surgeons should make every effort to achieve radical surgery at the initial operation [38]. In Matson’s experience, operating upon recurrent craniopharyngiomas was associated with unacceptable mortality and morbidity. The 1980s to 1990s represent the peak period of craniopharyngioma surgery, characterized by aggressive surgical management. In line with the benign histology of these tumors, surgeons were ideally striving for gross total resection (GTR) [37, 39,40,41,42].
However, achieving radical resection without significant damage to surrounding anatomical structures was, in many cases, surgically challenging, in part because of the calcified tumor capsule adhered to the surrounding vital structures [43]. During that period, it became clear that GTR was associated with decreased recurrence rate, but also with significantly higher morbidity [27, 42,43,44,45]. Yasargil et al. reported a GTR rate of approximately 90%, while acknowledging that it was associated with great morbidity [46]. Subsequently, Hoffman et al. reported their experience with GTR in pediatric patients, also with significant morbidity and mortality [47].
During this period, Elliott et al. showed that even with GTR, the recurrence rate was unacceptably high within a relatively short time period [48]. Nevertheless, they supported the radical resection approach for children aged 5 years or younger, claiming good disease control while avoiding the late effects of radiotherapy [49]. However, a systematic review by Clark et al. showed a high recurrence rate of 35% for pediatric patients treated with GTR only [50]. In parallel, Hetelekidis et al. reported their 20-year long-term follow-up showing that while overall survival was similar between all patient groups, patients who were treated with surgery alone had significantly worse progression-free survival rates and higher morbidity rates compared to children treated with radiation alone or in combination with surgery [51]. More recently, a 20-year long-term follow-up study showed that overall survival and progression-free survival are not correlated with the extent of resection and that the overall survival is lower when there is hypothalamic involvement [52]. Unlike most other childhood brain tumors, with craniopharyngioma, the extent of surgical resection is a poor predictor of both survival and quality of life.
Acknowledgment of Hypothalamic Involvement: Anatomical Classification
During the time period following the 1990s enthusiasm for GTR, it became clear that this management approach was associated with high postoperative morbidity [37, 46]. One of the main reasons for the shift in the paradigm for the management of pediatric craniopharyngiomas was the recognition of the importance of hypothalamic injury as a main cause of morbidity. Caldarelli et al., who argued for an aggressive surgical approach, admitted that extensive hypothalamic involvement should lead to a less aggressive attitude, as the postoperative complications are unacceptable and can even lead to death [40]. Among the many surgical risks, injury to the hypothalamus may result in hypothalamic syndrome, characterized by intractable severe morbid obesity, diverse endocrine abnormalities, and metabolic disorders. In addition, hypothalamic injury can lead to a variety of neurocognitive and neurobehavioral problems, as described below [12, 53, 54].
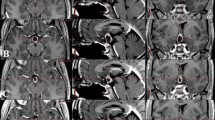
In the past, the classification systems used for craniopharyngiomas, as suggested by Yasargil et al., were based mainly on the anatomical involvement of the surrounding structures, with respect to the diaphragm and the third ventricle, and were used to identify the optimal surgical approach [37, 46]. In 2007, Puget et al. published their radiographic classification of craniopharyngiomas and hypothalamic involvement [55], using a different methodology. Puget et al. classified craniopharyngiomas, based on their physical proximity to the hypothalamus on preoperative MRI, into 3 grades (Fig. 1). Grade 0 means that the tumor has no contact with the floor of the third ventricle. Grade 1 means that the tumor has contact with the hypothalamus anterior to the mammillary bodies but the hypothalamus is still visible. Grade 2 indicates the greatest hypothalamic involvement, in which the tumor both compresses and is infiltrative to the hypothalamus, to a point where the hypothalamus is no longer visible [37, 55, 56]. This classification system was able to categorize tumor subgroups for which GTR was associated with increased hypothalamic morbidity, affecting the patient’s quality of life (QOL). Puget et al. demonstrated an improved outcome when surgical treatment goals were tailored to the tumor subgroup [37, 55, 56]. Today, it is accepted that treatment goals must include a strong emphasis on the QOL of the child, while not compromising tumor control. Treatment plans try to maximize QOL as well as the length of life, keeping in mind that craniopharyngiomas are usually slow-growing, low-grade tumors, and as such, should be addressed as a chronic illness [21, 22, 52, 56, 57].
Craniopharyngioma Puget’s grading system. Three grades of hypothalamic involvement have been defined. a Sagittal T2-weighted MRI of a preoperative grade 0 craniopharyngioma, with no hypothalamic involvement. The third ventricle floor in normal and the craniopharyngioma lies entirely below the sellar diaphragm (arrows). b Sagittal T2-weighted MRI of a preoperative grade 1 craniopharyngioma, with compression of the hypothalamus that can still be identified (from the mammillary bodies to the level of the infundibulum). A bulk and/or calcified portion of the tumor may have developed in the infundibulum. The sellar diaphragm is opened in its posterior part with an extension of the cystic part (arrow). c Sagittal T2-weighted MRI of a preoperative grade 2 CP in which the hypothalamus is unidentifiable. Adapted from Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5(1):75. https://doi.org/10.1038/s41572-019-0125-9
Acceptable Morbidity: Changing the Concept
When dealing with an individual child with a craniopharyngioma, one should consider the question of what is an acceptable morbidity. What are we willing to risk, while attempting tumor resection and control, vis-à-vis the alternatives. The potential morbidities of craniopharyngioma surgery and treatment can be divided into several categories:
-
1.
Neurological disorders: Severe neurological deficits such as refractory epilepsy, multiple cranial nerve deficits, or hemiparesis/plegia are considered non-acceptable. These are usually the result of vascular compromise [58,59,60].
-
2.
Visual disorders: Inflicting additional visual loss is considered unacceptable morbidity and is also associated with increased mortality [40, 61]. A unilateral visual loss might be considered acceptable, depending on the preoperative visual condition, and should be heavily weighed against the benefits of more radical surgery, keeping in mind that the vision may still deteriorate postoperatively following decompression [62, 63]. Between 30 and 50% of pediatric patients have some visual complaints as their presenting symptoms, and the reported rates of unilateral or bilateral blindness range between 3 and 13% [41, 63,64,65,66], values that are probably significantly underestimated due to young children’s inability to effectively appreciate or communicate visual complaints.
-
3.
Endocrinological disorders: The management of craniopharyngioma is complex and requires a multidisciplinary team and resources. Acceptable standards for endocrinological morbidity might differ between countries (i.e., standards could be geographically/culturally determined). These issues are also heavily reliant upon the general medical care at a location, which is largely influenced by the social-economic state prevailing in the country [67]. Some endocrinological disorders might be considered unacceptable in low-resource countries, where the availability of hormonal replacement therapy is beyond the reach of most patients [68]. In Western countries, or where pediatric endocrinological service is more readily available, receiving replacement therapy for panhypopituitarism is usually well coordinated, even when requiring frequent adjustment and tests. Growth hormone replacement is considered safe and has even been associated with lower recurrence rates and improved cognitive performance [69, 70•].
Diabetes insipidus (DI) requires a different dimension of care. Differentiation between DI with or without thirst mechanisms (i.e., adipsic DI) is important, as preservation of thirst mechanisms will simplify the maintenance of fluid and electrolyte balance [71, 72]. Those with hypothalamic damage that abolishes the thirst mechanisms will experience more fluid balance fluctuations, resulting in hypo/hyper-natremia that can lead to structural damage and death. Those patients should be closely monitored and followed, as they are in significant danger.
-
4.
Metabolic syndrome and hypothalamic obesity: Severe, morbid, and highly intractable obesity due to hyperphagia is part of hypothalamic syndrome. This might occur with damage to the posteriorly located hypothalamic nuclei, specifically from damage to the ventromedial hypothalamus [73,74,75]. The obesity is characterized by rapid and refractory weight gain, and is the result of an energy imbalance between uncontrollable increased food intake and decreased basal metabolic rate [72, 74]. Hypothalamic obesity occurs in about 40–60% of children with hypothalamic damage. It is this factor that most closely correlates with poorer quality of life and decreased overall lifetime survival [12, 21, 54, 56, 57, 73, 76]. Currently, there are no effective medical or surgical treatments for this resistant condition. The best treatment is prevention and early recognition [77, 78]. The severe obesity is also associated with impaired social-cognitive skills, disordered sleep patterns, and attentional/behavioral problems. Features of this metabolic syndrome include higher abdominal fat and adverse lipid profile, obstructive sleep apnea, decreased insulin sensitivity, diabetes mellitus, and hypertension, all of which increase the risks for cardiovascular co-morbidities in adolescents and adults [79,80,81,82].
-
5.
Neurocognitive/behavioral disorders: While not considered immediately life-threatening, many neurocognitive and neurobehavioral issues, including personality changes, hypothalamic obesity, memory impairment, attention deficits, reduced IQ, psychotic symptoms, and emotional lability, might appear following damage to the hypothalamus and mamillary bodies. All these issues highly influence the QOL of the child and may lead to greater dependence with age, when compared with the general population [12, 53, 54, 83, 84]. Thus, the presence of diabetes insipidus and hypothalamic obesity portends a patient who is likely to require long-term supervised care, is not likely able to maintain employment, and will have limited ability to contribute to society.
Alternative Treatments
In addition to surgical resection, other treatment modalities exist. These modalities can complement surgery and should be considered in each patient individually, especially in terms of aggressive surgery versus alternative options.
