Abstract
Purpose of Review
The aim of this review is to summarize the current literature on pediatric leukemia disparities with attention to not only racial and ethnic disparities, but also socioeconomic disparities. We focus on disparities in survival as well as other health-related outcomes, including end-of-life care and late effects.
Recent Findings
While progress has been made in decreasing some disparities, most notably in pediatric acute lymphoblastic leukemia, disparities along many axes persist. Proposed etiologies include differences in the genomic alterations of the leukemia itself to differences in access to care that operate through socioeconomic status, insurance, and geographic location.
Summary
As approaches to therapy become increasingly technical and complex, particular attention to the equitable distribution of these personalized therapeutic interventions is essential. Moving beyond simple descriptive studies to focus on mechanisms of existing disparities will allow for design of interventions to reduce or eliminate disparities in pediatric leukemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leukemia is the most common cancer in children and adolescents, accounting for almost 1 out of 3 pediatric cancers [1]. Despite tremendous progress in improving leukemia survival, a subset of patients will succumb to their disease [2]. Similarly, many patients will experience morbidity secondary to the treatments required to achieve and maintain remission as well as morbidity secondary to the leukemia itself. Unfortunately, leukemia morbidity and mortality is unevenly distributed with some groups affected differentially.
The study of these differential health-related outcomes among certain disadvantaged groups is the subject of this review. For the purposes of this review, we use the definition of health disparities put forth by the Healthy People 2020 subcommittee, in which progress towards the goal of health equity is measured by eliminating health disparities. Health disparities are “systematic, plausibly avoidable health differences adversely affecting socially disadvantaged groups… focus[ed] on the subset of health differences reflecting social injustice, distinguishing health disparities from other health differences” [3].
Most typically, the term “health disparities” is used to refer to racial or ethnic differences, whereas internationally the term “health inequalities” refers to differences operating along the socioeconomic axis. This difference between international and American terminology is likely rooted in the history of institutional racism and structural violence that is embedded in life in the USA. This storied history manifests in the remarkably increased risk of morbidity and mortality along racial lines from every leading cause including cardiovascular disease, stroke, diabetes, cancer, preterm delivery, low birth weight, and asthma [4]. It is essential to recognize that most studies use race as a proxy for a combination of environmental, behavioral, and genetic factors [5]. Unless explicitly defined as genetic ancestry using biologic data, we consider race to be a complex, heterogeneous, fluid sociocultural construct.
While much of the existing literature has focused on racial and ethnic disparities, we will review disparities along additional axes of social disadvantage, including socioeconomic status, insurance status, age, and location as they relate to access to care (as shown in Table 1). Socioeconomic status (SES), while often closely correlated with race and ethnicity, is itself a multifaceted concept that is comprised of factors such as income, education, and employment [6].
We will focus our attention first on survival outcome disparities and then subsequently delineate other health-related outcome disparities, including late effects. Differences in disease incidence and treatment will only be addressed as they relate to disparities in health-related outcomes. We will then briefly explore proposed etiologies and potential interventions resulting from patient, provider, and system-level factors in the discussion [7].
Survival
Acute Lymphoblastic Leukemia (ALL)
As the most common pediatric malignancy (representing 20% of all pediatric cancers) [1], acute lymphoblastic leukemia (ALL) survival disparities are among the most well studied. Black, Hispanic, and Native American children with ALL have all historically been shown to have lower overall survival compared to White non-Hispanic patients [8,9,10].
