Abstract
Over the past decade, scientific advancements have resulted in improved survival from acute leukemia. Continued advancements are expected given the attention to precision medicine and the resulting growth in development and adoption of risk-stratified, personalized therapies. While precision medicine has great potential to improve acute leukemia outcomes, there remain significant barriers to ensuring equitable access to these technologies and receipt of these prescribed targeted, personalized therapies. Over the past 3 years, studies report persistent outcome disparities among patients from specific racial and ethnic backgrounds, insurance and socioeconomic status, and other socio-demographic factors after a diagnosis of acute leukemia. A few recent studies examine etiologies for acute leukemia disparities and highlight the importance of ensuring access and equitable delivery of scientific advancements. In the context of continued scientific progress, future strategies require thoughtfully considered improvements in the delivery of care that can overcome the current challenges our patients face.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2015, the Institute of Medicine (IOM) convened a workshop regarding policy issues in the development and adoption of biomarkers for molecularly targeted cancer therapies, otherwise known as precision medicine, and currently efforts are actively underway to promote these technological, personalized advances [1••, 2]. Precision medicine holds great promise to improve cancer outcomes as the premise relies on risk-stratification of disease management either through the testing of biomarkers to identify proper therapy or recognizing genes linked to drug response [1••, 2]. Personalized and risk-based stratifications have long been used to optimize treatments for a wide array of cancer diagnoses. Such agents as imatinib, used in treatment of patients with chronic myeloid leukemia [3], traztuzumab, used for treatment of patients with breast cancer expression of Her2-neu [4], and erlotinib, used in non-small cell lung cancers that harbor epidermal growth factor receptor-activating mutations [5], are but a few older examples of therapeutic, precision-medicine discoveries that have resulted in dramatic outcomes for patients with these select molecular alterations in their tumors. Over the past 2 years, there have been continued rapid development and approval of newer targeted drugs for biomarker aberrations [6].
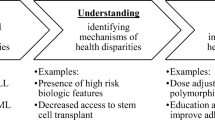
While these advancements have great potential to provide more effective treatment resulting in improved outcomes, continued barriers exist in equitable access and delivery of these scientific achievements given inequitable distribution of very basic, long-standing evidence-based therapies [7••, 8–11]. Figure 1 from Kilbourne et al. demonstrates a conceptual model for understanding the origins of health and health care disparities from a health services research perspective. This figure demonstrates the underlying individual, provider, and organizational/systems-level factors that contribute to health outcomes [12]. The IOM and the American Society of Clinical Oncology highlight similar challenges such as economic factors, cultural factors, and geographic diversity that may hinder public availability of the benefits that precision medicine has to offer [1••, 13]. To overcome these barriers, the IOM recommends to “promote equity in access to biomarker tests for molecularly targeted therapies and the expertise for effective use of the results in clinical decision making” [1••].
Understanding the origins of health and health care disparities from a health services research perspective (Adapted from Kilbourne et al. 2006 [12])
Among patients with cancer, studies, including our own work, over the past two decades consistently show that despite access to our currently available risk-stratification testing techniques, such as cytogenetic testing among patients with acute leukemia, survival disparities persist for patients from specific racial, ethnic, and socio-demographic backgrounds [7••, 14, 15••]. Furthermore, despite access to risk-stratification testing among these patient populations, as our previous studies show, discrepancies remain in ensuring receipt of treatments that target these actionable molecular and cytogenetic factors [15••]. Acute leukemia disparities research specifically confirms the concerns raised regarding inherent challenges of precision medicine to improve outcomes equitably. Below are key recent works published within the past 2 years that highlight persistent acute leukemia disparities despite recent scientific advancements. The articles highlight potentially widened disparities gaps in these heterogeneous diseases. We report on studies previously published and therefore did not conduct studies with human or animal subjects for this review.