Acknowledgment of the Effectiveness of Radiotherapy (RT), and Its Combination with Conservative Resection
The first report on the use of limited surgery combined with RT was by Kramer et al. in 1961 [85, 86]. In 1980, Richmond et al. shared their experience with 32 patients. Twelve patients had surgery alone (total or subtotal resection). Twenty patients had limited surgical management followed by RT, consisting of subtotal resection (STR) plus RT for 12 patients and biopsy with or without cyst aspiration followed by RT for 8 patients. They suggested that a conservative approach, for selected patients, resulted in outcomes comparable to those achieved with more aggressive treatment [87]. In 1984, Hoogenhout et al. reported their experience with 26 patients who underwent STR, half of whom were children. Thirteen of the patients received immediate postoperative RT, while the other group was followed. The irradiated group showed a much lower 5- and 10-year recurrence rate (11% vs. 45% and 25% vs. 71%, respectively), and no mortality [88]. In 1989, Weiss et al. published their series of 31 children, of whom 12 had STR, while the rest had GTR. Among these 12 STR patients, 5 patients received RT. Four (80%) of these patients were stable at a median follow-up of 89 months, while all 7 children who did not receive RT experienced recurrences at a median follow-up of 12 months [89]. However, it is important to recognize that, in that era, radiotherapy was administered by photons delivered using two opposing beam fields. As such, large volumes of normal frontal and temporal lobe tissue were included in the high-dose radiation field, with associated collateral damage.
Since that time, advancements in RT techniques allow more precise localization and less exposure of the adjacent normal brain [21, 86, 90,91,92]. In the early studies, disease control rates, using X-rays for treatment planning, were between 71 and 90%. Later, in the modern studies, the experience gained with 3D conformal radiation enabled control rates that were significantly higher [51, 88, 92,93,94]. Kiehna and Merchant have shown that, using conformal or intensity-modulated radiation therapy both for newly diagnosed and recurrent pediatric diseases, tumor control can be achieved with a reduced volume of irradiation which is comparable to most prior surgical series [86]. Stripp et al. have shown that there is no significant difference in overall survival between pediatric craniopharyngioma patients who had a GTR, and those undergoing STR with postoperative RT [93]. In addition, they showed that local control at 10-year follow-up was statistically higher in the STR + RT group when compared to the radical surgical group alone (Fig. 2) [93]. These results have been replicated by other groups, who have found that STR alone results in a higher 3-year tumor progression rate of 43–67% [21, 50, 57], as opposed to STR followed by RT, resulting in tumor progression rates of 15–35% [21, 50, 86, 95]. These tumor control rates were similar to those achieved through GTR, with many fewer neuro-endocrinological and hypothalamic risks [96]. More recently, Gorelyshev et al. also concluded from their large cohort of patients that the addition of adjuvant RT after STR or at the time of tumor progression results in similar control rates as those obtained by GTR [97].
Kaplan–Meier curves of local control and overall survival in craniopharyngioma patients. There is a significant difference in the local control (a) between patients treated with surgery only (solid line) compared to surgery + radiotherapy (dotted line), while not demonstrating similar difference in overall survival (b). The p values were determined by log–rank tests and are denoted on the graphs. Times when patients were censored are denoted by diamonds. Adapted from Stripp DCH, Maity A, Janss AJ, et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58(3):714–720. https://doi.org/10.1016/S0360-3016(03)01570-0
Proton vs. Photons
The proximity of the tumor to eloquent neural and vascular structures makes safe delivery of RT technically challenging [98]. Many modalities have been used over the years to deliver radiation, ranging from the conventional external beam RT (EBRT) to intra-cystic radioisotope injections, to more modern technologies, including three-dimensional conformal RT (3D-CRT), intensity-modulated RT (IMRT), and stereotactic irradiation (SRT) techniques, in addition to the emerging proton RT. Each has its own inherent advantages and disadvantages [94, 98,99,100,101]. Fractionated RT helps maximize tumor control while minimizing the damage to healthy tissues, emphasizing its importance in the pediatric population. At doses of approximately 54 Gy, delivered in fractionation of 1.8 Gy per fraction, RT achieves good long-term tumor control and functional results [90, 94, 102, 103]. Single-dose irradiation methods (radiosurgery) may be appropriate for small, circumscribed, tumor masses, situated away from the optic apparatus [90, 91, 94, 104]. Radiosurgery is, however, associated with inferior tumor control. Due to the inherent limitations, radiosurgery is currently used mainly in patients who have failed fractionated RT, and may in general be more suitable for adult craniopharyngiomas [90, 91, 94].
3D-CRT and IMRT have become the standard of care because of their ability to decrease radiation dose volume. Merchant et al. reported on their phase II clinical trial, demonstrating local control equivalent to conventional EBRT when using CRT with a 1-cm tumor margin, with 90% 3-year PFS with limited cognitive morbidity [94]. Greenfield et al. reported their experience with IMRT, showing about 70% 3-year PFS, with more favorable results in solid tumors compared to cystic tumors [100].
Protons have similar radiobiological effects as photons. However, due to the Bragg peak effect, a higher dose of energy can be delivered to a defined field, with limited scattered doses, thus sparing surrounding normal tissues [98, 101, 105,106,107, 108•]. Proton radiotherapy has been shown to be associated with fewer acute and chronic side effects in many brain tumor patients, especially those who underwent craniospinal irradiation [109,110,111,112,113].
These reported benefits have been clearly demonstrated for other types of malignancies; the situation is less straightforward in the management of pediatric craniopharyngioma. Initial results for craniopharyngioma have been promising [101, 102, 105, 114]. Fitzek et al. treated 15 patients with craniopharyngioma, among them 5 children, with combined photon-protons RT, and reported 0% recurrence rate among the children, followed for a median time of 13.1 years [114]. More recently, Jimenez et al. reported on their large cohort of pediatric patients, treated with adjuvant proton RT, achieving an excellent 90% local control rate at 5 years [108•]. Furthermore, Merchant et al. showed that protons reduced the overall dose distribution to the brain, cochlea, and hypothalamus, suggesting early on that it may minimize intelligence diminution after radiotherapy (Fig. 3) [107]. It is important to note that as we move to more focal treatment paradigms in an effort to reduce damage to normal tissues but maximize dose delivery to the tumor, highly circumscribed fields run the risk of incomplete coverage as the cystic components of craniopharyngiomas will usually change size over a 6-week treatment period. Thus, the original treatment plan developed at the beginning of therapy with 5–10-mm margins needs to be updated several times over the course of treatment with updated imaging. Merchant et al. have shown a significant increase in tumor control in patients whose treatment plans are updated with a weekly scan [103].
Protons minimization of intelligence diminution after radiotherapy. Estimated IQ for patients ages 5 and 9 with craniopharyngioma planned for treatment with scanning proton beam therapy and conformal photon radiation therapy. Adapted from Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008 Jul;51(1):110–7. https://doi.org/10.1002/pbc.21530
Bishop et al. compared proton RT to IMRT and failed to demonstrate the superiority of one technique over the other, in terms of overall survival, disease control, and late toxicity, including secondary malignancies and vascular complications (Fig. 4) [115]. In addition, they found no difference in the effectiveness of either RT method in terms of the initial tumor size [115]. Recently, Merchant et al. showed in a large prospectively study, of two groups of patients treated post-surgically with proton (94 patients) or photons (101 patients), with a median follow-up of 7.6 years, that RT side effects are similar between the modalities in terms of necrosis, vasculopathy, vision, or permanent neurologic deficits. In addition, 5-year PFS and OS were statistically similar (Fig. 5). The main difference, however, was that cognition was better preserved in the proton-treated group compared to the photon-treated group (Fig. 6) [116••].
Survival curves according to treatment. A Overall survival. Treatment modality, proton beam therapy (dotted line) vs intensity-modulated photon radiation therapy (IMRT) (solid line) did not affect survival outcomes. Adapted from Bishop AJ, Greenfield B, Mahajan A, et al. Proton Beam Therapy Versus Conformal Photon Radiation Therapy for Childhood Craniopharyngioma: Multi-institutional Analysis of Outcomes, Cyst Dynamics, and Toxicity. International Journal of Radiation Oncology*Biology*Physics. 2014;90(2):354–361. https://doi.org/10.1016/j.ijrobp.2014.05.051
Progression-free survival (A) and overall survival (B) of pediatric patients with craniopharyngioma treated with passively scattered proton therapy compared to photon conformal and intensity-modulated radiation therapy. In the proton therapy cohort, 3-year progression-free survival was 96.8% (95% CI 90.4–99.0) and 5-year progression-free survival was 93.6% (86.3–97.1). In the photon therapy cohort, 3-year progression-free survival was 96.0% (95% CI 89.7–98.5) and 5-year progression-free survival was 90.0% (82.2–94.5). The number of events for progression-free survival at 5 years was 6 (6%) of 94 for proton and 10 (10%) of 101 for photon. Progression was defined as an increase of 25% or more in the perpendicular tumor dimensions on two or more successive imaging evaluations 2–3 years after treatment and the date recorded as the earliest site of increase in tumor dimensions. Adapted from Merchant TE, Hoehn ME, Khan RB, Sabin ND, Klimo P, Boop FA, Wu S, Li Y, Burghen EA, Jurbergs N, Sandler ES, Aldana PR, Indelicato DJ, Conklin HM. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study. Lancet Oncol. 2023 May;24(5):523–534. https://doi.org/10.1016/S1470-2045(23)00146-8
Modeled longitudinal intellectual quotient (IQ) scores comparing pediatric patients with craniopharyngioma treated with passively scattered proton therapy to photon conformal and intensity-modulated radiation therapy. The patients, 81 in the proton cohort (solid line) and 67 in the photon cohort (dotted line), were evaluated at baseline and annually through year 5 after radiation therapy using age-appropriate Wechsler scale. The differences in the curves were statistically significant IQ (1.0939 points/year, p = 0.007). Normal IQ = 100 ± 15. Adapted from Merchant TE, Hoehn ME, Khan RB, Sabin ND, Klimo P, Boop FA, Wu S, Li Y, Burghen EA, Jurbergs N, Sandler ES, Aldana PR, Indelicato DJ, Conklin HM. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study. Lancet Oncol. 2023 May;24(5):523–534. https://doi.org/10.1016/S1470-2045(23)00146-8
Still, however, despite promising results, there are several limitations for protons use. There are limited numbers of proton centers available worldwide with the expertise and equipment to treat children. This makes proton accessibility challenging, causing a high impact on the cost of health care services and adversely affecting the development of high-quality multicenter trials [117,118,119,120]. Furthermore, in many cases, the limited availability forces the patient and family to seek medical care abroad, resulting in many logistical, emotional, and financial burdens. All these factors intensify the dilemma of whether or not to send a child for proton radiotherapy, which becomes even less clear as the child grows.
