Abstract
Introduction
The conservative management of upper tract urothelial carcinoma (UTUC) has historically been offered to patients with imperative indications. The recent International Consultation on Urologic Diseases (ICUD) publication on UTUC stratified treatment allocations based on high- and low-risk groups. This report updates the conservative management of the low-risk group.
Methods
The ICUD for low-risk UTUC working group performed a thorough review of the literature with an assessment of the level of evidence and grade of recommendation for a variety of published studies in this disease space. We update these publications and provide a summary of that original report.
Results
There are no prospective randomized controlled studies to support surgical management guidelines. A risk-stratified approach based on clinical, endoscopic, and biopsy assessment allows selection of patients who could benefit from kidney-preserving procedures with oncological outcomes potentially similar to radical nephroureterectomy with bladder cuff excision, with the added benefit of renal function preservation. These treatments are aided by the development of high-definition flexible digital URS, multi-biopsies with the aid of access sheaths and other tools, and promising developments in the use of adjuvant topical therapy.
Conclusions
Recent developments in imaging, minimally invasive techniques, multimodality approaches, and adjuvant topical regimens and bladder cancer prevention raise the hope for improved risk stratification and may greatly improve the endoscopic treatment for low-risk UTUC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Upper tract urothelial carcinoma (UTUC) is a rare disease. Approximately two-thirds of UTUCs present as high-grade invasive disease at the time of diagnosis, and multifocal disease has been reported in about 25–30 % of UTUCs [1, 2]. Radical nephroureterectomy (RNU) with excision of the bladder cuff is the gold standard surgical procedure for the treatment of UTUC in patients with a normal functional contralateral kidney with no evidence of metastatic disease [3]. The tumor characteristics can only be accurately determined by analysis of pathological specimens after RNU [4].
Low-risk cancers (defined for this section as pT0/pTa/pTis/pT1 low-grade tumors) are reported in approximately 40–56 % of UTUCs that undergo RNU, which may represent patients amenable to kidney preserving procedure (KPP) such as endoscopic management (ureteroscopic or percutaneous approach), partial nephrectomy and segmental ureterectomy [5, 6].
The management of UTUC should attempt to stratify patients in a preoperative setting, allowing identification of individuals who may benefit from conservative KPPs without compromising oncological outcomes while preserving renal function [7]. Several studies have reported KPP for the treatment of low-risk UTUC in selected patients, suggesting similar oncological outcomes as the gold standard RNU [8]. This stratification can be performed using various prognostic factors that have been shown to be predictive of outcomes in multiple studies [9].
The KPP approach in the management of UTUC has been offered to patients with normal contralateral renal function, for low-risk tumors as defined above, including selective cases of carcinoma in situ (CIS) or high-grade presumed noninvasive tumors. Conservative approaches can also be considered in imperative cases in those with renal insufficiency, bilateral UTUC, solitary kidney and associated severe morbid conditions that preclude fitness for surgery [1].
Strict surveillance is a prerequisite for follow-up after KPP allowing detection of recurrence and disease progression. The focus of this review is to provide an overview of the current indications and modalities of KPP in the management of low-risk UTUC, and assessing the recommendations based on the level of evidence and grade of recommendations.
Endoscopic treatment of low-risk UTUC
The objectives of the endoscopic management of low-risk UTUC are to control local tumor growth, prevent local recurrence (LR) and disease progression while preserving the renal function in selected patients. The endoscopic management of low-risk UTUC needs meticulous and stringent close follow-up due to limitations of clinical staging with ureteroscopic biopsy, imaging studies and risk of high recurrence rates in these patients. There have been no prospective randomized studies comparing endoscopic management with RNU in support of the management guidelines. Most available published data to date are limited to retrospective or pooled retrospective data from selected institutes or case reports. These retrospective cohort studies would fall under the category of level 3-evidence. In a recent systematic review, a 52 % recurrence of UTUC after endoscopic management and 37 % recurrence were reported in percutaneous management [10]. Tumor grade, multifocality, tumor size and history of bladder cancer have been reported as predictors of UTUC recurrence [11]. Whether or not the addition of topical therapy can improve these recurrence rates remains a topic of some debate and is discussed further below.
