Abstract
Uric acid is the final oxidation product of purine metabolism in circulation and has been associated with the occurrence of gout and kidney stones. Type 2 diabetes mellitus and hypertension are two important public health challenges, and both are linked to increased risk of cardiovascular events. Hyperuricemia has recently emerged as an independent risk factor in the development of type 2 diabetes mellitus and hypertension through several proposed mechanisms. Few clinical trials investigated the use of uric acid lowering agents in the management of these two disease entities; however, their results provided encouraging evidence to a potential role for these agents in fighting disease burden. Larger randomized controlled trials are therefore warranted to establish the role of uric acid as a promising target for novel therapeutic interventions in the management of type 2 diabetes mellitus and hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
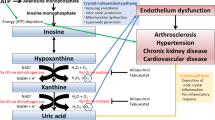
Uric acid (UA) is the final breakdown product of purine compounds. It is generated in the liver, and mainly excreted by the kidneys (65–75%) and the gastrointestinal tract (25–35%) [1, 2]. Hyperuricemia (HUA) is widely known to cause gout which is the most common inflammatory arthritis characterized by painful disabling acute attacks [3]. However, recent evidence suggests an emerging association between HUA, hypertension (HTN), and type 2 diabetes mellitus (T2DM) [4]. HTN remains a major cause of cardiovascular diseases and a leading risk factor for global disease burden, with almost 30% of the Western adult population affected with the disease [5]. Likewise, T2DM, a major lifestyle disease, has become a global burden, with more than 640 million adults expected to be affected by the disease by 2040 [6]. It is therefore necessary to develop new therapeutic interventions to limit the burden of both of these disease entities. Following an overview of UA homeostasis, on HUA and its prevalence, we will discuss recent evidence on the association between HUA, HTN, and T2DM with a focus on the effects of UA lowering therapies on cardiovascular events.
Uric Acid Homeostasis
UA is generated in the liver from the breakdown of dietary and endogenously synthesized purine nucleotides. Dietary intake, including purine rich products, glucose, and fructose, constitutes the exogenous pool of UA. The suggested association between fructose ingestion and HUA is of particular interest. Upon consumption of fructose, it is absorbed and phosphorylated resulting in a depletion of adenosine triphosphate and increase in adenosine monophosphate, leading to HUA. However, some trials attributed this association to the hypercaloric state rather than to fructose in particular, so additional studies are warranted to confirm the association [7]. On the other hand, the endogenous pool of UA is mostly regulated by the enzyme xanthine oxidase which irreversibly oxidizes xanthine into UA, a normal intracellular fluid compound [8, 9•]. Normal serum UA level is about 6.8 mg/dL [10], with adult males having higher levels than females [2].
UA degradation by the microbiota of the gut, known as intestinal uricolysis, eliminates almost one third of body urate. Complex interchange between secretion and reabsorbtion at the proximal tubules of the kidneys is responsible for the daily excretion of the remaining two thirds of total UA [9•, 10, 11]. The resultant effect of these mechanisms is a normal UA excretion of almost 10% [9•].
Several plasma membranes contribute to UA disposal by the kidneys. The ATP-binding cassette subfamily G membrane 2 (ABCG2) mediates urate entry to the intestines and is also found in the epithelium of proximal renal tubules. Furthermore, the major UA reabsorptive transporters in the renal tubule are the urate anion exchanger 1 (URAT1) and the glucose transporter type 9 (GLUT9). Any polymorphism involving the expression of these renal UA transporters may result in HUA and gout [10, 12, 13].
Current pharmacological agents aiming to decrease serum UA work by interfering with UA production via inhibition of the enzyme xanthine oxidase (such as allopurinol), decreasing UA reabsoption by targeting URAT1 (such as probenecid) or enhancing metabolism of UA into allantoin by uricase [14]. Moreover, SGLT2 inhibitors have been shown to decrease UA levels by interfering with GLUT9, and the angiotensin receptor blocker losartan was found to lower serum UA by inhibiting URAT1 [15].
Hyperuricemia Definition and Epidemiology
HUA is practically defined as UA levels greater than 6.8 mg/dL, which corresponds to its solubility point [16]. Some clinical trials define HUA as UA greater than 7 mg/dL in males and UA greater than 6 mg/dL in females [17••].