Today, it is clear that RT is an integral component of pediatric craniopharyngioma management protocols. Modern modalities maximize the delivered radiation dose, allowing for better local tumor control, while minimizing associated morbidity. Many studies have failed to demonstrate any advantage for early adjuvant RT postoperatively [91, 93, 121, 122]. Thus, following a limited surgical resection, RT may be delayed; yet, a too long delay is not recommended, as tumor progression may occur.
Side Effects of Radiation Therapy in Children
Surgical patients are more likely to experience acute, often permanent neuro-endocrinological deficits, which most likely result from mechanical disruption of surrounding anatomical structures (e.g., pituitary stalk and vascular injury). RT patients, on the other hand, are more likely to exhibit late sequelae, including endocrine, visual, cognitive, psychological, vasculopathy, and secondary malignancies, which may be devastating (especially in young children) [19, 83, 119, 120].
-
Endocrinological side effects: The exact extent of additional risks contributed by adjuvant RT to postoperative endocrinopathy is difficult to estimate, in part because many children may present with some deficiency, and additional deficiency may be caused by the surgical intervention [23, 101, 123]. Preoperative endocrinological deficits happen in children quite often, with growth hormone deficiency being the most common presentation, followed by gonadotropin deficiency [124]. Recently, Merchant et al. retrospectively evaluated the extent of endocrinopathy in pediatric craniopharyngioma patients, including those who had only limited surgery and photon-based therapy, with a 10-year follow-up [125]. They found variable results, influenced by cofactors such as hydrocephalus status, race, and the presence or absence of diabetes insipidus. Their findings included:
-
o
A 10-year cumulative incidence of growth hormone deficiency, in the range of 68–94%.
-
o
A cumulative incidence of thyroid-stimulating hormone deficiency, of approximately 70–91%.
-
o
A cumulative incidence of adrenocortical hormone deficiency, between 48 and 70%.
-
o
A cumulative incidence of LH/FSH deficiency, between 43 and 79% [125].
-
o
Posterior pituitary dysfunction (DI) is not considered to be a sequela of RT. Yet the incidence of anterior pituitary dysfunction appeared to be radiation dose-dependent [86, 125, 126]. Some deficits, related to the loss of homeostasis regulation, such as morbid obesity and hyperphagia, or diabetes insipidus with or without thirst mechanisms, are unique and usually directly related to surgical resection, and rarely seen after RT [123].
-
Visual side effects: The risk of visual acuity decline following RT for pediatric brain tumors in children is generally low [127]. Irreversible optic neuropathies, caused by progressive ischemia and necrosis of the optic apparatus, are dose-dependent [86, 101]. It appears that with a limiting dose of 56 Gy, optic neuropathy was significantly less common [86, 101, 123, 127,128,129,130].
-
Vasculopathy: Progressive occlusive vascular disease, which may present with ischemic stroke (Moya-Moya syndrome), can occur following radiation, surgical manipulation, or secondary to the pathology itself as a result of vascular encasement by the tumor [101, 131,132,133,134,135]. When considering radiation, this morbidity is related to the total dose provided to the internal carotid arteries and circle of Willis, rather than radiation type per se [101, 115, 131, 136]. Generally, true ischemic events are uncommon, and most events are subclinical, presenting only on imaging as stenosis [131, 137•]. Lucas et al. suggested that radiation probably does not present much of an additive risk for vascular injury in the surgical corridor or in areas of tumor invasion, but rather provides an additional risk for vascular injury in other non-operated surrounding regions with normal uninvolved vasculature [131]. Edmonston et al. retrospectively evaluated the extent of vasculopathy in 40 (out of 100) pediatric craniopharyngioma patients, including those who had limited surgery and photon-based therapy, using annual MR angiography (MRA) [137•]. They reported a cumulative 10-year incidence of vasculopathy at a rate of 7.9% (± 2.7%), which was higher in females than in males. Median time to abnormal MRA was 1.6 years (range 0.2–10.8 years). Only 3 patients underwent revascularization surgery, and 23 patients were treated with aspirin for various indications. Importantly, they demonstrated that a larger planning target volume was associated with an increased risk of vasculopathy and is likely the result of a larger exposure of the cerebral vessels. Furthermore, given that all of these children should be followed for years with imaging studies (given the recurrence risk), early evidence of vascular abnormalities identifies the “at risk” group for future stroke. Those with progressive stenosis may be placed on aspirin prophylactically, but the inclusion of regular MRA and perfusion imaging, coupled with educating the family of the possibility of TIAs, allows the treating physicians to intervene when imaging shows concerning perfusion defects or the child becomes clinically symptomatic. Those children are then treated with early vascular reinforcement (such as encephaloduroarteriomyosynangiosis (EDAS) surgery) to minimize the risk of disabling stroke, which is a significant advance in the management of craniopharyngioma children over the last decade [131].
The Intra-cystic Therapy Era
Almost all craniopharyngiomas include some fluid-filled cystic components. These associated cysts may sometimes appear with just small solid components, and with the cyst capsule containing tumor cells. Such cysts can sometimes serve as the main tumor component, causing the symptoms by direct pressure, or by causing hydrocephalus. Many intra-cavitary techniques have been suggested over the years. The intra-cystic therapies are usually delivered through an Ommaya (Rickham) reservoir, which allows both cyst aspirations and intra-cystic injection.
Brachytherapy for craniopharyngioma was first introduced by Leksell and Linden in 1952 [29, 138, 139]. It involves the insertion of radiation-emitting isotopes, namely pure β-emitters 32phosphate and 90yttrium, directly into the cystic cavity [21, 29, 138, 140,141,142,143,144]. In the past, 86rhenium and 198gold, which also emit γ-radiation, were tried, but without success and causing high toxicity [21, 29, 142]. These isotopes cause higher radiation doses in the immediate vicinity of the injection cavity when compared to conventional external beam RT.
Since each of the isotope alternatives possesses different physical characteristics, there is no consensus on the best agent, but 32phosphate has been identified as an effective treatment [21, 29, 138]. It is estimated that the brachytherapy anti-cystic effect is slow, achieved through the destruction of the secretory epithelial cystic lining, causing decreased fluid production and eventually leading to cyst shrinkage [21, 145]. Importantly, because of their short depth of penetration (3–4 mm), brachytherapy radioisotopes do not affect the solid portion of the tumor, thus only potentially providing a solution for the cystic portion. Therefore, this approach works best for predominantly cystic craniopharyngiomas but does not preclude the child from future surgeries and/or other treatments [21, 141, 146]. An additional concern is that following brachytherapy, the remaining cyst wall would be more adherent to the surrounding tissue [99, 144]. Today, brachytherapy has largely been abandoned for pediatric craniopharyngioma, since the dose calculations are more difficult and challenging to calculate compared to external radiation, and the results are equivocal, with an unpredictable morbidity profile, described as cyst reduction in 35 to 88%, stabilization in 3 to 19%, and growth in 5 to 15% [29, 99, 140, 141, 143].
Intra-cystic bleomycin was first described in children by Takahashi et al. in 1985 [147], as an antibiotic with antineoplastic properties. It interferes with the S-proliferation phase of the epithelial layer of the cyst wall [148]. It was thought that bleomycin not only has direct antineoplastic properties, but its administration also results in cysts with more established and thicker walls, which theoretically would make them more amendable for removal [149, 150]. Based on the currently available literature, however, although well-tolerated in most cases, the effectiveness of bleomycin still remains to be determined [21, 58, 149, 151,152,153,154,155]. Associated complications have been reported, especially if the drug leaks beyond the cyst to the subarachnoid space and to the ventricular system [58, 153].
Interferon-α (INF-α) acts as an antineoplastic agent by activating Fas-mediated apoptosis [156]. Initially, it was administered systemically in pediatric patients with recurrent craniopharyngioma. However, its systemic toxicity limited its use [156, 157]. Compared to bleomycin, INF-α has a safer toxicity profile, as spillage into CSF does not pose the same risks associated with bleomycin, such as blindness, hypothalamic injury, endocrinopathies, and cerebral ischemia [21, 99, 158]. Currently, intra-cystic INF-α administration may in some cases delay the disease progression. However, it seems that intra-cystic interferon does not significantly affect disease progression and the need for additional therapy. It may serve a role as a temporizing measure in selected cases, without long-term effects on disease progression and the need for further treatments [154, 157, 158].
