Abstract
Purpose of Review
Recent treatment advances in both acute myeloid leukemia and acute lymphoblastic leukemia have drastically improved outcomes for these diseases, but central nervous system (CNS) relapses still occur. Treatment of CNS disease can be challenging due to the impermeability of the blood–brain barrier to many systemic therapies.
Recent Findings
The diagnosis of CNS leukemia relies on assessment of clinical symptoms, cerebrospinal fluid sampling for conventional cytology and/or flow cytometry, and neuroimaging. While treatment of CNS leukemia with systemic or intrathecal chemotherapy and/or radiation can be curative in some patients, these modalities can also lead to serious toxicities. In the modern era, prophylaxis with intrathecal chemotherapy is the most important strategy to prevent CNS relapses in high risk patients.
Summary
Accurate risk stratification tools and the use of risk-adapted prophylactic therapy are imperative to improving the outcomes of patients with acute leukemias and preventing the development of CNS leukemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the improvement in current available therapies for acute leukemias, central nervous system (CNS) involvement remains a significant clinical challenge and can lead to serious complications and mortality. The diagnosis of CNS leukemia can be difficult, as neurologic symptoms can be subtle and widely range based on the anatomical site of infiltration. CNS leukemia can appear as leptomeningeal disease where leukemic cells infiltrate the cerebrospinal fluid (CSF) or rarely as a solid mass. In acute lymphoblastic leukemia (ALL), the CNS is a well-known site for disease infiltration; therefore, routine assessment of the CNS and CNS-directed prophylaxis are incorporated in standard ALL therapies. The incidence of CNS disease at diagnosis is 5–15% in adult ALL patients, and isolated CNS relapses after achieving remission are observed in 5% of patients of ALL [1,2,3]. In contrast, CNS involvement in adults with acute myeloid leukemia (AML) is less common, although it still can rarely be observed both at diagnosis and at the time of relapse. [4, 5]
Irrespective of the disease subtype, leukemic infiltration in the CNS can be difficult to treat due to the complexity and impermeability of the blood–brain barrier (BBB), which can impede optimal penetration of chemotherapy [6]. Additionally, the presence of adhesion molecules on leukemic cells can facilitate their adherence to the meningeal vasculature, allowing them to evade CNS-directed therapies. Despite recent advances in ALL and AML therapies, CNS relapses remain a therapeutic challenge underscoring the need to improve our understanding of its pathophysiology, identification of risk factors, and development of effective treatment strategies. Treatment options for CNS disease have drastically improved since the development of whole-brain radiation treatment (WBRT), with more effective therapies like intrathecal chemotherapy and more refined radiation techniques such as craniospinal irradiation, both of which have an improved efficacy and toxicity profile compared with prior therapies. However, acute and long-term toxicities still occur, especially if these treatments are not appropriately applied. Ultimately, understanding the unique biology of CNS leukemia, utilizing reliable diagnostic tools, and recognizing risk factors for CNS involvement in AML and ALL are key to design precise and effective CNS-directed therapies.
Pathophysiology
The brain and the spinal cord are enveloped by the dura, arachnoid, and pia matter, the latter two of which are referred as the leptomeninges. Behind the leptomeninges lies a subarachnoid space that holds the CSF. The CSF is produced by the choroid plexus and circulates through the ventricular system, spinal cord, and brain and then is absorbed into the blood by the arachnoid villi. Blood supplied from the periphery must pass through the BBB interface to enter the CNS. The BBB is composed of endothelial cells held together by tight, adherens junctions which consist of transmembrane proteins such as claudin-5 and VE-cadherin or PECAM-1 [7, 8]. Notably, physiologic changes, such as inflammation, and certain drugs can disrupt the BBB.
Leukemic cells can penetrate the CNS through multiple mechanisms. Leukemic cells can travel through the vasculature from the bone marrow to the vertebrae and the brain by crossing the BBB via endothelial disruption or trans-endothelial migration. Less commonly, leukemic cells can escape into the CSF after a traumatic LP, particularly in the presence of high circulating blasts [9,10,11]. Leukemic cells highly express adhesion molecules that aid in their migration, CNS infiltration, and chemoresistance. Both AML and ALL cells express numerous types and classes of adhesion molecules that interact with the bone marrow and CNS microenvironment. AML cells express CD56, CD44, CD34, VLA-4, VLA-5, LFA-1, E-selectin, ICAM-1, and MAC-1 [12, 13]. Similarly, ALL cells express ICAM-1, LFA-1, LFA-3, CD44, beta-1 integrin, beta-2 integrin, α6 integrin, and E-selectin, among others [13]. Several studies demonstrate a correlation between CD56 expression and extramedullary infiltration in AML [14,15,16,17]. In addition, high MAC-1 expression, a protein involved in transendothelial migration, is commonly observed in M4 and M5 AML subtypes, providing a potential mechanistic explanation for the clinical observation of an increased risk of extramedullary involvement with these monocytic leukemias [18]. In vivo, ALL cells expressing α6 integrin have been shown to penetrate the CNS by α6 integrin-laminin interaction, allowing migration through laminin-rich vessels into the CSF [19]. Increased expression of vascular endothelial growth factor (VEGF) has been detected in ALL blasts in the CNS as compared with those in the bone marrow, suggesting that VEGF may be a potential mediator of CNS migration and involvement in ALL [20]. Studies have also demonstrated that chemokine receptors including CCR7 and CXCR4 play an important role in cell adherence and trafficking into the CNS [19, 21, 22]. Targeting these receptors may therefore be potential future therapeutic strategy for the prevention or treatment of CNS involvement in acute leukemias.
Leukemic stem cells (LSCs) may also play a role in CNS involvement and persistence. LSCs may be present in the CNS at the time of leukemia diagnosis but remain quiescent in the meninges, leading to higher risk of isolated CNS relapse [23, 24]. Since LSCs exist in a quiescent state, they are inherently resistant to chemotherapy that require cells to be in an active cell cycle, such as commonly used CNS-directed treatment like high-dose methotrexate (HD-MTX) and high-dose cytarabine (HD-AraC) [25]. This may explain why some patients still develop CNS relapse even after receiving appropriate CNS-directed prophylaxis.
Risk Factors
Identifying CNS-related risk factors is essential in designing effective therapeutic and monitoring strategies to prevent CNS disease in both AML and ALL. In ALL, younger age, hyperleukocytosis, presence of high-risk cytogenetics such as KMT2A rearrangements, Philadelphia chromosome (Ph)-positive ALL, and mature B-cell or T-cell immunophenotypes are independent high-risk features for development of CNS disease based on multiple studies [26,27,28,29]. High proliferative index and leukocytosis at presentation may be contributing factors for higher CNS involvement in both Ph-positive and mature B cell ALL; hence, these entities required a higher total number of prophylactic doses of IT chemotherapy than does standard risk B- cell ALL. Presence of extramedullary disease such as mediastinal mass and lymphadenopathy, commonly present in T-cell ALL, also increases the risk for CNS involvement. [28, 30].
