Abstract
Purpose of Review
The purpose of this review is to summarize recent developments in PET imaging of neuropathologies underlying HIV-associated neurocognitive dysfunction (HAND). We concentrate on the recent post antiretroviral era (ART), highlighting clinical and preclinical brain PET imaging studies.
Recent Findings
In the post ART era, PET imaging has been used to better understand perturbations of glucose metabolism, neuroinflammation, the function of neurotransmitter systems, and amyloid/tau protein deposition in the brains of HIV-infected patients and HIV animal models. Preclinical and translational findings from those studies shed a new light on the complex pathophysiology underlying HAND.
Summary
The molecular imaging capabilities of PET in neuro-HIV are great complements for structural imaging modalities. Recent and future PET imaging studies can improve our understanding of neuro-HIV and provide biomarkers of disease progress that could be used as surrogate endpoints in the evaluation of the effectiveness of potential neuroprotective therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advent of antiretroviral therapy (ART) has decreased mortality and morbidity rates in HIV-positive (HIV+) patients and to a great extent diminished the incidence of HIV-associated dementia (HAD), the most severe form of neuro-HIV. Although the incidence of moderate or severe HAD fell from about 7% in 1989 to only 1% in 2000 [1], the frequency of milder forms of HIV-associated neurocognitive dysfunction (HAND), including mild neurocognitive disorder (MND) and asymptomatic neurocognitive impairment (ANI), remains high [2]. With the non-acute onset and relatively mild initial clinical presentation, HAND can often complicate the management of those patients and affect their quality of life. Considering the chronic nature of the disease associated with ART treatment, a change in research focus towards mild neurocognitive dysfunction is warranted [3] with a more detailed exploration of MND and ANI [4]. Understanding the pathophysiology of depression in HIV is another topic of interest considering recent reports of the role of depression in defining mortality and morbidity of HIV+ subjects [5]. Towards those goals, a better understanding of the molecular and functional neuropathologies underlying HAND becomes necessary.
Potential contributing factors to the occurrence of HAND and associated mood disorders include persistent latent HIV-1 reservoirs in the brain, irreversible CNS insult prior to ART initiation, toxicities related to antiretroviral drugs, amyloid and tau protein deposition, neuroinflammation [6, 7] as well as molecular damage of various neurotransmitter systems [8,9,10,11,12] (Fig. 1). Positron emission tomography (PET) is a molecular imaging modality that can non-invasively probe many of those pathologies in vivo. As such, PET can provide complementary information to conventional structural as well as novel magnetic resonance imaging (MRI) techniques such as diffusion tensor imaging (DTI) [13, 14], volumetric MRI [15,16,17], and magnetic resonance spectroscopy (MRS) [18, 19].
Since preclinical studies provide a practical platform for investigation of new imaging biomarkers that can eventually be translated into clinical applications, we structured this review to briefly describe neuroimaging research in various preclinical HIV models along with relevant/related clinical studies (Table 1).
Animal Models of HIV Infection
One way of controlling for heterogeneity in patient populations and to obtain longitudinal information is to perform imaging studies in animal models [33,34,35,36]. One major challenge in developing animal models for HIV is the availability of cellular proteins in those species such as CD4 and CCR5/CXCR4 that would support viral replication. This has limited the success of original attempts to infect small animals such as mice and rats with HIV-1 [37]. A naturally occurring virus, feline immunodeficiency virus, infects domestic cats and can result in a similar disease process to HIV infection in humans. Major differences however exist, including a different primary receptor (CD134 instead of CD4) with secondary additional infection of lymphocytes, and a slow protracted course of disease, rendering this model less practical and less widely used [37]. Among rodent models, transgenic (Tg) mice and rats have been developed. The HIV-1 Tg rat model expressing seven of the nine viral proteins including gp120, nef, and tat in lymphocytes, monocytes, and in the brain was developed in 2001 [38] and was found to develop neuropathologies reminiscent of HIV infection. As such, it has been used in multiple studies evaluating the effects of HIV viral proteins on the brain and in various imaging studies [39,40,41,42,43,44]. The “humanized mouse” model, on the other hand, is generated with immunocompromised mice with transplanted humanized immune system and is also a frequently used small animal model in HIV-1 studies [45, 46]. Among the various types of humanized mice, OD/scid-IL-2Rgcnull mice have been used for imaging using MRI techniques, but not PET imaging [47,48,49].
Rodents, however, remain suboptimal models of HIV, especially when compared to the simian immunodeficiency virus (SIV) and simian/human immunodeficiency syndrome (SHIV) infected monkey models, which are considered to be more appropriate animal models in HIV/AIDS research. Despite the associated costs and logistical limitations, SIV and SHIV-infected monkeys continue to provide valuable in vivo experimental data complementing human studies of transmission, pathogenesis, prevention, and treatment of HIV [50,51,52,53]. This is mainly because non-human primates (NHPs) are physiologically and immunologically similar to humans. This similarity results in the development of simian AIDS in those animals that is analogous in many aspects to HIV-1 AIDS in humans [37, 54]. SIV encephalitis (SIVE) is one of the aspects of SIV infection reminiscent of HIV CNS involvement in the early days of the epidemic [55,56,57], while newer models of non-accelerated diseases (e.g., SHIV model) seem to better reflect milder neurologic changes currently seen in infected treated patients [58]. Models of SIVE have already been used to investigate the pathophysiology of CNS involvement as well as in the evaluation of novel therapies [50, 55, 59, 60]. PET imaging studies have mostly used rhesus macaques [61,62,63,64,65,66] infected with SIV or SHIV, although there have been suggestions that pigtail macaques progress more rapidly to SIVE [50].