The Current Role of Surgery as Part of a Multimodal Approach
The understanding that complete surgical removal, while potentially curative, comes at the expense of unacceptable morbidity, and limited surgery alone results in unacceptable high recurrence rates, has brought us to the current era of multimodal management. In this context, the role of surgery needs to be better defined. RT alone, without surgery, may be appropriate for carefully selected patients, especially those with localized tumors with no or minimal surrounding mass effect. RT alone has shown, in selected cases, to provide good disease control and good functional outcomes [159, 160]. Thus, the extent of resection should be balanced with the individual potential risks, taking into account the comparable tumor control rates and decreased complications following a conservative resection supplemented by adjuvant radiotherapy [22, 57, 73, 93, 161]. Surgery, in this context, has several purposes, depending on the individual age and clinical and radiological status.
-
1.
Hydrocephalus management: Due to their anatomical origin near the floor of the third ventricle, it is estimated that up to 54% of pediatric patients with craniopharyngioma may present with obstructive hydrocephalus as a consequence of aqueductal or foramen of Monro occlusion, either from a large tumor mass itself or from an associated cyst [162,163,164,165]. Less commonly, even small tumor mass/cysts may cause nonobstructive hydrocephalus due to repeated subarachnoidal/intraventricular hemorrhage [166]. The role of surgery in these cases is to alleviate the hydrocephalus. The best technique depends on the individual anatomy and includes options such as stereotactic aspiration, stereotactic insertion of an Ommaya reservoir, endoscopic marsupialization of the cyst and/or insertion of an Ommaya, or placement of a ventriculoperitoneal shunt. Furthermore, treatment of the hydrocephalus may be performed as an initial preparatory step before other surgical options are considered, or performed during tumor surgery itself [167]. In cases where hydrocephalus is caused by the cystic component, cyst drainage, either by Ommaya reservoir placement or by endoscopic fenestration, can be attempted first [164]. In very young patients, or in those where the hydrocephalus is caused mainly by a large tumor mass that the surgeon determined beforehand as incurable by surgery, treatment of the hydrocephalus should be done regardless of the tumoral surgery [162]. Reports on the treatment of nonobstructive hydrocephalus are less conclusive and come from adult descriptions, with some patients requiring permanent CSF diversion, while others may achieve controlled hydrocephalus after tumor removal [166, 168].
-
2.
Relief of local pressure: Visual disturbances, resulting from local compression on the chiasm or tract, should prompt surgical decompression. In children, the chances of reversing the visual symptoms following decompression are higher compared to adults, due to improved perfusion and better remyelination recovery of the visual fibers, optimizing synaptic transmission and neuroplasticity [169]. The exact technique for optic apparatus decompression depends on the tumor component — cystic or solid — with more minimal surgical options suitable for cystic components.
-
3.
Separation from radio-sensitive structures: Given the gradual shift toward newer and safer radiation modalities, proximity of the tumor to radio-sensitive structures can be a significant issue, limiting options for adjuvant radiation postoperatively, and therefore requiring surgical intervention in selected cases. This is done in order to decrease tumoral volume, providing enough separation and/or distance from these structures, and eventually allowing higher RT doses to be delivered safely. As craniopharyngiomas are benign, and usually more sharply delineated from surrounding structures, they naturally permit a small irradiation safety margin [104, 170]. However, in cases of tumors situated very close to the brainstem, hypothalamus, or optical structures, the surgical option should be considered when there is a favorable separation plane seen on preoperative imaging.
-
4.
Aggressive surgery: In general, Puget type 0 and I should be approached with curative intent, albeit with a low threshold to halt the surgery should the tumor adhere to critical neuro-vascular structures. A big challenge, especially in pediatrics, is to identify the best candidates for more aggressive surgical approaches. For specific populations, including children under 5 years of age, or children with a small tumor that does not adhere to adjacent structures, we believe that there is a place to attempt a more radical resection, with safe preservation of visual apparatus and hypothalamus. In these cases, complete resection may be feasible, sometimes allowing radiation therapy to be avoided or delayed, minimizing or temporizing RT late side effects. This must be taken into consideration together with the expertise of the surgical team, and each surgical candidate should be considered individually. For children with recurrent craniopharyngioma, particularly if they have already had radiotherapy, aggressive surgical resection for cure is generally the best option if it can be done without impacting the patient’s quality of life.
Thus, it is of uttermost importance that craniopharyngioma surgery and decision-making be both tailored to the individual patient based upon their clinical condition, imaging characteristics, and access to medications. This can only be done by experienced centers, with both the surgical and medical abilities to optimize treatment and related decisions. Experience has a crucial role not only in the technical procedure but also in the decision-making.
Future Directions
The ongoing dilemmas in the management of craniopharyngiomas have highlighted the need for additional treatment options. While today’s general approach of less aggressive surgery followed by RT may provide long progression-free survival rates, it also leaves the developing child with the significant late consequences of the treatment.
Molecular research may lead to the identification of more targeted mutations which may have the potential to expand our therapeutic arsenal [171•, 172,173,174]. These include mainly the Wingless (Wnt)/β-catenin pathway, which is involved in pediatric aCP pathogenesis, and the BRAF (V600E) mutations involved in pCP pathogenesis [171•, 174]. Current studies have shown some potential for these targeted therapies. As of now, they achieve largely transient or limited tumor response, serving mainly as a neoadjuvant treatment prior to surgical removal, or as an adjuvant treatment following tumor recurrence [106, 171•, 172]. So far, however, no specific molecular or systemic immuno/chemo-therapy regimen has been approved or routinely used for craniopharyngioma management in children [171•]. Further studies are needed in this field to fully explore its potential.
Until then we advocate, in most cases, a minimalistic approach whenever possible, mainly for those with a predominantly cystic component. This approach includes either conservative surgery to rapidly decompress vital structures and simultaneously obtain a pathological specimen or biopsy only when rapid decompression is not needed, followed by postoperative RT. Ali et al. evaluated the quality-adjusted life years (QALYs) outcome of four surgical approaches in pediatric patients, demonstrating that the outcome at 5- and 10-year follow-up for biopsy followed by RT was associated with the highest mean QALY, while GTR was associated with the lowest [175].
Other conservative procedures, including endoscopically assisted cyst fenestration with or without placement of an Ommaya reservoir into a cystic cavity, might be considered. These options would be mainly for the younger patients, with a goal of controlling local tumor progression and thus postponing RT. Simple intermittent cyst aspiration via an Ommaya reservoir provides only temporary symptomatic relief, as the cyst fluid will inevitably reaccumulate. Therefore, this technique is only a temporary option, or sometimes a palliative option, when surgery is not possible and irradiation has failed [99, 176, 177]. In addition, cyst accumulation may continue during the period immediately after a successful RT. During that time, the existence of an Ommaya conduit can be extremely helpful.
Limitations
Much of the basic understanding of craniopharyngiomas came from studies of adults or mixtures of adult and pediatric patients. Therefore, extrapolation for the optimal management of children with craniopharyngiomas, whether surgical or including adjuvant measures such as radiation, should be taken very cautiously and explored further. Another limitation is the heterogenous nature of the reports from multiple studies on tumor characteristics, such as the size of its components (cystic vs. solid). Direct comparisons between management approaches are therefore difficult.
Conclusion
There is no clear consensus regarding the best management of craniopharyngioma in children. The shifting strategy toward a conservative approach in the management of pediatric craniopharyngioma reflects an increased understanding of the importance of better and longer QOL, and has been adopted by many centers worldwide [22, 23, 57, 95]. As opposed to the historic aggressive approaches that focused on GTR, current multimodal approaches support a more conservative and tailored surgical approach, coupled with radiation therapy. These approaches have led to similar tumor control rates, improved QOL, and decreased morbidity. This, in conjunction with decreased surgical experience worldwide, as a result of surgical dilution of cases among centers, leads us, among other centers, to adopt the conservative, multimodality, hypothalamic-sparing approach in the majority of cases. Extensive experience is also essential when evaluating treatment options, and given their rarity and ongoing need for expensive resources, treatment of these patients should be performed in large regional referral centers. Neurosurgeons should be included in local and international discussion groups, to increase awareness of alternative approaches as well as define surgical goals.
Data Availability
All materials and data supported the findings of our study are available at online repository databases.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jane JA, Laws ER. Craniopharyngioma. Pituitary. 2006;9:323–6.
Petito CK, DeGirolami U, Earle KM. Craniopharyngiomas: a clinical and pathological review. Cancer. 1976;37:1944–52.
Karavitaki N, Wass JAH. Craniopharyngiomas. Endocrinol Metab Clin North Am. 2008;37:173–93.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, The, et al. WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;2021:23.
Osborn AG. Osborn’s brain: imaging, pathology, and anatomy. Lippincott Williams & Wilkins, 2012.
Laws ER. Transsphenoidal microsurgery in the management of craniopharyngioma. J Neurosurg. 1980;52(5):661–6. https://doi.org/10.3171/jns.1980.52.5.0661
Rush JA, Younge BR, Campbell RJ, MacCarty CS. Optic glioma. Long-term follow-up of 85 histopathologically verified cases. Ophthalmology. 1982;89:1213–9.
Cashion EL, Young JM. Intraventricular craniopharyngioma report of two cases. J Neurosurg. 1971;34(1):84–7. https://doi.org/10.3171/jns.1971.34.1.0084
Bashir EM, Lewis PD, Edwards MR. Posterior fossa craniopharyngioma. Br J Neurosurg. 1996;10:613–6.
Bollati A, Giunta F, Lenzi A, Marini G. Third ventricle intrinsic craniopharingioma. Case report. J Neurosurg Sci. 1974;18:216–9.