Recent Advancements in Acute Leukemia
Acute leukemia, a group of heterogeneous diseases characterized by rapidly proliferating clonal malignancies, is highly responsive to chemotherapy, but fatal if not treated timely [16]. Scientific advancements in leukemia-directed treatments and supportive care, which improve tolerability to treatments such as chemotherapy and hematopoietic stem cell transplantation, have steadily increased survival from these diseases [17]. Risk-stratification advancements, such as identification of karyotype abnormalities and molecular aberrations, have led to prognostic-based treatment decisions that have also resulted in improved outcomes [18]. Recently, novel chemotherapeutics and combinations, such as chimeric antigen receptor-modified T cells [19, 20] and clofarabine [21], less toxic treatment protocols [22], and improved hematopoietic stem cell transplantation techniques [23] [24], have also improved outcomes from acute leukemia. Future progress in acute leukemia is inevitable given the attention to precision medicine and other advances that will greatly expand the identification of prognostic features and direct our treatment recommendations.
Scientific Advancements in Acute Leukemia May Not Reach Everyone
Over the past two decades, some patient populations have witnessed an increased overall increased survival from acute leukemia, while other patients, namely those from specific racial and ethnic backgrounds, continue to experience disparate outcomes [7••, 14, 15••, 25••, 26••]. These outcome disparities are similarly reported for patients with other cancer diagnoses [27], suggesting more systems-level barriers, such as inequitable delivery of acute leukemia advancements [7••]. Less access to highly conventional therapies that improve outcomes in clinical trials [28•], insurance status [29], and other socioeconomic challenges repeatedly are shown to impact survival outcomes among patients diagnosed with these and other malignancies [30, 31].
Recent Studies on Disparities Among Pediatric Patient Populations
Among the pediatric patient population, disparities have been extensively described. A study published within the past year showed that non-Hispanic black, Hispanic, and Asian children experienced worse outcomes after a diagnosis of acute leukemia as compared to non-Hispanic white children [26••]. The study demonstrated the influence of socioeconomic status on outcomes, showing that children living in low socioeconomic status neighborhoods also experienced disproportionately lower survival from acute leukemia as compared to children living in high socioeconomic status neighborhoods. Although the authors state that this study highlights the influence of precision medicine in helping to identify genomic predictors of racial and ethnic disparities, the concerns raised previously regarding the positive influence of precision medicine on disparities are again highlighted here. This particular study, along with the many others that describe disparate outcomes in acute leukemia, do not evaluate the etiology of these disparities, such as the influence of receipt of treatment on the disparities revealed [25••, 26••]. How can newer advances in treatment for these patient populations help to eliminate these disparities if there are other inherent systems-level factors that may be associated with the disparate outcomes the authors report? Identification of potential modifiable factors that contribute to these disparities could greatly help to validate the authors’ conclusions regarding the impact precision medicine has on improving acute leukemia outcomes equitably [7••].
Disparities Are Persistent Among Adult Populations With Acute Leukemia
Unlike studies from the pediatric population, as we have previously reported, there are few studies describing disparities among adults with acute leukemia [14]. Recently, a few studies reported survival disparities among non-Hispanic black and Hispanic adults as compared to patients from other racial and ethnic populations, corroborating earlier data [7••, 14, 15••, 25••]. Although survival advancements have improved among adults with acute leukemia, these advances do not equitably distribute across all populations. A recent study showed persistent disparities in survival over the past decade for adult minority populations as compared to non-Hispanic white patients [25••]. While non-Hispanic white patients were found to have increasingly improved survival over the past 10 years, these improvements in survival were not consistent among all patient populations and disproportionately less so for Hispanic and non-Hispanic black patients with acute lymphocytic leukemia (ALL). Among patients with acute myeloid leukemia (AML), survival increased substantially only for non-Hispanic white and Asian Pacific Islander populations [25••], thus widening the reported disparities gap in these diseases.
In our previous work, to uncover potential etiologies for worse survival outcomes among non-Hispanic black and Hispanic patients, we examined whether these populations have a higher proportion of poor-risk factors at diagnosis. Increasing age and specific cytogenetic abnormalities have consistently been shown to predict worse outcomes among patients in older [32, 33] and more recent studies conducted over the past 2 years [15••, 25••]. In our work, published last year, despite a higher proportion of better prognostic factors at diagnosis, survival disparities persisted for non-Hispanic black and Hispanic patients as compared to non-Hispanic white patients [15••]. In ours and other studies, minority populations are more likely to be diagnosed with acute leukemia at a younger ages, a positive prognostic factor [15••, 25••]; however, these patients continue to have worse survival outcomes as compared to non-Hispanic white patients after a diagnosis of both ALL and AML [14, 25••].