PET Imaging of Glucose Metabolism Using 18F-FDG
18F-fluorodeoxyglucose (FDG)-PET imaging can non-invasively quantify glucose metabolism within various tissues and can help detect brain activation patterns involved in normal and abnormal brain functioning. FDG brain imaging has been used in many CNS diseases such as Alzheimer’s dementia (AD) and Parkinson’s disease; however, limited literature is available on its use in SIV and HIV studies, especially in the post ART era.
In the setting of SIV and SHIV infection, FDG-PET imaging has mainly concentrated on peripheral patterns of immune activation rather than brain involvement [65, 66]. In a recent study, we used brain FDG-PET imaging in a group of SIV-infected macaques to longitudinally assess the effect of ART initiation and interruption by monitoring alterations in brain glucose metabolism. We observed increased brain glucose metabolism within 1 month of treatment cessation, which may reflect neuroinflammation in the setting of viral rebound. This was significantly associated with decreased CD4+ and CD8+ T cell counts and increased plasma/CSF viral load (VL). While we cannot assert neurologic damage in association with cerebral hypermetabolism, it is a concerning outcome of non-adherence to ART, even for short periods of time [67]. We did not find significant or consistent changes in FDG uptake when ART was initiated however, suggesting that abatement of neuroinflammatory changes associated with viral replication might take a long time to occur [67].
FDG-PET has been used more extensively in HIV+ patients, however. Early studies in the pre-ART era suggested early CNS HIV involvement with high FDG uptake consistent with increased glucose metabolism seen in the basal ganglia [68,69,70,71] and thalamus [71] as well as relative increased metabolism in subcortical regions for patients with AIDS dementia complex (ADC) [72]. Hypometabolism was found to eventually occur in infected patients suggesting neuronal damage/loss [70].
Among the few studies in the recent ART era, Andersen et al. studied the prevalence of cerebral metabolic abnormalities in an HIV+ patient group on ART, with at least 3 years of fully suppressed VLs. The authors observed a substantial fraction (55%) of the optimally treated patients displaying abnormally low mesial frontal FDG uptake [22]. The observed hypometabolism correlated with shorter history of known HIV infection, fewer years on ART and higher circulating levels of TNF-α and IL-6 [22]. In another study by Towgood and colleagues, the authors reported reduced metabolic activity in frontal brain regions with no significant interaction between HIV and aging [21]. However, in both study populations [21, 22], subjects had none of the co-morbidities commonly seen in HIV+ patients. Being able to assess the contribution of those co-morbidities to neuronal dysfunction in HIV has the potential of changing the clinical approach to HAND subjects. We have recently evaluated a large group of subjects (47 treated HIV+ patients with a long history of infection, 10 HIV− with co-morbidities similar to the HIV+ group, and 19 healthy volunteers). Interestingly, we observed abnormal global glucose metabolism in HIV+ and HIV− patients with co-morbidities that was best predicted by cardiovascular disease rather than HIV status [20]. This suggests a very important role for cardiovascular disease in neuronal loss/dysfunction, as measured by FDG-PET in this vulnerable patient population. In addition, however, we did see significant focal hypometabolism in the thalamus of HIV+ patients that was best predicted by HIV serostatus suggesting an HIV-related effect [20]. The exact pathophysiology underlying thalamic hypometabolism in HIV+ patients and its association with potential executive function dysfunction is unclear and warrants further evaluation.
Neuroinflammation PET Ligands in SIV/HIV
Despite successful control of HIV replication in the periphery and in the brain, low levels of persistent inflammation have been implicated in the pathophysiology of HAND [73]. Following HIV infection, the virus enters the brain and persists in perivascular macrophages and microglia, with the resulting cascade of viral neuropathogenesis assumed to be related to proinflammatory and cytotoxic products secreted by those cells [74,75,76]. PET imaging enables the in vivo quantification of microglial activation/neuroinflammation through radiolabeled ligands that target the translocator protein (TSPO), a naturally expressed receptor on the outer mitochondrial membrane of microglia, macrophages, and astrocytes. During microglial activation, TSPO is significantly upregulated [64] and as such can reflect the degree of neuroinflammation. The earliest TSPO radioligand to be used to assess neuroinflammation in various neurodegenerative diseases was 11C-PK11195, an isoquinoline carboxamide [77,78,79]. More recently however, multiple second-generation TSPO ligands have been developed with higher TSPO affinity and generally higher specific to non-specific binding compared to 11C-PK11195 [80,81,82].
In animal models, one of the second-generation ligands, 18F-DPA714, showed higher but not statistically significant uptake in Tg rats compared to wild-type rats. This raised the concern that microglial activation might not necessarily be the key mechanism for neuropathology in the Tg rat model [42] but rather chronic exposure to HIV viral proteins [40]. Earlier work in SIV-infected monkeys using 11C-PK11195, on the other hand, showed evidence of microglial activation in animals with SIVE compared to those without SIVE [64].