In AML, younger age, increased WBC and lactose dehydrogenase (LDH) at diagnosis, chromosomal 11q23 abnormalities, and FLT3-ITD mutations have consistently been found to be independent risk factors for CNS leukemia [4, 31,32,33]. Dabaja and colleagues also noted that core-binding factor (CBF) AML (inversion 16 and t[8;21]) and high peripheral blasts at diagnosis were historically associated with an increased risk of CNS relapse [4]. However, the use of HD-AraC-based regimens—which are capable of penetrating the BBB—for patients with CBF AML may mitigate this risk. In a large cohort of patients with non-CBF AML, age (< 64 years), elevated LDH, and FLT3-ITD mutation were independently associated with increased risk of CNS relapse [34•]. AML with M4 or M5 phenotype (monocytic) is known to have increased expression of adhesion molecules such as CD56 and MAC-1 and has been linked to higher rates of CNS relapse. [35, 36].
Diagnosis
The diagnosis of CNS disease involves three primary techniques which may be used independently or in combination: clinical evaluation of neurologic symptoms, assessment of CSF through lumbar puncture, and radiologic imaging. Patients with ALL have historically been divided into 3 groups based on the amount of CNS involvement by CSF sampling and/or imaging: CNS1, no blasts in CSF; CNS2, < 5 WBCs/µL in the CSF with blasts; and CNS3, ≥ 5 WBC/µL in the CSF with blasts, or cerebral mass, or cranial nerve palsy with leukemic cells in the CSF [37]. While these categories do have some prognostic impact, the main use of this classification is for purposes of uniform reporting of retrospective and prospective studies that includes patients with CNS involvement.
Clinical manifestations of CNS involvement vary based on burden of disease and anatomical location of the leukemic infiltration. General neurologic symptoms include headache, nausea/vomiting, dizziness, mood changes, irritability, and gait abnormalities. Patients with cranial nerve involvement may endorse aphasia, hearing loss, dysphagia, altered mental status, facial numbness or droop, visual changes such as vision loss or diplopia, and chin numbness [38]. Spinal involvement may present as back pain, focal weakness, radicular pain, or bowel and bladder dysfunction. Recognition and interpretation of these symptoms are important as these can be subtle and overlap with other neurologic conditions. Therefore, clinical evaluation accompanied with lumbar puncture and/or imagining is key to a more conclusive diagnosis.
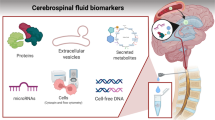
Evaluation of CSF by lumbar puncture (LP) is the standard diagnostic method used to detect CNS leukemia. Definitive diagnosis of leptomeningeal disease is based on presence of leukemic blasts in the CSF. Conventional cytology (CC) is used to examine the morphology of cells in order to distinguish malignant cells from benign. Although CC has a > 95% specificity, the sensitivity is relatively low (< 50%) leading to many false negatives [39, 40]. CSF specimens can have low cellularity making it possible to miss low levels of CNS involvement [39, 41]. Therefore, for patients with high clinical suspicion of CNS disease but without definitive evidence of involvement by CC, it may be necessary to repeat CC up to three times in order to rule out this diagnosis. Large-volume sampling can also increase the sensitivity of CC, albeit with an increased risk of post-LP headaches.
In contrast with CC, immunophenotyping by flow cytometry (FC) has higher sensitivity and specificity for detecting CNS leukemia even when cellularity is low [42]. However, FC requires expertise in handling, processing, and evaluating the sample in order to correctly distinguish neoplastic and non-neoplastic cells [41, 42]. Several studies have demonstrated superiority of FC over CC in detection of CNS disease [39, 42, 43••, 44,45,46,47]. A large multicenter study performed CC and FC on every CSF sample collected from 240 newly diagnosed ALL patients and found 43 patients had CNS disease that was identified by FC but not by CC [43••]. It is therefore recommended, whenever possible, to perform FC in conjunction with CC in order to provide adequate sensitivity for the detection of CNS leukemia. This is particularly important when the burden of disease is relatively low.
Radiologic imaging should also be considered when there is suspicion of CNS involvement and should be used as an adjunct to the other diagnostic tests previously discussed. The two most common neuroimaging modalities are cranial computed tomography (CT) and magnetic resonance imaging (MRI). MRI is more sensitive than CT in detecting smaller lesions or leptomeningeal involvement, which is the common area of leukemic infiltration [48,49,50]. Routine radiologic imaging is not indicated at the time of diagnosis in AML and ALL in the absence of clinical symptoms. However, for patients with neurologic deficits, MRI of the brain and/or spinal axis should be performed. For patients with CSF that is positive for leukemic involvement, imaging can help to identify mass lesions that may require more aggressive therapy (e.g., irradiation). In contrast, for those with negative CSF studies but a strong clinical suspicion for CNS involvement, MRI imaging of the brain and/or spinal cord can sometimes identify leukemic infiltration that is not appreciable with CSF analysis.
Prevention and Treatment Modalities
Systemic Therapy
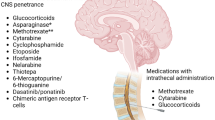
Systemic chemotherapy plays an integral role in preventing CNS disease. Utilizing drugs that penetrate the CNS such as HD-MTX (1–8 g/m2) and HD-AraC (1–3 g/m2) provides dual advantage due to their activity against both systemic and CNS disease. Methotrexate is an antifolate and antimetabolite that is hydrophilic and can penetrate the CNS when given at high doses, with higher concentrations achieved through bolus infusion [51]. It has shown to have significant activity in ALL cells, while AML cells remain intrinsically resistant [52, 53]. Doses of MTX up to 8 mg/m2 in lymphoma have been safely given with a folate rescue, leucovorin. Since AML has a relatively low incidence of CNS relapse, CNS-directed prophylaxis is not routinely used, aside from the use of HD-AraC in induction/consolidation therapy, which may have secondary benefit through its ability to penetrate the BBB [54, 55]. In contrast, both HD-MTX and HD-AraC are incorporated into widely used ALL regimens such as hyper-CVAD [56] and have been shown to be effective in preventing CNS relapses in ALL [57]. Corticosteroids, such as prednisone and dexamethasone, can also cross the BBB. Studies have shown higher CNS concentration and half-life with dexamethasone than prednisone. [58, 59].
