Abstract
Purpose of Review
Cardiac computed tomography (CT) is becoming a more widely applied tool in the diagnosis and management of a variety of cardiovascular conditions, including heart failure. The aim of this narrative review is to examine the role of cardiac CT in patients with heart failure.
Recent Findings
Coronary computed tomographic angiography has robust diagnostic accuracy for ruling out coronary artery disease. These data are reflected in updated guidelines from major cardiology organizations. New roles for cardiac CT in myocardial imaging, perfusion scanning, and periprocedural planning, execution, and monitoring are being implemented.
Summary
Cardiac CT is useful in ruling out coronary artery disease its diagnostic accuracy, accessibility, and safety. It is also intricately linked to invasive cardiac procedures that patients with heart failure routinely undergo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure is a widespread and burdensome disease affecting more than 37.5 million patients and costing an estimated US$108 billion globally in 2012 [1, 2]. Work in this field has led to advances in diagnostic tools and therapies to secure optimal outcomes for patients. Two areas of focus are non-invasive coronary artery disease (CAD) evaluation and periprocedural imaging. A suite of modalities can be used to obtain diagnostic information so that providers can deliver precise, personalized therapies. Cardiac computed tomography (CT), which includes coronary calcium scan and coronary computed tomographic angiogram (coronary CTA), has been increasingly incorporated into the care of this patient population for a variety of purposes. The purpose of this review is to highlight contemporary and future roles of cardiac CT in patients with heart failure. This paper will focus on the use of cardiac CT in ruling out CAD, imaging the myocardium, and assisting with the planning, execution, and monitoring of transcatheter procedures in this cohort.
Ruling Out Coronary Artery Disease
Coronary Calcium Scan
Cardiac CT has the capacity to detect and prognosticate on CAD. Initially, most studies on cardiac CT were performed using coronary calcium scans, which is a highly safe and sensitive modality to evaluate for obstructive CAD in low–intermediate-risk patients with congestive heart failure. Regarding accuracy, Shemesh et al. published a study on coronary calcium scans in patients who have heart failure of unknown origin that showed a sensitivity of 100%, specificity of 92%, and total accuracy of 97% in diagnosis of ischemic type cardiomyopathy [3]. Correspondingly, a prospective, blinded, subsequent study in a multi-ethnic patient population showed similar results with sensitivity of 99% and overall accuracy of 92% in patients with left ventricular ejection fraction < 40% [4]. In the past, the core tenet of coronary calcium scans was to be able to rule out CAD in patients with calcium scores of 0. Newer data suggest that very high coronary artery calcium scores (≥ 1,000) have robust prognostic information. Coronary artery calcium scores of ≥ 1,000 have double the all-cause and CVD mortality rates, and more than double the CAD mortality rate, of patients with coronary artery calcium scores between 400 and 999 [5]. Another study found comparable results for all-cause 30-day and 1-year mortality status post-transaortic valve replacement (TAVR) for coronary artery calcium scores ≥ 1,000 as compared to scores of 400–999 [6].
The accuracy of coronary calcium scans has also been compared to other imaging modalities. A 2008 investigation comparing coronary calcium scans to nuclear myocardial perfusion imaging (nMPI) in patients with cardiomyopathy concluded that coronary calcium scanning was significantly more accurate than nMPI (84% vs 64%, P = 0.009). In addition, coronary artery calcium scans are not “… affected by left bundle branch block, abnormal perfusion patterns, or breast or diaphragmatic artifacts” [7]. When compared with echocardiography, the presence of coronary calcification on CT was significantly more sensitive and accurate than the presence of wall motion abnormalities in distinguishing ischemic and nonischemic etiology of heart failure [8].
One of the benefits of a coronary calcium scan is its safety profile, which includes an extremely low radiation dose (~ 1 mSv) and contrast avoidance [9]. Moreover, coronary calcium scans may help patients avoid invasive testing. To illustrate, data on 76 newly diagnosed patients with cardiomyopathy from our public hospital shows that 49% of patients had a coronary artery calcium score of zero, effectively ruling out CAD without the risks of invasive angiography with near perfect negative predictive values.
In general, coronary calcium scans do not have the ability to reliably assess detailed anatomical structures such as myocardial tissue or valvular structure. Coronary calcium scans also have poor resolution of the coronary artery tree and lack of direct visualizations of lesions [10]. Coronary calcium scanning has proven to be a useful screening tool in the appropriate setting, and it may continue to have a role in low- to intermediate-risk patients with heart failure when providers want to avoid contrast and minimize radiation exposure. Other imaging options with superior diagnostic performance have made coronary calcium scans less relevant to this patient population.
Coronary CTA
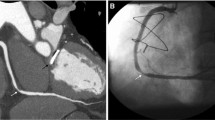
In comparison to coronary calcium scans, coronary CTA is an even more adept screening tool for CAD, given its ability to assess stenosis as well as myocardial disease and presence of infarction (Figs. 2 and 3). A 2009 comparison of coronary CTA to invasive coronary angiography showed sensitivity of 100%, specificity of 93%, positive predictive value (PPV) of 95%, negative predictive value (NPV) of 100%, and accuracy of 97% on a per-patient analysis in the general population for excluding CAD of greater than 50% luminal stenosis [11]. Other investigations by Pontone et al. and Maruyama et al. on coronary CTA in the general population have had comparable results [12, 13]. A fourth report did not complete a per-patient data analysis, but did describe similar sensitivity, specificity, accuracy, PPV, and NPV on a per-segment basis as 84.3%, 98.6%, 96.1%, 92.2%, and 96.9% [14]. With regard to the diagnostic performance and utility in patients with heart failure, a 2009 investigation concluded sensitivity of 100%, specificity of 96%, PPV of 0.9, and NPV 1.0 for > 50% luminal stenosis on a per-patient basis [15]. Papers by Andreini et al. (in 2007 and again in 2009), Ghostine et al., Polain De Warux et al., and Boulmier et al. have been published with concurrent results [16,17,18,19,20]. One possible complaint with these prior investigations is the relatively small patient population in the study. Yet, more recently, a larger (n = 537), multicentered prospective trial demonstrated that coronary CTA has sensitivity, specificity, NPV, and PPV of 92–100%, 83–93%, 88–100%, and 81–92%, respectively, for detection of > 50% luminal stenosis on per-patient model in the heart failure population [21].