HUA has been associated with several known risk factors including alcohol consumption, diet rich in fructose, meat or seafood, certain medications, namely, diuretics, angiotensin converting enzyme, as well as obesity and hypertension [18, 19]. The prevalence of HUA in the USA attained 13% of the general population as defined by a serum UA >7 mg/dL and 21% as defined by a serum UA >7 mg/dL in males and >5.7 mg/dL in females [20]. HUA in females is more common after menopause due to the inhibitory effect of estrogen on urate reabsorption [21]. This finding is further corroborated by other studies showing decreased UA levels among postmenopausal women on hormone replacement therapy [22].
Hyperuricemia and Type 2 Diabetes Mellitus
Emerging evidence suggests that HUA is an independent risk factor for developing T2DM. In a systematic review of eight prospective cohort studies, including a total of 32,016 participants and 2930 incident T2DM, the risk of T2DM was increased by 6% for every 1 mg/dL increase in serum UA [23••]. A meta-analysis of 12 cohort studies assessed the correlation between UA levels and incident T2DM and impaired fasting glucose (IFG). The study included 6340 cases, and a total of 62,834 participants were analyzed. Findings from this meta-analysis revealed that a positive nonlinear relationship exists between serum UA levels and incident T2DM and IFG. The findings were also applicable to the incidence of T2DM alone [24]. Kim et al. prospectively examined the incidence of T2DM among 54,075 gout and 162,225 osteoarthritis patients over a mean follow-up of 1.9 years. Incidence rate of T2DM was almost 2% among gout patients versus 0.98% among osteoarthritis patients after controlling for potential confounders including age, comorbidities, medications, and health care use patterns [25]. In another prospective cohort study investigating the association between serum UA and incident IFG, 13,328 women and 41,350 men without diabetes were followed up for 4 years. Results suggested that any abnormality in UA concentrations is associated with an increased risk for the development of IFG in men, independent of other known risk factors. In women, such association was not present [26]. Although the reason for this gender difference is still uncertain, sex hormones may play a role, especially that estrogen facilitates excretion of UA during the reproductive period [27]. A retrospective cohort study examined the risk of new-onset diabetes among 1923 male patients with HUA and no prior evidence of diabetes. The risk was positively correlated with serum UA levels, reaching up to 27% for serum UA > 9 mg/dL, after adjusting for confounding factors [28].
Data from experimental studies reveal that HUA mediates increased insulin resistance and decreased insulin release, eventually leading to T2DM through different potential pathogenic factors. Indeed, UA emerged as a pro-inflammatory substance: Infusion of UA into mice increases TNF-α levels and activates phospholipase A2 and nuclear factor kappa-light-chain-enhancer of activated B cells [29]. UA also activates inflammasome in mice, which leads to a decrease in insulin sensitivity [30]. Furthermore, UA enhances production of reactive oxygen species which can lead to the loss of transcription factors needed for insulin gene expression, leading to decreased insulin production and secretion [31].
A small number of studies assessed the effects of pharmacologic lowering of UA on T2DM.
In a randomized open parallel-controlled study, 176 patients with T2DM and asymptomatic HUA were randomly assigned to the conventional or allopurinol treatment groups. After a 3-year follow-up period, the homeostasis assessment for insulin resistance (HOMA-IR) index mean value was significantly lower in the allopurinol group as compared to the conventional group (3.49 in the allopurinol group versus 3.57 in the conventional group), although there were no statistically significant differences in the baseline values between the two treatment groups. The authors therefore concluded that long-term effective control of serum UA by allopurinol may actually ameliorate insulin resistance [32]. This finding was also seen in a prospective cohort study involving 73 subjects with asymptomatic HUA placed on allopurinol or control with a follow-up period of 3 months. Allopurinol-treated subjects exhibited a significant improvement in HOMA-IR index, fasting blood glucose, fasting insulin as compared to the control group [33]. An experimental study investigating the effects of allopurinol in experimentally induced insulin-resistant rats showed that allopurinol plays a protective role against vascular complications of insulin resistance partly attributed to its effect on increasing insulin secretion [34].