In Ph-positive ALL, the BCR-ABL1 tyrosine kinase inhibitors dasatinib and ponatinib have both been shown to cross the BBB [60]. Higher doses of dasatinib (150 mg) appear to achieve adequate concentration for CNS activity, although optimal CNS concentrations for these tyrosine kinase inhibitors have not been defined. Recently, there is a trend towards reduced intensity or chemotherapy-free regimens for Ph-positive ALL. In a phase II study, blinatumomab plus dasatinib with 12 doses of IT chemotherapy in Ph-positive ALL was highly effective with promising and durable responses. However, among the 63 patients treated, 9 relapsed (4 of which were in the CNS) [61, 62]. Longer-term follow-up is still needed from this study, although this suggests that more doses of IT chemotherapy may be required when using these chemotherapy-free regimens in Ph-positive ALL.
Nelarabine, a drug that is approved for the treatment of relapsed/refractory T cell ALL, has demonstrated good CNS penetration [63]. In a phase III study, nelarabine improved disease-free survival, driven mostly by decrease in CNS relapse. T-ALL patients receiving nelarabine had significantly lower CNS relapses than those who did not receive nelarabine (1.3% versus 6.9%, respectively; P = 0.0001). [64•].
Intrathecal Chemotherapy
In the absence of prophylactic IT chemotherapy for ALL, more than half of patients may develop CNS disease [57]. Similarly, in AML, CNS disease has been observed in patients despite receiving HD-AraC as part of standard therapy [35, 65,66,67]. IT chemotherapy is essential to prevent and treat CNS leukemia due to its direct CSF penetration and sustained exposure, aided by the slow metabolism and clearance of drugs in the CSF [68, 69]. Routine prophylactic IT chemotherapy is an integral part of ALL therapy, while generally only those with significant CNS-related risk factors should receive IT chemotherapy in AML, as the rate of CNS involvement is low. In a recent large retrospective study of 3240 newly diagnosed AML patients, the incidence of CNS leukemia was only 1.1%. [70•].
IT chemotherapy can be administered through two routes: LP or intraventricularly through Ommaya reservoir placement. Several studies report better outcomes with Ommaya reservoirs than LPs [71,72,73]. Ommaya reservoirs allow for direct access to the CSF and cerebral ventricles for optimal and even distribution of chemotherapy, whereas LPs may lead to suboptimal distribution of chemotherapy through the neuroaxis. Chemotherapy delivery through an Ommaya reservoir is also associated with less discomfort than repetitive LPs. However, Ommaya reservoirs require surgical placement and may lead to serious and even life-threatening complications such as catheter migration or obstruction, device failure, infection, and subdural hematoma or hygroma [74,75,76,77,78]. These complications can be mitigated by skillful surgical technique, pre-operative imaging to view catheter placement and flow, and preoperative prophylactic antibiotics to reduce infection risk. Patients with intravascular coagulation, tumor at the site of intended reservoir placement, scalp infection, brain abscess, or allergy to silicone may not be candidates for Ommaya placement [74, 75]. In our own practice, we avoid Ommaya reservoirs, whenever possible, due to the aforementioned potential complications, which are particularly prevalent in patients with acute leukemia who experience repeated periods of chemotherapy-induced neutropenia. While LPs can be sometimes associated with discomfort for the patient, if done by an experienced practitioner and premedicated with anxiolysis and local analgesic, pain can be mitigated and complications such as post-LP headaches due to CSF leak and traumatic taps can be avoided.
The most utilized IT chemotherapies include MTX and AraC; liposomal AraC, thiotepa, and topotecan are rarely used but may be considered refractory cases. IT MTX and AraC may be given individually (usually when given as prophylaxis) or together for synergy (usually when given as treatment). Corticosteroids may be added to attenuate arachnoiditis associated with IT MTX and AraC, or all three agents may be combined, sometimes referred to as “triple IT therapy.” In adults, MTX is commonly given at a flat dose of 12 mg by LP and 6 mg intraventricularly, while AraC is given at a dose of 100 mg. The liposomal formulation of AraC allows for exposure that is 40 times that of standard cytarabine [79, 80] and results in sustained levels in the CSF for ≥ 14 days, as compared with < 24 h with standard AraC [80, 81]. Consequently, liposomal cytarabine is associated with an increased risk of neurotoxicity, which limits its use as a prophylactic agent [82, 83]. Topotecan has also demonstrated activity in meningeal malignancies at a dose of 400 mcg intrathecally. [84, 85].
Routine prophylaxis with IT MTX and IT AraC is an integral part of ALL therapy. Early integration of IT chemotherapy has been shown to reduce CNS relapses and improve survival [86, 87]. The number of doses of IT chemotherapy that should be administered varies on risk stratification. With the Hyper-CVAD regimen, standard IT prophylaxis for pre-B ALL and T-cell ALL includes 8 ITs (alternating doses of MTX and AraC, given 2 per cycle for the first 4 cycles). Given the higher rate of CNS relapse in patients with Ph-positive ALL, 12 doses of IT prophylaxis are routinely given [88], and patients with mature B- cell ALL (Burkitt leukemia) have the higher risk of CNS relapse and should receive 16 doses [29]. Concomitant administration of systemic HD-MTX and HD-AraC with ITs should be avoided, if possible, due to potential overlapping CNS toxicity. In the Hyper-CVAD regimen, during odd cycles with HD-MTX and HD-AraC, IT AraC should be given on approximately day 2 and IT MTX on approximately day 8 in order to avoid concomitant administration and increased risk of neurotoxicity. [89•].
If IT chemotherapy is administered by LP, it is recommended to delay the procedure until peripheral blasts are undetectable in order to prevent accidental infiltration of leukemic blasts into the CSF, although this cannot always be avoided in patients with active systemic and CNS disease [90]. For patients with ALL and CNS involvement, our practice is to administer triple IT chemotherapy (i.e., the combination of MTX, AraC and corticosteroids) twice weekly until documented clearance of the CSF by CC and/or FC. The frequency of IT chemotherapy is then decreased to weekly for 4 weeks, then every other week for 4 weeks, and then monthly for approximately 4 months. For patients who also require radiotherapy (RT), it is best to avoid concomitant MTX as it acts as a radiosensitizer and can increase radiation-related CNS toxicity. [91••, 92, 93].
In AML, routine CNS prophylaxis is unnecessary except in those with high-risk features for CNS involvement. In our own practice, we reserve prophylactic IT chemotherapy (generally 2 doses of IT cytarabine) for those patients with AML who present with high WBC (≥ 50 × 109/L) or elevated LDH or have a FLT3-ITD mutation.