Endoscopic management of UTUC is deemed a failure if clinical or radiological evidence of locally advanced or metastatic disease or pathological up staging or up grading is found on subsequent RNU specimen. Cutress et al. [10] reported pooled data of failure rates for ureteroscopic treatment of around 24 % and for the percutaneous approach around 32 %. A follow-up biopsy of the tumor base after ablation of the lesions might be helpful to determine whether additional modalities of treatment are required (Table 1).
Diagnosis
Imaging
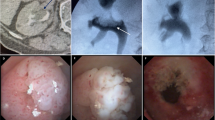
The imaging modality identifying soft tissue density within the pelvicalyceal cavities and ureteral lumen has been most commonly used for the diagnosis of UTUC. CT urography (CTU) is a standard imaging study for the diagnosis of UTUC. Both nephrographic and excretory phases of CTU are complementary for the diagnosis of UTUC [12]. CTU using thin slices (<2 mm) to visualize the entire urinary tract through multiplanar reformatted imaging (MPR) offers accuracy in diagnosing UTUC (Fig. 1).
Sagittal view of a computed tomography scan showing a filling defect representing a soft tissue mass in the upper pole of the left collecting system, in a patient with prior endoscopic therapy for low-grade left ureteral tumors. Biopsy in this case showed high-grade papillary tumor. Nephroureterectomy showed parenchymal invasion (stage pT3)
A filling defect or soft tissue density in the renal collecting system that enhances after the administration of contrast is highly suspicious for UTUC. CTU limitations leading to unclear findings might include flat lesions or focal wall thickening or sub-centimeter lesions for which attenuation measurements are difficult to characterize the lesion, or may be nonspecific findings [13]. A meta-analysis and systematic review of CTU for UTUC reported pooled sensitivity of 96 % (95 % CI 88–100 %) and specificity of 99 % (95 % CI 98–100 %) [14]. Another systematic review showed similar high sensitivity and specificity for CTU (96 and 99 %) and retrograde urography (96 and 96 %) in detecting the UTUC [15].
MR urography (MRU) had a high specificity of 97 % with a rather low sensitivity of 69 % [1]. Excretory urography had a low specificity of 81 % and low sensitivity of 80 %. It has been noted that sensitivities are lower with lower tumor burden for all imaging modalities [1].
Diagnostic ureteroscopy (URS)
The development of high definition flexible fiber optic and now digital URS has greatly improved visualizing the entire upper urinary tract and ureters.
URS evaluation of UTUC should assess for tumor location, number, size and architecture. URS assessment under direct vision of these parameters influences the treatment approach and outcomes. Ureteral access sheaths have been shown to increase the diagnostic efficacy of URS. Diagnostic URS should assess the ureter before the placement of ureteral access sheath. URS significantly missed concomitant CIS when compared to RNU specimens (9.7 vs. 43.3 %) [16]. URS is invaluable in those cases where renal preservation may be paramount, such as individuals with renal insufficiency, solitary kidney and multiple comorbidities. URS facilitates selective ureteral sampling for cytology from the renal pelvicalyceal system and ureters, which provides prognostic information. URS findings combining with biopsy grade, urinary/selective cytology and imaging findings may help in determining if the patient would benefit from endoscopic management of UTUC (Table 1). The accuracy of URS biopsies for diagnosis and grading of UTUC is summarized in Table 2.
Biopsy
The difficulties in predicting accurate clinical staging result from limitations in biopsy specimen size and restriction of depth of resection. The tumor grade of UTUC is thus a primary driver in making treatment decisions, as grade is used to infer stage. Low-grade disease on biopsy specimen has a positive predictive value of 80–90 % in predicting low-stage disease, while high-grade disease has lower predictive value for invasiveness. It is often helpful for the pathologist to have more than one biopsy since nondiagnostic tissue materials are found in URS biopsy specimens up to 25–31.5 % [16, 23, 25].