In a recent study using the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database, Black (relative risk (RR) 1.8; 95% CI 1.5, 2.2), Hispanic (RR 1.9; 95% CI 1.7, 2.1), and Native American (RR 2.26; 95% CI 1.51, 3.38) children continued to have significantly inferior survival compared to White non-Hispanic patients [11]. They also found that most East Asian Americans, particularly those of Vietnamese descent had significantly worse survival (RR 2.4; 95% CI 1.5, 4.0), with the exception of Chinese patients [11]. These nuanced findings among patients of Asian ancestry highlight the heterogeneity within racial groups and the impact of grouping dissimilar patients into a single category. Similar patterns of survival disparities were observed using population-based data from the California Cancer Registry (CCR): increased hazard of death among Black (by 57%), Hispanic (by 38%), and Asian children (by 33%) compared with White children [12•]. In the context of the Dana-Farber Cancer Institute (DFCI) ALL Consortium, Hispanic patients had significantly worse event-free survival (EFS) (HR 1.7; 95% CI 0.9, 2.9) and overall survival (OS) (HR 1.6; 95% CI 1.1, 2.5) than non-Hispanic patients [13], suggesting that Hispanic patients have a higher risk of relapse than their non-Hispanic White counterparts.
The increased relapse risk among Hispanic patients has been partially attributed to the high Native American genetic ancestry among self-reported Hispanics. Independent of known prognostic factors, including minimal residual disease, Native American genetic ancestry was associated with the cumulative incidence of ALL relapse [14]. The inclusion of the delayed intensification phase of therapy abrogated this increased risk of relapse among those with greater than 10% Native American genetic ancestry, suggesting ethnicity-related genetic variation could be incorporated into the risk-adapted approach to chemotherapy that has already been quite successful in improving leukemia outcomes over time [14].
In a meta-analysis, Petridou et al. [15••] found that children with ALL from low SES families suffered nearly twofold higher death rates (pooled RR, 1.8; 95% CI 1.0, 3.3). When SES was evaluated using area-level indices, the risk of death was also higher among children living in lower SES areas (RR ranged from 1.2 to 1.3) [15••]. Within the California Cancer Registry, ALL patients residing in the lowest SES neighborhoods at diagnosis had a 39% increased risk of death relative to those living in higher SES neighborhoods [12•]. On DFCI ALL Consortium trials, OS among children living in high-poverty zip codes (85%) was significantly lower compared to children living in low-poverty zip codes (92%). In addition, children living in high-poverty zip codes (13%) more frequently experienced early relapse than those from low-poverty zip codes (6%) [16]. Given the known poor prognosis associated with early relapse relative late relapse, the increased frequency of early relapse likely partially explains the poverty-related disparity in overall survival.
In addition to race, ethnicity, and socioeconomic status, the hospital where an ALL patient receives treatment has been shown to impact mortality. Receiving cancer care outside of a Comprehensive Cancer Center (CCC) or Children’s Oncology Group (COG) site was associated with inferior survival among adolescents (HR 1.9; 95% CI 1.2, 1.8) in Los Angeles County relative to younger children who received care at a CCC/COG site [17•]. Notably, some of the well-documented inferior survival among adolescents was mitigated by treatment at a specialized cancer center. The role that access to comprehensive cancer care plays was further substantiated in a study where overall survival among Black and White children in SEER was compared with overall survival at St. Jude Children’s Research Hospital (SJCRH). While ALL overall survival disparities were present in SEER (82% v. 89%, p < 0.01), the disparity between Black and White children was narrower and not statistically significant at SJCRH (89% v. 93%, p = 0.41) [18]. These findings suggest that with equal access to effective risk-directed therapy and supportive care, widely observed survival disparities can be lessened and high cure rates can be achieved across racial groups.
In a study using a nationally representative cohort of children’s hospitals, ALL induction mortality increased linearly with hospital-wide proportion of patients with public insurance (Spearman correlation ρ 0.36; p = 0.03) [19]. The mechanism by which hospital payer mix influences mortality is not completely clear; perhaps, hospital payer mix reflects the degree of at risk and vulnerable patients served or maybe it correlates with the financial health of the hospital and the resources available to provide patient care.
Acute Myeloid Leukemia (AML)
As a less frequent form of acute leukemia occurring in children and adolescents, survival disparities in acute myeloid leukemia (AML) are less well studied; nonetheless, racial and ethnic disparities in pediatric acute myeloid leukemia have been consistently observed as well. In Children’s Cancer Group (CCG) studies, inferior overall survival (OS) was observed among Black and Hispanic children compared to White non-Hispanic children [20]. Using longitudinal cancer registry data from California, Black patients were again shown to have inferior OS compared with White patients (Hazard Ratio (HR) 1.3; 95% CI 1.1, 1.5) [21].
