Abstract
Purpose
Disparities in solid tumors have been well studied. However, disparities in hematologic malignancies have been relatively unexplored on population-based levels. The purpose of this study is to examine the relationship between race/ethnicity and acute leukemia mortality.
Methods
All patients with acute leukemia [acute lymphoblastic leukemia (ALL) and acute myelogenous leukemia (AML)] were identified in the Surveillance Epidemiology and End Results database, 1999–2008. Kaplan–Meier curves were generated to reflect survival probabilities by race/ethnicity. Multivariable Cox proportional hazard models estimated hazard of mortality by race with adjustment for individual (age, gender, year of diagnosis) and select genetic factors.
Results
A total of 39,002 patients with acute leukemia were included in the study. Overall, there was a mortality disparity in acute leukemia for blacks (HR 1.17, p < 0.0001) and Hispanics (HR 1.13, p < 0.0001) compared with non-Hispanic whites. In stratified analysis, disparities in ALL were greater than AML; blacks (HR[ALL]1.45, p < 0.0001; HR[AML]1.12, p < 0.0011); Hispanics (HR[ALL]1.46, p < 0.0001; HR[AML]1.06, p < 0.0001). Adjustment for individual patient and select genetic factors did not explain disparities.
Conclusions
Blacks and Hispanics suffer decreased survival in acute leukemia as compared to others. Further investigation is needed to understand the drivers of poor cancer outcomes in these populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute leukemia is a malignant disorder resulting in impairment of blood cell production and unrestrained proliferation of immature blood cells. The disease is sub-classified into two broad categories: acute myeloid leukemia (AML) which accounts for 12,330 new cases each year and acute lymphoblastic/lymphocytic leukemia (ALL) which accounts for about 5,330 new cases each year [1]. Disparities in cancer are well documented in the literature, and the elimination of these differences has become an important part of the national health agenda [2–12]. Although disparities in solid tumors such as lung, breast, and colorectal cancers have been well studied [3–5], racial-/ethnically based outcomes in hematologic malignancies, especially acute leukemia, have received much less scholarly attention.
Disparities in acute leukemia mortality have been previously described in the pediatric literature. These studies have shown that black and Hispanic children have worse outcomes when compared with whites [6–12]. There are a handful of studies in the adult population assessing outcomes in acute leukemia by race/ethnicity, and these reveal conflicting results [10, 13–19]. While some studies demonstrate disparities [10, 13, 15, 16], others do not [15, 16, 18]. All of these studies are limited in the use of relatively small datasets. A recent study examined outcomes in one subtype of acute leukemia for children less than 20 years of age and demonstrated worse outcomes among black and Hispanic children as well as among some Asian subgroups [12]. The study, however, only focused on one subtype of acute leukemia and limited analysis to the pediatric population. No studies to date have assessed cohorts large enough to distinguish the outcomes between subtypes as well as differences in outcome for both children and adults with both subtypes of the disease.
Disparities in cancer are thought to result from a complex interplay of factors. This has been demonstrated in studies of solid tumor outcomes [3–5, 22, 24, 25]. In order to address disparities in acute leukemia, we need a better understanding of the factors that influence outcomes by race. The purpose of the current investigation is to use a national database to characterize differences in outcomes in AML versus ALL and to describe the relationship between race/ethnicity and mortality in all patients with both subtypes of acute leukemia. Given that the treatment course for ALL is more intensive than for AML [1], the hypothesis is that overall outcomes and disparities are worse for ALL as compared with AML; the secondary hypothesis is that racial/ethnic minorities suffer worse outcomes than whites with both subtypes of disease.
Methods
Sources of data
After obtaining approval from the Stanford University Institutional Review Board, data from SEER 17 database were obtained through enrollment in the SEER*Stat program, Version 4.2 (NCI, Bethesda, MD; last accessed 7/5/11). Data contained in the SEER database is sampled data from 17 registries across the United States capturing a little more than 25 % of the US patient population [20]. The set includes information about new malignancies, basic clinical data, and patient demographic information.