The primary objective of tumor biopsy is obtaining a proper grade rather than adequate staging. About 68–100 % of G1 tumors on biopsy are noninvasive on final histology while 62–100 % of G3 tumors are invasive. Results for G2 tumors vary significantly from 17 to 80 %, again reflecting likely inclusion of both low- and high-grade disease in this histological subgroup. Grade is one of the most important predictive factors for oncologic outcome of endoscopic treatment. High-grade UTUC has worse oncological outcomes. Gillan et al. [16] in a retrospective multicenter study reported URS biopsy grade matched with final RNU histopathology on 43.4 %, only 32.6 % had concordance between URS biopsy and final pathology for both grade and stage of UTUC. Concomitant CIS was found in 21.6 % of cases in final RNU pathology, with finding of discordance between URS biopsy specimens and RNU specimens in the diagnosis of concomitant CIS in UTUC (Tables 1, 3).
The multibiopsy approach has been proposed to improve diagnosis. Biopsy grade was identical in 43.4–78 % of cases to surgical pathology [16, 23]. The combined use of access sheath, cup biopsies and baskets (particularly for papillary tumors) can yield substantially more tissue than has been historically possible (Fig. 2).
Cytology
Cytological examination is thought to play a significant role in diagnosis of high-grade urothelial cancer (UC) and CIS of the bladder; however, its role in the detection and management of UTUC is poorly investigated and controversy exists in the utility of routine cytology testing in the absence of radiographic or direct visual endoscopic evidence of a tumor. In cases where an upper tract source is suspected, selective ureteral samples (by catheterization or with brushing) are performed for lateralizing the source of the finding but should be confirmed endoscopically when possible. Recent interest in urinary biomarker studies for malignancies has developed [18]. The sensitivity of selective ureteral cytology for UTUC ranged from 43 to 78 % [20, 26], with false-negative results as high as 50 % for low-grade neoplasms [12]. Several studies have evaluated the role of cytology for UTUC, with a few highlighted below [26–34]
Messer et al. [28] evaluated patients who had undergone RNU or distal ureterectomy without previous history of bladder cancer and concluded the positive urine cytology was not predictive of either muscle invasive disease or high-grade urothelial lesions. Selective upper tract cytology was more frequently positive than voiding urine cytology (60.3 vs. 33.6 %, p < 0.001). Sensitivity was 45.0 % for low-grade UTUC, 66.3 % for high-grade UTUC and 78.6 % for isolated CIS [14]. A multi-institutional retrospective study using Johns Hopkins Hospital template of cytopathology criteria reported sensitivity, specificity, PPV and NPV of UT urine cytology for high-grade UTUC were 71.4, 91.9, 66.7 and 93.4 %, respectively [30]. UT urine cytology has low sensitivity and specificity for low-grade UTUC [15–17].
The UroVysion test (Abbott Molecular, Des Plaines, IL, USA) is a multitarget multicolor fluorescent in situ hybridization (FISH) assay. This test has high occurrence of specific chromosomal abnormalities in UCs.
UroVysion showed abnormalities in 91 % of CIS and all invasive cancers and about 30 % of nonneoplastic lesions in patients with concomitant urothelial carcinoma. The sensitivity of UroVysion ranges from 39 to 97 % (average 74 %) but is significantly lower for low-grade and low-stage tumors [18].
The UroVysion/FISH combined with UT urine cytology may improve the sensitivity of detecting low-grade UTUC [34]. Table 1 shows the recommendations for cytology and markers.
Surgical techniques
A Cochrane review of the surgical management of UTUC concluded that there is no high-quality evidence available to determine the best surgical management [35].
Endoscopic treatment of low-risk UTUC
Ureteroscopic management
Digital flexible URS is a most valuable instrument to evaluate the intrarenal collecting system and ureter under direct vision that enables complete ablation of the tumor. Advanced laser technology using holmium, holmium:yttrium aluminum garnet (YAG) and the neodymium:YAG lasers is efficient for treating neoplasms and can be delivered through a flexible ureteroscope.
The holmium:YAG laser energy tissue penetration is <0.5 mm, which enables excellent tumor ablation with a reduced risk of upper urinary tract perforation.