We evaluated early mortality in a national cohort and found an increased risk of early death among Black patients (HR 2.8; 95% CI 1.2, 6.4). We also found that Black patients had a significantly higher risk of intensive care unit (ICU)-level utilization within the first 72 h after initial presentation (RR 1.5; 95% CI 1.0, 2.2). Sixty-one percent of the relative excess early mortality among Black patients was mediated by acuity at initial presentation [22•]. Following the initial course of induction chemotherapy, mortality and ICU-level utilization between Black and White patients on therapy is similar [23].
SEER data confirmed our findings that Black race was associated with a higher risk of early mortality compared with White race among patients with hematologic malignancies (odds ratio (OR) 1.7; 95% CI 1.1, 2.5). These SEER data also showed Hispanic children had a higher risk of early death compared with non-Hispanic children (OR 1.5; 95% CI 1.1, 2.0) [24••]. The higher incidence of early mortality may contribute to lower enrollment of Black patients onto clinical trials [25]. On the most recent COG clinical trial for AML, we found substantially reduced enrollment of Black patients (RR 0.53; 95% CI 0.38, 0.74) relative to non-Hispanic White patients; however, we did not identify any association with on therapy mortality [25].
In evaluating county-level income using SEER data, disadvantaged patients were at increased risk for early mortality (OR 1.5; 95% CI 1.1, 2.1) [24••]. Similarly among patients in the California Cancer Registry, patients living in the lowest SES neighborhoods had a significantly greater risk of early mortality than patients living in the highest SES neighborhoods (OR 1.6; 95% CI 1.1, 2.3) [21]. The CCR study also demonstrated that uninsured patients had three times greater risk of early mortality than privately insured patients [21].
End-of-Life Care
Children with hematologic malignancies were more than three times as likely as patients with solid tumors to die in the hospital and were twice as likely to receive high-intensity care at the end of life [26]. While this is not a disparity in itself (as the population of patients with hematologic malignancies are not inherently socially disadvantaged), the increased frequency of intense end-of-life care among pediatric leukemia patients makes end-of-life care of particular interest in leukemia patients. Black, Hispanic, and Asian American patients were more likely to die in the hospital relative to White patients. Conversely patients who live more than 25 miles from the hospital were 36% less likely to die in the hospital [26]. Among those that received stem cell transplant (the majority of whom are leukemia patients), adolescents (15–21 years) more frequently receive medically intense interventions relative to older adult patients [27]. Ideally, patients should receive end-of-life care consistent with their expressed wishes. More research is needed to understand if these differences in pediatric end-of-life care are concordant with family goals [28].
Subsequent Neoplasm
Second malignant neoplasms are the leading cause of nonrelapse-related late mortality after childhood cancer [29]. The incidence of subsequent neoplasms among Black pediatric patients was lower than White pediatric patients [30••]. This difference was consistent with the lower rates of neoplasm among the Black population more generally. Similarly, the cumulative incidence over 30 years of subsequent malignant neoplasm was higher among White non-Hispanic patients (8.1%) than Black (5.1%) or Hispanic (6.7%) cancer survivors [30••]. While these data are not specific to leukemia survivors, leukemia survivors do comprise the largest component of the population evaluated.
Cardiotoxicity
Anthracyclines, such as doxorubicin, daunorubicin, and mitoxantrone, are a mainstay of chemotherapy regimens for acute leukemia. Furthermore, the risk of anthracycline cardiotoxicity is higher in children than in adults, owing to the developing heart muscle. Cardiovascular disease (CVD) is the second leading cause of long-term morbidity and mortality among cancer survivors. Among cancer survivors, those with a history of leukemia have the highest risk of developing CVD (adjusted incidence rate ratio 4.2; 95% CI 1.7, 10.3) compared to patients without cancer [31].