In humans, early studies using 11C-PK11195 in HIV+ patients had conflicting results [26, 27] which could be due to the heterogeneity of HIV patient populations assessed and the inherent high non-specific binding of the ligand. In a more recent study, also using 11C-PK11195, clusters of significantly increased 11C-PK11195 binding suggestive of the presence of focal cortical regions of activated microglia were observed by Garvey et al. in a group of asymptomatic subjects with chronic HIV infection on suppressive ART [25]. Using second-generation TSPO ligands by two different groups also showed microglial activation in treated HIV+ patients [23, 24]. In the paper by Coughlin et al., the authors uncovered suboptimal test-retest reproducibility of TSPO distribution volume values (Vt) in healthy controls using a second-generation ligand, 11C-DPA713. As a result, they resorted to a different approach in which the ratios of Vt relative to overall gray matter (VtGM) were calculated relative to overall gray matter (VtGM). Higher VtGM values were then observed in treated HIV+ patients compared to HIV-negative subjects in the white matter, cingulate cortex, and supramarginal gyrus. Increased binding in the frontal cortex was specifically seen in patients with dementia [24]. Similar positive results were found by Vera et al. using a different second-generation TSPO ligand, 11C-PBR28, in a cohort of cognitively healthy treated HIV+ individuals: increased uptake was found globally as well as regionally, in the parietal and occipital lobes and in the globus pallidus [23]. The findings of both papers seem to support glial cell activation that is persistent in the course of HIV infection, despite treatment and virological suppression.
Imaging Neurotransmitter Systems in SIV/HIV
Dopaminergic System
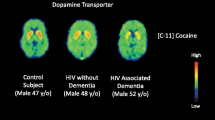
Among the neurotransmitter systems, the vulnerability of the dopaminergic system to the effects of the virus has been well documented, with the basal ganglia being most affected, resulting in a Parkinsonian-like symptomology [83]. These findings were supported by decreased neuronal number and neuronal density in the globus pallidus and substantia nigra in SIV-infected monkeys compared to controls [84].
In animal models, we evaluated the dopaminergic system in the Tg rat [44, 85], based on reports of dopaminergic dysfunction in this model [86, 87]. Using two different radioligands, 18F-FPCMT for presynaptic dopaminergic transporter (DAT) and 18F-fallypride for postsynaptic D2/D3 receptors, we observed significant loss of both DAT and D2/D3 receptors in older (15–18 months) Tg rats compared to controls [85]. Dopaminergic dysfunction observed with this Tg rat model is probably related to viral protein exposure, considering that rats with higher serum gp120 had lower mean binding potential values for both ligands [85]. No similar reported work has been published in SIV-infected animals however.
A few human PET studies targeted the dopaminergic system in the setting of HIV infection. In an early study with 11C-cocaine targeting DAT and 11C-raclopride targeting D2 receptors, a significant decrease in DAT was seen in HIV+ patients with HAD compared to HIV− controls especially in the putamen and ventral striatum. Mild but non-significant decreases in D2 receptor availability were observed within the same subject groups [28]. A subsequent study using the same PET ligands with a larger cohort of HIV+ patients (n = 35) including a subcohort of HIV+ patients with continued cocaine abuse (11 out of 35) again found significantly decreased DAT in the putamen, compared to HIV seronegative controls [11]. In the same study, only the HIV + Coc subgroup had significantly lower DAT in the caudate compared to controls. The authors concluded that reduced dopaminergic function may contribute to cognitive dysfunction in HIV+ patients with or without additional cocaine abuse [11]. There have been no additional clinical PET studies targeting the dopaminergic system in HIV+ patients published since those two papers.
Serotonergic System
More recently, there has been a renewed interest in mood disorders, mainly depression, in HIV+ patients, mainly due to the associated deleterious effects on treatment adherence with secondary increased mortality and morbidity [5, 88, 89]. One related neurotransmitter system, the serotonergic system, is thus of interest, especially considering prior reports suggesting involvement in the pathophysiology of neuro-HIV [8, 9, 90]. Similar to the dopaminergic system, multiple ligands have been developed targeting various components of the serotonergic system. However, one of the most common targets remains the serotonin transporter (SERT) with 11C-DASB, a specific PET SERT ligand, being used to assess SERT changes in the setting of depression in multiple previous studies [91,92,93].
In the only clinical study where 11C-DASB was used to image serotonin dysfunction in a cohort of HIV+ patients with depression, HIV+ patients had generally lower 11C-DASB binding than HIV− controls. Depressed HIV+ patients however had higher 11C-DASB binding compared to non-depressed subjects, suggesting a role of the serotonergic system in depression associated with HIV [10]. More recently, we used 11C-DASB PET to longitudinally image NHPs (rhesus macaques) infected with the neurotropic SIV strain (SIVsm804E) [94]. Interestingly, we found higher 11C-DASB binding in 85% of the infected animals compared to baseline. Increased 11C-DASB binding reflective of serotonergic upregulation in the midbrain in infected animals correlated significantly with the duration of infection and DASB binding in the thalamus correlated significantly with CSF cytokines [94]. Our findings suggest inherent involvement of the serotonergic system in SIV pathophysiology. Whether these results can be reproduced and correlated to depressive symptomatology in optimally treated HIV+ patients remains to be seen.
Imaging Amyloid and Tau Deposition in HIV
Histopathological similarities between AD and HIV brain involvement were suggested more than two decades ago, with deposition of amyloid-β plaques and tau proteins shown in postmortem brain tissues of HIV+ patients [95,96,97,98,99]. This issue became more relevant after the advent of ART and secondary prolonged survival of infected patients with increased concerns that the aging HIV population could be more prone to the risk of developing AD. Similar to what has been described in AD patients, decreased levels of CSF amyloid beta 42 (Aβ42) have been described in HIV+ patients with neurocognitive dysfunction by a few groups, potentially reflecting increased Aβ42 deposition in brain parenchyma [100,101,102]. Other studies however did not support the findings [30, 103, 104].