Prieto R, Pascual JM, Subhi-Issa I, Jorquera M, Yus M, Martínez R. Predictive factors for craniopharyngioma recurrence: a systematic review and illustrative case report of a rapid recurrence. World Neurosurg. 2013;79(5–6):733–49. https://doi.org/10.1016/j.wneu.2012.07.033
Sweeney KJ, Mottolese C, Villanueva C, Beuriat PA, Szathmari A, di Rocco F. Adult Versus Paediatric Craniopharyngiomas: Which Differences? Adult Craniopharyngiomas. Cham: Springer International Publishing; 2020. p. 187–207.
Sofela AA, Hettige S, Curran O, Bassi S. Malignant transformation in craniopharyngiomas. Neurosurgery. 2014;75(3):306–14. https://doi.org/10.1227/neu.0000000000000380
Gupta K, Kuhn MJ, Shevlin DW, Wacaser LE. Metastatic craniopharyngioma. AJNR Am J Neuroradiol. 1999;20(6):1059–60.
Cushing H. The craniopharyngiomas. Intracranial tumors. 1932;93–8.
Pascual JM, Prieto R. Harvey Cushing and pituitary Case Number 3 (Mary D.): the origin of this most baffling problem in neurosurgery. Neurosurg Focus. 2016;41:E6.
Pascual JM, Prieto R, Barrios L. Harvey Cushing’s craniopharyngioma treatment: Part 1. Identification and clinicopathological characterization of this challenging pituitary tumor. J Neurosurg. 2019;131:949–63.
Lober RM, Harsh GR. A perspective on craniopharyngioma. World Neurosurg. 2013;79:645–6.
Ohmori K, Collins J, Fukushima T. Craniopharyngiomas in children. Pediatr Neurosurg. 2007;43:265–78.
• Pang JC, Chung DD, Wang J, Abiri A, Lien BV, Himstead AS, et al. Characteristics and outcomes in pediatric versus adult craniopharyngiomas: A systematic review and meta-analysis. Neurosurgery. 2023;92:1112–29. (This is a systematic review and meta-analysis, comparing treatments and outcomes between adult and pediatric craniopharyngiomas, emphasizing the fundamental differences in clinical presentation and functional outcomes.)
Winn HR. Youmans and Winn neurological surgery E-Book: 4 - Volume Set. Elsevier Health Sciences. 2022;236:1786-94.e4.
Schoenfeld A, Pekmezci M, Barnes MJ, Tihan T, Gupta N, Lamborn KR, et al. The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neurooncol. 2012;108:133–9.
Cohen M, Guger S, Hamilton J. Long term sequelae of pediatric craniopharyngioma ? literature review and 20 years of experience. Front Endocrinol (Lausanne). 2011;2:81.
Banna M. Craniopharyngioma: based on 160 cases. Br J Radiol. 1976;49:206–23.
Karavitaki N. Craniopharyngiomas: Natural History and Clinical Presentation. Diagnosis and Management of Craniopharyngiomas. Cham: Springer International Publishing; 2016. p. 1–11.
Raimondi AJ, Rougerie J. A Critical Review of Personal Experiences with Craniopharyngioma: Clinical History, Surgical Technique and Operative Results. Pediatr Neurosurg. 1994;21:134–54.
Barkhoudarian G. Laws ER. Craniopharyngioma: history. Pituitary. 2013;16:1–8.
Der LH. Gehirnhang und die Steissdruese des Menschen. Berlin: G Reimer; 1860.
Karavitaki N, Cudlip S, Adams CBT, Wass JAH. Craniopharyngiomas. Endocr Rev. 2006;27:371–97.
Lewis D (1910) A contribution to the subject of tumors of the hypophysis. JAMA. 55(12):1002. https://doi.org/10.1001/jama.1910.04330120024010
Mihalkovics V. Wirbelsaite und hirnanhang. Archiv für mikroskopische Anatomie. 1875;11(Suppl 1):389–441.
Saxer F. Ependymepithel, gliome und epitheliale Geschwülste des Centralnervensystems. 1902.
Erdheim J. Über Hypophysengangsgeschwül- ste und Hirncholesteatome. Sitz Kais Akad Wissen Math Naturw Klin. 1904;113:537–726.
Susman W. Embryonic epithelial rests in the pituitary. Br J Surg. 1932;19:571–6.
Hardy J, Wigser SM. Trans-sphenoidal surgery of pituitary fossa tumors with televised radiofluoroscopic control. J Neurosurg. 1965;23(6):612–9. https://doi.org/10.3171/jns.1965.23.6.0612.
Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH. The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg. 2008;108:1043–7.
Sainte-Rose C, Puget S, Wray A, Zerah M, Grill J, Brauner R, et al. Craniopharyngioma: the pendulum of surgical management. Child’s Nerv Syst. 2005;21:691–5.
Matson DD, Crigler JF. Management of Craniopharyngioma in Childhood. J Neurosurg. 1969;30:377–90.
Caldarelli M, di Rocco C, Papacci F, Colosimo C Jr. Management of Recurrent Craniopharyngioma. Acta Neurochir (Wien). 1998;140:447–54.
Caldarelli M, Massimi L, Tamburrini G, Cappa M, di Rocco C. Long-term results of the surgical treatment of craniopharyngioma: the experience at the Policlinico Gemelli, Catholic University, Rome Child’s. Nerv Syst. 2005;21:747–57.
Chakrabarti I, Amar AP, Couldwell W, Weiss MH. Long-term neurological, visual, and endocrine outcomes following transnasal resection of craniopharyngioma. J Neurosurg. 2005;102:650–7.
Fouda MA, Scott RM, Marcus KJ, Ullrich N, Manley PE, Kieran MW, et al. Sixty years single institutional experience with pediatric craniopharyngioma: between the past and the future. Child’s Nerv Syst. 2020;36:291–6.
Gordy PD, Peet MM, Kahn EA. The surgery of the craniopharyngiomas. J Neurosurg. 1949;6(6):503–17. https://doi.org/10.3171/jns.1949.6.6.0503.
Symon L, Pell M, Habib A. Radical excision of craniopharyngioma by the temporal route: a review of 50 patients. Br J Neurosurg. 1991;5(6):539–49. https://doi.org/10.3109/02688699109002877.
Epstein FJ. Meeting Summary. Pediatr Neurosurg. 1994;21:129–30.
Yaşargil MG, Curcić M, Kış M, Siegenthaler G, Teddy PJ, Roth P. Total removal of craniopharyngiomas. J Neurosurg. 1990;73(1):3–11. https://doi.org/10.3171/jns.1990.73.1.0003.
Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI. Aggressive surgical management of craniopharyngiomas in children. J Neurosurg. 1992;76(1):47–52. https://doi.org/10.3171/jns.1992.76.1.0047.
Elliott RE, Moshel YA, Wisoff JH. Minimal residual calcification and recurrence after gross-total resection of craniopharyngioma in children. J Neurosurg. 2009;3(4):276–83. https://doi.org/10.3171/2009.1.peds08335.
Elliott RE, Wisoff JH. Successful surgical treatment of craniopharyngioma in very young children. J Neurosurg Pediatr. 2009;3:397–406.
Clark AJ, Cage TA, Aranda D, Parsa AT, Sun PP, Auguste KI, et al. A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Child’s Nervous System. 2013;29:231–8.
Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, et al. 20-Year experience in childhood craniopharyngioma. Int J Radiat* Oncol* Biol Phys. 1993;27:189–95.
Sterkenburg AS, Hoffmann A, Gebhardt U, Warmuth-Metz M, Daubenbüchel AM, Müller HL. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro-oncology. 2015;17(7):1029–38. https://doi.org/10.1093/neuonc/nov044.
Memmesheimer RM, Lange K, Dölle M, Heger S, Mueller I. Psychological well-being and independent living of young adults with childhood-onset craniopharyngioma. Dev Med Child Neurol. 2017;59:829–36.
Crom DB, Smith D, Xiong Z, Onar A, Hudson MM, Merchant TE, et al. Health Status in Long-Term Survivors of Pediatric Craniopharyngiomas. J Neurosci Nurs. 2010;42:323–8.
Puget S, Garnett M, Wray A, Grill J, Habrand J-L, Bodaert N, et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg Pediatr. 2007;106:3–12.
Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera J-P, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5:75.
Müller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M, Pietsch T, Pohl F, Sörensen N, Calaminus G. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial Kraniopharyngeom 2000 after 3-year follow-up. Eur J Endocrinol. 2011;165(1):17–24. https://doi.org/10.1530/eje-11-0158.
Hukin J, Steinbok P, Lafay-Cousin L, Hendson G, Strother D, Mercier C, et al. Intracystic bleomycin therapy for craniopharyngioma in children. Cancer. 2007;109:2124–31.
Singh A, Salunke P, Rangan V, Ahuja CK, Bhadada S. Vasospasm After Craniopharyngioma Surgery: Can We Prevent It? World Neurosurg. 2017;101:208–15.
Pereira AM, Schmid EM, Schutte PJ, Voormolen JHC, Biermasz NR, van Thiel SW, et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol (Oxf). 2005;62:197–204.
McCarty CA. Vision impairment predicts 5 year mortality. Br J Ophthalmol. 2001;85:322–6.
Abrams LS, Repka MX. Visual outcome of craniopharyngioma in children. J Pediatr Ophthalmol Strabismus. 1997;34:223–8.
Goldenberg-Cohen N, Ehrenberg M, Toledano H, Kornreich L, Snir M, Yassur I, et al. Preoperative visual loss is the main cause of irreversible poor vision in children with a brain tumor. Front Neurol. 2011;2:62.
Wan MJ, Zapotocky M, Bouffet E, Bartels U, Kulkarni AV, Drake JM. Long-term visual outcomes of craniopharyngioma in children. J Neurooncol. 2018;137:645–51.