The neodymium:YAG laser uses an alternative source of energy that has a tissue penetration of up to 5–6 mm and works by coagulative necrosis with eventual sloughing of the necrotic tumor. Laser technology for UTUC ablation through flexible URS has the advantage of lower morbidity compared to electrocautery.
Other approaches include using a flat wire basket or tumor grasping forceps to debulk the tumor burden, with the tumor base treated with either electrocautery delivered through small Bugbee electrode (2 or 3 Fr) or laser ablation using flexible fibers (200 or 365 μm) that easily fit through the working channel of the URS [21, 22].
The outcomes of URS management of UTUC have been reported in various retrospective studies. The accuracy of URS grading is summarized in Table 2. URS management of UTUC can be associated with a high LR and intravesical recurrence (IVR) rate. Table 3 shows these outcomes of URS management of UTUC.
Percutaneous management
The percutaneous antegrade approach can be considered for low-grade, large volume UTUCs that may not be anatomically accessible with flexible URS. After establishing percutaneous access, the tumor can be ablated using resectoscopes, cold cup biopsy forceps or laser ablation. Percutaneous approach allows antegrade instillation of topical adjuvant agents if indicated after successful tumor ablation [43]. Retrospective studies have reported that the percutaneous approach had a lower LR rates and lower IVR when compared to URS approach in the management of UTUC [44].
The oncological outcomes of the percutaneous approach in the management of UTUC are summarized in Table 4.
Adjuvant topical therapies
Recurrence rates in the upper tract following endoscopic treatment of UTUC have been reported in 30–70 % of patients [66, 67]. Topical adjuvant agents might decrease the risk of LR as suggested by several reported case series [68]. In theory, there should be a role of topical adjuvant therapies based on what is observed in patients with bladder cancer [9]. The role of intravesical therapy after RNU, however, is well established. Patients with UTUC treated with RNU subsequently develop IVR in approximately 30 % [9]. Two randomized clinical trials have demonstrated a decreased risk of IVR after RNU when using a single dose of early intravesical instillation of chemotherapy [69, 70]. The risk of IVR is even greater in patients managed with KPP, and such patients might logically benefit from the adjuvant intravesical instillation of chemotherapy. Future studies are needed to evaluate the benefit of single dose intravesical chemotherapy after KPP.
Adjuvant topical installation can be accomplished in either antegrade instillation through nephrostomy tube or retrograde instillation through an open-ended, multiple side hole ureteral catheter (Fig. 3). Use of double-J stents promoting reflux is not a reliable delivery method and its use is discouraged.
Topical treatment of UTUC is made more complicated by the need for reliable approach of accessing the upper tract, mode of delivery and lack of dwell time for the therapeutic agent. A single-institution retrospective study of 28 cases was recently presented, showing improved results of adjuvant topical chemotherapy to the upper tract when given as induction and maintenance therapy [71]. Lifshitz et al. recently reported a novel hydrogel polymer with reverse thermal gelation properties, solid at body temperature and liquid at cold temperature, which might promote high-dose delivery of mitomycin C into the upper urinary tract [72]. This product is expected to enter a phase 2/3 trial in the USA by 2016 for intended use as chemoablation of low-grade UTUC.
For isolated CIS of the upper tract (Fig. 4), in the absence of any papillary tumors, topical therapy with BCG can be considered the primary mode of treatment, given the field-effect nature of the disease. The available data pertaining to BCG instillation in patients with CIS are quite promising but are limited to small series of retrospective studies.
Conservative surgery
Partial pyelectomy or partial nephrectomy
Partial pyelectomy or partial nephrectomy is rarely performed for renal pelvic tumors, in particular with advent of newer digital URS technologies. Partial nephrectomy has a very narrow indication for treatment of UTUC and is rarely undertaken due to the uncommon nature of the disease as well as the much higher technical complexity than partial nephrectomy for parenchymal tumors (Fig. 5). Generally it is reserved for low-grade, unifocal polar tumors in the setting of solitary kidneys and has had variably successful results [60, 73].