The aforementioned studies largely excluded patients with decompensated heart failure, and many of those patients were on beta-blockers as part of their guideline-directed medical therapy or as a part of the imaging protocol, ensuring low heart rate. In general, there has been some question as to which patient population coronary CTA may be suitable for. This is particularly relevant in the heart failure population because their presentation may resemble that of unstable angina; therefore, there may be a sense of urgency in initiating their workup. It would be ideal if these patients could undergo a single test to rule out CAD prior to fully stabilizing them on guideline-directed medical therapy.
In contrast to prior studies examining patients with heart failure only once stabilized, Srichai et al. showed that coronary CTA retained accuracy (96.7%), although sensitivity (62.5%) was decreased, in patients who are in acutely decompensated heart failure with average heart rate of 75 beats per minute [22]. These findings are supported by a separate report in the general population, which found no changes to diagnostic values across patients in three different heart rate groups [14]. Oncel et al. found that coronary CTA retained sensitivity (100%), but did have decreased specificity (88.9%) in patients with atrial fibrillation [23]. Follow-up papers in patient populations in Europe and China also showed high sensitivity (90–100%), with slightly decreased specificity (75–84%) for patients in atrial fibrillation [24,25,26]. In addition, coronary CTA is reliable in patients with known CAD and coronary artery stents, as well as patients with end-stage renal disease (Figs. 1 and 2) [27, 28]. In short, coronary CTA is a suitable tool for distinguishing ischemic from dilated cardiomyopathy in a large and diverse patient population.
In addition to performing well over a variety of cohorts, coronary CTA is a relatively comfortable, fast, and safe examination. Coronary CTA is non-invasive and has a relatively low radiation dose at 3.5msV [29]. It has also been shown to be safer than invasive coronary angiography [17]. One area of concern could be the risk of contrast-induced nephropathy, especially as patients with heart failure frequently experience comorbid kidney disease. Other limitations of cardiac CT will be discussed later.
Recently, clinicians have had to decide between multiple imaging tools to rule out CAD and ischemic causes of heart failure. Often, the choice comes down to coronary CTA and stress testing, particularly nMPI. The 2021 AHA guidelines on chest pain, while not specific to the population of patients with HF, give a class 1A recommendation to both coronary CTA and nMPI in intermediate-risk patients with acute chest pain and no known CAD, after a negative or inconclusive evaluation for acute coronary syndrome [30]. The 2021 guidelines for the diagnosis and treatment of acute and chronic heart failure from the European Society of Cardiology (ESC) report that coronary CTA is considered a “key element” in the initial workup of heart failure and should be considered in patients with low to intermediate pre-test probability of CAD [31•]. These same guidelines also indicate that nMPI is another recommended study to determine the underlying etiology of heart failure. The Society of Cardiac Computed Tomography states in a consensus document that coronary CTA is an appropriate test to exclude CAD in those with suspected nonischemic heart failure [32•].
Per these guidelines, coronary CTA and nMPI are both reasonable options for noninvasively ruling out CAD and ischemia, respectively, in patients with heart failure. Multiple studies have been done to assess the comparative performance of coronary CTA and nMPI in this setting. Although these studies are not specifically focused on patients with heart failure, they still provide useful insights. With regard to the ability to detect CAD, one retrospective study showed that coronary CTA has 98.9% sensitivity, 74.2% specificity, 91.8% PPV, and 95.8% NPV as compared to nMPI (56% sensitivity, 38.7% specificity, 72.9% PPV, and 23% NPV) on a per-patient level for lesions of > 50% stenosis [33]. Multiple other studies, including a prospective, randomized, and multicentered investigation, show similar findings [34,35,36].
Evaluation of the Myocardium
Imaging of the myocardium can be useful in identifying regions of fibrosis. Fibrotic changes represent the end effect of a variety of pathologic cardiac conditions including cardiac valvular disease, hypertension, chronic kidney disease, infiltrative diseases (i.e., amyloid, sarcoid, Fabry), hypertrophic cardiomyopathy, and coronary artery disease/myocardial ischemia. These imaging findings may carry diagnostic and prognostic information.
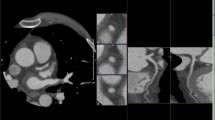
One of the main ways to assess cardiac fibrosis is by measuring extracellular volume in the myocardium, which can be done with both late gadolinium enhancement with MRI and myocardial CT delayed enhancement (CTDE). The gold standard of non-invasive evaluation of myocardial scar from ischemia is cardiac MRI using a late gadolinium enhancement protocol with sensitivity, specificity, PPV, and NPV of 97.1%, 88.9%, 97.1%, and 88.9%, respectively [37]. Assessment of myocardial fibrosis via quantification of extracellular volume is possible with myocardial CTDE results which show strong correlations with both cardiac MRI and histological measures of fibrosis [38, 39]. Myocardial CTDE assessment of myocardial scar related to ischemia has an accuracy of 93–96% when compared with late gadolinium enhancement [37, 40, 41].