Cases of acute leukemia were identified by International Classification of Disease-Oncology 3rd edition (ICD-O3) codes. Acute lymphoblastic leukemia codes: 9727, 9728, 9729, 9835, 9836, 9837, 9805 identified patients with ALL; and acute myelogenous leukemia codes 9840, 9861, 9866–9867, 9870–9874, 9891, 9895–9897, 9910, 9931, 9930, 9920 identified patients diagnosed with AML during the years between 1999 and 2008, inclusive.
In addition to primary diagnosis, the dataset contains demographic (race/ethnicity, gender, age at the time of diagnosis, and year of diagnosis) and other clinical predictor variables (date at last follow-up, the presence of specific cytogenetic factors). Genetic mutations t(8;21) and t(11q23), and the acute promyelocytic leukemia (APL) subtype of acute myelogenous leukemia were the genetic mutations available in SEER and also identified by ICD-O3 code. Records with missing race/ethnicity data and those with American Indian/Alaskan Native (AI/AN) race were excluded from analysis due to small numbers and potentially unreliable estimates. Records with incomplete categorization of acute leukemia were also excluded. The primary outcome for the study was overall survival.
Data analysis
SAS software, version 9.2 (Cary, NC. March 2008), was used to perform all analyses. Chi-squared analysis was used to compare distribution of ALL, AML, and genetic translocations across demographic categories. Kaplan–Meier survival curves were generated to compare survival across various subgroups (race/ethnicity, age) and to compare survival in each subtype of acute leukemia. The Kaplan–Meier curves capture patients up to 10 years after diagnosis.
Cox proportional hazard models were constructed to predict the hazard of 1- and 5-year mortality. The models were adjusted for age, gender, and year of diagnosis. Year of diagnosis was included in the models to account for historical changes in treatment protocols that may confer improved outcome for those treated after 1995 [20]. After assessing baseline mortality risk, the models were further adjusted for the specified genetic mutations. Patients without death records were censored at the last date of known information regarding the patient (the last date of follow-up). Results were considered significant when point estimates were not equal to 1, the associated confidence intervals excluded 1, and when the associated p value was <0.05. All tests of significance were two-tailed. The proportionality assumption was tested by a thorough analysis of the Schoenfield residuals [21].
Results
Cohort demographics
A total of 40,951 cases of acute leukemia were identified during the years under study. Of those, 1,949 (4.76 %) records were excluded due to unknown race/ethnicity, AI/AN race/ethnicity, and incomplete categorization of subtype of leukemia. A final cohort of 39,002 records were retained for analysis. Among survivors, the median follow-up was 39 months as compared to the entire cohort where follow-up was 8 months.
Table 1 shows the demographics of the study cohort. There were 27,525 (71 %) patients with AML and 11,477 (29 %) patients with ALL. In both subtypes, the majority of patients were male (53.9 % with AML and 56.2 % with ALL). There were differences in the distribution of disease by age group. There were approximately 60 % of patients with AML above age 60, compared to 13 % of patients with ALL. There were a higher proportion of patients with ALL under age 21 (61.8 %) compared with AML (6.7 %). By race, there were a higher proportion of whites with both subtypes of disease (73.6 % of AML and 54.2/5 % of ALL).
Distribution of genetic factors in AML
Table 2 shows the distribution of the genetic factors under study. The most prevalent of the three factors was the APL subtype of AML. Approximately 7 % of patients with AML had this mutation. APL was more prevalent among females (8.1 %) than males (6.7 %). By age, APL was more prevalent in patients younger than 41 (33 %). APL was more prevalent in blacks (8.9 %) and Hispanics (13.4 %), compared to other races/ethnicities.
Translocations (8;21) and (11q23) occurred in 2 % of AML cases. There were no gender-based differences noted for the translocation t(8;21) and t(11q23). These mutations occurred at higher rates in patients less than age 41 and in Asian/Pacific Islanders (API) subgroups.
