Abstract
Pouchitis is an inflammatory complication after restorative proctocolectomy and ileal pouch-anal anastomosis (IPAA). IPAA is the surgical treatment of choice in patients with ulcerative colitis (UC) who require colectomy. Initial episodes of acute pouchitis generally respond to antibiotics but significant numbers of cases eventually become dependent on or refractory to antibiotics. Management of chronic antibiotic refractory pouchitis is challenging and can ultimately lead to pouch failure. The etiopathogenesis is unknown though recent studies have implicated bacterial dysbiosis of the pouch microbiota, NOD2 polymorphism, and Clostridium difficile infection in the development of severe pouchitis. Early identification of risk factors can help in tailoring therapy and reducing cases of chronic pouchitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) involves two major disorders: ulcerative colitis (UC) and Crohn’s disease (CD). A recent large population based study in the USA reported prevalence of UC and CD at 238 and 201 per 100,000 adults, respectively [1]. Improved medical therapy for IBD, including anti-TNF biologics, has reduced the need for surgical intervention, but 20–30 % of patients with UC will eventually require surgical management [2]. Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is currently the surgical procedure of choice for complicated UC requiring operative intervention. IPAA involves a total proctocolectomy with construction of an ileal reservoir anastomosed to the anus; this restores intestinal continuity, preserves sphincter function, and maintains continence.
Between 23 and 46 % of patients with UC who have undergone IPAA subsequently develop inflammation of the ileal reservoir, called pouchitis [3, 4, 5••]. The symptoms and severity of pouchitis will vary from patient to patient, but typically include increased stool frequency and urgency, loose watery stools, and abdominal cramping. More severe inflammation may be associated with blood in the stool, fever, tenesmus, and incontinence.
At present, there are no accepted guidelines to classify the severity of pouch inflammation; however, the pouch disease activity index (PDAI), which incorporates a combination of clinical, endoscopic, and histological findings, is used at many centers to score pouch inflammation [6]. Despite the frequent occurrence of pouchitis, there remains limited evidence-based data available regarding optimal treatment. Oral antibiotics are routinely used for management of initial episodes of pouchitis, but approximately 5–19 % of patients with IPAA for UC will become antibiotic dependent or develop chronic antibiotic refractory pouchitis (CARP) [4]. These patients may require treatment with corticosteroids, immunomodulators, anti-TNF biologics, or even pouch excision. This update will review the most recent information available related to the pathophysiology and management of pouchitis.
Etiology and Pathogenesis
The pathophysiology of pouchitis is incompletely understood and is likely multifactorial. The effectiveness of oral antibiotics in the treatment of acute pouchitis, along with the absence of inflammation in patients with an end ileostomy, implies a role for changes in pouch flora in the development of pouchitis. It is hypothesized that surgical construction of the ileal reservoir leads to fecal stasis, which in turn leads to a change in gut microenvironment adapting to this new fecal storage function. Pouch inflammation can be a result of direct inflammatory action of hitherto unknown gut microbe(s) or a response to accumulation of toxic bacterial metabolic products; on the other hand, it can also be an abnormal immune response to the changed commensal flora. There is likely an inherent epithelial barrier defect in UC patients that allows inflammation to develop, as the incidence of pouchitis is much higher in patients who have had IPAA for UC compared to patients with an IPAA for familial adenomatous polyposis (FAP).
Bacterial Flora and Pouchitis
Approximately 99 % of the pouch microbiota is made up of Firmicutes, Proteobacteria, Bacteriodetes, and Actinobacteria. Two recent reports examined mucosal biopsy specimens from inflamed pouches in UC patients compared to healthy pouches of FAP patients [7••, 8]. These studies reported an overall decreased microbial diversity in the pouchitis group. At the phylum level, significant decreases in Bacteroidetes and increases in Proteobacteria were found in inflamed pouches; at the genus level, members of Clostridia group XIVa (Blautia, Moryella, and Dorea), Bacteroides, and Parabacteroides were present in greater abundance in healthy pouches indicating a possible anti-inflammatory role of these organisms. Whether this is a direct bacterial effect or related to modulation of host immune response needs further investigation. Interestingly, Faecalibacterium prausnitzii and adherent-invasive Escherichia coli (AIEC), which have both been reported to be associated with IBD, were not associated with pouch inflammation [7••, 8, 9].