Among patients in survivorship care, Black patients were nearly twice as likely to report cardiac conditions (RR 1.8; 95% CI 1.1, 2.7) [30••]. However, the risk of cardiac conditions was attenuated after adjusting for cardiovascular risk factors. Black patients with AML also had an 87% higher risk of early-onset cardiotoxicity than White patients, much of which was attributable to infection-associated cardiac effects [32]. Importantly, early-onset cardiotoxicity was also associated with significantly reduced EFS and OS, suggesting that dose adjustments due to toxicity may potentially contribute to some of the known racial disparities in survival.
Other Complications and Health Conditions
Over the course of treatment, patients are exposed to a variety of insults that cause organ and tissue damage. The kidneys are vulnerable to nephrotoxic medications as well as injury following hypoperfusion from sepsis or heart failure. Within the first year after a new diagnosis of AML, Black children were significantly more likely to experience acute kidney failure than White patients (HR 1.7; 95% CI 1.1, 2.6) [33]. We also demonstrated that Black patients received significantly more anti-hypertensive medications while on therapy (RR 2.3; 95% CI 1.8, 2.9) [23] consistent with greater incidence of hypertension in Black patients.
Using data from the Dana-Farber Cancer Institute ALL Consortium, Hispanic patients less than 10 years of age were shown to have a lower cumulative incidence of bone fracture (HR 0.24; 95% CI 0.10–0.54). Likewise, among patients greater than 10 years of age, the hazard of osteonecrosis was significantly lower in Hispanic patients (HR 0.28; 95% CI 0.10–0.76) relative to non-Hispanic patients [13]. The lower incidence of skeletal toxicity was attributed to reduced steroid exposure. In another multi-institutional study, Hispanic children were shown to have a greater risk of neurotoxicity secondary to methotrexate (HR 2.4; 95% CI 1.1, 5.6). These patients had lower intrathecal methotrexate exposure as a result of neurotoxicity and this was associated with increased relapse risk [34].
Two of three cancer survivors will develop a chronic health condition [35]. Data from the Childhood Cancer Survivor Study among survivors of at least 5 years (which were predominantly leukemia patients) showed that Black patients have higher all-cause mortality (relative rate 1.4; 95% CI 1.1, 1.9). Notably, once SES was accounted for the racial disparity resolved, suggesting that the disparity in all-cause mortality operates more along the SES axis than the racial axis [30••]. Higher prevalence of obesity and hypertension were noted among the Black and Hispanic cancer survivors relative to the White non-Hispanic patients. In addition, even after accounting for obesity, Black and Hispanic patients had a significantly increased risk of diabetes [30••]. To date, SES has not been evaluated as an independent predictor of chronic health conditions following leukemia survival.
Conclusions and Future Directions
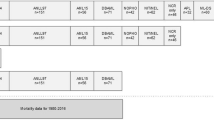
According to the conceptual framework put forth by Kilbourne et al. (adapted in Fig. 1) the first phase in disparities research is detecting, describing, and measuring disparities [7]. We are at a phase now where survival disparities in pediatric acute leukemia are well recognized, although they remain understudied in the rarer subtypes of leukemia, such as juvenile myelomonocytic leukemia (JMML). Leukemia disparities extend across the entire spectrum of care from initial diagnosis throughout treatment to end-of-life care and survivorship following therapy completion. Several of the complications associated with chemotherapy are more prevalent among minority patients.
Three sequential phases of health disparities research, each informing the subsequent phase, based on the conceptual framework from Kilbourne and colleagues [7]
The field is just starting to venture into the second phase of disparities research (Fig. 1), in which the determinants of the disparities are identified. Most studies have focused on individual patient/disease genomics or area-based SES as possible etiologies. However, it is important to note that when incorporated into biomedical research, SES is inconsistently defined between studies and often utilizes area-level data or composite measures [36]. The use of inconsistent definitions makes comparison between studies difficult. Moreover, the use of composite measures often inhibits identification of targetable drivers of disparities.