Outside of imaging of myocardial scar related to ischemia, other uses of myocardial CTDE include detecting fibrosis in a variety of disease states including aortic stenosis, hypertrophic cardiomyopathy, cardiac sarcoidosis, and cardiac amyloidosis [42,43,44,45]. Specifically regarding cardiac amyloidosis, both the European Society of Cardiology and the American Heart Association include cardiac scintigraphy as a part of the diagnostic pathway, but both suggest CMR may be helpful [46, 47]. Per the Society of Cardiac Computed Tomography (SCCT), it may be appropriate to use myocardial CTDE to rule out infiltrative diseases such as amyloidosis [32•]. Although this is not the gold standard for detecting myocardial scar and perfusion, CT scans are relatively cheap, fast, easy to access, and have comparable performance to MRI. Therefore, cardiac CT is a reasonable substitute to cardiac MRI for imaging of myocardial fibrosis when MRI is not possible or local expertise and or equipment is lacking.
Myocardial CTDE has excellent temporal and spatial resolution, and the image acquisition is very rapid [48]. But, myocardial CTDE has higher signal-to-noise ratio as compared to cardiac MRI and standardized cutoff values are less developed among CT imaging as compared to MRI. Meanwhile, cardiac MRI with late gadolinium enhancement has the distinct ability to “null” the myocardium, and thereby increase the ability to spot hyper-enhancing fibrotic regions [49]. Classically, cardiac MRI has been hampered by the long image acquisition time and difficulty in assessing diffuse fibrosis (which does not provide healthy myocardium to compare to diseased tissue). Fortunately, cardiac MRI imaging acquisition time has decreased over the last decade [50]. Plus, in recent years, advances in MRI techniques such as T1 mapping, feature-tracking MRI, and MRI tagging, have overcome some of the issues with MRI and diffuse fibrosis, but these technologies are currently not widely implemented [48]. Similar myocardial CTDE techniques are also evolving, but they are not as well validated.
In addition to performing comparably to late gadolinium enhancement for evaluation of myocardial scar, a myocardial CT perfusion technique can detect hemodynamically significant CAD (Fig. 3) [51]. Myocardial CT perfusion can perform similarly to MRI stress perfusion, positron emission tomography, and single-photon emission computed tomography for detecting hemodynamically significant coronary artery lesions [52].
To conclude, cardiac CT can directly visualize atherosclerotic lesions, evaluate myocardial fibrosis, and assess myocardial perfusion. Some of these capabilities are being used now to assist with invasive cardiac procedures that are pertinent to patients with heart failure.
Periprocedural Uses of Cardiac CT
Beyond the initial heart failure workup, many of these patients will require interventional therapies like valve replacement/repair, left atrial appendage occlusion, and electrophysiology procedures, among others. Echocardiography, including transthoracic, intra-cardiac, and transesophageal approaches, remains a staple of periprocedural diagnosis and planning. Now, cardiac CT has also become integral in the pre- and peri-interventional time periods. It allows for sophisticated, highly detailed three-dimensional mapping of valves, coronary arteries, and the cardiac venous system [53, 54•, 55]. These images have given cardiac interventionalists more options in the way they precisely plan their approach and monitor patients post-procedurally.
Regarding mitral valve interventions, echocardiography remains the reference standard, but cardiac CT has comparable ability to discern mitral valve geometry [56]. Natarajan et al. further describe the granular anatomical information made available via cardiac CT such as “mitral annular shape, dimensions, and angiographic coordinates, presence and extent of annular calcification, coronary sinus anatomy and spatial relationship, leaflet anatomy, thickening, calcification, tenting height, tethering angles compared to annulus plane, and papillary muscle structure” [53]. These data have been used in transcatheter mitral valve replacement to assist in device selection [53]. Another application revolves around left ventricular outflow obstruction (LVOT) status post transcatheter mitral valve replacement, which is associated with higher procedural mortality [57]. Yoon et al. found that cardiac CT can successfully predict post transcatheter mitral valve replacement LVOT, which may help identify patients at high risk of LVOT obstruction prior to procedure, and therefore influence patient eligibility [57]. Patients with heart failure have relatively high rates of atrial fibrillation, and left atrial appendage occlusion is a relevant procedure in this population [58]. Transesophageal echocardiography is the gold standard for imaging in this setting [59]. A recent expert recommendation states that cardiac CT offers improved imaging and three-dimensional reconstruction modeling when compared to transesophageal echocardiography for pre-procedural planning of left atrial appendage occlusion [59]. Prosper et al. (2020) in a review article stated coronary CTA “…can serve as a 3D patient avatar, providing detailed characterization of the left atrial appendage and serving as a useful adjunct for pre-procedural decision-making, intraprocedural planning, and follow-up, given the complex morphology and variations of the left atrial appendage and myriad procedural options” [54•]. Pre-procedurally, transesophageal echocardiogram is typically used to rule out left atrial appendage thrombus. Cardiac CT has similar diagnostic performance to transesophageal echocardiography for ruling out left atrial appendage thrombus, with sensitivity and specificity of 100% and 99%, respectively (Fig. 4) [60]. Another benefit of pre-procedure cardiac CT includes improved device selection, which then translates to decreased procedure time [61]. Post-procedurally, cardiac CT is a suitable alternative to transesophageal echocardiogram for device surveillance status post left atrial appendage occlusion [62].