Although imaging of Aβ42 in AD patients has been very successful using ligands such as 11C-labeled Pittsburg compound B (PIB), 18F-florbetaben, and 18F-florbetapir [105], clinical Aβ42 imaging studies in HIV+ subjects have not shown increased amyloid accumulation regardless of the degree of neurocognitive impairment and despite lower levels of CSF Aβ42 in some HIV+ subjects [29, 30, 106]. The exact reasons for negative imaging results in view of documented amyloid deposition in HIV by pathology are unclear. One possible explanation is a difference in structural composition/location of amyloid plaques between HIV and AD patients: while amyloid plaques are generally extracellular in AD, they are more likely to be intracellular in HIV [98, 107]. Also, extracellular amyloid plaques sometimes seen in HAND are more diffuse [107, 108], while in AD, they tend to be fibrillar [109]. Since amyloid radiotracers have generally high affinity and selectivity for fibrillar Αβ in plaques [105], this could account for lower binding in HIV+ subjects. Another possible difference in amyloid pathology between AD and HIV could be related to amyloid metabolism with downregulation of upstream pathways involved in amyloid precursor protein production [106]. Interestingly, a case report by Turner et al. recently demonstrated increased 18F-florbetaben reflecting amyloid deposition in an older (71-year old) HIV+ individual [31]. It is unclear however, in the absence of post mortem tissues, whether this patient’s dementia is due to HIV, co-incidental AD, or a combination of both. Additional studies are thus needed to better evaluate amyloid deposition in an older HIV population. Another issue that needs better investigation is the potential interaction between ART and amyloid clearance from the brain which could possibly increase the risk of developing AD in the treated aging HIV patient population [98, 107].
Tau protein deposition is another pathological hallmark of AD that has been seen in HIV brains [110] with the highest levels of phosphorylated tau (p-tau) deposition seen in HAART-treated patients [97]. Multiple groups have attempted to measure total tau (t-tau) and p-tau protein levels in the CSF; however, the findings have not always been in agreement: while some groups showed no changes in p- or t-tau levels, others found changes in t-tau [111]. Despite the uncertainty, there is increased interest in utilizing tau-specific PET ligands to image HIV+ patients. In one case report, a 70-year-old subject presenting with HIV encephalitis had increased binding of 18F-THK 5117 (tau ligand) in the periventricular and deep white matter regions [32]. More recently, however, PET imaging with another Tau ligand (18F-AV-1451) showed similar binding for HIV+ and HIV-negative control individuals [112]. This raises the possibility that PET with tau ligands could be used in older HIV+ individuals to differentiate AD from cognitive impairment due to HIV.
Conclusions and Future Directions
In conclusion, PET imaging remains underutilized in the evaluation of neuro-HIV, especially in the post-ART era. PET imaging targeting novel neuroinflammation biomarkers besides TSPO, such as cannabinoid receptors or cyclooxygenases 1 and 2, might be helpful in better assessing the exact role of neuroinflammation in the pathophysiology of HAND in treated subjects. In addition to the dopaminergic and serotonergic systems, other neurotransmitter systems such as the cholinergic and GABAergic systems could be assessed in HIV for possible system-specific effects of the virus. PET imaging of amyloid and tau deposition in older HIV+ subjects might provide new insights into the exact connection/interaction between HIV and AD and the role of ART in amyloid and tau deposition. Finally, in a recent immunoPET study using a 64Cu-labeled SIV Gp120-specific antibody, the authors were able to detect viral dynamics and localization in the lymphoid tissues, gastrointestinal, and respiratory tracts in SIV monkeys, before and after treatment [62]. Although such ligands could be potentially useful in the detection of latent viral reservoirs in the whole body of treated HIV+ subjects, there is still the caveat of the labeled antibodies not crossing the blood brain barrier (BBB) to reveal potential sites of HIV persistence in the CNS. Developing radiolabeled ligands that can target SIV/HIV and cross the BBB would help us measure CNS reservoirs in HIV animal models and eventually in HIV+ subjects.
References
McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol. 2010;67(6):699–714.
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. https://doi.org/10.1007/s13365-010-0006-1.
Gates TM, Cysique LA. The chronicity of HIV infection should drive the research strategy of NeuroHIV treatment studies: a critical review. CNS Drugs. 2016;30(1):53–69. https://doi.org/10.1007/s40263-015-0302-7.
Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS (London, England). 2004;18(Suppl 1):S75–8.
Pence BW, Mills JC, Bengtson AM, Gaynes BN, Breger TL, Cook RL, et al. Association of increased chronicity of depression with HIV appointment attendance, treatment failure, and mortality among HIV-infected adults in the United States. JAMA Psychiatry. 2018;75:379–85. https://doi.org/10.1001/jamapsychiatry.2017.4726.
Spudich SS, Ances BM. CROI 2017: neurologic complications of HIV infection. Top Antivir Med. 2017;25(2):69–76.
Farhadian S, Patel P, Spudich S. Neurological complications of HIV infection. Curr Infect Dis Rep. 2017;19(12):50. https://doi.org/10.1007/s11908-017-0606-5.
Keegan MR, Chittiprol S, Letendre SL, Winston A, Fuchs D, Boasso A, et al. Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. Int J Tryptophan Res. 2016;9:79–88. https://doi.org/10.4137/ijtr.s36464.