A partial nephrectomy specimen performed in a patient with a solitary kidney and polar tumor (T). Blue ink indicates the urothelial margin. As opposed to partial nephrectomy for parenchymal tumors, the urothelial margin needs to be planned and examined in addition when performing a partial nephrectomy for UTUC. Indications for partial nephrectomy are very narrow and include an endoscopically unmanageable tumor, no multifocality, polar location and imperative indications for kidney preservation
Segmental ureterectomy
Ureteral cancers occur in the distal ureter about two-thirds of the time, and these may be managed with endoscopic ablation or segmental resection with ureteroneocystostomy in highly selected patients. High-risk proximal or mid-ureteral tumors are managed with RNU with bladder cuff excision. For low-risk tumors, these can invariably be managed endoscopically in most cases.
Follow-up
The European Association of Urology (EAU) guidelines recommend close oncological follow-up for ≥5 years [61]: after conservative management, cystoscopy, ureteroscopy and cytology at 3 and 6 months, and then every 6 months for 2 years, then yearly, and urinary cytology and CTU at 3 and 6 months, and then yearly.
Low-risk UC have a low risk of progression to invasion (<3 %), metastasis and death, but often recur as noninvasive lesions elsewhere within the urinary tract [3, 74]. Five-year disease specific survival for pT0/pTa/pTis is 100 % and pT1 92 %, respectively [4]. Noting that the majority of UTUC cases are high grade [4], the greatest potential lies in improving clinical risk stratification, which is highly limited in this disease.
Cystoscopy
IVR has been reported in 20–50 % of patients after RNU for UTUC [1]. Recurrence is common within the first 2 years after the management of UTUC; thus, strict follow-up with scheduled cystoscopic evaluation is prudent in detecting IVR [9].
Urinary cytology
Urine cytology preferably collected with selective washings might be helpful in assessing tumor recurrence after conservative treatment of low-risk UTUC.
Imaging
CTU is a standard imaging study for surveillance for early detection of potential recurrence following KPP approach for low-grade, low-stage UTUC.
MRU is indicated in patients with renal insufficiency or allergic to iodine-based IV contrast media. However, gadolinium is contraindicated in patients with severe renal insufficiency (GFR <30 ml/min), although in these cases T2-weighted imaging without gadolinium may still be performed.
Ureteroscopy
Patients treated with KPP require close monitoring owing to the high risk of recurrence [1]. URS has become most valuable to evaluate these patients while assessing ipsilateral as well contralateral renal units for recurrence and selective collection of urine cytology.
Conclusions
The management of UTUC requires a risk-adopted approach, which determines who would benefit from KPP. This strategy has demonstrated oncological outcomes in selected patients, which appear to be comparable with gold standard RNU with bladder cuff excision in patients with low-risk UTUC, albeit only within the context of retrospective, single institutional data. Adjuvant topical therapies, particularly with novel agents, raise the hope of decreasing the risk of tumor recurrence and disease progression in those undergoing KPP. Recent developments in genomics, tumor biology and molecular profiling of UTUCs hold promise in providing a better risk-adopted approach and development for future therapies.