Nuijts MA, Veldhuis N, Stegeman I, van Santen HM, Porro GL, Imhof SM, et al. Visual functions in children with craniopharyngioma at diagnosis: A systematic review. PLoS ONE. 2020;15:e0240016.
Bogusz A, Müller HL. Childhood-onset craniopharyngioma: latest insights into pathology, diagnostics, treatment, and follow-up. Expert Rev Neurother. 2018;18:793–806.
Amayiri N, Spitaels A, Zaghloul M, Figaji A, Cavalheiro S, Muller HL, Elhassan M, Parkes J, Mushtaq N, El Beltagy M, Yousef YA. SIOP PODC adapted treatment guidelines for craniopahryngioma in low-and middle-income settings. Pediatr Blood Cancer. 2020;70(11). https://doi.org/10.1002/pbc.28493.
Oluwayemi IO, Olatunya OS, Ogundare EO, Ajite AB, Babatola AO, Adeniyi AT, Komolafe AK. Paediatric hypopituitarism: a case report and management challenges in a resource poor setting. Pan Afr Med J. 2020;37. https://doi.org/10.11604/pamj.2020.37.170.23656.
Alotaibi N, Noormohamed N, Coté DJ, Alharthi SS, Doucette J, Zaidi HA, Mekary RA, Smith TR. Physiologic growth hormone-replacement therapy and craniopharyngioma recurrence in pediatric patients: a meta-analysis. World Neurosurg. 2018;109:487-96.e1. https://doi.org/10.1016/j.wneu.2017.09.164.
• Merchant TE, Dangda S, Hoehn ME, Wu S, Li Y, Wang F, et al. Pediatric Craniopharyngioma: The Effect of Visual Deficits and Hormone Deficiencies on Long-Term Cognitive Outcomes After Conformal Photon Radiation Therapy. Int J Radiat Oncol*Biol*Phys. 2023;115:581–91. (In this article, they investigated the cognitive outcomes after conformal photon radiation therapy and the effect of visual deficits and hormone deficiencies.•)
Pérez MA, Millán HA, Naranjo JA, Flórez RA. Adipsic diabetes insipidus secondary to craniopharyngioma resection. BMJ Case Rep. 2019;12:e225903.
Smith D, Finucane F, Phillips J, Baylis PH, Finucane J, Tormey W, et al. Abnormal regulation of thirst and vasopressin secretion following surgery for craniopharyngioma. Clin Endocrinol (Oxf). 2004;61:273–9.
Eveslage M, Calaminus G, Warmuth-Metz M, Kortmann R-D, Pohl F, Timmermann B, et al. The Postoperative Quality of Life in Children and Adolescents with Craniopharyngioma. Dtsch Arztebl Int. 2019;116(18):321.
Bereket A, Kieß W, Lustig RH, Müller HL, Goldstone AP, Weiss R, Yavuz Y, Hochberg Z. Hypothalamic obesity in children. Obes Rev. 2012;13(9):780–98. https://doi.org/10.1111/j.1467-789x.2012.01004.x.
Rachmasari K, Strauß S, Phillips CD, Lantos J, An A, Cisse B, Ramakrishna R, Schwartz TH, Dobri G. Posterior hypothalamic involvement on pre-operative MRI predicts hypothalamic obesity in craniopharyngiomas. Pituitary. 2022;26(1):105–14. https://doi.org/10.1007/s11102-022-01294-0.
Erfurth E-M. Diagnosis, Background, and Treatment of Hypothalamic Damage in Craniopharyngioma. Neuroendocrinology. 2020;110:767–79.
Müller HL, Tauber M, Lawson EA, Özyurt J, Bison B, Martinez-Barbera JP, Puget S, Merchant TE, Van Santen HM. Hypothalamic syndrome. Nat Rev Dis Primers. 2022;8(1). https://doi.org/10.1038/s41572-022-00351-z.
Shoemaker AH, Tamaroff J. Approach to the patient with hypothalamic obesity. J Clin Endocrinol Metab. 2022;108(5):1236–42. https://doi.org/10.1210/clinem/dgac678.
Srinivasan S, Ogle GD, Garnett SP, Briody JN, Lee JW, Cowell CT. Features of the Metabolic Syndrome after Childhood Craniopharyngioma. J Clin Endocrinol Metab. 2004;89:81–6.
Steinbok P. Craniopharyngioma in Children: Long-term Outcomes. Neurol Med Chir (Tokyo). 2015;55:722–6.
Sahakitrungruang T, Klomchan T, Supornsilchai V, Wacharasindhu S. Obesity, metabolic syndrome, and insulin dynamics in children after craniopharyngioma surgery. Eur J Pediatr. 2011;170:763–9.
Özyurt J, Mehren A, Boekhoff S, Müller HL, Thiel CM. Social cognition in patients with hypothalamic-pituitary tumors. Front Oncol. 2020;10. https://doi.org/10.3389/fonc.2020.01014.
Müller HL. Consequences of Craniopharyngioma Surgery in Children. J Clin Endocrinol Metab. 2011;96:1981–91.
Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, et al. Craniopharyngioma: the St. Jude Children’s Research Hospital experience 1984–2001. Int J Radiat Oncol*Biol*Phys. 2002;53:533–42.
Kramer S, McKissock W, Concannon JP. Craniopharyngiomas. J Neurosurg. 1961;18:217–26.
Kiehna EN, Merchant TE. Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus. 2010;28:E10.
Richmond IL, Wara WM, Wilson CB. Role of Radiation Therapy in the Management of Craniopharyngiomas in Children. Neurosurgery. 1980;6:513–7.
Hoogenhout J, Otten BJ, Kazem I, Stoelinga GBA, Walder AHD. Surgery and radiation therapy in the management of craniopharyngiomas. Int J Radiat Oncol*Biol*Phys. 1984;10:2293–7.
Weiss M, Sutton L, Marcial V, Fowble B, Packer R, Zimmerman R, et al. The role of radiation therapy in the management of childhood craniopharyngioma. Int J Radiat Oncol*Biol*Phys. 1989;17:1313–21.
Minniti G, Esposito V, Amichetti M, Maurizi ER. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev. 2009;32:125–32.
Kortmann R. Different approaches in radiation therapy of craniopharyngioma. Front Endocrinol. 2011;2. https://doi.org/10.3389/fendo.2011.00100.
Jimenez R, Ahmed S, Johnson A, Thomas H, Depauw N, Horick N, Tansky J, Evans CL, Pulsifer MB, Ebb D, Butler WE, Fullerton BC, Tarbell NJ, Yock TI, MacDonald SM. Proton radiation therapy for pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys. 2021;110(5):1480–7. https://doi.org/10.1016/j.ijrobp.2021.02.045.
Stripp D, Maity A, Janss AJ, Belasco JB, Tochner Z, Goldwein JW, Moshang T, Rorke LB, Phillips PC, Sutton LN, Shu H. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58(3):714–20. https://doi.org/10.1016/s0360-3016(03)01570-0.
Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg Pediatr. 2006;104:94–102.
Tan TSE, Patel L, Gopal-Kothandapani JS, Ehtisham S, Ikazoboh EC, Hayward R, et al. The neuroendocrine sequelae of paediatric craniopharyngioma: a 40-year meta-data analysis of 185 cases from three UK centres. Eur J Endocrinol. 2017;176:359–69.
Clark AJ, Cage TA, Aranda D, Parsa AT, Auguste KI, Gupta N. Treatment-related morbidity and the management of pediatric craniopharyngioma. J Neurosurg Pediatr. 2012;10:293–301.
Gorelyshev S, Savateev AN, Mazerkina N, Medvedeva O, Konovalov AN. Craniopharyngiomas: surgery and radiotherapy. In Springer eBooks 2022;97–137. https://doi.org/10.1007/978-3-030-99166-1_3.
Boehling NS, Grosshans DR, Bluett JB, Palmer MT, Song X, Amos RA, et al. Dosimetric Comparison of Three-Dimensional Conformal Proton Radiotherapy, Intensity-Modulated Proton Therapy, and Intensity-Modulated Radiotherapy for Treatment of Pediatric Craniopharyngiomas. Int J Radiat Oncol*Biol*Phys. 2012;82:643–52.
Graffeo C, Perry A, Link M, Daniels D. Pediatric Craniopharyngiomas: A Primer for the Skull Base Surgeon. J Neurol Surg B Skull Base. 2018;79:065–80.
Greenfield BJ, Okcu MF, Baxter PA, Chintagumpala M, Teh BS, Dauser RC, et al. Long-term disease control and toxicity outcomes following surgery and intensity modulated radiation therapy (IMRT) in pediatric craniopharyngioma. Radiother Oncol. 2015;114:224–9.
Kalapurakal JA. Radiation therapy in the management of pediatric craniopharyngiomas—a review. Child’s Nerv Syst. 2005;21:808–16.
Luu QT, Loredo LN, Archambeau JO, Yonemoto LT, Slater JM, Slater JD. Fractionated Proton Radiation Treatment for Pediatric Craniopharyngioma: Preliminary Report. Cancer J. 2006;12:155–9.
Merchant TE, Kun LE, Hua C, Wu S, Xu X, Sanford RA, Boop FA. Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys. 2013;85(4):e187–92. https://doi.org/10.1016/j.ijrobp.2012.10.030.
Aggarwal A, Fersht N, Brada M. Radiotherapy for craniopharyngioma. Pituitary. 2013;16:26–33.