Studies report similar findings in terms of other prognostic factors, such as cytogenetic abnormalities. Identification of cytogenetic subtypes through technologies such as karyotype is one of the most powerful prognostication tools in acute leukemia and can direct personalized, risk-adapted treatment approaches [34]. For example, identification of the core-binding factor leukemias such as t(8;21), through karyotype, has directly resulted in favorable treatment decisions, with patient receiving more doses of cytarabine therapy, thereby resulting in improved outcomes [35]. Similarly, patients with the identified acute promyelocytic leukemia (APL) subtype have benefitted from better survival outcomes due to targeted treatment with all-trans retinoic acid (ATRA), a therapy specifically indicated for patients with this cytogenetic subtype [36]. In our previous work, among other studies, we showed that these cytogenetic AML subtypes are independently associated with improved survival [15••]. We also showed that non-Hispanic black and Hispanic patients, when diagnosed with AML, have a higher proportion of these cytogenetic subtypes (i.e., t(8;21) and APL). Despite the higher proportion of these favorable subtypes among non-Hispanic black and Hispanic patients, the survival disparities we previously described previously persisted [15••].
To evaluate whether systems-level factors may contribute to these continued acute leukemia outcome disparities among these populations, given that these populations had higher proportion of positive prognostic factors at diagnosis, we examined whether these populations faced discrepancies in the receipt of evidence-based treatment and the impact of treatment discrepancies on AML survival outcomes [7••]. In a work published this past year, we revealed that Hispanic and non-Hispanic black populations have lower receipt of evidence-based treatment, including both chemotherapy and hematopoietic stem cell transplant. Other studies have similarly shown systems-level factors influencing receipt of hematopoietic stem cell transplant [37]. However, when high quality care is delivered, including targeted treatment when indicated, survival outcomes are equalized among all racial and ethnic populations [7••]. The impact of high quality care delivery on outcomes has also been shown among pediatric populations [38]. Despite targeted therapies conferring better outcomes among patients with these cytogenetic subtypes [36], studies, including ours [7••, 39], demonstrate the consistent inequitable distribution of these evidence-based strategies.
Despite availability of already established personalized, precision medicine techniques, such as ATRA for APL, disparities remain in how these therapies are deployed to the subpopulations of patients who may derive the most benefit. Furthermore, other modifiable factors, such as improving differential adherence to treatment [40], can also ensure that these technological advances are complied with to achieve equitable outcomes. Unless scientific advancements are implemented with assurance that all patients can reap the benefits of appropriate, high quality, evidence-based, and personalized treatments, the acute leukemia disparity gaps, that others and we repeatedly show, have immense potential to widen.
Cost Implications of Precision Medicine
In the setting of rising health expenditures, and as with many other technological advances, costs of novel approaches to improving care are often another challenge to ensuring equitable access to these therapies. Although outcomes are expected to improve with these personalized therapies with a potential for reduction in downstream healthcare expenditures, the associated high costs of these treatments remain an overwhelming challenge. As technology advances, however, many stakeholders believe that the costs of these advances will significantly decrease, as exemplified with rapid whole genome sequencing where costs, initially nearly $3 billion, have now decreased due to recent developments to approximately $1000 [41]. Skeptics remain concerned, given other examples, such as the OncoType DX 21-gene assay for estrogen-receptor positive breast cancer, which continues to cost nearly $4000 [42]. While paid for by insurance companies, due to a potential downstream reduction in chemotherapy costs for approximately 16 % of patients with early stage diseases [43], the cost of this technology prohibits equitable access to all patients [44]. The continued access disparities due to costs lead to a widening of outcome disparities gaps among patients. Given these implications of potential widening of disparities gaps due to costs and access to these novel advances, President Obama suggested during the White House Precision Medicine Initiative Summit on February 25, 2016, that all-stakeholder approaches are needed to reduce the costs and ensure equitable access to these novel advances. Greater attention to the balance of costs and quality requires collaborations between the public and private sectors, payers, and providers alike. Alternative payment models are one way that stakeholders can collaborate to ensure improved outcomes and access to technology. These models incentivize the use of care protocols, clinical pathways, and other clinical decision support tools such as those offered by precision medicine, to improve population-based outcomes. Efforts by advocacy groups, such as the Personalized Medicine Coalition, are another way to encourage continued education regarding the benefits of personalized medicine and advocacy of public policies and reimbursement that encourage investment in these technological advances that, if equitably implemented and accessible, can improve outcomes for all.