Hyperuricemia and Hypertension
Several studies have demonstrated an association between serum UA and HTN. Incident HTN increases by 13% per 1 mg/dL increment in serum UA level based on the results of a systematic review of 18 prospective cohort studies involving 55,607 subjects [35••]. In a retrospective study including 315 hypertensive patients and 181 individuals without hypertension, HUA was observed in 6.7% of patients with newly diagnosed hypertension. A positive correlation between serum creatinine and UA and negative correlation between UA and eGFR were also demonstrated in this study [36]. In a longitudinal cohort of healthy subjects, a baseline UA level greater than 6.5 mg/dL was associated with a 25% increase in the risk of HTN [37]. A prospective study including subjects without any baseline cardiovascular disease showed that increased serum UA was highly associated with more intima media thickness leading to the incidence of HTN [38].
Different pathophysiological mechanisms have been proposed to understand the association between HTN and HUA.
A HUA rat model was created by adding oxonic acid, a uricase inhibitor, to the diet leading to almost twofold increase in serum UA. Three weeks later, blood pressure measurements were significantly elevated among hyperuricemic rats in comparison to controls. Interestingly, this finding was not dependent on oxonic acid, since lowering UA by a uricosuric agent or a xanthine oxidase inhibitor could not prevent HTN. Moreover, these rats demonstrated increased renin levels and decreased nitric oxide synthase in the macula densa, as well as tubulointerstitial injury, highlighting important pathological findings relating HUA to HTN and kidney disease [39•]. In another experimental study on human endothelial cells, HUA was associated with an increase in reactive oxygen species and angiotensin II, which causes efferent arteriole constriction leading to HTN, as well as in reactive oxygen species, known to significantly contribute to the incidence of HTN, cardiovascular diseases, and kidney diseases [40].
Few clinical trials looked for a possible role for UA reduction in the management of HTN.
In a randomized crossover study involving 30 adolescents (aged 11–17 years) with HUA and newly diagnosed essential HTN, treatment with allopurinol resulted in a 6.8 mmHg decrease in the 24-h systolic blood pressure [41••]. In another randomized, double-blinded, placebo-controlled trial, prehypertensive adolescents, aged 11 to 17 years, were randomized to receive the allopurinol, probenecid, or placebo. Subjects treated with urate-lowering therapy experienced a marked reduction in clinic systolic blood pressure of 10.2 mmHg and diastolic blood pressure of 9.0 mmHg [42]. In a prospective trial investigating the benefits of allopurinol treatment in hyperuricemic patients with normal renal function, allopurinol 300 mg/day was given to 48 patients with HUA and included 34 patients with HTN. Three months later, it was seen that noted that allopurinol resulted in a significant decrease in mean blood pressure from 135.4/80.2 to 131.5/78.3 mmHg [43••].
Conclusion
Substantial data from epidemiologic and experimental studies indicate an emerging association between HUA, T2DM, and HTN. Pharmacologic agents lowering serum UA proved to play a promising role in the management of T2DM and HTN. Nevertheless, large prospective randomized, controlled trials are warranted to determine direct effects between UA lowering and long-term clinical outcomes. Moreover, further studies are required to validate the utility of UA levels for the primary prevention of T2DM and HTN and to clarify the serum UA level at which intervention is needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rock KL, Kataoka H, Lai J-J. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9(1):13–23.
de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetol Metab Syndr. 2012;4:12.
Aung T, Myung G, FitzGerald JD. Treatment approaches and adherence to urate-lowering therapy for patients with gout. Patient Prefer Adherence. 2017;11:795–800.
Kuwabara M. Hyperuricemia, cardiovascular disease, and hypertension. Pulse. 2016;3(3–4):242–52.
Mortada I. Hyperbilirubinemia, hypertension, and CKD: the links. Curr Hypertens Rep. 2017;19(7):58. https://doi.org/10.1007/s11906-017-0756-8
Pradeepa R, Mohan V. Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. Eur J Clin Nutr. 2017;71(7):816–24.
Wang C, et al. Quercetin and allopurinol ameliorate kidney injury in STZ-treated rats with regulation of renal NLRP3 inflammasome activation and lipid accumulation. PLoS One. 2012;7(6):e38285.
Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9(1):13–23.
• Jalal DI, et al. Uric acid as a target of therapy in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(1):134–46. A review focusing on uric acid as a potential therapeutic target to prevent kidney disease onset and progression.
Maiuolo J, et al. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14.
Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19(6):358–71.
Liote F. Hyperuricemia and gout. Curr Rheumatol Rep. 2003;5(3):227–34.
Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19(6):358–71.