Beltran C, Roca M, Merchant TE. On the Benefits and Risks of Proton Therapy in Pediatric Craniopharyngioma. Int J Radiat Oncol Biol Phys. 2012;82:e281-7.
Qi S, Pan J, Lu Y, editors. Craniopharyngiomas - Classification and Surgical Treatmen. UAE: Bentham Science Publishers Ltd.; 2017.
Merchant TE, Hua C, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: Comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51:110–7.
• Jimenez RB, Ahmed S, Johnson A, Thomas H, Depauw N, Horick N, et al. Proton Radiation Therapy for Pediatric Craniopharyngioma. Int J Radiat Oncol*Biol*Phys. 2021;110:1480–7. (This study describes the author’s experience with a large cohort of pediatric craniopharyngioma patients, treated with adjuvant proton RT, achieving an excellent local control rate.•)
Rieber J, Kessel KA, Witt O, Behnisch W, Kulozik AE, Debus J, Combs SE. Treatment tolerance of particle therapy in pediatric patients. Acta Oncol. 2015;54(7):1049–55. https://doi.org/10.3109/0284186x.2014.998273.
Yoo GS, Yu JI, Cho S, Han Y, Oh Y, Lim DH, Nam HY, Lee J, Sung K, Shin HJ. Chronological analysis of acute hematological outcomes after proton and photon beam craniospinal irradiation in pediatric brain tumors. Cancer Res Treat. 2022;54(3):907–16. https://doi.org/10.4143/crt.2021.332.
Uemura S, Demizu Y, Hasegawa D, Fujikawa T, Inoue S, Nishimura A, Tojyo R, Nakamura S, Kozaki A, Saito A, Kishimoto K, Ishida T, Mori T, Koyama J, Kawamura A, Akasaka Y, Yoshida M, Fukumitsu N, Soejima T, Kosaka Y. The comparison of acute toxicities associated with craniospinal irradiation between photon beam therapy and proton beam therapy in children with brain tumors. Cancer Med. 2022;11(6):1502–10. https://doi.org/10.1002/cam4.4553.
Nelson B, Lamba M, Ewart S, Ike N, Lewis L, Pater L. Normal tissue exposure and second malignancy risk in vertebral-body-sparing craniospinal irradiation. Med Dosim. 2022;47:142–5.
Aldrich KD, Horne VE, Bielamowicz K, Sonabend R, Scheurer ME, Paulino AC, Mahajan A, Chintagumpala M, Okcu MF, Brown AL. Comparison of hypothyroidism, growth hormone deficiency, and adrenal insufficiency following proton and photon radiotherapy in children with medulloblastoma. J Neuro-Oncol. 2021;155(1):93–100. https://doi.org/10.1007/s11060-021-03847-y.
Fitzek MM, Linggood RM, Adams J, Munzenrider JE. Combined proton and photon irradiation for craniopharyngioma: Long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int J Radiat Oncol*Biol*Phys. 2006;64:1348–54.
Bishop AJ, Greenfield B, Mahajan A, Paulino AC, Okcu MF, Allen PK, et al. Proton Beam Therapy Versus Conformal Photon Radiation Therapy for Childhood Craniopharyngioma: Multi-institutional Analysis of Outcomes, Cyst Dynamics, and Toxicity. Int J Radiat Oncol*Biol*Phys. 2014;90:354–61.
•• Merchant TE, Hoehn ME, Khan RB, Sabin ND, Klimo P, Boop FA, Wu S, Li Y, Burghen E, Jurbergs N, Sandler E, Aldana PR, Indelicato DJ, Conklin HM. Proton therapy and limited surgery for paediatric and adolescent patients with craniopharyngioma (RT2CR): a single-arm, phase 2 study. Lancet Oncol. 2023;24(5):523–34. https://doi.org/10.1016/s1470-2045(23)00146-8. (The researchers showed in a large prospective study that photon therapy and proton therapy have similar survival outcomes and severe complication rates; however, cognitive outcomes with proton therapy were better when compared to photon therapy.)
Rombi B, Vennarini S, Vinante L, Ravanelli D, Amichetti M. Proton radiotherapy for pediatric tumors: review of first clinical results. Ital J Pediatr. 2014;40:74.
Ontario Health (Quality). Proton beam therapy for cancer in children and adults: a health technology assessment. Ontario Health Technology Assessment Series, 2021;21(1):1–142.
Lawell MP, Bajaj BVM, Gallotto SL, Hess CB, Patteson BE, Nartowicz JA, et al. Increased distance from a treating proton center is associated with diminished ability to follow patients enrolled on a multicenter radiation oncology registry. Radiother Oncol. 2019;134:25–9.
Bishop AJ, Livingston JA, Ning MS, Valdez ID, Wages CA, McAleer MF, et al. Young Adult Populations Face Yet Another Barrier to Care With Insurers: Limited Access to Proton Therapy. Int J Radiat Oncol*Biol*Phys. 2021;110:1496–504.
Moon SH, Kim IH, Park SW, Kim I, Hong S, Park CI, et al. Early adjuvant radiotherapy toward long-term survival and better quality of life for craniopharyngiomas—a study in single institute. Child’s Nerv Syst. 2005;21:799–807.
Tomita T, Bowman RM. Craniopharyngiomas in children: surgical experience at Children’s Memorial Hospital. Child’s Nerv Syst. 2005;21:729–46.
Evans JJ, Kenning TJ. Craniopharyngiomas: comprehensive diagnosis, treatment and outcome. Academic Press, 2015.
Sklar CA. Craniopharyngioma: endocrine abnormalities at presentation. Pediatr Neurosurg. 1994;21(1):18–20. https://doi.org/10.1159/000120856.
•• Merchant TE, Edmonston DY, Wu S, Li Y, Boop FA, Lustig RH. Endocrine outcomes after limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: Long-term results from the RT1 protocol. Neuro Oncol. 2022;24:2210–20. (The investigators estimated the long-term endocrine outcome of pediatric patients with craniopharyngioma after limited surgery and photon-based radiation.)
Regine WF, Kramer S. Pediatric craniopharyngiomas: Long term results of combined treatment with surgery and radiation. Int J Radiat Oncol*Biol*Phys. 1992;24:611–7.
Bates JE, Indelicato DJ, Morris CG, Rotondo RL, Bradley JA. Visual decline in pediatric survivors of brain tumors following radiotherapy. Acta Oncol. 2020;59(10):1257–62. https://doi.org/10.1080/0284186x.2020.1803500.
Flickinger JC, Lunsford LD, Singer J, Cano ER, Deutsch M. Megavoltage external beam irradiation of craniopharyngiomas: Analysis of tumor control and morbidity. Int J Radiat Oncol*Biol*Phys. 1990;19:117–22.
Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJG, et al. Craniopharyngioma — long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26:1–10.
Acharya S, Quesada S, Coca K, Richardson C, Hoehn ME, Chiang J, Qaddoumi I, Boop FA, Gajjar A, Merchant TE. Long-term visual acuity outcomes after radiation therapy for sporadic optic pathway glioma. J Neuro-Oncol. 2019;144(3):603–10. https://doi.org/10.1007/s11060-019-03264-2.
Lucas JT, Faught AM, Hsu CY, Wilson L, Guo Y, Li Y, Khan RB, Becksfort J, Levine DA, Ismael Y, Darrow K, Moskvin V, Pirlepesov F, Klimo P, Elijovich L, Indelicato DJ, Boop FA, Merchant TE. Pre- and posttherapy risk factors for vasculopathy in pediatric patients with craniopharyngioma treated with surgery and proton radiation therapy. Int J Radiat Oncol Biol Phys. 2022;113(1):152–60. https://doi.org/10.1016/j.ijrobp.2021.12.17.
Sutton LN. Vascular complications of surgery for craniopharyngioma and hypothalamic glioma. Pediatr Neurol. 1994;21(1):124–8. https://doi.org/10.1159/000120874.
Jamshidi A, Soldozy S, Elarjani T, Burks JD, Luther E, Starke RM. Fusiform dilatation of the internal Carotid artery in Childhood-Onset Craniopharyngioma: a systematic review. World Neurosurg. 2022;162:77–84. https://doi.org/10.1016/j.wneu.2021.09.058.
Nordstrom M, Felton E, Sear K, Tamrazi B, Torkildson J, Gauvain K, Haas-Kogan DA, Chen J, Del Buono B, Banerjee A, Samuel D, Saloner D, Tian B, Roddy E, Hess CP, Fullerton HJ, Mueller S. Large vessel arteriopathy after cranial radiation therapy in pediatric brain tumor survivors. J Child Neurol. 2018;33(5):359–66. https://doi.org/10.1177/0883073818756729.
Sandvik U, Ohlsson M, Edström E. Vascular complications in pediatric craniopharyngioma patients: a case-based update. Childs Nerv Syst. 2019;35(12):2273–8. https://doi.org/10.1007/s00381-019-04394-8.
Morris B, Partap S, Yeom KW, Gibbs IC, Fisher PG, King AA. Cerebrovascular disease in childhood cancer survivors: a children’s oncology group report. Neurol. 2009;73(22):1906–13. https://doi.org/10.1212/wnl.0b013e3181c17ea8.
• Edmonston D, Wu S, Li Y, Khan RB, Boop FA, Merchant TE. Limited surgery and conformal photon radiation therapy for pediatric craniopharyngioma: long-term results from the RT1 protocol. Neuro-oncology. 2022;24(12):2200–09. https://doi.org/10.1093/neuonc/noac124. (This study estimated the long-term disease control and complications in pediatric craniopharyngioma patients after limited surgical and photon-based radiotherapy and showed excellent tumor control rates.)