The issue underlying these inconsistencies is that individual-level socioeconomic data are rarely collected. Perhaps more surprising, race and ethnicity are also still not consistently collected or reported on clinical trials, in administrative data, or in electronic health records. In an analysis of high profile clinical trials, 46% of trials that provided race/ethnicity data reported only one or two racial/ethnic categories and only 1% reported differences in outcomes by race/ethnicity [37]. As additional data emerge that link SES to health-related outcomes, it is our hope that the prospective collection of these data will become more common if for no other reason than because race, ethnicity, and SES bear consideration as important confounders in clinical studies. Moreover, for clinicians to evaluate the generalizability of clinical trial results to their patient population, the racial/ethnic makeup of the population included in the trial is essential information. In addition, the omission of these data represents a lost opportunity to evaluate for the presence of disparities. Because much of race/ethnicity data is gathered from the hospital medical record, ensuring that patients have the opportunity to self-report race and ethnicity is essential to accurate race and ethnicity data.
Once self-reported race and ethnicity are effectively captured, studies to compare self-reported race/ethnicity with genetic ancestry are an important part of teasing apart the complex interactions between host biology and sociocultural and environmental influences. Tying the details of the leukemia biology into this mosaic will further help to elucidate the source of observed outcome disparities.
Within pediatric oncology, relatively little has been done in the third phase of disparities research (Fig. 1), which focuses on reducing disparities through interventions. There are a few notable exceptions where ongoing efforts may eventually yield approaches that ameliorate existing disparities. For example, there is a Children’s Oncology Group study (ACCL1033), which randomized pediatric ALL patients to a comprehensive approach to improve medication adherence [38], that enrolled almost 600 patients and closed to accrual in 2018. The intervention involves an interactive multimedia educational program as well as a personalized mercaptopurine schedule and automated customized text message reminders. Because there are documented disparities in non-adherence among vulnerable populations [39•], this intervention has the potential to lessen existing survival disparities in pediatric ALL.
Another instance in which an ongoing COG study may preferentially benefit minority patients is the Phase 2 study of ruxolitinib in CRLF2-rearranged and/or JAK pathway-mutant ALL (AALL1521) [40]. Philadelphia-like ALL with CRLF2 rearrangements is over-represented in self-reported Hispanic children (35.3%) compared with non-Hispanic children (7.1%) [41]. A portion of the survival outcome disparities among Hispanic patients with ALL is likely explained by this difference in leukemia biology. If ruxolitinib proves effective in this setting, the component of the outcome disparity attributable to these CRLF2 lesions will be ameliorated. This study is currently ongoing and its results are much anticipated as a solution for this chemotherapy-resistant and often refractory leukemia.
Advances in the use of alternative donor sources for stem cell transplant, such as cord blood transplants and haploidentical stem cell transplants [42], have made stem cell transplant a viable option for almost all patients, including racial and ethnic minorities who may not have an HLA-matched related or unrelated donor available [43]. If access to stem cell transplant mediates some of the racial and ethnic disparities seen in pediatric leukemia, these technical advances may help to resolve differential access to transplant, thereby decreasing leukemia outcome disparities.
Over the last several years, we have seen major advances in the treatment of pediatric ALL with the advent of chimeric antigen receptor (CAR) T cells directed against CD19 [44] and use of antibody-mediated immunotherapies, such as blinatumomab [45, 46] and inotuzumab [47, 48]. Our ability to salvage patients following ALL relapse has substantially improved as a result. With higher rates of relapse in minority and socioeconomically disadvantaged populations, specific attention should be directed at equitable access to these novel therapies. To date, no studies dedicated to evaluating the distribution of these novel therapeutic approaches have been conducted.