Leukemia survival by subtype
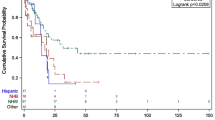
Patients with AML had a worse overall survival than patients with ALL across the entire cohort (Fig. 1). Overall, for all ages, the unadjusted survival in AML was worse for whites (Fig. 2). In comparison with AML, black and Hispanic patients with ALL have worse survival as compared to whites, across all age categories (Fig. 3).
Leukemia mortality and race
Cox Proportional Hazard models, adjusted for gender, age, race/ethnicity, and year of diagnosis, predict the hazard of mortality (HR) in both AML and ALL. Models for 1-year mortality did not differ from models for 5-year mortality (not shown). Table 3 shows estimates of 5-year mortality, as well as stratified outcomes by leukemia subtype. There was a small survival advantage associated with female gender (HR 0.95, 95 % CI 0.93–0.97, p < 0.01). The risk of mortality increased with increasing age. Overall, black race and Hispanic ethnicity were associated with a 17 and 13 % increase risk of death, respectively. There was no significant mortality difference between white and aggregated API populations.
In a subsequent analysis, stratified by gender, age, race, and year of diagnosis, the survival disparity for blacks and Hispanics persisted. However, the degree of disparity between the AML and ALL was marked. Table 3 shows that in comparison with whites, blacks, and Hispanics with AML had a 12 and 6 % increased hazard of death, respectively. The probability of death for these same groups with ALL was nearly 50 % greater than that for whites [(HRblk 1.45; 95 % CIblk 1.28–1.65; HRHisp 1.46; 95 % CIHisp 1.36–1.57)]. There was no significant difference in outcome for API groups as compared to whites.
AML, Genetic mutations, survival, and race
Table 4 shows results of testing the relationship between genetic mutations and survival. There is no significant association between mutation 11q23 and mortality. However, a significant and protective association was noted for t(8;21) (HR 0.75; 95 % CI 0.66–0.86) and t(15;17), APL (HR 0.47; 95 % CI 0.44–0.51). Although a higher percentage of blacks and Hispanics had these positive prognostic genetic mutations, adjustment for these factors did not decrease the high mortality rates for those groups with AML. The mortality disparity in AML was increased for blacks and Hispanics with APL as compared with whites.
Discussion
In the current study, we described the correlation between race/ethnicity and mortality in acute leukemia, with attention to stratified outcomes in two distinct disease subtypes (AML and ALL). This is the first national study to address this question in all populations on a national level. We have several novel findings from this investigation. First, to our surprise, we found that despite the less intensive course of therapy, a diagnosis of AML was associated with decreased overall survival as compared to ALL. We also found a disparity in survival for blacks and Hispanics with both subtypes of disease. Despite the better outcomes overall for patients with ALL, the disparities gap was much wider for patients with a diagnosis of ALL than AML. We also found that despite the higher prevalence of favorable genetic markers in blacks and Hispanics, there was no survival advantage, and no positive impact on disparities by accounting for these factors.
Our study is consistent with previous findings in the pediatric literature that show lower survival after a diagnosis of acute leukemia among black and Hispanic children compared with whites [7, 9, 12]. These results are also consistent with the few studies in the adult literature that find similar survival disparities in this age group [13, 14, 17]. Thus, racial and ethnic outcomes in acute leukemia are similar to those found in solid tumors. Our study has also uncovered an epidemiologic paradox that has been observed in other malignancies. While the incidence of acute leukemia in US black and Hispanic populations is lower relative to whites, the mortality rates are notably higher by comparison. This has been best studied in African American populations with breast cancer [22]. It is unclear from the current study, why this paradox exists.
Our findings are in contrast to studies that do not show a disparity in AML [15] or ALL [23]. Previous studies in acute leukemia largely reflect single-institution experience, in trial-based populations [15], or have focused on outcomes only among children [23]. By comparison, our work is novel because of the use of all-age inclusive national data. In light of our findings at the national level, previous studies that do not show disparities within hospitals may suggest a stronger effect of differences in outcome between hospitals.