Aortic valvular disease is another common pathologic entity for this population. A consensus document from the SCCT reports that cardiac CT should be a fully integrated component of any TAVR program [63]. Part of this consensus is that cardiac CT is now the gold standard tool for aortic annular sizing, determining the risk of annular injury and coronary occlusion, and providing coplanar fluoroscopic angle prediction [63]. Additionally, cardiac CT may be useful in the subset of patients that have a “low-flow” gradient aortic stenosis with severely reduced aortic valve area. In those situations, the decision to intervene on the aortic valve or not can be perplexing. The aortic valve calcification seen on cardiac CT carries important prognostic implications for survival even beyond information gleaned from Doppler echocardiography, including prediction of eventual aortic valve replacement as well as mortality [6, 64, 65]. These data can be used as a decision aid to determine whether to proceed with aortic valve intervention (Fig. 5). Status post-TAVR, cardiac CT can assess for thrombosis of the replaced aortic valve [66, 67]. This complication has historically been diagnosed via transesophageal echocardiography which has drawbacks related to prosthetic shadowing, invasive risks, and discomfort [68]. Cardiac CT is an established and integral part of TAVR programs for determining complex anatomy, calcification load, and valvular function status post-TAVR.
In comparison to mitral and aortic valvular diseases, CT imaging of the tricuspid valve is a relatively less evolved area of interest [69]. Both the ESC and the AHA guidelines agree that transthoracic echocardiogram is the preferred imaging modality for tricuspid regurgitation, but the AHA does acknowledge that cardiac CT “... may provide more accurate information on the status of the right ventricle” [70, 71]. Some of the strengths of CT imaging include the capacity to measure tricuspid annulus dimensions, distance from the tricuspid annulus to the right ventricular apex, right ventricular size, and distance from right coronary artery to annulus [72]. Further work on imaging of tricuspid regurgitation with cardiac CT showed that moderate to severe tricuspid regurgitation is more likely to have an unfavorable distance from right coronary artery to the annulus which may confer higher risk of injury during transcatheter interventions [73]. Cardiac MRI shares the same capabilities as cardiac CT in this context, and thus, cardiac CT can be thought of as an option in scenarios where access, cost, or safety of MRI is prohibitive.
In cardiac electrophysiology, cardiac CT has been used to image myocardial scar, evaluate for mechanical dyssynchrony, and assess coronary venous anatomy [74]. These abilities, in conjunction with MRI and echocardiography, can assist in the positioning of electrical leads for cardiac resynchronization therapy [75]. One study showed that when used concomitantly with single-photon emission computed tomography and echocardiography, there was improved response to left ventricular lead placement as compared to late electrical activation protocols [76]. Further, cardiac CT can also be used to detect lead position post-procedurally, and assess for possible lead perforation [77].
Future Roles of Cardiac CT
Hardware improvements are in progress. More advanced CT scanners will likely improve diagnostic accuracy. Specifically, dual energy (high and low kilovoltage peaks) may better assess structures, plaque, infiltrative diseases, and better deal with calcification. Higher numbers of detector arrays should allow for better assessment of perfusion imaging. Better detectors may allow for more accurate assessment of viability, stenosis severity, and functional significance of stenosis. With increased performance, it is likely that cardiac CT will be used more routinely in patients with heart failure to rule out CAD, assess the myocardium, and plan transcatheter procedures.
One active area of research is coronary CTA-derived fractional flow reserve. Invasive coronary angiography-derived fractional flow reserve is an established parameter for assessing coronary artery stenoses, but it carries the invasive risks of the procedure. A 2019 meta-analysis showed non-invasive coronary CTA-derived fractional flow reserve has similar sensitivity and higher specificity than invasive coronary angiography-derived fractional flow reserve, but it remains unclear how to implement its use in patients with heart failure [78].
Another proposed use of cardiac CT is the determination of the viability of the myocardium. The end goal for this branch of research is to be able to successfully predict which areas of myocardium will benefit from revascularization. This concept has gained traction with cardiac MRI [79]. Given similar properties between gadolinium and iodine-based contrast, cardiac CT may have the ability to differentiate myocardial scar and viable tissue [80].
A second possible application of myocardial scar imaging involves planning for radiofrequency catheter ablation in the treatment of ventricular tachycardia. To date, imaging prior to radiofrequency catheter ablation has focused on assessing areas of myocardial fibrosis, which likely have a role in ventricular arrhythmias [81]. Cardiac MRI with late gadolinium enhancement has dominated research in this area, and pre-ablation cardiac MRI has been associated with better outcomes [37, 82]. Unfortunately, these patients may not be stable enough to obtain spend time in the MRI machine (although MRI acquisition times are decreasing and real-time, “free-breathing” MRI is beginning to show promising results) [50, 83]. Cardiac CT is a faster, more accessible, ICD-friendly approach to RFCA planning while comparably evaluating myocardial scar [37, 40]. While studies incorporating cardiac CT in radiofrequency catheter ablation are published, this practice is not yet widely adopted [84].
Finally, cardiac CT may develop a more active role in guiding procedures in the catheterization laboratory. A contemporary state-of-the-art article provides an in-depth description of how operators may use coronary CTA in real time with technologies that are readily available [85]. Three-dimensional images from CT can be synchronized with two-dimensional fluoroscopic images in the catheterization laboratory. In this way, proceduralists will have three-dimensional images to guide their procedures.
Limitations of Cardiac CT
There are important limitations to using cardiac CT. Radiation exposure from these imaging protocols, although it has decreased in the last 10 years, is non-negligible. Likewise, coronary CTA is limited by renal function given the need for iodine-based contrast use, which many physicians are reluctant to use in patients at high risk for kidney injury. Unfortunately, contrast-induced nephropathy is a frequent concern, as renal disease is frequently a comorbid condition in this patient population. Next, coronary CTA does have diminished sensitivity in patients with heart rate > 65 or in an irregular rhythm. For best results, nitroglycerin and beta-blockers are regularly used, which may be contraindicated in some patients.