Zhao R, Deng J, Liang X, Zeng J, Chen X, Wang J. Treatment of cystic craniopharyngioma with phosphorus-32 intracavitary irradiation. Child’s Nervous System. 2010;26:669–74.
Leksell L, Liden K. A therapeutic trial with radioactive isotopes in cystic brain tumour. Radioisotope techniques I. Med Physiol Appl. 1952;1–4.
Pollock BE, Lunsford LD, Kondziolka D, Levine G, Flickinger JC. Phosphorus-32 intracavitary irradiation of cystic craniopharyngiomas: Current technique and long-term results. Int J Radiat Oncol*Biol*Phys. 1995;33:437–46.
Voges J, Sturm V, Lehrke R, Treuer H, Gauss C, Berthold F. Cystic Craniopharyngioma: Long-term Results after Intracavitary Irradiation with Stereotactically Applied Colloidal ??-Emitting Radioactive Sources. Neurosurgery. 1997;40:263–70.
Bond WH, Richards D, Turner E. Experiences with radioactive gold in the treatment of craniopharyngioma. J Neurol Neurosurg Psychiatry. 1965;28:30–8.
van den Berge JH, Blaauw G, Breeman WAP, Rahmy A, Wijngaarde R. Intracavitary brachytherapy of cystic craniopharyngiomas. J Neurosurg. 1992;77:545–50.
Blackburn TP, Doughty D, Plowman PN. Stereotactic intracavitary therapy of recurrent cystic craniopharyngioma by instillation of 90yttrium. Br J Neurosurg. 1999;13:359–65.
Szeifert GT, Julow J, Slowik F, Balint K, Lanyi F, Pasztor E. Pathological changes in cystic craniopharyngiomas following intracavital90yttrium treatment. Acta Neurochir (Wien). 1990;102:14–8.
Julow JV. Intracystic irradiation for craniopharyngiomas. Pituitary. 2013;16:34–45.
Takahashi H, Nakazawa S, Shimura T. Evaluation of postoperative intratumoral injection of bleomycin for craniopharyngioma in children. J Neurosurg. 1985;62:120–7.
Broggi G, Franzini A, Cajola L, Pluchino F. Cell Kinetic Investigations in Craniopharyngioma: Preliminary Results and Considerations. Pediatr Neurosurg. 1994;21:21–3.
Cavalheiro S, de Castro Sparapani FV, Franco JOB, da Silva MC, Braga FM. Use of bleomycin in intratumoral chemotherapy for cystic craniopharyngioma. J Neurosurg. 1996;84:124–6.
Broggi G, Giorgi C, Franzini A, Leocata F, Riva D. Therapeutic role of intracavitary bleomycin administration in cystic craniopharyngioma. In: Craniopharyngioma: Surgical Treatment. Springer Milan: Milano; 1995. p. 113–9.
Broggi G, Franzini A. Bleomycin for cystic craniopharyngioma. J Neurosurg. 1996;84(6). https://doi.org/10.3171/jns.1996.84.6.1080.
Si Z, Yuan F, Cai BW, Xu J, You C. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Libr 2016;2016(7). https://doi.org/10.1002/14651858.cd008890.pub4.
Park DH, Park JY, Kim JH, Chung YG, Lee HK, Lee KC, et al. Outcome of Postoperative Intratumoral Bleomycin Injection for Cystic Craniopharyngioma. J Korean Med Sci. 2002;17:254.
Steinbok P, Hukin J. Intracystic treatments for craniopharyngioma. Neurosurg Focus. 2010;28:E13.
Jiang R, Liu Z, Zhu C. Preliminary Exploration of the Clinical Effect of Bleomycin on Craniopharyngiomas. Stereotact Funct Neurosurg. 2002;78:84–94.
Jakacki RI, Cohen BH, Jamison C, Mathews VP, Arenson E, Longee DC, et al. Phase II evaluation of interferon α-2a for progressive or recurrent craniopharyngiomas. J Neurosurg. 2000;92:255–60.
Bartels U, Laperrière N, Bouffet É, Drake JM. Intracystic therapies for cystic craniopharyngioma in childhood. Front Endocrinol. 2012;3. https://doi.org/10.3389/fendo.2012.00039.
Kilday J, Caldarelli M, Massimi L, Chen RH, Lee YY, Liang M, Parkes J, Naiker T, Van Veelen M, Michiels E, Mallucci C, Pettorini B, Meijer L, Dorfer C, Czech T, Diezi M, Meeteren AYNS, Holm S, Gustavsson B, Bartels U. Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro-oncol. 2017;19(10):1398–407. https://doi.org/10.1093/neuonc/nox056.
Zhang C, Verma V, Lyden ER, Horowitz DP, Zacharia BE, Lin C, et al. The Role of Definitive Radiotherapy in Craniopharyngioma. Am J Clin Oncol. 2018;41:807–12.
Young M, Delaney A, Jurbergs N, Pan H, Wang F, Boop FA, et al. Radiotherapy alone for pediatric patients with craniopharyngioma. J Neurooncol. 2022;156:195–204.
Yang I, Sughrue ME, Rutkowski MJ, Kaur R, Ivan ME, Aranda D, Barani IJ, Parsa AT. Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus. 2010;28(4):E5. https://doi.org/10.3171/2010.1.focus09307.
Zuccaro G. Radical resection of craniopharyngioma. Child’s Nerv Syst. 2005;21:679–90.
Wong T-T, Liang M-L, Chen H-H, Chang F-C. Hydrocephalus with brain tumors in children. Child’s Nerv Syst. 2011;27:1723–34.
Thompson D, Aquilina K. Surgical approaches. In Elsevier eBooks. 2015. https://doi.org/10.1016/b978-0-12-416706-3.00016-7.
Balogun JA, Rutka JT. Surgery of intracranial gliomas in children. In Progress in neurological surgery. 2017:204–17. https://doi.org/10.1159/000464437.
Kawaguchi T, Ogawa Y, Watanabe M, Tominaga T. Craniopharyngiomas Presenting with Nonobstructive Hydrocephalus: Underlying Influence of Subarachnoidal Hemorrhage. Two Case Reports. J Neurol Surg A Cent Eur Neurosurg. 2014;76:418–23.
Pierre-Kahn A, Sainte-Rose C, Renier D. Surgical Approach to Children with Craniopharyngiomas and Severely Impaired Vision: Special Considerations. Pediatr Neurosurg. 1994;21:50–6.
Shoji T, Kawaguchi T, Ogawa Y, Watanabe M, Fujimura M, Tominaga T. Continuous Minor Bleeding from Tumor Surface in Patients with Craniopharyngiomas: Case Series of Nonobstructive Hydrocephalus. J Neurol Surg A Cent Eur Neurosurg. 2018;79:436–41.
Starkr KL, Kaufman B, Lee BCP, Primack J, Tychsen L. Visual recovery after a year of craniopharyngioma-related amaurosis: Report of a nine-year-old child and a review of pathophysiologic mechanisms. J Am Assoc Pediatr Ophthalmol Strabismus. 1999;3:366–71.
Müller HL. Craniopharyngioma – Pediatric management. In Elsevier eBooks 2015;429–58. https://doi.org/10.1016/b978-0-12-416706-3.00027-1.
• Alexandraki KI, Xekouki P. Medical therapy for craniopharyngiomas. Eur Endocrinol. 2021;17:121. (A summarizing article of the latest available medical treatment for craniopharyngioma.)
Alexandraki KI, Kaltsas GA, Karavitaki N, Grossman AB. The medical therapy of craniopharyngiomas: The way ahead. J Clin Endocrinol Metab. 2019;104:5751–64.
Brastianos PK, Taylor-Weiner A, Manley PE, Jones RT, Dias-Santagata D, Thorner AR, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46:161–5.
Hussain I, Eloy JA, Carmel PW, Liu JK. Molecular oncogenesis of craniopharyngioma: current and future strategies for the development of targeted therapies. J Neurosurg. 2013;119:106–12.
Ali ZS, Bailey RL, Daniels LB, Vakhshori V, Lewis DJ, Hossain AT, et al. Comparative effectiveness of treatment options for pediatric craniopharyngiomas. J Neurosurg Pediatr. 2014;13:178–88.
Wisoff JH. Surgical Management of Recurrent Craniopharyngiomas. Pediatr Neurosurg. 1994;21:108–13.
Gutin PH, Klemme WM, Lagger RL, MacKay AR, Pitts LH, Hosobuchi Y. Management of the unresectable cystic craniopharyngioma by aspiration through an Ommaya reservoir drainage system. J Neurosurg. 1980;52:36–40.
Acknowledgements
We thank Mrs. Adina Sherer for her editorial assistance.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article and made substantial contributions to discussions of content. S.G., and S.C., wrote the manuscript. All authors reviewed and edited the manuscript before final submission.
Corresponding author
Ethics declarations
Competing Interests
There are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• There is no consensus for the best management of pediatric craniopharyngioma. However, there is a shifting strategy toward a more conservative, hypothalamic-sparing, approach, emphasizing quality of life as a main goal.
• Limited surgical resection, coupled with post-radiation proton therapy, provides a similar control rate when compared to gross total resection, albeit minimizing morbidity.
• Pediatric craniopharyngioma patients require a multimodality team, and an individualized surgical-medical approach.
• Extensive experience is essential when evaluating treatment options; thus, management should be held and congregated in large regional referral centers of excellence.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gabay, S., Merchant, T.E., Boop, F.A. et al. Shifting Strategies in the Treatment of Pediatric Craniopharyngioma. Curr Oncol Rep 25, 1497–1513 (2023). https://doi.org/10.1007/s11912-023-01471-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01471-9