Sperner-Unterweger B, Kohl C, Fuchs D. Immune changes and neurotransmitters: possible interactions in depression? Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;48:268–76. https://doi.org/10.1016/j.pnpbp.2012.10.006.
Hammoud DA, Endres CJ, Hammond E, Uzuner O, Brown A, Nath A, et al. Imaging serotonergic transmission with [11C]DASB-PET in depressed and non-depressed patients infected with HIV. NeuroImage. 2010;49(3):2588–95. https://doi.org/10.1016/j.neuroimage.2009.10.037.
Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42(2):869–78.
Nagano-Saito A, Liu J, Doyon J, Dagher A. Dopamine modulates default mode network deactivation in elderly individuals during the tower of London task. Neurosci Lett. 2009;458(1):1–5.
Haynes BI, Pitkanen M, Kulasegaram R, Casey SJ, Schutte M, Towgood K, et al. HIV: ageing, cognition and neuroimaging at 4-year follow-up. HIV Med. 2018;19:376–85. https://doi.org/10.1111/hiv.12598.
Wright PW, Vaida FF, Fernandez RJ, Rutlin J, Price RW, Lee E, et al. Cerebral white matter integrity during primary HIV infection. AIDS (London, England). 2015;29(4):433–42. https://doi.org/10.1097/qad.0000000000000560.
Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, et al. Structural brain alterations can be detected early in HIV infection. Neurology. 2012;79(24):2328–34. https://doi.org/10.1212/WNL.0b013e318278b5b4.
Correa DG, Zimmermann N, Ventura N, Tukamoto G, Doring T, Leite SC, et al. Longitudinal evaluation of resting-state connectivity, white matter integrity and cortical thickness in stable HIV infection: preliminary results. Neuroradiol J. 2017;30(6):535–45. https://doi.org/10.1177/1971400917739273.
Guha A, Brier MR, Ortega M, Westerhaus E, Nelson B, Ances BM. Topographies of cortical and subcortical volume loss in HIV and aging in the cART era. J Acquir Immune Defic Syndr. 2016;73(4):374–83. https://doi.org/10.1097/qai.0000000000001111.
Descamps M, Hyare H, Stebbing J, Winston A. Magnetic resonance imaging and spectroscopy of the brain in HIV disease. J HIV Ther. 2008;13(3):55–8.
Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, et al. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PLoS One. 2013;8(4):e61738. https://doi.org/10.1371/journal.pone.0061738.
Hammoud DA, Sinharay S, Steinbach S, Wakim PG, Geannopolous K, Traino K, et al. Global and regional brain hypometabolism on FDG-PET in treated HIV-infected individuals. Neurology. 2018;91(17):e1591-e601. https://doi.org/10.1212/wnl.0000000000006398.
Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Soni S, Sibtain N, et al. Regional cerebral blood flow and FDG uptake in asymptomatic HIV-1 men. Hum Brain Mapp. 2013;34(10):2484–93. https://doi.org/10.1002/hbm.22078.
Andersen AB, Law I, Krabbe KS, Bruunsgaard H, Ostrowski SR, Ullum H, et al. Cerebral FDG-PET scanning abnormalities in optimally treated HIV patients. J Neuroinflammation. 2010;7:13. https://doi.org/10.1186/1742-2094-7-13.
Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology. 2016;86(15):1425–32. https://doi.org/10.1212/wnl.0000000000002485.
Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, et al. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol. 2014;20(3):219–32. https://doi.org/10.1007/s13365-014-0239-5.
Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS (London, England). 2014;28(1):67–72. https://doi.org/10.1097/01.aids.0000432467.54003.f7.
Wiley CA, Lopresti BJ, Becker JT, Boada F, Lopez OL, Mellors J, et al. Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol. 2006;12(4):262–71.
Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC, et al. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol. 2005;11(4):346–55.
Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127(Pt 11):2452–8.
Ances BM, Christensen JJ, Teshome M, Taylor J, Xiong C, Aldea P, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case-control study. Neurology. 2010;75(2):111–5. https://doi.org/10.1212/WNL.0b013e3181e7b66e.
Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. 2012;69(1):72–7. https://doi.org/10.1001/archneurol.2011.761.
Turner RS, Chadwick M, Horton WA, Simon GL, Jiang X, Esposito G. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amsterdam, Netherlands). 2016;4:1–5. https://doi.org/10.1016/j.dadm.2016.03.009.
Tripathi M, Yadav S, Kumar V, Kumar R, Tripathi M, Gaikwad S, et al. HIV encephalitis with subcortical tau deposition: imaging pathology in vivo using F-18 THK 5117. Eur J Nucl Med Mol Imaging. 2016;43(13):2456–7. https://doi.org/10.1007/s00259-016-3473-7.
Beck SE, Queen SE, Metcalf Pate KA, Mangus LM, Abreu CM, Gama L, et al. An SIV/macaque model targeted to study HIV-associated neurocognitive disorders. J Neurovirol. 2018;24(2):204–12. https://doi.org/10.1007/s13365-017-0582-4.
Honeycutt JB, Garcia JV. Humanized mice: models for evaluating neuroHIV and cure strategies. J Neurovirol. 2018;24(2):185–91. https://doi.org/10.1007/s13365-017-0567-3.
Nixon CC, Mavigner M, Silvestri G, Garcia JV. In vivo models of human immunodeficiency virus persistence and cure strategies. J Infect Dis. 2017;215(suppl_3):S142–s51. https://doi.org/10.1093/infdis/jiw637.