With FDA approval, Kymriah® (tisagenlecleucel) is available at a subset of centers that have been designated as well-equipped to manage the complexities of cytokine release syndrome (CRS). This regionalized care model combined with the high costs associated with obtaining and administering the CAR T cell product commercially risk widening disparities as insurance and treating hospital determine who has access to therapy among the population of patients with relapsed leukemia. The difference in the cost of administering this complex therapy from the amount reimbursed by insurance, particularly among Medicaid-insured patients, often falls to treating hospitals [49]. As a result, hospitals that serve largely Medicaid-insured populations may face difficulties in sustainably providing tisagenlecleucel. Such differences in treatment availability force patients to be referred to the specialized centers that are able to provide these advanced therapies, requiring them to travel from their home institution and remain away from home and work for an extended period. While programs that offer financial assistance for travel are in place, the benefits of specialized and expensive therapies may remain elusive to those patients who are unable to travel due to other non-financial barriers such as employment, family structure, or immigration status, thus further widening disparities for these patients.
While striving towards generating evidence on how to reduce disparities on a broader scale with system-wide interventions, each healthcare provider can work towards recognizing and reducing disparities at the individual patient level. Data shows that implicit bias among oncologists leads to shorter patient interactions and less supportive and patient-centered communication. These interactions were associated with less patient confidence and more difficulty with adherence to oncologists’ recommendations [50]. It is likely that these adult data also extend into the pediatric realm [51]. As clinicians, being aware of our own implicit biases, how they impact patients and their care, and taking steps to reduce unconscious biases are a concrete approach we can each take to improve care and lessen disparities.
There are a variety of angles from which to work on leukemia disparities: describing them using population-based cohorts, delving into their etiology at the institutional or societal level, or attempting to address them through interventions at the biologic, patient, or provider level. In any of these forms, applying a disparities lens to the full spectrum of work we do on leukemia will allow us to eventually improve care across diverse patient populations and promote health equity.
Funding/Support
Dr. Winestone’s time is supported by a Young Investigator Award from Alex’s Lemonade Stand Foundation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer. 2008;112(2):416–32.
Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(14):1663–9.
Braveman PA, Kumanyika S, Fielding J, Laveist T, Borrell LN, Manderscheid R, et al. Health disparities and health equity: the issue is justice. Am J Public Health. 2011;101(Suppl 1):S149–55.
Gravlee CC. How race becomes biology: embodiment of social inequality. Am J Phys Anthropol. 2009;139(1):47–57.
Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348(12):1170–5.
Galobardes B, Shaw M, Lawlor DA, Lynch JW, Davey Smith G. Indicators of socioeconomic position. J Epidemiol Community Health. 2006;60(1):7–12.
Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113–21.
Pollock BH, DeBaun MR, Camitta BM, Shuster JJ, Ravindranath Y, Pullen DJ, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. J Clin Oncol. 2000;18(4):813–23.
Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–64. https://doi.org/10.1182/blood-2002-02-0395.
Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008–14. https://doi.org/10.1001/jama.290.15.2008.
Goggins WB, Lo FFK. Racial and ethnic disparities in survival of US children with acute lymphoblastic leukemia: evidence from the SEER database 1988–2008. Cancer Causes Control. 2012;23(5):737–43.
• Abrahão R, Lichtensztajn DY, Ribeiro RC, Marina NM, Keogh RH, Marcos-Gragera R, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988–2011: a population-based observational study. Pediatr Blood Cancer. 2015;62(10):1819–25. Cohort study in California showing worse survival in minorities and children from the lowest SES neighborhoods with ALL.
Kahn JM, Cole PD, Blonquist TM, Stevenson K, Jin Z, Barrera S, et al. An investigation of toxicities and survival in Hispanic children and adolescents with ALL: results from the Dana-Farber Cancer Institute ALL consortium protocol 05-001. Pediatr Blood Cancer. 2018;65(3):e26871.
Yang JJ, Cheng C, Devidas M, Cao X, Fan Y, Campana D, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43(3):237–41.