Conclusion
Cardiac CT is a robust, multi-faceted tool for patients with heart failure. It has strong performance for ruling out CAD, assessing functional significance of CAD, and rendering detailed three-dimensional imaging for assistance in peri-interventional planning.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association. Circulation. 2020;141.https://doi.org/10.1161/CIR.0000000000000757.
Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–76. https://doi.org/10.1016/j.ijcard.2013.12.028.
Shemesh J, Tenenbaum A, Fisman EZ, et al. Coronary calcium as a reliable tool for differentiating ischemic from nonischemic cardiomyopathy. Am J Cardiol. 1996;77:191–4. https://doi.org/10.1016/S0002-9149(96)90596-2.
Budoff MJ, Shavelle DM, Lamont DH, et al. Usefulness of electron beam computed tomography scanning for distinguishing ischemic from nonischemic cardiomyopathy. J Am Coll Cardiol. 1998;32:1173–8. https://doi.org/10.1016/S0735-1097(98)00387-8.
Peng AW, Mirbolouk M, Orimoloye OA, et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ≥1,000: Results From the CAC Consortium. JACC Cardiovasc Imaging. 2020;13:83–93. https://doi.org/10.1016/j.jcmg.2019.02.005.
Eberhard M, Hinzpeter R, Schönenberger ALN, et al. Incremental Prognostic Value of Coronary Artery Calcium Score for Predicting All-Cause Mortality after Transcatheter Aortic Valve Replacement. Radiology. 2021;301:105–12. https://doi.org/10.1148/radiol.2021204623.
Budoff MJ, Jacob B, Rasouli ML, et al. Comparison of Electron Beam Computed Tomography and Technetium Stress Testing in Differentiating Cause of Dilated Versus Ischemic Cardiomyopathy. J Comput Assist Tomogr. 2005;29:699–703. https://doi.org/10.1097/01.rct.0000175503.87578.0d.
Le T, Ko JY, Tina Kim H, et al. Comparison of echocardiography and electron beam tomography in differentiating the etiology of heart failure. Clin Cardiol. 2000;23:417–20. https://doi.org/10.1002/clc.4960230608.
Patel AA, Fine J, Naghavi M, Budoff MJ. Radiation exposure and coronary artery calcium scans in the society for heart attack prevention and eradication cohort. Int J Cardiovasc Imaging. 2019;35:179–83. https://doi.org/10.1007/s10554-018-1431-0.
Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. Circulation. 2012;126:e354–471. https://doi.org/10.1161/CIR.0b013e318277d6a0.
Stolzmann P, Goetti R, Baumueller S, et al. Prospective and retrospective ECG-gating for CT coronary angiography perform similarly accurate at low heart rates. Eur J Radiol. 2011;7.
Pontone G. Diagnostic Accuracy of Coronary Computed Tomography Angiography. 2009;54:10.
Maruyama T, Takada M, Hasuike T, et al. Radiation Dose Reduction and Coronary Assessability of Prospective Electrocardiogram-Gated Computed Tomography Coronary Angiography. J Am Coll Cardiol. 2008;52:1450–5. https://doi.org/10.1016/j.jacc.2008.07.048.
Sun M, Lu B, Wu R, et al. Diagnostic accuracy of dual-source CT coronary angiography with prospective ECG-triggering on different heart rate patients. Eur Radiol. 2011;21:1635–42. https://doi.org/10.1007/s00330-011-2107-5.
Cornily J-C, Gilard M, Gal GL, et al. Accuracy of 16-detector Multislice Spiral Computed Tomography in the initial evaluation of dilated cardiomyopathy. Eur J Radiol. 2007;61:84–90. https://doi.org/10.1016/j.ejrad.2006.08.010.
Andreini D, Pontone G, Pepi M, et al. Diagnostic Accuracy of Multidetector Computed Tomography Coronary Angiography in Patients With Dilated Cardiomyopathy. J Am Coll Cardiol. 2007;49:2044–50. https://doi.org/10.1016/j.jacc.2007.01.086.
Andreini D, Pontone G, Bartorelli AL, et al. Sixty-Four–Slice Multidetector Computed Tomography: An Accurate Imaging Modality for the Evaluation of Coronary Arteries in Dilated Cardiomyopathy of Unknown Etiology. Circ Cardiovasc Imaging. 2009;2:199–205. https://doi.org/10.1161/CIRCIMAGING.108.822809.
Ghostine S, Caussin C, Habis M, et al. Non-invasive diagnosis of ischaemic heart failure using 64-slice computed tomography. Eur Heart J. 2008;29:2133–40. https://doi.org/10.1093/eurheartj/ehn072.
Le Polain de Waroux J-B, Pouleur A-C, Goffinet C, et al. Combined coronary and late-enhanced multidetector-computed tomography for delineation of the etiology of left ventricular dysfunction: comparison with coronary angiography and contrast-enhanced cardiac magnetic resonance imaging. Eur Heart J. 2008;29:2544–51. https://doi.org/10.1093/eurheartj/ehn381.
Boulmier D, Audinet C, Heautot J-F, et al. Clinical contributions of 64-slice computed tomography in the evaluation of cardiomyopathy of unknown origin. Arch Cardiovasc Dis. 2009;102:685–96. https://doi.org/10.1016/j.acvd.2009.06.004.
Lee D, Li D, Jug B, et al. Diagnostic accuracy of 64 slice multidetector coronary computed tomographic angiography in left ventricular systolic dysfunction. IJC Heart Vasc. 2015. https://doi.org/10.1016/j.ijcha.2015.04.007
Srichai MB. Dual source computed tomography coronary angiography in new onset cardiomyopathy. World J Radiol. 2012;4:258. https://doi.org/10.4329/wjr.v4.i6.258.