Williams K, Lackner A, Mallard J. Non-human primate models of SIV infection and CNS neuropathology. Curr Opin Virol. 2016;19:92–8. https://doi.org/10.1016/j.coviro.2016.07.012.
Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10(12):852–67. https://doi.org/10.1038/nrmicro2911.
Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci. 2001;98(16):9271–6.
Casas R, Muthusamy S, Wakim PG, Sinharay S, Lentz MR, Reid WC, et al. MR brain volumetric measurements are predictive of neurobehavioral impairment in the HIV-1 transgenic rat. NeuroImage Clin. 2018;17:659–66. https://doi.org/10.1016/j.nicl.2017.11.018.
Reid WC, Ibrahim WG, Kim SJ, Denaro F, Casas R, Lee DE, et al. Characterization of neuropathology in the HIV-1 transgenic rat at different ages. J Neuroimmunol. 2016;292:116–25. https://doi.org/10.1016/j.jneuroim.2016.01.022.
Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun. 2015;48:336–49. https://doi.org/10.1016/j.bbi.2015.02.020.
Lee DE, Yue X, Ibrahim WG, Lentz MR, Peterson KL, Jagoda EM, et al. Lack of neuroinflammation in the HIV-1 transgenic rat: an [(18)F]-DPA714 PET imaging study. J Neuroinflammation. 2015;12(1):171. https://doi.org/10.1186/s12974-015-0390-9.
Lentz MR, Peterson KL, Ibrahim WG, Lee DE, Sarlls J, Lizak MJ, et al. Diffusion tensor and volumetric magnetic resonance measures as biomarkers of brain damage in a small animal model of HIV. PLoS One. 2014;9(8):e105752. https://doi.org/10.1371/journal.pone.0105752.
Lee DE, Reid WC, Ibrahim WG, Peterson KL, Lentz MR, Maric D, et al. Imaging dopaminergic dysfunction as a surrogate marker of neuropathology in a small-animal model of HIV. Mol Imaging. 2014;13:1–10.
Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9(3):208–14.
Kieffer C, Ladinsky MS, Ninh A, Galimidi RP, Bjorkman PJ. Longitudinal imaging of HIV-1 spread in humanized mice with parallel 3D immunofluorescence and electron tomography. elife. 2017;6:e23282. https://doi.org/10.7554/eLife.23282.
Boska MD, Dash PK, Knibbe J, Epstein AA, Akhter SP, Fields N, et al. Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV-1 infection of humanized mice. Mol Neurodegener. 2014;9:58. https://doi.org/10.1186/1750-1326-9-58.
Epstein AA, Narayanasamy P, Dash PK, High R, Bathena SP, Gorantla S, et al. Combinatorial assessments of brain tissue metabolomics and histopathology in rodent models of human immunodeficiency virus infection. J Neuroimmune Pharmacol. 2013;8(5):1224–38. https://doi.org/10.1007/s11481-013-9461-9.
Dash PK, Gorantla S, Gendelman HE, Knibbe J, Casale GP, Makarov E, et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J Neurosci. 2011;31(9):3148–57. https://doi.org/10.1523/jneurosci.5473-10.2011.
Beck SE, Kelly KM, Queen SE, Adams RJ, Zink MC, Tarwater PM, et al. Macaque species susceptibility to simian immunodeficiency virus: increased incidence of SIV central nervous system disease in pigtailed macaques versus rhesus macaques. J Neurovirol. 2015;21(2):148–58. https://doi.org/10.1007/s13365-015-0313-7.
Garcia-Lerma JG, Heneine W. Animal models of antiretroviral prophylaxis for HIV prevention. Curr Opin HIV AIDS. 2012;7(6):505–13. https://doi.org/10.1097/COH.0b013e328358e484.
Schmitz JE, Korioth-Schmitz B. Immunopathogenesis of simian immunodeficiency virus infection in nonhuman primates. Curr Opin HIV AIDS. 2013;8(4):273–9. https://doi.org/10.1097/COH.0b013e328361cf5b.
Sui Y, Gordon S, Franchini G, Berzofsky JA. Nonhuman primate models for HIV/AIDS vaccine development. Curr Protoc Immunol. 2013;102:Unit 12.4. https://doi.org/10.1002/0471142735.im1214s102.
Evans DT, Silvestri G. Nonhuman primate models in AIDS research. Curr Opin HIV AIDS. 2013;8(4):255–61. https://doi.org/10.1097/COH.0b013e328361cee8.
Dang Q, Whitted S, Goeken RM, Brenchley JM, Matsuda K, Brown CR, et al. Development of neurological disease is associated with increased immune activation in simian immunodeficiency virus-infected macaques. J Virol. 2012;86(24):13795–9. https://doi.org/10.1128/jvi.02174-12.
Matsuda K, Brown CR, Foley B, Goeken R, Whitted S, Dang Q, et al. Laser capture microdissection assessment of virus compartmentalization in the central nervous systems of macaques infected with neurovirulent simian immunodeficiency virus. J Virol. 2013;87(16):8896–908. https://doi.org/10.1128/jvi.00874-13.
Matsuda K, Dang Q, Brown CR, Keele BF, Wu F, Ourmanov I, et al. Characterization of simian immunodeficiency virus (SIV) that induces SIV encephalitis in rhesus macaques with high frequency: role of TRIM5 and major histocompatibility complex genotypes and early entry to the brain. J Virol. 2014;88(22):13201–11. https://doi.org/10.1128/jvi.01996-14.