•• Petridou ET, Sergentanis TN, Perlepe C, Papathoma P, Tsilimidos G, Kontogeorgi E, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann Oncol. 2015;26(3):589–97. Meta-analysis demonstrating increased mortality among ALL patients with low SES at the individual- and area-levels.
Bona K, Blonquist TM, Neuberg DS, Silverman LB, Wolfe J. Impact of socioeconomic status on timing of relapse and overall survival for children treated on Dana-Farber Cancer Institute ALL consortium protocols (2000-2010). Pediatr Blood Cancer. 2016;63(6):1012–8. https://doi.org/10.1002/pbc.25928.
• Wolfson J, Sun C-L, Wyatt L, Stock W, Bhatia S. Adolescents and young adults with acute lymphoblastic leukemia and acute myeloid leukemia: impact of Care at Specialized Cancer Centers on survival outcome. Cancer Epidemiol Biomark Prev. 2017;26(3):312–20. Cohort study in LA County demonstrating a survival benefit to being treated at a specialized cancer center.
Pui CH, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol. 2012;30(16):2005–12. https://doi.org/10.1200/jco.2011.40.8617.
Seif AE, Fisher BT, Li Y, Torp K, Rheam DP, Huang Y-SV, et al. Patient and hospital factors associated with induction mortality in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(5):846–52.
Aplenc R, Alonzo TA, Gerbing RB, Smith FO, Meshinchi S, Ross JA, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108(1):74–80. https://doi.org/10.1182/blood-2005-10-4004.
Abrahão R, Keogh RH, Lichtensztajn DY, Marcos-Gragera R, Medeiros BC, Coleman MP, et al. Predictors of early death and survival among children, adolescents and young adults with acute myeloid leukaemia in California, 1988-2011: a population-based study. Br J Haematol. 2016;173(2):292–302. https://doi.org/10.1111/bjh.13944.
• Winestone LE, Getz KD, Miller TP, Wilkes JJ, Sack L, Li Y, et al. The role of acuity of illness at presentation in early mortality in black children with acute myeloid leukemia. Am J Hematol. 2017;92(2):141–8. https://doi.org/10.1002/ajh.24605. Cohort study demonstrating that acuity at presentation mediates 61% of excess early mortality in Black pediatric AML patients.
Li Y, Newton JG, Getz KD, Huang YS, Seif AE, Fisher BT, et al. Comparable on-therapy mortality and supportive care requirements in Black and White patients following initial induction for pediatric acute myeloid leukemia. Pediatr Blood Cancer. 2019;66(4):e27583. https://doi.org/10.1002/pbc.27583.
•• Green AL, Furutani E, Ribeiro KB, Rodriguez-Galindo C. Death within 1 month of diagnosis in childhood cancer: an analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320–7. https://doi.org/10.1200/JCO.2016.70.3249. Cohort study using SEER data demonstrating Black and Hispanic patients and patients in lower income counties are at higher risk for early death across multiple pediatric cancers including leukemia.
Winestone LE, Getz KD, Rao P, Li Y, Hall M, Huang YV, et al. Disparities in pediatric acute myeloid leukemia (AML) clinical trial enrollment. Leuk Lymphoma. 2019:1–9. https://doi.org/10.1080/10428194.2019.1574002.
Johnston EE, Alvarez E, Saynina O, Sanders L, Bhatia S, Chamberlain LJ. Disparities in the intensity of end-of-life care for children with cancer. Pediatrics. 2017;140(4):e20170671.
Johnston EE, Muffly L, Alvarez E, Saynina O, Sanders LM, Bhatia S et al. End-of-life care intensity in patients undergoing allogeneic hematopoietic cell transplantation: a population-level analysis. J Clin Oncol. 2018;JCO.2018.78.095.
Bona K, Wolfe J. Disparities in pediatric palliative care: an opportunity to strive for equity. Pediatrics. 2017;140(4):e20171662.
Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the childhood cancer survivor study. JNCI Journal of the National Cancer Institute. 2008;100(19):1368–79.