Hinata and colleagues examined the effluent from healthy pouches of patients undergoing IPAA for UC and followed them for 1-year post ileostomy closure. They observed a time-dependent shift in pouch flora from ileum predominant to colonic predominant. Lactobacillus species were most abundant soon after surgery, transitioning to colonic type of flora consisting of anaerobic bacteria belonging to Clostridium, Coccoides group, Clostridium leptum subgroup, Bacteroides fragilis group, and Atopobium cluster [10].
Lim and colleagues reported similar alterations in bacterial diversity when comparing stool from healthy and inflamed pouches. Interestingly, of 17 bacterial populations exclusively found in inflamed pouches, only 6 of them were identifiable by 16s RNA analysis, belonging to genus Leptospira, Pseudoalteromonas, Desulfosporosinus, Microcystis, Methylobacter, and Proteobacterium [11]. Desulfosporosinus is a sulfate-reducing bacterium (SRB); previous reports have hypothesized a role of hydrogen sulfide, a by-product of SRB in causing pouchitis, via abnormal epithelial proliferation [12, 13].
The application of advanced molecular techniques to the investigation of the pouch microflora has currently raised more questions than provided answers. Many of the novel bacteria identified by molecular techniques are not cultivable and therefore difficult to study. To date, the available data suggest a role for dysbiosis in patients with pouchitis; however, it is unclear at this time weather changes in the pouch bacterial population are primary etiologic events or secondary response to inflammation caused by an aberrant host immune response to pouch bacteria.
Risk Factors for Pouchitis
Although the cause of pouchitis is unknown, certain patient characteristics increase its risk. An elevated white blood cell count preoperatively, steroid use, and the extent of colonic involvement are all associated with increased risk of subsequent histological pouchitis [14, 15].
Risk factors for chronic pouchitis include the presence of large joint arthritis [14, 16••], S-pouch construction [14], and primary sclerosing cholangitis [16••]. NOD2insC and TNFSF15 polymorphism have also shown a strong association with chronic pouchitis [16••, 17]. Serological markers, such as ASCA and ANCA, do not appear to affect acute pouchitis risk; however, ANCA-positivity increases risk of chronic pouchitis [18]. There are conflicting reports about the association of smoking and family history of IBD with pouch complications, with some earlier studies reporting an association, but a recent large prospective study finding no association [16••, 19]. No significant difference in outcome after IPAA was identified between Hispanic and non-Hispanic white patients [20].
Cytomegalovirus Infection
Isolated small series have reported an association between cytomegalovirus (CMV) infection (diagnosed histologically) and pouchitis, with symptom improvement upon anti-viral treatment. CMV pouch infection should be suspected in immunosuppressed patients with fever and increased stool frequency and who have been unresponsive to antibiotic therapy [21, 22•].
Clostridium Difficile Infection
A high index of suspicion is warranted for Clostridium difficile infection (CDI) in patients with pouchitis who were recently hospitalized and have increased bowel frequency. A recent prospective study identified CDI by PCR assay in approximately 10 % of patients with active pouchitis. Importantly, however, almost half of these patients had recurrence of CDI after antibiotic therapy or were refractory to treatment with oral vancomycin [23•].
Diagnosis
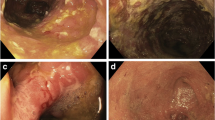
There is considerable heterogeneity in the presentation of pouchitis, ranging from acute, antibiotic responsive, pouchitis to chronic antibiotic refractory pouchitis. Patients with a healthy pouch usually have ≤6 soft bowel movements a day; by comparison, a patient with pouchitis can have 10–20 urgent stools in 24 h, with incontinence and abdominal cramps. However, a diagnosis of pouchitis should not rely solely on clinical symptoms as they are non-specific, and previous studies have not shown a correlation between severity of clinical symptoms and endoscopic or histologic findings [24]. The differential diagnosis for pouchitis includes infectious diarrhea, irritable pouch syndrome, CD of the pouch, and cuffitis (inflammation at the site of the pouch-anal surgical anastomosis). Pouchoscopy with biopsy is warranted in patients with clinical symptoms suggestive of pouchitis. Endoscopic findings can help in differentiating pouchitis from CD of the pouch and can identify postoperative pouch complications, including pouch strictures and fistula. Histopathology can further confirm the diagnosis and help in grading the severity of pouch inflammation. In patients with CARP, levels of serum IgG4 levels, ANA, and antimicrosomal antibodies can help in identifying an autoimmune process as a cause for persistent symptoms [25, 26].