Our findings are also novel in that we could not show a significant impact on disparities after adjusting the models for certain genetic factors which are associated with a more positive prognosis. The current findings are somewhat surprising, because despite the higher prevalence of the APL mutation in black and Hispanic subgroups of all ages, no protective effect was noted for either group. This is concerning, in part, because the protective effect is conferred by enhanced susceptibility to appropriate treatment. This may suggest that the treatment of acute leukemia, such as treatment for other cancers (hepatocellular; colorectal, head, and neck), may be delivered differentially to black and Hispanic patients in the United States [22–25].
Limitations
This study has some limitations that should be considered when interpreting the results. First, the study is a retrospective, cross-sectional analysis. As such, we cannot make any claims about causation. There are also limitations related to the use of the SEER database. Because it is a national dataset, assignation of race and ethnicity has not been uniform. There are no current studies critiquing the acquisition of race data in SEER, nor with any methods to impute self-identified race. The SEER set also lacks certain relevant information such as details about treatment and patient level socioeconomic status (SES) information. These data are not routinely collected (treatment data), or are not collected at the patient level. Thus, our ability to further describe the effects of these factors on outcomes in also limited. As with many other cancers, SES is an important factor that has been demonstrated to play a large role in outcomes. This is very important because there is a growing recognition of the conflation of minority race with low SES and that socioeconomic factors alone may explain observed disparities by race/ethnicity. This may also be true for acute leukemia, and therefore, the effect of these factors should be studied in the future.
Patients lost to follow-up in our analysis were 6 % at 1 year and approximately 15 % at 5 years. Hispanic and A/PIs had the highest attrition rates—approximately 4 % higher than whites and blacks at 1 year and 6 % higher at 5 years. In general, the patients who were censored tended to be younger, thus the mortality hazards for Hispanics and A/PIs, who account for many of the younger patients in the cohort, may be slightly overestimated. For blacks and whites, attrition rates were the lowest and therefore the results reported for blacks are not influenced by attrition bias. Despite such attrition rates, the study was still well powered, given the large sample of the study, to detect differences in hazards of mortality between racial/ethnic groups. Further, Schoenfeld residual tests of proportionality showed that the models did not violate the proportionality assumption. For APL, however, the slight violation was minor and has a very minimal impact on the overall estimate considering that APL shows a highly significant difference.
Finally, there may be other important genetic factors in addition to those we analyzed in the current study including NPM1, FLT3, inversion or trisomy 13. These factors were not assessed because currently, they are not routinely collected by SEER registries. Thus, the factors selected for study were limited to those available in SEER and as guided by the World Health Organization classification scheme for acute leukemia. The recorded cases of t(8;21) and t(11q23) were small (2 %) compared to the national reported prevalence (4–8 %) [26, 27]. Capture of cases with APL (7 %) was consistent with previously reported prevalence represented the national epidemiological average (5–7 %). This is a known limitation of the SEER database and calls attention to the need for improved clinical coding in US cancer registry data systems [20, 26, 27].
Conclusions
Despite these limitations, our work is important because while solid tumor disparities have been previously described, there has been little published literature investigating disparities in hematologic malignancies. Our findings emphasize that disparities may not be just clinically driven, but likely represent a complex interplay of factors. This is highly important for future research to investigate the reasons associated with worse outcomes among black and Hispanic populations with acute leukemia.