McDonagh EM, et al. PharmGKB summary: uric acid-lowering drugs pathway, pharmacodynamics. Pharmacogenet Genomics. 2014;24(9):464–76.
Mende C. Management of chronic kidney disease: the relationship between serum uric acid and development of nephropathy. Adv Ther. 2015;32:1177–91.
Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6(1):30–8.
•• Li L, et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: a systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. This systematic review and meta-analysis shows that elevated serum uric acid levels showed an increased risk for the development of chronic renal dysfunction.
Lapi F, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ : British Medical Journal. 2013;346:e8525.
Zhu J-N, et al. Dietary factors associated with hyperuricemia and glycolipid metabolism disorder in middle-aged and elderly people. Sichuan da xue xue bao Yi xue ban = Journal of Sichuan University Medical science edition. 2016;47(1):68–72.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41.
Anton FM, et al. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35(4):343–8.
Hak AE, Choi HK. Menopause, postmenopausal hormone use and serum uric acid levels in US women—the Third National Health and Nutrition Examination Survey. Arthritis Res Ther. 2008;10(5):R116.
•• Lv Q, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One. 2013;8(2):e56864. This meta-analysis of prospective cohort studies provides strong evidence that high level of serum uric acid is independent of other established risk factors, for developing type 2 diabetes in middle-aged and older people.
Jia Z, et al. Serum uric acid levels and incidence of impaired fasting glucose and type 2 diabetes mellitus: a meta-analysis of cohort studies. Diabetes Res Clin Pract. 2013;101(1):88–96.
Kim SC, Liu J, Solomon DH. Risk of incident diabetes in patients with gout: a cohort study. Arthritis & rheumatology (Hoboken, NJ). 2015;67(1):273–80.
Liu Y, et al. Serum uric acid levels and the risk of impaired fasting glucose: a prospective study in adults of north China. PLoS One. 2013;8(12):e84712.
Lee S-H, Kim K-M, Kim K-N. Combined effect of serum gamma-glutamyltransferase and uric acid on incidence of diabetes mellitus: a longitudinal study. Medicine. 2017;96(19):e6901.
Krishnan E, et al. Relative and attributable diabetes risk associated with hyperuricemia in US veterans with gout. QJM: An International Journal of Medicine. 2013;106(8):721–9.
Maahs DM, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13(4):550–9.
Chaudhary K, et al. Uric acid—key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 2013;3(3):208–20.
Matsuoka T, et al. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Investig. 1997;99(1):144–50.
Liu P, et al. The effects of allopurinol on the carotid intima-media thickness in patients with type 2 diabetes and asymptomatic hyperuricemia: a three-year randomized parallel-controlled study. Intern Med. 2015;54(17):2129–37.
Takir M, et al. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Investig Med. 2015;63(8):924–9.
El-Bassossy HM, et al. Ameliorative effect of allopurinol on vascular complications of insulin resistance. Journal of Diabetes Research. 2015;2015:10.
•• Grayson PC, et al. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63(1):102–10. This systematic review and meta-analysis show that hyperuricemia is associated with an increased risk for incident hypertension, independent of traditional hypertension risk factors.
Komendarek-Kowalska M. The assessment of renal function in patients with newly diagnosed hypertension—the role of hyperuricemia as a risk factor for chronic kidney disease—preliminary study. Pol Merkur Lekarski. 2017;42(251):193–6.
Perlstein TS, et al. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031–6.
Cicero AF, et al. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis: data from the Brisighella Heart Study. J Hypertens. 2014;32(1):57–64.
• Mazzali M, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–6. The study reveals that elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism.
Yu MA, et al. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–42.
•• Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–32. This randomized trial shows that treatment with allopurinol reduces blood pressure in adolescents with newly diagnosed hypertension.
Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60(5):1148–56.
•• Kanbay M, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39(4):1227–33. This study brings indirect evidence that management of hyperuricemia may prevent the progression of renal disease, even in patients with normal renal function.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Mortada declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Hypertension and the Kidney
Rights and permissions
About this article
Cite this article
Mortada, I. Hyperuricemia, Type 2 Diabetes Mellitus, and Hypertension: an Emerging Association. Curr Hypertens Rep 19, 69 (2017). https://doi.org/10.1007/s11906-017-0770-x
Published:
DOI: https://doi.org/10.1007/s11906-017-0770-x