References
Roupret M et al (2015) European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol 68(5):868–879
Andersen JR, Kristensen JK (1994) Ureteroscopic management of transitional cell tumors. Scand J Urol Nephrol 28(2):153–157
Roupret M et al (2013) European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 63(6):1059–1071
Margulis V et al (2009) Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer 115(6):1224–1233
Fajkovic H et al (2013) Results and outcomes after endoscopic treatment of upper urinary tract carcinoma: the Austrian experience. World J Urol 31(1):37–44
Sverrisson EF et al (2014) The merits of cytology in the workup for upper tract urothelial carcinoma—a contemporary review of a perplexing issue. Int Braz J Urol 40(4):493–498
Roupret M, Colin P, Yates DR (2014) A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: low-risk versus high-risk UTUCs. Eur Urol 66(2):181–183
Yakoubi R et al (2014) Radical nephroureterectomy versus endoscopic procedures for the treatment of localised upper tract urothelial carcinoma: a meta-analysis and a systematic review of current evidence from comparative studies. Eur J Surg Oncol 40(12):1629–1634
Seisen T et al (2015) A systematic review and meta-analysis of clinicopathologic factors linked to intravesical recurrence after radical nephroureterectomy to treat upper tract urothelial carcinoma. Eur Urol 67(6):1122–1133
Cutress ML et al (2012) Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int 110(5):614–628
Thompson RH et al (2008) Elective endoscopic management of transitional cell carcinoma first diagnosed in the upper urinary tract. BJU Int 102(9):1107–1110
Takeuchi M et al (2015) CT urography for diagnosis of upper urinary tract urothelial carcinoma: are both nephrographic and excretory phases necessary? Am J Roentgenol (AJR) 205(3):W320–W327
Potretzke AM et al. (2015) Is ureteroscopy needed prior to nephroureterectomy? An evidence-based algorithmic approach. Urology 88:43–48
Chlapoutakis K et al (2010) Performance of computed tomographic urography in diagnosis of upper urinary tract urothelial carcinoma, in patients presenting with hematuria: systematic review and meta-analysis. Eur J Radiol 73(2):334–338
Razavi SA et al (2012) Comparative effectiveness of imaging modalities for the diagnosis of upper and lower urinary tract malignancy: a critically appraised topic. Acad Radiol 19(9):1134–1140
Gillan A et al (2015) Carcinoma in situ is significantly underdetected by prenephroureterectomy ureteroscopy in the management of upper tract urothelial cancers. Biomed Res Int 2015:547586
Vashistha V, Shabsigh A, Zynger DL (2013) Utility and diagnostic accuracy of ureteroscopic biopsy in upper tract urothelial carcinoma. Arch Pathol Lab Med 137(3):400–407
Wang J et al (2012) Distinguishing urothelial carcinoma in the upper urinary tract from benign diseases with hematuria using FISH. Acta Cytol 56(5):533–538
Smith AK et al (2011) Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology 78(1):82–86
Williams SK et al (2008) Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol 22(1):71–76
Shiraishi K et al (2003) Role of ureteroscopic biopsy in the management of upper urinary tract malignancy. Int J Urol 10(12):627–630
Skolarikos A et al (2003) Cytologic analysis of ureteral washings is informative in patients with grade 2 upper tract TCC considering endoscopic treatment. Urology 61(6):1146–1150
Guarnizo E et al (2000) Ureteroscopic biopsy of upper tract urothelial carcinoma: improved diagnostic accuracy and histopathological considerations using a multi-biopsy approach. J Urol 163(1):52–55
Keeley FX et al (1997) Diagnostic accuracy of ureteroscopic biopsy in upper tract transitional cell carcinoma. J Urol 157(1):33–37
Tavora F et al (2009) Small endoscopic biopsies of the ureter and renal pelvis: pathologic pitfalls. Am J Surg Pathol 33(10):1540–1546
Renshaw AA (2006) Comparison of ureteral washing and biopsy specimens in the community setting. Cancer 108(1):45–48
Sedlock DJ, MacLennan GT (2004) Urine cytology in the evaluation of upper tract urothelial lesions. J Urol 172(6 Pt 1):2406
Messer J et al (2011) Urinary cytology has a poor performance for predicting invasive or high-grade upper-tract urothelial carcinoma. BJU Int 108(5):701–705