Radiation
With the successful combination of IT with high-dose systemic chemotherapy, prophylaxis with cranial radiation therapy (RT) is no longer warranted, as the added toxicity outweighs benefit. In a meta-analysis of 16,623 newly diagnosed children with ALL receiving both systemic and IT chemotherapy, the addition of prophylactic cranial RT did not impact the risk of CNS relapse overall [94••]. In contrast with its limited benefit as prophylaxis, cranial RT has demonstrated benefit in improving symptoms and decreasing disease burden in those with CNS relapse, especially isolated CNS relapse [95, 96]. In a study with 163 adults with CNS involvement and neurologic symptoms, comprehensive WBRT or craniospinal irradiation (CSI) provided symptom resolution or improvement in two-thirds of the patients [95]. As a result, CNS-directed RT is generally reserved for patients with relapsed CNS leukemia, those with CNS disease that is refractory to systemic and/or IT chemotherapy, or those with CNS disease and who plan to undergo allogeneic stem cell transplant (HSCT) [97].
In CNS-directed radiation, normal tissue complication probability, tumor control probability, location of involvement, patient age and performance status, and treatment goal (palliation, bridge to HSCT, etc.) are all taken into consideration when designing and determining dose of RT. CNS radiation in adults is generally recommended at a dose of 23.4 Gy in 1.8 Gy fractions [91••]. CSI can be photon- or proton-based, although the latter is preferred due to better dose distribution and less toxicity [98]. Proton beam RT uses charged particles for tumor killing and spares normal tissue, thus reducing acute and chronic toxicities. In contrast, photon beam RT uses high-energy X-rays and can damage both normal cells and tissues as it exits the body. If CSI is combined with total body irradiation as a preparatory regimen for HSCT, the cumulative dose should not surpass 24 Gy [91••].
Toxicities
Systemic and IT Chemotherapy
The advantage of CNS penetration with HD-MTX and HD-AraC must be balanced with their potential for neurotoxicity. MTX can lead to CNS toxicity through direct neuronal damage or disruption of CNS folate homeostasis [99,100,101]. MTX-induced neurotoxicity can be acute or chronic. Acute toxicities generally occur 2 days to weeks after exposure and manifest as seizures, headache, stroke-like symptoms, dysarthria, aphasia, leukoencephalopathy, and/or myelopathy. In contrast, chronic toxicity can take months to years to become evident and often presents as cognitive decline and behavioral abnormalities. Several germline variants and polymorphisms including MTX metabolism, such as thymidylate synthase, SLCO1B1, methylenetetrahydrofolate reductase (MTHFR), GSTP1, and SHMT1, predispose patients to increased toxicity [102,103,104]. A high plasma MTX-to-leucovorin ratio and increased serum homocysteine levels have also been linked to increased risk of neurotoxicity [105, 106]. Delayed MTX clearance due to pleural effusions, decreased renal function, or drug-drug interactions should be avoided if possible. Re-exposure of MTX after transient neurotoxicity, such as acute encephalopathy, has been successful in some reports but should be attempted with caution [105, 107]. Folate rescue with leucovorin should be used to mitigate MTX-induced toxicity. In addition, dextromethorphan, a homocysteine antagonist, has also suggested to improve symptoms in pediatric patients with subacute MTX toxicity, although its efficacy remains controversial [108]. Management of toxicity includes discontinuing MTX, administering leucovorin rescue, and considering empiric dextromethorphan and/or vitamin B12.
HD-AraC (1–3 g/m2) can cause cerebellar and ocular toxicity. Most cerebellar toxicity, such as delirium, ataxia, dysarthria, nystagmus, and somnolence can be reversible; in contrast, conjunctivitis and corneal toxicity may be irreversible [109]. Toxicity may occur via direct cytotoxic effect or by an immune-mediated mechanism [110, 111]. Risk factors for neurotoxicity include: a dose > 1 g/m2, older age, renal dysfunction, IT dose of > 100 mg per week, liposomal IT administration, and concurrent use of HD-MTX [112,113,114,115]. In one retrospective analysis, creatinine ≥ 1.2 mg/dL and alkaline phosphatase ≥ 3 times above the upper limit of normal were found to be independent risk factors for AraC toxicity [115]. All patients treated with HD-AraC should also receive prophylactic corticosteroid eye drops (prednisolone or dexamethasone) starting the day prior to administration and until 2 days after completion in order to prevent conjunctivitis. Patients may also derive benefit from topical nonsteroidal anti-inflammatory drugs or cold compresses to the eye [116, 117]. Discontinuation of cytarabine and administration of a corticosteroid may help resolve or attenuate cerebellar symptoms. [111].
Occurrence of neurologic toxicity after IT chemotherapy varies largely in the time of onset and degree of symptoms. IT chemotherapy can cause arachnoiditis that can be mitigated with concurrent corticosteroid use. MTX-induced myelopathy and encephalopathy have been reported with IT administration as well. [118,119,120,121]. IT chemotherapy-induced neurotoxicity generally correlates with higher cumulative dose [122]. Currently, no patient-related risk factors predicting for CNS toxicity from IT chemotherapy have been identified [123].
Radiation
RT complications can vary based on the radiation field, dose, and length of therapy. Common signs and symptoms include pituitary dysfunction, neurocognitive decline, brain necrosis, leukoencephalopathy, and demyelination of spinal cord. Onset of RT complications can be defined as acute (during or up to 6 weeks), early delayed (6 weeks to 6 months), and late (≥ 6 months). While acute and early-delayed symptoms are often reversible, late effects may be irreversible. Although some IT and intravenous chemotherapies may have synergy and higher tumoricidal capacity when combined with RT, in the context of CNS leukemia, it is generally recommended to avoid their concurrent administration in order to prevent overlapping and serious neurotoxicity, such as necrotizing leukoencephalopathy. RT should be delayed by at least 2 weeks from last intravenous and intrathecal administration of MTX and AraC, but in emergent cases separation by 48–72 h can be considered [91••]. Patients who receive WBRT should receive prophylactic memantine as it is shown to decrease cognitive decline and be neuroprotective. [124,125,126].