Stool fecal coliform culture with antibiotic sensitivity testing can be useful in identifying appropriate antibiotic therapy in CARP. Ciprofloxacin and metronidazole are typically the first line of antibiotics for treatment of acute pouchitis; however, McLaughlin et al. found 100 % ciprofloxacin resistance on antibiotic coliform sensitivity testing of 15 patients with CARP. After treatment with a non-quinolone, sensitive antibiotic remission was achieved in 80 % of these patients [27].
Small studies have evaluated the use of fecal lactoferrin and calprotectin in patients with pouchitis and have shown utility in both diagnosing pouchitis as well as predicting resolution of inflammation [28, 29]. Anti-glycoprotein 2 antibody has been reported to be elevated in serum of patients with inflamed pouch, more so in patients with CD like phenotype [30]. These tests need further validation before they can be recommended for use in diagnosis and monitoring of disease activity.
Treatment
Given the variable clinical presentation, course, and etiology, effective treatment of pouchitis can be challenging. Approximately 33–46 % of patients will have an episode of pouchitis after IPAA with a further 5–19 % of patients developing relapsing or treatment refractory disease [5••, 31•]. Pouchitis is often classified as responsive, dependent, and refractory based on the effect of anitbiotics [32]. In the acute setting, an initial episode of pouchitis generally responds well to broad-spectrum antibiotics. A recent Cochrane systematic review from 2010 reported ciprofloxacin to be more effective in inducing remission in acute pouchitis then metronidazole, while budesonide and metronidazole were equally effective. On the other hand, rifaximin or the probiotic Lactobacillus GG was not able to achieve a level of significance in comparison to placebo in inducing remission [33]. The mechanism of action of probiotics in pouchitis is unknown, but probiotics may work in pouchitis through restoration of mucosal barrier function [34•]. The effectiveness of the probiotic VSL#3 (6 g oral per day) for the maintenance of remission in chronic pouchitis is supported by two randomized controlled trials (RCTs). However, the prevention of pouchitis with VSL#3 is uncertain, with one study showing a significant difference compared to placebo, but another not showing any significant difference [33]. A favorable result from a small pilot study using AST-120 (a spherical carbon adsorbent) as a treatment for acute pouchitis awaits confirmation in a larger trial [35].
In the disease spectrum of pouchitis, treatment of CARP is the most challenging; unfortunately, only limited data from open-label studies and a few small RCTS are available. RCTs using glutamine suppositories, butyrate suppositories, and bismuth carbomer foam enemas were not shown to be more effective than placebo [33]. Uncontrolled data suggest treatment using prolonged courses of combination antibiotic therapy (ciprofloxacin 1 g/day in combination with rifaximin 2 g/day or metronidazole 1 g/day for 4 weeks) can be temporarily effective [36]. Anecdotal findings suggest oral ertapenem as another antibiotic treatment option for CARP [37•]. Small case series looking at the effectiveness of elemental diet and topical tacrolimus enemas in CARP have shown some benefit but further study is needed [38]. The anti-TNF-alpha agents, infliximab and adalimumab, and budesonide (topical and oral) have also shown efficacy in the treatment of CARP as well as CD of pouch [39–41, 42•]. Infliximab has recently been shown to have long-term effectiveness in treatment of CARP [43•]. CARP can also arise as a complication of untreated CMV or C. difficile infection. It is prudent to check for both in patients with antibiotic refractory disease. No data is available at this time on fecal transplant in CARP.