Comploj E, Babjuk M, Capitanio U, Cha E, Colin P, Fritsche HM, Herrmann T, Hübner W, Klatte T, Merseburger A, Montorsi F, Pycha A, Roscigno M, Rouprêt M, Shariat S, Zigeuner R, Remzi M (2013) Role of conventional cytology in the treatment of upper tract urothelial carcinoma (OSS-UTUC): results from the multi-institutional organ-sparing-UTUC collaboration. Eur Urol Suppl 1(12):e601–e602
Chen L et al (2015) Upper tract urinary cytology to detect upper tract urothelial carcinoma: using the Johns Hopkins Hospital template and evaluation of its feasibility. Cytojournal 12:17
Tanaka N et al (2014) The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: a multi-institutional study. Urol Oncol 32(1):48.e19–48.e26
Wang L (2015) Diagnsosis of upper tract urothelial carcinoma-A comparative study of urine cytology and surgical biopsy. J Am Soc Cytopathol 4:3–9
Daniely M et al (2007) Combined morphologic and fluorescence in situ hybridization analysis of voided urine samples for the detection and follow-up of bladder cancer in patients with benign urine cytology. Cancer 111(6):517–524
Reynolds JP et al (2014) Comparison of urine cytology and fluorescence in situ hybridization in upper urothelial tract samples. Cancer Cytopathol 122(6):459–467
Rai BP et al (2011) Surgical management for upper urinary tract transitional cell carcinoma. Cochrane Database Syst Rev (4):CD007349
Kalaitzis C et al (2013) Ureteroscopic laser treatment of upper urinary tract urothelial cell carcinomas: can a tumour free status be achieved? Adv Urol 2013:429585
Hoffman A et al (2014) Oncologic results of nephron sparing endoscopic approach for upper tract low grade transitional cell carcinoma in comparison to nephroureterectomy—a case control study. BMC Urol 14:97
Cutress ML et al (2012) Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int 110(11):1608–1617
Grasso M et al (2012) Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int 110(11):1618–1626
Gadzinski AJ et al (2010) Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol 183(6):2148–2153
Cornu JN et al (2010) Oncologic control obtained after exclusive flexible ureteroscopic management of upper urinary tract urothelial cell carcinoma. World J Urol 28(2):151–156
Pak RW, Moskowitz EJ, Bagley DH (2009) What is the cost of maintaining a kidney in upper-tract transitional-cell carcinoma? An objective analysis of cost and survival. J Endourol 23(3):341–346
Lucas SM et al (2008) Conservative management in selected patients with upper tract urothelial carcinoma compares favourably with early radical surgery. BJU Int 102(2):172–176
Painter DJ et al (2008) Ureteroscopic management of upper-tract urothelial cancer: an exciting nephron-sparing option or an unacceptable risk? J Endourol 22(6):1237–1239
Krambeck AE et al (2007) Endoscopic management of upper tract urothelial carcinoma in patients with a history of bladder urothelial carcinoma. J Urol 177(5):1721–1726
Reisiger K et al (2007) Office-based surveillance ureteroscopy after endoscopic treatment of transitional cell carcinoma: technique and clinical outcome. Urology 70(2):263–266
Roupret M et al (2006) Comparison of open nephroureterectomy and ureteroscopic and percutaneous management of upper urinary tract transitional cell carcinoma. Urology 67(6):1181–1187
Johnson GB, Grasso M (2005) Ureteroscopic management of upper urinary tract transitional cell carcinoma. Curr Opin Urol 15(2):89–93
Iborra I et al (2003) Conservative elective treatment of upper urinary tract tumors: a multivariate analysis of prognostic factors for recurrence and progression. J Urol 169(1):82–85
Matsuoka K et al (2003) Transurethral endoscopic treatment of upper urinary tract tumors using a holmium:YAG laser. Lasers Surg Med 32(5):336–340
Daneshmand S, Quek ML, Huffman JL (2003) Endoscopic management of upper urinary tract transitional cell carcinoma: long-term experience. Cancer 98(1):55–60
Chen GL, Bagley DH (2001) Ureteroscopic surgery for upper tract transitional-cell carcinoma: complications and management. J Endourol 15(4):399–404 (discussion 409)
Engelmyer EI, Belis JA (1996) Long-term ureteroscopic management of low-grade transitional cell carcinoma of the upper urinary tract. Tech Urol 2(2):113–116