Conclusion
CNS involvement of acute leukemia has serious implications on outcomes and requires swift identification and treatment. FC is more specific and sensitive than CC in detecting leukemic cells in the CSF; it should be included in the diagnostic work-up of suspected CNS disease. Routine incorporation of prophylactic IT chemotherapy is a fundamental component of ALL therapy and has significantly reduced the rate of CNS relapses in this disease. In contrast, only those patients with AML who harbor significant CNS-related risk factors require CNS-directed prophylactic therapy. For patients with relatively low-burden CNS disease, aggressive IT chemotherapy (alongside appropriate systemic chemotherapy) may be adequate to eradicate the CNS leukemia. For more refractory cases or for patients with gross disease by imaging, radiation may be required. For all these treatments, an appropriate risk–benefit assessment should be performed, and the potential for both acute and chronic toxicities should be considered. Given the many challenges of treating CNS leukemia, prevention is of the utmost importance. Accurate risk stratification tools and algorithms for delivery of risk-adapted prophylactic therapy are both imperative to improving the outcomes of patients with both ALL and AML.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gokbuget N, Hoelzer D. Meningeosis leukaemica in adult acute lymphoblastic leukaemia. J Neurooncol. 1998;38:167–80. https://doi.org/10.1023/a:1005963732481.
Lazarus HM, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 2006;108:465–72. https://doi.org/10.1182/blood-2005-11-4666.
Kreuger A, et al. Central nervous system disease in childhood acute lymphoblastic leukemia: prognostic factors and results of treatment. Pediatr Hematol Oncol. 1991;8:291–9. https://doi.org/10.3109/08880019109028802.
Shihadeh F, et al. Cytogenetic profile of patients with acute myeloid leukemia and central nervous system disease. Cancer. 2012;118:112–7. https://doi.org/10.1002/cncr.26253.
Vermeire, T. et al. Sera from different age cohorts in Belgium show limited cross-neutralization between the mumps vaccine and outbreak strains. Clin Microbiol Infect. 2019;907 e901–907.e906, https://doi.org/10.1016/j.cmi.2018.11.016
Si M, et al. The role of cytokines and chemokines in the microenvironment of the blood-brain barrier in leukemia central nervous system metastasis. Cancer Manag Res. 2018;10:305–13. https://doi.org/10.2147/CMAR.S152419.
Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. https://doi.org/10.1016/j.bbamem.2007.09.003.
Taddei A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–34. https://doi.org/10.1038/ncb1752.
Price RA, Johnson WW. The central nervous system in childhood leukemia. I. The arachnoid. Cancer. 1973;31:520–33. https://doi.org/10.1002/1097-0142(197303)31:3%3c520::aid-cncr2820310306%3e3.0.co;2-2.
Thomas LB. Pathology of leukemia in the brain and meninges: postmortem studies of patients with acute leukemia and of mice given inoculations of L1210 leukemia. Cancer Res. 1965;25:1555–71.
Pui CH, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol. 2008;9:257–68. https://doi.org/10.1016/S1470-2045(08)70070-6.
Alegretti AP, et al. The expression of CD56 antigen is associated with poor prognosis in patients with acute myeloid leukemia. Rev Bras Hematol Hemoter. 2011;33:202–6. https://doi.org/10.5581/1516-8484.20110054.
Deak D, et al. A narrative review of central nervous system involvement in acute leukemias. Ann Transl Med. 2021;9:68. https://doi.org/10.21037/atm-20-3140.
Chang H, et al. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004;28:1007–11. https://doi.org/10.1016/j.leukres.2004.01.006.
Ravandi F, et al. CD56 expression predicts occurrence of CNS disease in acute lymphoblastic leukemia. Leuk Res. 2002;26:643–9. https://doi.org/10.1016/s0145-2126(01)00188-6.
Zaidi SZ, Motabi IH, Al-Shanqeeti A. CD56 and RUNX1 isoforms in AML prognosis and their therapeutic potential. Hematol Oncol Stem Cell Ther. 2016;9:129–30. https://doi.org/10.1016/j.hemonc.2015.11.006.
Hu W, et al. Expression of CD56 is a risk factor for acute lymphocytic leukemia with central nervous system involvement in adults. Hematology. 2017;22:81–7. https://doi.org/10.1080/10245332.2016.1238183.
Hyun YM, Choe YH, Park SA, Kim M. LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) distinctly regulate neutrophil extravasation through hotspots I and II. Exp Mol Med. 2019;51:1–13. https://doi.org/10.1038/s12276-019-0227-1.
Yao H, et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018;560:55–60. https://doi.org/10.1038/s41586-018-0342-5.
Munch V, et al. Central nervous system involvement in acute lymphoblastic leukemia is mediated by vascular endothelial growth factor. Blood. 2017;130:643–54. https://doi.org/10.1182/blood-2017-03-769315.
Buonamici S, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459:1000–4. https://doi.org/10.1038/nature08020.
Alsadeq A, et al. The role of ZAP70 kinase in acute lymphoblastic leukemia infiltration into the central nervous system. Haematologica. 2017;102:346–55. https://doi.org/10.3324/haematol.2016.147744.
Roboz GJ, Guzman M. Acute myeloid leukemia stem cells: seek and destroy. Expert Rev Hematol. 2009;2:663–72. https://doi.org/10.1586/ehm.09.53.
Ford AM, et al. Protracted dormancy of pre-leukemic stem cells. Leukemia. 2015;29:2202–7. https://doi.org/10.1038/leu.2015.132.
Jonart LM, et al. Disrupting the leukemia niche in the central nervous system attenuates leukemia chemoresistance. Haematologica. 2020;105:2130–40. https://doi.org/10.3324/haematol.2019.230334.
Cortes J. Central nervous system involvement in adult acute lymphocytic leukemia. Hematol Oncol Clin North Am. 2001;15:145–62. https://doi.org/10.1016/s0889-8588(05)70203-3.
Sancho JM, et al. Central nervous system recurrence in adult patients with acute lymphoblastic leukemia: frequency and prognosis in 467 patients without cranial irradiation for prophylaxis. Cancer. 2006;106:2540–6. https://doi.org/10.1002/cncr.21948.
Kantarjian HM, et al. Identification of risk groups for development of central nervous system leukemia in adults with acute lymphocytic leukemia. Blood. 1988;72:1784–9.
Samra B, et al. Long term outcome of Hyper-CVAD-R for Burkitt leukemia/lymphoma and high-grade B-cell lymphoma: focus on CNS relapse. Blood Adv. 2021. https://doi.org/10.1182/bloodadvances.2021004427.
Reman O, et al. Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis and/or at first relapse: results from the GET-LALA group. Leuk Res. 2008;32:1741–50. https://doi.org/10.1016/j.leukres.2008.04.011.
Rozovski U, et al. Incidence of and risk factors for involvement of the central nervous system in acute myeloid leukemia. Leuk Lymphoma. 2015;56:1392–7. https://doi.org/10.3109/10428194.2014.953148.
Cassileth PA, Sylvester LS, Bennett JM, Begg CB. High peripheral blast count in adult acute myelogenous leukemia is a primary risk factor for CNS leukemia. J Clin Oncol. 1988;6:495–8. https://doi.org/10.1200/JCO.1988.6.3.495.