References
The American Cancer Society www.acs.org. Accessed 18 Nov 2011
The President’s Cancer Panel www.deainfo.nci.nih.gov. Accessed 18 Nov 2011
White A, Vernon SW, Franzini L et al (2011) Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer 116:4622–4631
Vainshtein J (2008) Disparities in breast cancer incidence across racial/ethnic strata and socioeconomic status: a systematic review. J Natl Med Assoc 100:833–839
Underwood JM, Townsend JS, Tai E et al. (2011).Racial and regional disparities in lung cancer incidence. Cancer
Rubnitz JE, Lensing S, Razzouk BI et al (2007) Effect of race on outcome of white and black children with acute myeloid leukemia: the St. Jude experience. Pediatr Blood Cancer 48:10–15
Kadan-Lottick NS, Ness KK, Bhatia S et al (2003) Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA 290:2008–2014
Foucar K, Duncan MH, Stidley CA et al (1991) Survival of children and adolescents with acute lymphoid leukemia. A study of American Indians and Hispanic and non-Hispanic whites treated in New Mexico (1969 to 1986). Cancer 67:2125–2130
Bhatia S, Sather HN, Heerema NA et al (2002) Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood 100:1957–1964
Kent EE, Sender LS, Largent JA et al (2009) Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes Control 20:1409–1420
Aplenc R, Alonzo TA, Gerbing RB et al (2006) Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood 108:74–80
Goggins WB, Lo FF (2012) Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: evidence from the SEER database: 1988–2008. Cancer Causes Control 23:737–743
Pullarkat ST, Danley K, Bernstein L et al (2009) High lifetime incidence of adult acute lymphoblastic leukemia among Hispanics in California. Cancer Epidemiol Biomarkers Prev 18:611–615
Sekeres MA, Peterson B, Dodge RK et al (2004) Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia. Blood 103:4036–4042
Rodriguez CP, Baz R, Jawde RA et al (2008) Impact of socioeconomic status and distance from treatment center on survival in patients receiving remission induction therapy for newly diagnosed acute myeloid leukemia. Leuk Res 32:413–420
Byrne MM, Halman LJ, Koniaris LG et al (2011) Effects of poverty and race on outcomes in acute myeloid leukemia. Am J Clin Oncol 34:297–304
Bradley CJ, Dahman B, Jin Y, et al. (2011) Acute myeloid leukemia: How the Uninsured Fare. Cancer
Brady AK, Fu AZ, Earl M et al (2011) Race and intensity of post-remission therapy in acute myeloid leukemia. Leuk Res 35:346–350
Pulte D, Gondos A, Brenner H (2008) Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica 93:594–600
Surveillance, Epidemiology, and End Results http://www.seer.gov. Accessed 11 Aug 2011
Shoenfield Residuals. Available at http://biomet.oxfordjournals.org/content/69/1/239.short. Accessed 27 July 2012
Gray GE, Henderson BE, Pike MC (1980) Changing ratio of breast cancer incidence rates with age of black females compared with white females in the United States. J Natl Cancer Inst 64:461–463
Wilkinson JD, Fleming LE, MacKinnon J et al (2001) Lymphoma and lymphoid leukemia incidence in Florida children: ethnic and racial distribution. Cancer 91:1402–1408
Zak Y, Rhoads KF, Visser BC (2011) Predictors of surgical intervention for hepatocellular carcinoma: race, socioeconomic status, and hospital type. Arch Surg 146:778–784
Rhoads KF, Cullen J, Ngo JV et al. (2011) Racial and ethnic differences in lymph node examination after colon cancer resection do not completely explain disparities in mortality. Cancer
Caligiuri MA, Stout MP, Gilliland DG (1997) Molecular biology of acute myeloid leukemia. Semin Oncol 24:32–44
National Cancer Institute http://www.cancer.org. Accessed 11 Aug 2011
Jaffe ES, Harris NL, Diebold J, Muller-Hermelink HK (1998) World Health Organization classification of lymphomas: a work in progress. Ann Oncol 9(Suppl 5):S25–S330
Acknowledgments
Our manuscript contains original data. There was full access to all the data in the study, and all authors take full responsibility for the integrity of the data and the accuracy of the data analysis. There are no conflicts of interest to disclose for any author listed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, M.I., Ma, Y., Mitchell, B.S. et al. Understanding disparities in leukemia: a national study. Cancer Causes Control 23, 1831–1837 (2012). https://doi.org/10.1007/s10552-012-0062-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-012-0062-3