Conclusions
While impressive therapeutic advancements continue in acute leukemia, resulting in improved survival rates after diagnosis, striking outcomes disparities remain by race and ethnicity and socioeconomic status. Fair access to healthcare for acute leukemia continues to be challenged by socioeconomic status, insurance status, and the locations in which care is delivered [30]. Among populations with acute leukemia, previously and recently published research consistently demonstrate inequitable distribution of our current evidence-based treatments [7••, 26••, 29]. In the context of precision medicine, which holds great promise for improving risk-stratification evidence-based treatments, among patients with acute leukemia and other cancers, questions remain regarding how these personalized treatment advances may influence disparities. The recent studies published over the past 2 years, showing persistent disparities in acute leukemia despite scientific advancements in survival outcomes overall, validate the grave concerns previously raised regarding the influence of precision medicine to widen disparities gaps.
The fundamental linkage of personalized treatments and cancer disparities should, therefore, be at the forefront of our research agenda, especially as scientific advancements are made in acute leukemia treatments. Research questions should help to address how to ensure equitable dissemination of precision, personalized medicine. How can we ensure fair reach of scientific advancements to all populations, specifically among those who are currently underserved? As demonstrated in the studies highlighting persistent disparities in acute leukemia, population-wide access to provision of standard evidence-based treatments is unattainable presently. The current challenges in equitable delivery of high quality care raise many questions regarding how we can sustainably and appropriately overcome barriers in access, capital, health literacy, and other socio-demographic features that influence appropriate receipt of our evidence-based treatment options. How can we ensure equitable availability and application of precision medicine, and, further, ensure that the targets identified by precision medicine will result in appropriate receipt of the indicated treatments?
The IOM recommendations are but one step in raising awareness of the influence of precision medicine to further widened our already established disparities gaps [1••]. The passing of the Affordable Care Act is also an important component that can improve access and serves as a giant step forward in the journey to eliminating disparities [45]. Despite these advances, however, an all-stakeholder approach is needed to truly realize the elimination of cancer disparities through equitable distribution of our basic, standard of care and newer scientific advancements. Emerging alternative payment models that reward population-based, appropriate, equitable, high-value treatment, such as the ones proposed by Medicare [46], can financially align application of scientific advancements to eliminate disparities. Likewise, research priorities with aligned funding and supportive infrastructure can also help us to rigorously move from merely describing disparities to the identification of interventions that can modify the etiologies. The elimination of health disparities, especially among patients with acute leukemia, will take a village of individuals who vigilantly seek to deeply understand current challenges in equitable population-wide distribution of appropriate treatment and who will lead thoughtful interventions and influence policy to improve equitable access and implementation of scientific advancements for all patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
National Academies of Sciences, Engineering, and Medicine. Biomarker tests for molecularly targeted therapies: key to unlocking precision medicine. Washington, DC: The National Academies Press; 2016. doi:10.17226/21860. This report by the Institute of Medicine convening describes the benefits and challenges of precision medicine with recommendations of how to address future clinical practice, regulatory and reimbursement policy, and data challenges.
Precision Medicine Initiative Cohort Program. https://www.nih.gov/precision-medicine-initiative-cohort-program. Accessed February 22 2016.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17. doi:10.1056/NEJMoa062867.
Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi:10.1056/NEJMra043186.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32. doi:10.1056/NEJMoa050753.
Hematology/Oncology (Cancer) Approvals and Safety Notifications. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. Accessed February 22 2016.
Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344–9. doi:10.1158/1055-9965.EPI-14-0963. Using the California Cancer Registry linked to hospital discharge abstracts from the Office of Statewide Health Planning and Development, investigators demonstrated lower receipt of treatment among non-Hispanic black and Hispanic as compared to non-Hispanic white patients. Treatment receipt among these populations was associated with equitable survival outcomes.