Johnston DL, Alonzo TA, Gerbing RB, Lange BJ, Woods WG. Risk factors and therapy for isolated central nervous system relapse of pediatric acute myeloid leukemia. J Clin Oncol. 2005;23:9172–8. https://doi.org/10.1200/jco.2005.02.7482.
Jabbour E, et al. Factors associated with risk of central nervous system relapse in patients with non-core binding factor acute myeloid leukemia. Am J Hematol. 2017;92:924–928. https://doi.org/10.1002/ajh.24799. This paper identified risk factors for CNS relapse in patients with AML (i.e., older age, elevated LDH, and FLT3-ITD mutations).
Martinez-Cuadron D, et al. Central nervous system involvement at first relapse in patients with acute myeloid leukemia. Haematologica. 2011;96:1375–9. https://doi.org/10.3324/haematol.2011.042960.
Grier HE, et al. Prognostic factors in childhood acute myelogenous leukemia. J Clin Oncol. 1987;5:1026–32. https://doi.org/10.1200/JCO.1987.5.7.1026.
Smith M, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. https://doi.org/10.1200/JCO.1996.14.1.18.
Sasaki M, et al. Bilateral numb chin syndrome leading to a diagnosis of Burkitt’s cell acute lymphocytic leukemia: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e11-16. https://doi.org/10.1016/j.tripleo.2010.09.066.
Bromberg JE, et al. CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology. 2007;68:1674–9. https://doi.org/10.1212/01.wnl.0000261909.28915.83.
Kaplan JG, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. 1990;9:225–9. https://doi.org/10.1007/BF02341153.
Craig FE, Ohori NP, Gorrill TS, Swerdlow SH. Flow cytometric immunophenotyping of cerebrospinal fluid specimens. Am J Clin Pathol. 2011;135:22–34. https://doi.org/10.1309/AJCPANA7ER1ABMZI.
Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941–67. https://doi.org/10.1182/blood-2007-11-120535.
Del Principe, M. I. et al. Clinical significance of occult central nervous system disease in adult acute lymphoblastic leukemia. A multicenter report from the Campus ALL Network. Haematologica. 2021;106;39–45. https://doi.org/10.3324/haematol.2019.231704. This multicenter study showed the superior sensitivity of flow cytometry (as compared with conventional cytology) for the detection of CNS disease in patients with ALL.
Zeiser R, et al. Clinical follow-up indicates differential accuracy of magnetic resonance imaging and immunocytology of the cerebral spinal fluid for the diagnosis of neoplastic meningitis - a single centre experience. Br J Haematol. 2004;124:762–8. https://doi.org/10.1111/j.1365-2141.2004.04853.x.
Quijano S, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin’s lymphoma: improved sensitivity of flow cytometry. J Clin Oncol. 2009;27:1462–9. https://doi.org/10.1200/JCO.2008.17.7089.
Di Noto R, et al. Critical role of multidimensional flow cytometry in detecting occult leptomeningeal disease in newly diagnosed aggressive B-cell lymphomas. Leuk Res. 2008;32:1196–9. https://doi.org/10.1016/j.leukres.2007.12.016.
Subira D, et al. Flow cytometry and the study of central nervous disease in patients with acute leukaemia. Br J Haematol. 2001;112:381–4. https://doi.org/10.1046/j.1365-2141.2001.02505.x.
Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–19. https://doi.org/10.1053/ctrv.1999.0119.
Baytan B, Evim MS, Guler S, Gunes AM, Okan M. Acute Central Nervous System Complications in Pediatric Acute Lymphoblastic Leukemia. Pediatr Neurol. 2015;53:312–8. https://doi.org/10.1016/j.pediatrneurol.2015.03.006.
Shen H, et al. The diagnostic and prognostic value of MRI in central nervous system involvement of acute myeloid leukemia: a retrospective cohort of 84 patients. Hematology. 2020;25:258–63. https://doi.org/10.1080/16078454.2020.1781500.
Lassman AB, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78:255–60. https://doi.org/10.1007/s11060-005-9044-6.
Farber S. Some observations on the effect of folic acid antagonists on acute leukemia and other forms of incurable cancer. Blood. 1949;4:160–7.
Freeman AI, Wang JJ, Sinks LF. High-dose methotrexate in acute lymphocytic leukemia. Cancer Treat Rep. 1977;61:727–31.
Kadia TM, et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2021;8:e552–61. https://doi.org/10.1016/S2352-3026(21)00192-7.
DiNardo CD, et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J Clin Oncol. 2021;39:2768–78. https://doi.org/10.1200/JCO.20.03736.
Kantarjian HM, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547–61.
Cortes J, et al. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86:2091–7.
Bostrom BC, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101:3809–17. https://doi.org/10.1182/blood-2002-08-2454.
Jones B, et al. Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol. 1991;19:269–75. https://doi.org/10.1002/mpo.2950190411.
Porkka K, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112:1005–12. https://doi.org/10.1182/blood-2008-02-140665.
Foa R, et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383:1613–23. https://doi.org/10.1056/NEJMoa2016272.
Sabina Chiaretti RB, Vitale A, Elia L, Messina M, Viero P, Annunziata M, Lunghi M, Fabbiano F, Bonifacio M, Fracchiolla N, Di Bartolomeo P, Renzulli LG, De Propris MS, Vignetti M, Guarini A, Rambaldi A, Foà R. In European Hematology Association (EHA). 2021.
Berg SL, et al. Plasma and cerebrospinal fluid pharmacokinetics of nelarabine in nonhuman primates. Cancer Chemother Pharmacol. 2007;59:743–7. https://doi.org/10.1007/s00280-006-0328-0.
Dunsmore KP, et al. Children's Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2020;38:3282–3293. https://doi.org/10.1200/JCO.20.00256. This randomized study showed EFS benefit for the addition of nelarabine in T-cell ALL. This was driven by a decrease in CNS relapses in the nelarabine arm.
Abbott BL, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution’s experience. Leukemia. 2003;17:2090–6. https://doi.org/10.1038/sj.leu.2403131.
Bisschop MM, et al. Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukaemia. Leukemia. 2001;15:46–9. https://doi.org/10.1038/sj.leu.2401971.
Pui CH, et al. Central nervous system leukemia in children with acute nonlymphoblastic leukemia. Blood. 1985;66:1062–7.
DeAngelis L, Batchelor T. Primary CNS lymphoma: is there a role for prophylaxis against lymphomatous meningitis? Expert Rev Neurother. 2004;4:S19-24. https://doi.org/10.1586/14737175.4.4.S19.
Hodozuka A, et al. Intrathecal infusion of the antineoplastic agents for meningeal dissemination. Gan To Kagaku Ryoho. 2008;35:900–5.