Oncel D, Oncel G, Tastan A. Effectiveness of Dual-Source CT Coronary Angiography for the Evaluation of Coronary Artery Disease in Patients with Atrial Fibrillation: Initial Experience1. Radiology. 2007. https://doi.org/10.1148/radiol.2453070094.
Rist C, Johnson TR, Müller-Starck J, et al. Noninvasive Coronary Angiography Using Dual-Source Computed Tomography in Patients With Atrial Fibrillation. Invest Radiol. 2009;44:159–67. https://doi.org/10.1097/RLI.0b013e3181948b05.
Marwan M, Pflederer T, Schepis T, et al. Accuracy of dual-source computed tomography to identify significant coronary artery disease in patients with atrial fibrillation: comparison with coronary angiography. Eur Heart J. 2010;31:2230–7. https://doi.org/10.1093/eurheartj/ehq223.
Zhang J-J, Liu T, Feng Y, et al. Diagnostic Value of 64-Slice Dual-Source CT Coronary Angiography in Patients with Atrial Fibrillation: Comparison with Invasive Coronary Angiography. Korean J Radiol. 2011;8.
Abdelkarim MJ, Ahmadi N, Gopal A, et al. Noninvasive quantitative evaluation of coronary artery stent patency using 64-row multidetector computed tomography. J Cardiovasc Comput Tomogr. 2010;4:29–37. https://doi.org/10.1016/j.jcct.2009.10.014.
Jug B, Papazian J, Gupta M, et al. Diagnostic performance of computed tomographic coronary angiography in patients with end-stage renal disease. Coron Artery Dis. 2013;24:135–41. https://doi.org/10.1097/MCA.0b013e32835be39a.
Menke J, Unterberg-Buchwald C, Staab W, et al. Head-to-head comparison of prospectively triggered vs retrospectively gated coronary computed tomography angiography: Meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am Heart J. 2013;165:154-163.e3. https://doi.org/10.1016/j.ahj.2012.10.026.
Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021:CIR0000000000001030. https://doi.org/10.1161/CIR.0000000000001030.
• McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368. 2021 AHA guidelines on chest pain give a class 1A recommendation to both coronary CTA in intermediate-risk patients with acute chest pain and no known CAD, after a negative or inconclusive evaluation for acute coronary syndrome. This is a large shift from the prior 2012 class IIb recommendation, which represents the influx of new data surrounding coronary CTA in the last 10 years.
• Narula J, Chandrashekhar Y, Ahmadi A, et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2021;15:192–217. https://doi.org/10.1016/j.jcct.2020.11.001. 2021 ESC guidelines on heart failure call coronary CTA a “key element” in the workup of patients with heart failure of unknown etiology. This strong, affirmative language from a respected multi-national organization legitimizes the utility of coronary CTA in this patient population.
Hamirani YS, Isma’eel H, Larijani V, et al. The Diagnostic Accuracy of 64-Detector Cardiac Computed Tomography Compared With Stress Nuclear Imaging in Patients Undergoing Invasive Cardiac Catheterization. J Comput Assist Tomogr. 2010;34:645–51. https://doi.org/10.1097/RCT.0b013e3181e3d0b1.
Budoff MJ, Rasouli ML, Shavelle DM, et al. Cardiac CT Angiography (CTA) and Nuclear Myocardial Perfusion Imaging (MPI)—A Comparison in Detecting Significant Coronary Artery Disease. Acad Radiol. 2007;14:252–7. https://doi.org/10.1016/j.acra.2006.11.006.
Budoff MJ, Li D, Kazerooni EA, et al. Diagnostic Accuracy of Noninvasive 64-row Computed Tomographic Coronary Angiography (CCTA) Compared with Myocardial Perfusion Imaging (MPI). Acad Radiol. 2017;24:22–9. https://doi.org/10.1016/j.acra.2016.09.008.
Danad I, Raijmakers PG, Driessen RS, et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017;2:1100. https://doi.org/10.1001/jamacardio.2017.2471.
Ohta Y, Kitao S, Yunaga H, et al. Myocardial Delayed Enhancement CT for the Evaluation of Heart Failure: Comparison to MRI. Radiology. 2018;288:682–91. https://doi.org/10.1148/radiol.2018172523.
Scully PR, Bastarrika G, Moon JC, Treibel TA. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr Cardiol Rep. 2018;20:15. https://doi.org/10.1007/s11886-018-0961-3.
Bandula S, White SK, Flett AS, et al. Measurement of Myocardial Extracellular Volume Fraction by Using Equilibrium Contrast-enhanced CT: Validation against Histologic Findings. Radiology. 2013;269:396–403. https://doi.org/10.1148/radiol.13130130.
Chang S, Han K, Youn J-C, et al. Utility of Dual-Energy CT-based Monochromatic Imaging in the Assessment of Myocardial Delayed Enhancement in Patients with Cardiomyopathy. Radiology. 2018;287:442–51. https://doi.org/10.1148/radiol.2017162945.
Wichmann JL, Bauer RW, Doss M, et al. Diagnostic accuracy of late iodine-enhancement dual-energy computed tomography for the detection of chronic myocardial infarction compared with late gadolinium-enhancement 3-T magnetic resonance imaging. Invest Radiol. 2013;48:851–6. https://doi.org/10.1097/RLI.0b013e31829d91a8.