Conclusion
Pouchitis is a common complication after IPAA in patients with UC. Recent data suggest an important role of dysbiosis of the pouch flora in the development of pouchitis. Decreased microbial diversity in the setting of an inherent immune defect likely triggers an inflammatory mucosal response leading to clinical symptoms. Whether this is inflammation is secondary to a specific, yet to be identified organism, or due to immune intolerance to changes in commensal pouch flora needs further investigation. Broad-spectrum antibiotics remain the mainstay of treatment in acute pouchitis. Treatment of pouchitis with antibiotics non-selectively eradicates pouch bacterial populations thereby removing the inflammatory stimulus, while therapy with probiotics likely acts to restore pouch flora balance and mucosal barrier function. The management of chronic recurrent or antibiotics refractory pouchitis continues to be challenging, with a growing role for anti-TNF biologic therapy. Overall a patient-tailored approach, with a thorough evaluation of risk factors and comorbidities, is helpful in stratifying treatment of this complicated disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kappelman MD et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–9.
Leijonmarck CE, Persson PG, Hellers G. Factors affecting colectomy rate in ulcerative colitis: an epidemiologic study. Gut. 1990;31(3):329–33.
Penna C et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38(2):234–9.
Ferrante M et al. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis. 2008;14(1):20–8.
Fazio VW et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257(4):679–85. An important paper from one of the leading centers for pouch surgery describing long term outcome of patients with IPAA in a large cohort of prospectively followed patients.
Sandborn WJ et al. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69(5):409–15.
Tyler AD et al. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One. 2013;8(9):e66934. This recent paper details changes in the pouch microbiota at phylum and genus level in patients with pouchitis. Knowledge of these changes can help in understanding the etiology of pouchitis and providing a direction to future therapies.
McLaughlin SD et al. The bacteriology of pouchitis: a molecular phylogenetic analysis using 16S rRNA gene cloning and sequencing. Ann Surg. 2010;252(1):90–8.
Scarpa M et al. TLR2 and TLR4 up-regulation and colonization of the ileal mucosa by Clostridiaceae spp. in chronic/relapsing pouchitis. J Surg Res. 2011;169(2):e145–54.
Hinata M et al. A shift from colon- to ileum-predominant bacteria in ileal-pouch feces following total proctocolectomy. Dig Dis Sci. 2012;57(11):2965–74.
Lim M et al. An assessment of bacterial dysbiosis in pouchitis using terminal restriction fragment length polymorphisms of 16S ribosomal DNA from pouch effluent microbiota. Dis Colon Rectum. 2009;52(8):1492–500.
Christl SU et al. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa: a potential role for these agents in the pathogenesis of ulcerative colitis. Dig Dis Sci. 1996;41(12):2477–81.
Duffy M et al. Sulfate-reducing bacteria colonize pouches formed for ulcerative colitis but not for familial adenomatous polyposis. Dis Colon Rectum. 2002;45(3):384–8.
Lipman JM et al. Perioperative factors during ileal pouch-anal anastomosis predict pouchitis. Dis Colon Rectum. 2011;54(3):311–7.
Hashavia E et al. Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis. 2012;14(11):1365–71.
Tyler AD et al. The NOD2insC polymorphism is associated with worse outcome following ileal pouch-anal anastomosis for ulcerative colitis. Gut. 2013;62(10):1433–9. Novel association of genetic factor (NOD2inc polymorphism) in the development of chronic pouchitis.
Sehgal R et al. Genetic risk profiling and gene signature modeling to predict risk of complications after IPAA. Dis Colon Rectum. 2012;55(3):239–48.
Singh S et al. Meta-analysis: serological markers and the risk of acute and chronic pouchitis. Aliment Pharmacol Ther. 2013;37(9):867–75.
Abdelrazeq AS et al. Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2008;10(8):805–13.
Lian L et al. Different clinical characteristics in Hispanic and non-Hispanic whites with ileal pouch-anal anastomosis: a case-control study. Inflamm Bowel Dis. 2011;17(4):1003–7.
Araujo Miguez A et al. Pouchitis associated with cytomegalovirus infection: a case study. Inflamm Bowel Dis. 2013;19(5):E65–6.
McCurdy JD et al. Cytomegalovirus infection of the ileoanal pouch: clinical characteristics and outcomes. Inflamm Bowel Dis. 2013;19(11):2394–9. This paper describes the risk factors and clinical features associated with CMV infection of the pouch.