Hsu DC, Sunyakumthorn P, Wegner M, Schuetz A, Silsorn D, Estes JD, et al. Central nervous system inflammation and infection during early, nonaccelerated simian-human immunodeficiency virus infection in rhesus macaques. J Virol. 2018;92(11). https://doi.org/10.1128/jvi.00222-18.
Lee KM, Chiu KB, Renner NA, Sansing HA, Didier PJ, MacLean AG. Form follows function: astrocyte morphology and immune dysfunction in SIV neuroAIDS. J Neurovirol. 2014;20(5):474–84. https://doi.org/10.1007/s13365-014-0267-1.
Meulendyke KA, Pletnikov MV, Engle EL, Tarwater PM, Graham DR, Zink MC. Early minocycline treatment prevents a decrease in striatal dopamine in an SIV model of HIV-associated neurological disease. J Neuroimmune Pharmacol. 2012;7(2):454–64. https://doi.org/10.1007/s11481-011-9332-1.
Santangelo PJ, Cicala C, Byrareddy SN, Ortiz KT, Little D, Lindsay KE, et al. Early treatment of SIV+ macaques with an alpha4beta7 mAb alters virus distribution and preserves CD4(+) T cells in later stages of infection. Mucosal Immunol. 2018;11(3):932–46. https://doi.org/10.1038/mi.2017.112.
Santangelo PJ, Rogers KA, Zurla C, Blanchard EL, Gumber S, Strait K, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods. 2015;12(5):427–32. https://doi.org/10.1038/nmeth.3320.
Freeman ZT, Rice KA, Soto PL, Pate KA, Weed MR, Ator NA, et al. Neurocognitive dysfunction and pharmacological intervention using guanfacine in a rhesus macaque model of self-injurious behavior. Transl Psychiatry. 2015;5:e567. https://doi.org/10.1038/tp.2015.61.
Venneti S, Lopresti BJ, Wang G, Bissel SJ, Mathis CA, Meltzer CC, et al. PET imaging of brain macrophages using the peripheral benzodiazepine receptor in a macaque model of neuroAIDS. J Clin Invest. 2004;113(7):981–9. https://doi.org/10.1172/jci20227.
Wallace M, Pyzalski R, Horejsh D, Brown C, Djavani M, Lu Y, et al. Whole body positron emission tomography imaging of activated lymphoid tissues during acute simian-human immunodeficiency virus 89.6PD infection in rhesus macaques. Virology. 2000;274(2):255–61.
Scharko AM, Perlman SB, PWN H, Hanson JM, Uno H, Pauza CD. Whole body positron emission tomography imaging of simian immunodeficiency virus-infected rhesus macaques. Proc Natl Acad Sci U S A. 1996;93(13):6425–30.
Schreiber-Stainthorp W, Srinivasula S, Sinharay S, Shah S, Wang J, Dodd LE, et al., editors. Brain 18F-FDG PET of SIV-infected macaques after treatment interruption or initiation. Boston: CROI; 2018.
Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med. 1996;37(7):1133–41.
Hinkin CH, van Gorp WG, Mandelkern MA, Gee M, Satz P, Holston S, et al. Cerebral metabolic change in patients with AIDS: report of a six-month follow-up using positron-emission tomography. J Neuropsychiatry Clin Neurosci. 1995;7(2):180–7. https://doi.org/10.1176/jnp.7.2.180.
von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Arch Neurol. 2000;57(11):1601–7.
van Gorp WG, Mandelkern MA, Gee M, Hinkin CH, Stern CE, Paz DK, et al. Cerebral metabolic dysfunction in AIDS: findings in a sample with and without dementia. J Neuropsychiatry Clin Neurosci. 1992;4(3):280–7. https://doi.org/10.1176/jnp.4.3.280.
Rottenberg DA, Moeller JR, Strother SC, Sidtis JJ, Navia BA, Dhawan V, et al. The metabolic pathology of the AIDS dementia complex. Ann Neurol. 1987;22(6):700–6. https://doi.org/10.1002/ana.410220605.
Rappaport J, Volsky DJ. Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J Neurovirol. 2015;21(3):235–41. https://doi.org/10.1007/s13365-015-0346-y.
Lipton SA, Gendelman HE. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332(14):934–40. https://doi.org/10.1056/nejm199504063321407.
Boven LA. Macrophages and HIV-1-associated dementia. Arch Immunol Ther Exp. 2000;48(4):273–9.
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202(1–2):13–23.
Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord. 2007;22(13):1852–6.
Verma P, Asopa RV. Incidental global hypometabolism in the brain of patient with AIDS-related dementia seen on 18F-Fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med. 2018;33(1):73–5. https://doi.org/10.4103/ijnm.IJNM_108_17.
Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ, et al. Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain. 2007;130(Pt 7):1759–66.
Endres CJ, Pomper MG, James M, Uzuner O, Hammoud DA, Watkins CC, et al. Initial evaluation of 11C-DPA-713, a novel TSPO PET ligand, in humans. J Nucl Med. 2009;50(8):1276–82.
Chauveau F, Van Camp N, Dolle F, Kuhnast B, Hinnen F, Damont A, et al. Comparative evaluation of the translocator protein radioligands 11C-DPA-713, 18F-DPA-714, and 11C-PK11195 in a rat model of acute neuroinflammation. J Nucl Med. 2009;50(3):468–76.
Brown AK, Fujita M, Fujimura Y, Liow JS, Stabin M, Ryu YH, et al. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J Nucl Med. 2007;48(12):2072–9. https://doi.org/10.2967/jnumed.107.044842.
Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol (Oxford, England). 2000;14(3):222–7.
Marcario JK, Manaye KF, SantaCruz KS, Mouton PR, Berman NE, Cheney PD. Severe subcortical degeneration in macaques infected with neurovirulent simian immunodeficiency virus. J Neurovirol. 2004;10(6):387–99.
Sinharay S, Lee D, Shah S, Muthusamy S, Papadakis GZ, Zhang X, et al. Cross-sectional and longitudinal small animal PET shows pre and post-synaptic striatal dopaminergic deficits in an animal model of HIV. Nucl Med Biol. 2017;55:27–33. https://doi.org/10.1016/j.nucmedbio.2017.08.004.
Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–47. https://doi.org/10.1016/j.expneurol.2012.10.008.
Webb KM, Aksenov MY, Mactutus CF, Booze RM. Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2010;16(2):168–73. https://doi.org/10.3109/13550281003690177.
Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci. 2014;68(2):96–109. https://doi.org/10.1111/pcn.12097.
Mills JC, Pence BW, Todd JV, Bengtson AM, Breger TL, Edmonds A, et al. Cumulative burden of depression and all-cause mortality in women living with HIV. Clin Infect Dis. 2018;67:1575–81. https://doi.org/10.1093/cid/ciy264.
Greeson JM, Gettes DR, Spitsin S, Dube B, Benton TD, Lynch KG, et al. The selective serotonin reuptake inhibitor citalopram decreases human immunodeficiency virus receptor and coreceptor expression in immune cells. Biol Psychiatry. 2016;80(1):33–9. https://doi.org/10.1016/j.biopsych.2015.11.003.
Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, et al. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62(8):870–7.
Bhagwagar Z, Murthy N, Selvaraj S, Hinz R, Taylor M, Fancy S, et al. 5-HTT binding in recovered depressed patients and healthy volunteers: a positron emission tomography study with [11C]DASB. Am J Psychiatry. 2007;164(12):1858–65.
Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61(12):1271–9.
Shah S, Sinharay S, Lee D, Reid WC, Wakim P, Matsuda K, et al., editors. Longitudinal PET imaging of the serotonergic system in SIV-infected nonhuman primates. Boston: CROI; 2018.
An SF, Giometto B, Groves M, Miller RF, Beckett AA, Gray F, et al. Axonal damage revealed by accumulation of beta-APP in HIV-positive individuals without AIDS. J Neuropathol Exp Neurol. 1997;56(11):1262–8.
Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65(1):29–33.
Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111(6):529–38. https://doi.org/10.1007/s00401-006-0037-0.
Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS (London, England). 2005;19(4):407–11.
Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS (London, England). 2012;26(18):2327–35. https://doi.org/10.1097/QAD.0b013e32835a117c.
Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65(9):1490–2. https://doi.org/10.1212/01.wnl.0000183293.95787.b7.
Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73(23):1982–7. https://doi.org/10.1212/WNL.0b013e3181c5b445.
Krut JJ, Zetterberg H, Blennow K, Cinque P, Hagberg L, Price RW, et al. Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol. 2013;260(2):620–6. https://doi.org/10.1007/s00415-012-6688-y.
Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207(11):1703–12. https://doi.org/10.1093/infdis/jit088.
Steinbrink F, Evers S, Buerke B, Young P, Arendt G, Koutsilieri E, et al. Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur J Neurol. 2013;20(3):420–8. https://doi.org/10.1111/ene.12006.
Villemagne VL, Dore V, Burnham SC, Masters CL, Rowe CC. Imaging tau and amyloid-beta proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol. 2018;14(4):225–36. https://doi.org/10.1038/nrneurol.2018.9.
Ortega M, Ances BM. Role of HIV in amyloid metabolism. J Neuroimmune Pharmacol. 2014;9(4):483–91. https://doi.org/10.1007/s11481-014-9546-0.
Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer’s disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009;4(2):200–12. https://doi.org/10.1007/s11481-008-9136-0.
Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS (London, England). 2005;19(2):127–35.
Ubhi K, Masliah E. Alzheimer’s disease: recent advances and future perspectives. J Alzheimer's Dis. 2013;33(Suppl 1):S185–94. https://doi.org/10.3233/jad-2012-129028.
Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang S, Marshak DR, et al. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer’s disease. J Neuropathol Exp Neurol. 1994;53(3):231–8.
Brown LA, Scarola J, Smith AJ, Sanberg PR, Tan J, Giunta B. The role of tau protein in HIV-associated neurocognitive disorders. Mol Neurodegener. 2014;9:40. https://doi.org/10.1186/1750-1326-9-40.
Cooley SA, Strain JF, Beaumont H, Boerwinkle AH, Doyle J, Morris JC et al. Tau positron emission tomography binding is not elevated in HIV-Infected individuals. J Infect Dis. 2018. https://doi.org/10.1093/infdis/jiy663.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies with human subjects performed by the authors have been previously published and complied with all applicable ethical standards. All reported studies with animal subjects performed by the authors were approved by the Institutional Animal Care and Use Committee of the National Institutes of Health and were performed in accordance with the guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Central Nervous System and Cognition
Rights and permissions
About this article
Cite this article
Sinharay, S., Hammoud, D.A. Brain PET Imaging: Value for Understanding the Pathophysiology of HIV-associated Neurocognitive Disorder (HAND). Curr HIV/AIDS Rep 16, 66–75 (2019). https://doi.org/10.1007/s11904-019-00419-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-019-00419-8