Rhoads KF, Patel MI, Ma Y, Schmidt LA. How do integrated health care systems address racial and ethnic disparities in colon cancer? J Clin Oncol. 2015;33(8):854–60. doi:10.1200/JCO.2014.56.8642.
Rhoads KF, Ackerson LK, Ngo JV, Gray-Hazard FK, Subramanian SV, Dudley RA. Adequacy of lymph node examination in colorectal surgery: contribution of the hospital versus the surgeon. Med Care. 2013;51(12):1055–62. doi:10.1097/MLR.0b013e3182a53d72.
Morris AM, Billingsley KG, Baxter NN, Baldwin LM. Racial disparities in rectal cancer treatment: a population-based analysis. Arch Surg. 2004;139(2):151–5. doi:10.1001/archsurg.139.2.151. discussion 156.
Revels SL, Morris AM, Reddy RM, Akateh C, Wong SL. Racial disparities in esophageal cancer outcomes. Ann Surg Oncol. 2013;20(4):1136–41. doi:10.1245/s10434-012-2807-3.
Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113–21. doi:10.2105/AJPH.2005.077628.
American Society of Clinical, O. (2016). The State of Cancer Care in America, 2016: a report by the American Society of Clinical Oncology. J Oncol Pract, doi:10.1200/JOP.2015.010462.
Patel MI, Ma Y, Mitchell BS, Rhoads KF. Understanding disparities in leukemia: a national study. Cancer Causes Control. 2012;23(11):1831–7. doi:10.1007/s10552-012-0062-3.
Patel MI, Ma Y, Mitchell BS, Rhoads KF. Age and genetics: how do prognostic factors at diagnosis explain disparities in acute myeloid leukemia? Am J Clin Oncol. 2015;38(2):159–64. doi:10.1097/COC.0b013e31828d7536. Using the Surveillance, Epidemiology, and End Results Database, investigators identified that non-Hispanic black and Hispanic patients are diagnosed at younger ages and with more favorable cytogenetic subtypes.
Overview of leukemia. http://www.merckmanuals.com/professional/hematology-and-oncology/leukemias/overview-of-leukemia. Accessed Feb 22 2016.
Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007;110(2):695–708. doi:10.1182/blood-2006-11-051672.
Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78. doi:10.1016/j.cell.2012.06.023.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi:10.1056/NEJMoa1215134.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi:10.1056/NEJMoa1407222.
Burnett AK, Russell NH, Hunter AE, Milligan D, Knapper S, Wheatley K, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122(8):1384–94. doi:10.1182/blood-2013-04-496596.
Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):61–73. doi:10.1016/j.pcl.2014.09.006.
Kalashetty M, Dalal B, Roland K, Mourad Y, Barnett M, Broady R, et al. Improved survival in adults with mixed-phenotype acute leukemia following stem cell transplantation (SCT): a single centre experience. Blood. 2013;21:122.
Kreutzman A, Ilander M, Porkka K, Vakkila J, Mustjoki S. Dasatinib promotes Th1-type responses in granzyme B expressing T-cells. Oncoimmunology. 2014;3, e28925. doi:10.4161/onci.28925.
Pulte D, Redaniel MT, Jansen L, Brenner H, Jeffreys M. Recent trends in survival of adult patients with acute leukemia: overall improvements, but persistent and partly increasing disparity in survival of patients from minority groups. Haematologica. 2013;98(2):222–9. doi:10.3324/haematol.2012.063602. Investigators used the Surveillance Epidemiology and End Results database to describe survival trends for patients with acute leukemia. Investigators reported the improved survival for non-Hispanic white patients after a diagnosis of acute leukemia with a smaller increase in survival among patient populations from other racial and ethnic backgrounds.
Abrahao R, Lichtensztajn DY, Ribeiro RC, Marina NM, Keogh RH, Marcos-Gragera R, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988–2011: a population-based observational study. Pediatr Blood Cancer. 2015;62(10):1819–25. doi:10.1002/pbc.25544. Investigators used the California Cancer Registry to evaluate outcomes among patients ages 0–19 years to evaluate the influence of neighborhood socioeconomic status on survival outcomes. Investigators showed an association of increased risk of death among children living in low socioeconomic status neighborhoods as compared to children living in higher socioeconomic status neighborhoods.