Li Y et al. Risk factors and outcome of PCR-detected Clostridium difficile infection in ileal pouch patients. Inflamm Bowel Dis. 2013;19(2):397–403. A prospective study which describes the increased incidence of C. difficile infection and associated risk factors in IBD patients who have undergone IPAA.
Shen B et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001;121(2):261–7.
Navaneethan U et al. Elevated serum IgG4 is associated with chronic antibiotic-refractory pouchitis. J Gastrointest Surg. 2011;15(9):1556–61.
Navaneethan U et al. Prevalence and clinical implications of positive serum anti-microsomal antibodies in symptomatic patients with ileal pouches. J Gastrointest Surg. 2011;15(9):1577–82.
McLaughlin SD et al. Fecal coliform testing to identify effective antibiotic therapies for patients with antibiotic-resistant pouchitis. Clin Gastroenterol Hepatol. 2009;7(5):545–8.
Johnson MW et al. Faecal calprotectin: a noninvasive diagnostic tool and marker of severity in pouchitis. Eur J Gastroenterol Hepatol. 2008;20(3):174–9.
Gonsalves S et al. Fecal lactoferrin: a noninvasive fecal biomarker for the diagnosis and surveillance of pouchitis. Dis Colon Rectum. 2013;56(6):733–7.
Werner L et al. Antibodies against glycoprotein 2 are novel markers of intestinal inflammation in patients with an ileal pouch. J Crohns Colitis. 2013;7(11):e522–32.
Shen B. Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol. 2013;11(12):1538–49. A recent comprehensive review of pouchitis by one of the world’s leading pouch researchers.
Mahadevan U, Sandborn WJ. Diagnosis and management of pouchitis. Gastroenterology. 2003;124(6):1636–50.
Holubar SD et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev. 2010;6:CD001176.
Persborn M et al. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38(7):772–83. This paper provides insight into the possbile mechanisms for the beneficial effects of probiotics in pouchitis.
Shen B et al. The efficacy and tolerability of AST-120 (spherical carbon adsorbent) in active pouchitis. Am J Gastroenterol. 2009;104(6):1468–74.
Gionchetti P et al. Management of pouch dysfunction or pouchitis with an ileoanal pouch. Best Pract Res Clin Gastroenterol. 2004;18(5):993–1006.
Ham M, Moss A. Oral ertapenem for refractory pouchitis. J Crohns Colitis. 2013;7(10):e501–2. This brief report describes a potential novel treatment for refractory pouchitis.
McLaughlin SD et al. Exclusive elemental diet impacts on the gastrointestinal microbiota and improves symptoms in patients with chronic pouchitis. J Crohns Colitis. 2013;7(6):460–6.
Barreiro-de Acosta M et al. Efficacy of adalimumab rescue therapy in patients with chronic refractory pouchitis previously treated with infliximab: a case series. Eur J Gastroenterol Hepatol. 2012;24(7):756–8.
Barreiro-de Acosta M et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm Bowel Dis. 2012;18(5):812–7.
Gionchetti P et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment Pharmacol Ther. 2007;25(10):1231–6.
Li Y et al. Adalimumab therapy in Crohn’s disease of the ileal pouch. Inflamm Bowel Dis. 2012;18(12):2232–9. Both the short and long term use of adalimimab for Crohn’s disease of pouch are reported.
Viazis N et al. Long term benefit of one year infliximab administration for the treatment of chronic refractory pouchitis. J Crohns Colitis. 2013;7(10):e457–60. Biologic anti-TNF therapy is becoming an important treatment in chronic or refractory pouchitis. This report details the utility of maintenance infliximab infusions in chronic antibiotic refractory pouchitis.
Compliance with Ethics Guidelines
Conflict of Interest
Saleem Chowdhry has no conflicts of interest. Jeffry Katz gave expert testimony for Tucker, Ellis, LLC. Dr. Katz received honoraria from AbbVie Inc. and Janssen Biotech Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Intra-abdominal Infections, Hepatitis, and Gastroenteritis
Rights and permissions
About this article
Cite this article
Chowdhry, S., Katz, J.A. Update on the Pathogenesis and Management of Pouchitis. Curr Infect Dis Rep 16, 442 (2014). https://doi.org/10.1007/s11908-014-0442-9
Published:
DOI: https://doi.org/10.1007/s11908-014-0442-9