Ganzel C, et al. CNS Involvement in AML at Diagnosis is Rare and does not Affect Response or Survival: Data from 11 ECOG-ACRIN Trials. Blood Adv. 2021. https://doi.org/10.1182/bloodadvances.2021004999. This large study shows that CNS involvement in AML at the time of diagnosis is relatively rare (1.1% of cases) and did not impact prognosis.
Glantz MJ, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394–402.
Chamberlain MC, Kormanik PA. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996;46:1674–7. https://doi.org/10.1212/wnl.46.6.1674.
Montes de Oca Delgado M, et al. The Comparative Treatment of Intraventricular Chemotherapy by Ommaya Reservoir vs. Lumbar Puncture in Patients With Leptomeningeal Carcinomatosis. Front Oncol. 2018;8:509. https://doi.org/10.3389/fonc.2018.00509.
Obbens EA, Leavens ME, Beal JW, Lee YY. Ommaya reservoirs in 387 cancer patients: a 15-year experience. Neurology. 1985;35:1274–8. https://doi.org/10.1212/wnl.35.9.1274.
Perrin RG, et al. Experience with Ommaya reservoir in 120 consecutive patients with meningeal malignancy. Can J Neurol Sci. 1990;17:190–2. https://doi.org/10.1017/s0317167100030432.
Mead PA, Safdieh JE, Nizza P, Tuma S, Sepkowitz KA. Ommaya reservoir infections: a 16-year retrospective analysis. J Infect. 2014;68:225–30. https://doi.org/10.1016/j.jinf.2013.11.014.
Szvalb AD, et al. Ommaya reservoir-related infections: clinical manifestations and treatment outcomes. J Infect. 2014;68:216–24. https://doi.org/10.1016/j.jinf.2013.12.002.
Bin Nafisah S, Ahmad M. Ommaya reservoir infection rate: a 6-year retrospective cohort study of Ommaya reservoir in pediatrics. Childs Nerv Syst. 2015;31:29–36. https://doi.org/10.1007/s00381-014-2561-x.
Bomgaars L, et al. Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J Clin Oncol. 2004;22:3916–21. https://doi.org/10.1200/JCO.2004.01.046.
Kim S, et al. Extended CSF cytarabine exposure following intrathecal administration of DTC 101. J Clin Oncol. 1993;11:2186–93. https://doi.org/10.1200/JCO.1993.11.11.2186.
Chamberlain MC, Kormanik P, Howell SB, Kim S. Pharmacokinetics of intralumbar DTC-101 for the treatment of leptomeningeal metastases. Arch Neurol. 1995;52:912–7. https://doi.org/10.1001/archneur.1995.00540330094020.
Jabbour E, et al. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood. 2007;109:3214–8. https://doi.org/10.1182/blood-2006-08-043646.
Parasole R, et al. Efficacy and safety of intrathecal liposomal cytarabine for the treatment of meningeal relapse in acute lymphoblastic leukemia: experience of two pediatric institutions. Leuk Lymphoma. 2008;49:1553–9. https://doi.org/10.1080/10428190802216749.
Blaney SM, et al. Phase I clinical trial of intrathecal topotecan in patients with neoplastic meningitis. J Clin Oncol. 2003;21:143–7. https://doi.org/10.1200/JCO.2003.04.053.
Groves MD, et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10:208–15. https://doi.org/10.1215/15228517-2007-059.
Pui CH, et al. Early intensification of intrathecal chemotherapy virtually eliminates central nervous system relapse in children with acute lymphoblastic leukemia. Blood. 1998;92:411–5.
Dekker AW, et al. Intensive postremission chemotherapy without maintenance therapy in adults with acute lymphoblastic leukemia. Dutch Hemato-Oncology Research Group. J Clin Oncol 1997;15:476–482. https://doi.org/10.1200/JCO.1997.15.2.476.
Paul S, et al. Title: 12 Versus 8 Prophylactic Intrathecal (IT) Chemotherapy Administration Decrease Incidence of Central Nervous System (CNS) Relapse in Patients (pts) with Newly Diagnosed Philadelphia (Ph)-Positive Acute Lymphocytic Leukemia (ALL). Blood. 2019;134:3810–3810. https://doi.org/10.1182/blood-2019-130284.
Rausch CR, Jabbour EJ, Kantarjian HM, Kadia TM. Optimizing the use of the hyperCVAD regimen: Clinical vignettes and practical management. Cancer. 2020;126;1152–1160. https://doi.org/10.1002/cncr.32606. This paper provides practical guidelines for the use of hyper-CVAD regimens in ALL, including how to sequence and dose intrathecal chemotherapy within this regimen.
Liu HC, et al. Triple intrathecal therapy alone with omission of cranial radiation in children with acute lymphoblastic leukemia. J Clin Oncol. 2014;32:1825–9. https://doi.org/10.1200/JCO.2013.54.5020.
Pinnix CC, Yahalom J, Specht L, Dabaja BS. Radiation in Central Nervous System Leukemia: Guidelines From the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2018;102:53–58. https://doi.org/10.1016/j.ijrobp.2018.05.067. This paper from the International Lymphoma Radiation Oncology Group (ILROC) provides consensus guidelines for the use of radiation in acute leukemias, as well as the timing of intrathecal chemotherapy relative to radiation administration.
Kim A, et al. A combination of methotrexate and irradiation promotes cell death in NK/T-cell lymphoma cells via down-regulation of NF-kappaB signaling. Leuk Res. 2012;36:350–7. https://doi.org/10.1016/j.leukres.2011.07.027.
Shehata WM, Meyer RL. The enhancement effect of irradiation by methotrexate. Report of three complications. Cancer. 1980;46:1349–52. https://doi.org/10.1002/1097-0142(19800915)46:6%3c1349::aid-cncr2820460609%3e3.0.co;2-c.
Vora, A. et al. Influence of Cranial Radiotherapy on Outcome in Children With Acute Lymphoblastic Leukemia Treated With Contemporary Therapy. J Clin Oncol. 2016;34:919–926. https://doi.org/10.1200/jco.2015.64.2850. This paper shows that in the contemporary era, cranial radiotherapy does not significantly impact relapse rates when given as prophylaxis in patients with ALL.
Walker GV, et al. Comprehensive craniospinal radiation for controlling central nervous system leukemia. Int J Radiat Oncol Biol Phys. 2014;90:1119–25. https://doi.org/10.1016/j.ijrobp.2014.08.004.