Zhao L, Ma X, Delano MC, et al. Assessment of myocardial fibrosis and coronary arteries in hypertrophic cardiomyopathy using combined arterial and delayed enhanced CT: comparison with MR and coronary angiography. Eur Radiol. 2013;23:1034–43. https://doi.org/10.1007/s00330-012-2674-0.
Aikawa T, Oyama-Manabe N, Naya M, et al. Delayed contrast-enhanced computed tomography in patients with known or suspected cardiac sarcoidosis: A feasibility study. Eur Radiol. 2017;27:4054–63. https://doi.org/10.1007/s00330-017-4824-x.
Deux J-F, Mihalache C-I, Legou F, et al. Noninvasive detection of cardiac amyloidosis using delayed enhanced MDCT: a pilot study. Eur Radiol. 2015;25:2291–7. https://doi.org/10.1007/s00330-015-3642-2.
Bing R, Cavalcante JL, Everett RJ, et al. Imaging and Impact of Myocardial Fibrosis in Aortic Stenosis. JACC Cardiovasc Imaging. 2019;12:283–96. https://doi.org/10.1016/j.jcmg.2018.11.026.
Kittleson MM, Maurer MS, Ambardekar AV, et al. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e7–22. https://doi.org/10.1161/CIR.0000000000000792.
Garcia-Pavia P, Rapezzi C, Adler Y, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2021;42:1554–68. https://doi.org/10.1093/eurheartj/ehab072.
Mandoli GE, D’Ascenzi F, Vinco G, et al. Novel Approaches in Cardiac Imaging for Non-invasive Assessment of Left Heart Myocardial Fibrosis. Front Cardiovasc Med. 2021;8:614235. https://doi.org/10.3389/fcvm.2021.614235.
Liguori C, Farina D, Vaccher F, et al. Myocarditis: imaging up to date. Radiol Med (Torino). 2020;125:1124–34. https://doi.org/10.1007/s11547-020-01279-8.
Attili AK, Schuster A, Nagel E, et al. Quantification in cardiac MRI: advances in image acquisition and processing. Int J Cardiovasc Imaging. 2010;26 Suppl 1:27–40. https://doi.org/10.1007/s10554-009-9571-x.
Li Y, Yu M, Dai X, et al. Detection of Hemodynamically Significant Coronary Stenosis: CT Myocardial Perfusion versus Machine Learning CT Fractional Flow Reserve. Radiology. 2019;293:305–14. https://doi.org/10.1148/radiol.2019190098.
Takx Richard AP, Blomberg BA, El AH, et al. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography With Fractional Flow Reserve Meta-Analysis. Circ Cardiovasc Imaging. 2015;8:e002666. https://doi.org/10.1161/CIRCIMAGING.114.002666.
Natarajan N, Patel P, Bartel T, et al. Peri-procedural imaging for transcatheter mitral valve replacement. Cardiovasc Diagn Ther. 2016;6:144–59. https://doi.org/10.21037/cdt.2016.02.04.
• Prosper A, Shinbane J, Maliglig A, et al. Left Atrial Appendage Mechanical Exclusion: Procedural Planning Using Cardiovascular Computed Tomographic Angiography. J Thorac Imaging. 2020;35:W107–18. https://doi.org/10.1097/RTI.0000000000000504. SCCT consensus document on the TAVR solidifies the role of coronary CTA in TAVR programs.
Van de Veire NR, Marsan NA, Schuijf JD, et al. Noninvasive Imaging of Cardiac Venous Anatomy With 64-Slice Multi-Slice Computed Tomography and Noninvasive Assessment of Left Ventricular Dyssynchrony by 3-Dimensional Tissue Synchronization Imaging in Patients With Heart Failure Scheduled for Cardiac Resynchronization Therapy. Am J Cardiol. 2008;101:1023–9. https://doi.org/10.1016/j.amjcard.2007.11.052.
Shanks M, Delgado V, Ng ACT, et al. Mitral valve morphology assessment: three-dimensional transesophageal echocardiography versus computed tomography. Ann Thorac Surg. 2010;90:1922–9. https://doi.org/10.1016/j.athoracsur.2010.06.116.
Yoon S-H, Bleiziffer S, Latib A, et al. Predictors of Left Ventricular Outflow Tract Obstruction After Transcatheter Mitral Valve Replacement. JACC Cardiovasc Interv. 2019;12:182–93. https://doi.org/10.1016/j.jcin.2018.12.001.
Batul SA, Gopinathannair R. Atrial Fibrillation in Heart Failure: a Therapeutic Challenge of Our Times. Korean Circ J. 2017;47:644–62. https://doi.org/10.4070/kcj.2017.0040.
Korsholm K, Berti S, Iriart X, et al. Expert Recommendations on Cardiac Computed Tomography for Planning Transcatheter Left Atrial Appendage Occlusion. JACC Cardiovasc Interv. 2020;13:277–92. https://doi.org/10.1016/j.jcin.2019.08.054.
Romero J, Husain SA, Kelesidis I, et al. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging. 2013;6:185–94. https://doi.org/10.1161/CIRCIMAGING.112.000153.
Eng MH, Wang DD, Greenbaum AB, et al. Prospective, randomized comparison of 3-dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter Cardiovasc Interv. 2018;92:401–7. https://doi.org/10.1002/ccd.27514.
Qamar SR, Jalal S, Nicolaou S, et al. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention. 2019;15:663–70. https://doi.org/10.4244/EIJ-D-18-01107.
Blanke P, Weir-McCall JR, Achenbach S, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2019;13:1–20. https://doi.org/10.1016/j.jcct.2018.11.008.
Clavel M-A, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202–13. https://doi.org/10.1016/j.jacc.2014.05.066.