•• Liu Q, Leisenring WM, Ness KK, Robison LL, Armstrong GT, Yasui Y, et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the childhood cancer survivor study. J Clin Oncol. 2016;34(14):1634–43. Cohort study showing Black pediatric cancer survivors have higher all-cause mortality, attirbutable to SES, and higher risk of diabetes and cardiac conditions.
Chao C, Xu L, Bhatia S, Cooper R, Brar S, Wong FL, et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol. 2016;34(14):1626–33.
Getz KD, Sung L, Ky B, Gerbing RB, Leger KJ, Leahy AB, et al. Occurrence of treatment-related cardiotoxicity and its impact on outcomes among children treated in the AAML0531 clinical trial: a report from the Children’s Oncology Group. J Clin Oncol. 2019;37(1):12–21.
Fisher BT, Zaoutis TE, Leckerman KH, Localio R, Aplenc R. Risk factors for renal failure in pediatric patients with acute myeloid leukemia: a retrospective cohort study. Pediatr Blood Cancer. 2010;55(4):655–61. https://doi.org/10.1002/pbc.22601.
Taylor OA, Brown AL, Brackett J, Dreyer ZE, Moore IK, Mitby P, et al. Disparities in neurotoxicity risk and outcomes among pediatric acute lymphoblastic leukemia patients. Clin Cancer Res. 2018;24(20):5012–7.
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82.
Polite BN, Adams-Campbell LL, Brawley OW, Bickell N, Carethers JM, Flowers CR, et al. Charting the future of cancer health disparities research: a position statement from the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. CA Cancer J Clin. 2017;67(5):353–61.
Corbie-Smith G, St George DMM, Moody-Ayers S, Ransohoff DF. Adequacy of reporting race/ethnicity in clinical trials in areas of health disparities. J Clin Epidemiol. 2003;56(5):416–20.
Bhatia S. A comprehensive approach to improve medication adherence in pediatric ALL. Children’s Oncology Group; ClinicalTrials.gov Identifier: NCT01503632.
• Bhatia S, Landier W, Hageman L, Kim H, Chen Y, Crews KR, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–53. https://doi.org/10.1182/blood-2014-01-552166. Prospective cohort study identifying poor adherence to oral chemotherapy among Black and Asian Americans and implicating adherence as a mediator of relapse in ALL.
Tasian S. A phase 2 study of ruxolitinib with chemotherapy in Children with acute lymphoblastic leukemia. Children’s Oncology Group; ClinicalTrials.gov Identifier: NCT02723994.
Harvey RC, Mullighan CG, Chen I-M, Wharton W, Mikhail FM, Carroll AJ, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–21.
El-Cheikh J, Crocchiolo R, Furst S, Bramanti S, Sarina B, Granata A, et al. Unrelated cord blood compared with haploidentical grafts in patients with hematological malignancies. Cancer. 2015;121(11):1809–16.
Joshua TV, Rizzo JD, Zhang M-J, Hari PN, Kurian S, Pasquini M, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer. 2010;116(14):3469–76.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–9.
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53.
Bhojwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. 2018.
Bach PB. National coverage analysis of CAR-T therapies - policy, evidence, and payment. N Engl J Med. 2018;379(15):1396–8.
Penner LA, Dovidio JF, Gonzalez R, Albrecht TL, Chapman R, Foster T, et al. The effects of oncologist implicit racial bias in racially discordant oncology interactions. J Clin Oncol. 2016;34(24):2874–80.
Johnson TJ, Winger DG, Hickey RW, Switzer GE, Miller E, Nguyen MB, et al. Comparison of physician implicit racial bias toward adults versus children. Acad Pediatr. 2017;17(2):120–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lena E. Winestone and Richard Aplenc declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Health Economics
Rights and permissions
About this article
Cite this article
Winestone, L.E., Aplenc, R. Disparities in Survival and Health Outcomes in Childhood Leukemia. Curr Hematol Malig Rep 14, 179–186 (2019). https://doi.org/10.1007/s11899-019-00515-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-019-00515-x