Hiniker SM, et al. Survival and neurocognitive outcomes after cranial or craniospinal irradiation plus total-body irradiation before stem cell transplantation in pediatric leukemia patients with central nervous system involvement. Int J Radiat Oncol Biol Phys. 2014;89:67–74. https://doi.org/10.1016/j.ijrobp.2014.01.056.
Patel N, et al. Emergent Radiotherapy for Leukemia-Induced Cranial Neuropathies Refractory to Intrathecal Therapy. Cureus. 2021;13: e15212. https://doi.org/10.7759/cureus.15212.
Armenian SH, et al. Cardiovascular Disease in Survivors of Childhood Cancer: Insights Into Epidemiology, Pathophysiology, and Prevention. J Clin Oncol. 2018;36:2135–44. https://doi.org/10.1200/JCO.2017.76.3920.
Cole PD, et al. Folate homeostasis in cerebrospinal fluid during therapy for acute lymphoblastic leukemia. Pediatr Neurol. 2009;40:34–41. https://doi.org/10.1016/j.pediatrneurol.2008.09.005.
Vezmar S, Schusseler P, Becker A, Bode U, Jaehde U. Methotrexate-associated alterations of the folate and methyl-transfer pathway in the CSF of ALL patients with and without symptoms of neurotoxicity. Pediatr Blood Cancer. 2009;52:26–32. https://doi.org/10.1002/pbc.21827.
Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. 2003;49:92–104. https://doi.org/10.1159/000069773.
Kishi S, et al. Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood. 2007;109:4151–7. https://doi.org/10.1182/blood-2006-10-054528.
Radtke S, et al. Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood. 2013;121:5145–53. https://doi.org/10.1182/blood-2013-01-480335.
Vagace JM, et al. Methotrexate-induced subacute neurotoxicity in a child with acute lymphoblastic leukemia carrying genetic polymorphisms related to folate homeostasis. Am J Hematol. 2011;86:98–101. https://doi.org/10.1002/ajh.21897.
Bhojwani D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949–59. https://doi.org/10.1200/JCO.2013.53.0808.
Pinnix CC, et al. Dorsal column myelopathy after intrathecal chemotherapy for leukemia. Am J Hematol. 2017;92:155–60. https://doi.org/10.1002/ajh.24611.
Murtomaa H, Saxen L. [Juvenile periodontitis. Report of a case (author's transl)]. Proc Finn Dent Soc 1976;72:135–138.
Afshar M, Birnbaum D, Golden C. Review of dextromethorphan administration in 18 patients with subacute methotrexate central nervous system toxicity. Pediatr Neurol. 2014;50:625–9. https://doi.org/10.1016/j.pediatrneurol.2014.01.048.
Ritch PS, Hansen RM, Heuer DK. Ocular toxicity from high-dose cytosine arabinoside. Cancer. 1983;51:430–2. https://doi.org/10.1002/1097-0142(19830201)51:3%3c430::aid-cncr2820510313%3e3.0.co;2-5.
Hilgendorf I, et al. Neurological complications after intrathecal liposomal cytarabine application in patients after allogeneic haematopoietic stem cell transplantation. Ann Hematol. 2008;87:1009–12. https://doi.org/10.1007/s00277-008-0546-0.
Malhotra P, et al. Cytarabine-induced neurotoxicity responding to methyl prednisolone. Am J Hematol. 2004;77:416. https://doi.org/10.1002/ajh.20171.
Baker WJ, Royer GL Jr, Weiss RB. Cytarabine and neurologic toxicity. J Clin Oncol. 1991;9:679–93. https://doi.org/10.1200/JCO.1991.9.4.679.
Gottlieb D, et al. The neurotoxicity of high-dose cytosine arabinoside is age-related. Cancer. 1987;60:1439–41. https://doi.org/10.1002/1097-0142(19871001)60:7%3c1439::aid-cncr2820600705%3e3.0.co;2-f.
Damon LE, Mass R, Linker CA. The association between high-dose cytarabine neurotoxicity and renal insufficiency. J Clin Oncol. 1989;7:1563–8. https://doi.org/10.1200/JCO.1989.7.10.1563.
Rubin EH, et al. Risk factors for high-dose cytarabine neurotoxicity: an analysis of a cancer and leukemia group B trial in patients with acute myeloid leukemia. J Clin Oncol. 1992;10:948–53. https://doi.org/10.1200/JCO.1992.10.6.948.
Higa GM, Gockerman JP, Hunt AL, Jones MR, Horne BJ. The use of prophylactic eye drops during high-dose cytosine arabinoside therapy. Cancer. 1991;68:1691–3. https://doi.org/10.1002/1097-0142(19911015)68:8%3c1691::aid-cncr2820680805%3e3.0.co;2-w.
Matteucci P, et al. Topical prophylaxis of conjunctivitis induced by high-dose cytosine arabinoside. Haematologica. 2006;91:255–7.
Gosavi T, Diong CP, Lim SH. Methotrexate-induced myelopathy mimicking subacute combined degeneration of the spinal cord. J Clin Neurosci. 2013;20:1025–6. https://doi.org/10.1016/j.jocn.2012.06.018.
Joseph PJ, Reyes MR. Dorsal column myelopathy following intrathecal chemotherapy for acute lymphoblastic leukemia. J Spinal Cord Med. 2014;37:107–13. https://doi.org/10.1179/2045772312Y.0000000081.
Lu CH, Yao M, Liu HM, Chen YF. MR findings of intrathecal chemotherapy-related myelopathy in two cases: mimicker of subacute combined degeneration. J Neuroimaging. 2007;17:184–7. https://doi.org/10.1111/j.1552-6569.2007.00094.x.
McLean DR, et al. Myelopathy after intrathecal chemotherapy. A case report with unique magnetic resonance imaging changes. Cancer. 1994;73, 3037–40. https://doi.org/10.1002/1097-0142(19940615)73:12<3037::aid-cncr2820731223>3.0.co;2-6.
Bleyer WA, Drake JC, Chabner BA. Neurotoxicity and elevated cerebrospinal-fluid methotrexate concentration in meningeal leukemia. N Engl J Med. 1973;289:770–3. https://doi.org/10.1056/NEJM197310112891503.
Mahoney DH Jr, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy–a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–22. https://doi.org/10.1200/JCO.1998.16.5.1712.
Brown PD, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–37. https://doi.org/10.1093/neuonc/not114.
Nakanishi N, et al. Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci. 2009;29:5260–5. https://doi.org/10.1523/JNEUROSCI.1067-09.2009.
Chen HS, et al. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Paul, S., Short, N.J. Central Nervous System Involvement in Adults with Acute Leukemia: Diagnosis, Prevention, and Management. Curr Oncol Rep 24, 427–436 (2022). https://doi.org/10.1007/s11912-022-01220-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01220-4