Pawade T, Clavel M-A, Tribouilloy C, et al. Computed Tomography Aortic Valve Calcium Scoring in Patients With Aortic Stenosis. Circ Cardiovasc Imaging. 2018;11:e007146. https://doi.org/10.1161/CIRCIMAGING.117.007146.
Leetmaa T, Hansson NC, Leipsic J, et al. Early aortic transcatheter heart valve thrombosis: diagnostic value of contrast-enhanced multidetector computed tomography. Circ Cardiovasc Interv. 2015;8:e001596. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001596.
Pache G, Schoechlin S, Blanke P, et al. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J. 2016;37:2263–71. https://doi.org/10.1093/eurheartj/ehv526.
Blanke P, Schoepf UJ, Leipsic JA. CT in transcatheter aortic valve replacement. Radiology. 2013;269:650–69. https://doi.org/10.1148/radiol.13120696.
Godoy Cardiac Computed Tomography (CT) Evaluation of Valvular Heart Disease in Transcatheter Interventions - PubMed. https://library.harbor-ucla.org:3285/31768770/. Accessed 26 Dec 2020.
Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease. J Am Coll Cardiol. 2021;77:e25–197. https://doi.org/10.1016/j.jacc.2020.11.018.
Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. https://doi.org/10.1093/eurheartj/ehx391.
Naoum C, Blanke P, Cavalcante JL, Leipsic J. Cardiac Computed Tomography and Magnetic Resonance Imaging in the Evaluation of Mitral and Tricuspid Valve Disease. Circ Cardiovasc Imaging. 2017;10:e005331. https://doi.org/10.1161/CIRCIMAGING.116.005331.
van Rosendael PJ, Kamperidis V, Kong WKF, et al. Computed tomography for planning transcatheter tricuspid valve therapy. Eur Heart J. 2017;38:665–74. https://doi.org/10.1093/eurheartj/ehw499.
Liddy S, Buckley U, Kok HK, et al. Applications of cardiac computed tomography in electrophysiology intervention. Eur Heart J Cardiovasc Imaging. 2018;19:253–61. https://doi.org/10.1093/ehjci/jex312.
Nguyên UC, Cluitmans MJM, Strik M, et al. Integration of cardiac magnetic resonance imaging, electrocardiographic imaging, and coronary venous computed tomography angiography for guidance of left ventricular lead positioning. EP Eur. 2019;21:626–35. https://doi.org/10.1093/europace/euy292.
Sommer A, Kronborg MB, Nørgaard BL, et al. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial: Imaging-guided LV lead placement in CRT. Eur J Heart Fail. 2016;18:1365–74. https://doi.org/10.1002/ejhf.530.
Pang BJ, Lui EH, Joshi SB, et al. Pacing and Implantable Cardioverter Defibrillator Lead Perforation As Assessed by Multiplanar Reformatted ECG-Gated Cardiac Computed Tomography and Clinical Correlates: PACEMAKER AND ICD LEAD PERFORATION BY CT. Pacing Clin Electrophysiol. 2014;37:537–45. https://doi.org/10.1111/pace.12307.
Zhuang B, Wang S, Zhao S, Lu M. Computed tomography angiography-derived fractional flow reserve (CT-FFR) for the detection of myocardial ischemia with invasive fractional flow reserve as reference: systematic review and meta-analysis. Eur Radiol. 2020;30:712–25. https://doi.org/10.1007/s00330-019-06470-8.
Arrighi JA, Dilsizian V. Multimodality Imaging for Assessment of Myocardial Viability: Nuclear, Echocardiography, MR, and CT. Curr Cardiol Rep. 2012;14:234–43. https://doi.org/10.1007/s11886-011-0242-x.
Jacquier A, Revel D, Croisille P, et al. Mécanismes du rehaussement tardif myocardique et apport des produits de contraste en IRM et scanner dans le diagnostic de viabilité myocardique11Ce travail a été rendu possible grâce à la Bourse de la Société Française de Radiologie. J Radiol. 2010;91:751–7. https://doi.org/10.1016/S0221-0363(10)70112-8.
Disertori M, Rigoni M, Pace N, et al. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1046–55. https://doi.org/10.1016/j.jcmg.2016.01.033.
Siontis KC, Kim HM, Sharaf Dabbagh G, et al. Association of preprocedural cardiac magnetic resonance imaging with outcomes of ventricular tachycardia ablation in patients with idiopathic dilated cardiomyopathy. Heart Rhythm. 2017;14:1487–93. https://doi.org/10.1016/j.hrthm.2017.06.003.
Li YY, Rashid S, Craft J, et al. Temporospatial characterization of ventricular wall motion with real-time cardiac magnetic resonance imaging in health and disease. Sci Rep. 2022;12:4070. https://doi.org/10.1038/s41598-022-08094-3.
Conte E, Mushtaq S, Carbucicchio C, et al. State of the art paper: Cardiovascular CT for planning ventricular tachycardia ablation procedures. J Cardiovasc Comput Tomogr. 2021;15:394–402. https://doi.org/10.1016/j.jcct.2021.01.002.
Collet C, Sonck J, Leipsic J, et al. Implementing Coronary Computed Tomography Angiography in the Catheterization Laboratory. JACC Cardiovasc Imaging. 2021;14:1846–55. https://doi.org/10.1016/j.jcmg.2020.07.048.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging in Heart Failure
Rights and permissions
About this article
Cite this article
Kitchin, S.S., Manubolu, V.S., Roy, S.K. et al. The Role of Cardiac Computed Tomography in Heart Failure. Curr Heart Fail Rep 19, 213–222 (2022). https://doi.org/10.1007/s11897-022-00553-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-022-00553-2