Cancer facts & figures (2015). http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Accessed Feb 22 2016.
Abrahao R, Ribeiro RC, Medeiros BC, Keogh RH, Keegan TH. Disparities in early death and survival in children, adolescents, and young adults with acute promyelocytic leukemia in California. Cancer. 2015;121(22):3990–7. doi:10.1002/cncr.29631. Investigators used the California Cancer Registry to describe 7-day and 30-day mortality among patients diagnosed with acute leukemia ages 0 to 39 and show that patients with lack of insurance had a higher early death and lower survival.
Borate UM, Mineishi S, Costa LJ. Nonbiological factors affecting survival in younger patients with acute myeloid leukemia. Cancer. 2015;121(21):3877–84. doi:10.1002/cncr.29436.
Yung RL, Chen K, Abel GA, Gesten FC, Roohan PJ, Boscoe FP, et al. Cancer disparities in the context of Medicaid insurance: a comparison of survival for acute myeloid leukemia and Hodgkin’s lymphoma by Medicaid enrollment. Oncologist. 2011;16(8):1082–91. doi:10.1634/theoncologist.2011-0126.
Brunner AM, Blonquist TM, Sadrzadeh H, Perry AM, Attar EC, Amrein PC, et al. Population-based disparities in survival among patients with core-binding factor acute myeloid leukemia: a SEER database analysis. Leuk Res. 2014;38(7):773–80. doi:10.1016/j.leukres.2014.04.001.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5. doi:10.1182/blood-2005-09-3724.
Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051–62. doi:10.1056/NEJM199909303411407.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–65. doi:10.1182/blood-2009-11-254441.
Byrd JC, Dodge RK, Carroll A, Baer MR, Edwards C, Stamberg J, et al. Patients with t(8;21)(q22;q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J Clin Oncol. 1999;17(12):3767–75.
Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021–8. doi:10.1056/NEJM199710093371501.
Pidala J, Craig BM, Lee SJ, Majhail N, Quinn G, Anasetti C. Practice variation in physician referral for allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(1):63–7. doi:10.1038/bmt.2012.95.
Pui CH, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol. 2012;30(16):2005–12. doi:10.1200/JCO.2011.40.8617.
Patel MI, Rhoads KF. Integrated health systems and evidence-based care: standardizing treatment to eliminate cancer disparities. Future Oncol. 2015;11(12):1715–8. doi:10.2217/fon.15.90.
Bhatia S, Landier W, Shangguan M, Hageman L, Schaible AN, Carter AR, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol. 2012;30(17):2094–101. doi:10.1200/JCO.2011.38.9924.
Mardis ER. Anticipating the 1,000 dollar genome. Genome Biol. 2006;7(7):112. doi:10.1186/gb-2006-7-7-112.
Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11(5):313–24.
McVeigh TP, Hughes LM, Miller N, Sheehan M, Keane M, Sweeney KJ, et al. The impact of Oncotype DX testing on breast cancer management and chemotherapy prescribing patterns in a tertiary referral centre. Eur J Cancer. 2014;50(16):2763–70. doi:10.1016/j.ejca.2014.08.002.
Jasem, J., Amini, A., Rabinovitch, R., Borges, V. F., Elias, A., Fisher, C. M., et al. (2016). 21-Gene recurrence score assay as a predictor of adjuvant chemotherapy administration for early-stage breast cancer: an analysis of use, therapeutic implications, and disparity profile. J Clin Oncol, doi:10.1200/JCO.2015.65.0887.
Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the Affordable Care Act. Med Care. 2016;54(2):140–6. doi:10.1097/MLR.0000000000000467.
Burwell SM. Setting value-based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–9. doi:10.1056/NEJMp1500445.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Health Economics
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 265 kb)
Rights and permissions
About this article
Cite this article
Patel, M.I. Scientific Achievements May Not Reach Everyone: Understanding Disparities in Acute Leukemia. Curr Hematol Malig Rep 11, 265–270 (2016). https://doi.org/10.1007/s11899-016-0329-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-016-0329-y