Gaboardi F et al (1994) Conservative treatment of upper urinary tract tumors with Nd:YAG laser. J Endourol 8(1):37–41
Schmeller NT, Hofstetter AG (1989) Laser treatment of ureteral tumors. J Urol 141(4):840–843
Rastinehad AR et al (2009) A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncologic benefit with adjuvant bacillus Calmette Guerin therapy? Urology 73(1):27–31
Palou J et al (2004) Percutaneous nephroscopic management of upper urinary tract transitional cell carcinoma: recurrence and long-term followup. J Urol 172(1):66–69
Motamedinia P et al. (2015) The expanded use of percutaneous resection for upper tract urothelial carcinoma: a 30-year comprehensive experience. J Endourol 30(3):262–267
Roupret M et al (2007) Upper urinary tract transitional cell carcinoma: recurrence rate after percutaneous endoscopic resection. Eur Urol 51(3):709–713 (discussion 714)
Goel MC, Mahendra V, Roberts JG (2003) Percutaneous management of renal pelvic urothelial tumors: long-term followup. J Urol 169(3):925–929 (discussion 929–930)
Clark PE, Streem SB, Geisinger MA (1999) 13-year experience with percutaneous management of upper tract transitional cell carcinoma. J Urol 161(3):772–775 (discussion 775–776)
Patel A et al (1996) Long-term outcome after percutaneous treatment of transitional cell carcinoma of the renal pelvis. J Urol 155(3):868–874
Plancke HR, Strijbos WE, Delaere KP (1995) Percutaneous endoscopic treatment of urothelial tumours of the renal pelvis. Br J Urol 75(6):736–739
Fuglsig S, Krarup T (1995) Percutaneous nephroscopic resection of renal pelvic tumors. Scand J Urol Nephrol Suppl 172:15–17
Tasca A, Zattoni F, Garbeglio A, Villi G, Bassi P, Meneghini A (1992) Endourologic treatment of transitional cell carcinoma of the upper urinary tract. J Endourol 6(3):253–256
Johnson GB, Fraiman M, Grasso M (2005) Broadening experience with the retrograde endoscopic management of upper urinary tract urothelial malignancies. BJU Int 95(Suppl. 2):110–113
Keeley FX Jr, Bibbo M, Bagley DX (1997) Ureteroscopic treatment and surveillance of upper urinary tract transitional cell carcinoma. J Urol 157(5):1560–1565
Aboumarzouk OM et al (2013) Mitomycin C instillation following ureterorenoscopic laser ablation of upper urinary tract carcinoma. Urol Ann 5(3):184–189
O’Brien T et al (2011) Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol 60(4):703–710
Ito A et al (2013) Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol 31(11):1422–1427
Wagenheim GN, Papadopoulos J, Navai N, Davis JW, Karam JA, Kamat AM, Wood CG, Dinney CP, Matin SF (2016) Mitomycin-c induction and maintenance topical therapy for upper tract urothelial carcinoma. In: Presented at GU symposium, January 8, San Francisco, CA, USA
Lifshitz D (2014) Hydrogel based drug retention system for the treatment of upper tract urothelial carcinoma. Eur Urol Suppl 1(13):e26
Goel MC et al (2006) Partial nephrectomy for renal urothelial tumors: clinical update. Urology 67(3):490–495
Linton KD et al (2013) Disease specific mortality in patients with low risk bladder cancer and the impact of cystoscopic surveillance. J Urol 189(3):828–883
Acknowledgments
The senior author would like to recognize support from the Monteleone Family Foundation for Research in Kidney and Bladder Cancer.
Authors’ contributions
Mandalapu, Matin was involved in protocol/project development, data collection or management, data analysis and manuscript writing/editing. Remzi, De Reijke, Margulis, Palou, Kapoor, Yossepowitch, Coleman, Traxer, Anderson, Catto, de la Rosette, O’Brien and Zlotta took part in data collection or management, data analysis and manuscript writing/editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest by any author directly related to the topic.
Ethical standards
This review does not involve human subjects and meets Helsinki Declaration for protection of human subjects.
Rights and permissions
About this article
Cite this article
Mandalapu, R.S., Remzi, M., de Reijke, T.M. et al. Update of the ICUD-SIU consultation on upper tract urothelial carcinoma 2016: treatment of low-risk upper tract urothelial carcinoma. World J Urol 35, 355–365 (2017). https://doi.org/10.1007/s00345-016-1859-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1859-6